Abstract
Purpose: Radiation treatment patterns in patients with brain metastases from non-small cell lung cancer (NSCLC) have not been well elucidated. The National Cancer Database (NCDB) was used to evaluate trends in the use of whole brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) for brain metastasis from NSCLC.
Methods: This NCDB study included patients > 18 years old with metastatic NSCLC treated with single-fraction SRS or WBRT between 2004 and 2014. Chi-square, t-test, and multivariable logistic regression analyses were used to identify predictors of SRS versus WBRT.
Results: Of 40,803 patients, 34,183 (83.8%) received WBRT and 6,620 (16.2%) received SRS. SRS utilization increased from 7% (157 cases) in 2004 to 37% (1,346 cases) in 2014 (p < .001). SRS was utilized more by academic than community facilities (22% versus 13%, p < .001). The strongest independent predictors of SRS included year of diagnosis in 2010-2014 versus 2004-2009 (odds ratio [OR] 2.62, 95% CI 2.46-2.79, p < .0001), metropolitan versus rural (OR 2.26, CI 1.79-2.85, p < .0001), distance from cancer-reporting facility of ≥ 30 versus < 30 miles (OR 2.36, CI 2.18-2.56, p < .0001), private insurance versus non-insured patients (OR 1.96, CI 1.68-2.29, p < .0001), and academic versus community facility (OR 1.76, CI 1.66-1.87, p < .0001).
Conclusion: SRS for NSCLC brain metastases has steadily increased in the United States; however, WBRT remains the most commonly used. Wide geographic and socioeconomic variations exist in the utilization of SRS and WBRT for this patient population.
Keywords: brain metastasis, radiation, utilization, radiosurgery
Introduction
Brain metastases are the most common type of intracranial tumor and affect up to 40% of all patients with cancer. More than half of these metastases come from lung cancer histologies [1-2]. Survival from lung cancer continues to improve, which could be explained by improvements in systemic therapies including chemotherapy, targeted agents and immunotherapies [3]. Despite these advancements, the primary modalities of treatment for brain metastasis remain surgery and radiation, with a large portion of these patients receiving whole brain radiation therapy (WBRT) [4].
The value of WBRT in the treatment of brain metastasis from non-small cell lung cancer (NSCLC) has recently been questioned. The Quality of Life after Treatment for Brain Metastases trial randomized patients with brain metastasis from NSCLC who were unsuitable for surgery or stereotactic radiosurgery (SRS) to WBRT or best supportive care. With no difference found in overall survival and a small difference in the quality of life, the authors concluded WBRT provided little additional clinical benefit over supportive care [5]. WBRT also has been found to have more detrimental side effects than more focally delivered radiation, such as SRS. A recent Alliance trial randomized patients with one to three brain metastases (mostly from NSCLC) to SRS with or without WBRT. The group of patients treated with SRS alone had fewer cognitive deficits but at the expense of intracranial control; both arms had similar overall survival [6].
Prior randomized evidence, such as the two Patchell et al. studies, give credibility to treat these patients with WBRT, which is technically easy to plan and deliver safely to almost all patients [7-8]. While Gamma Knife (Elekta Instrument AB, Stockholm, Sweden) radiosurgery was the first platform to deliver SRS starting in 1951, SRS became widely available after refinement of linear accelerator (LINAC)-based SRS systems [9]. SRS, however, requires extensive therapy and physics staff support to deliver successfully and safely. It remains unknown if these technical challenges limit access to SRS; radiation treatment patterns in these patients are not well understood. We used the National Cancer Database (NCDB) to evaluate trends in the use of WBRT and SRS in the treatment of brain metastasis from NSCLC and factors that may reveal limitations to SRS accessibility.
Materials and methods
The NCDB is a nationwide joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, serving as a powerful surveillance and quality improvement mechanism for participating cancer programs. This clinical oncology outcomes database is sourced from hospital registry data that are collected in more than 1500 Commission on Cancer accredited facilities, presenting nearly 70% of all new invasive cancer diagnoses in the United States each year [10]. The data used in the study were derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigators. This dataset was used in our analysis and was exempt from institutional review board authorization.
The NCDB was used to identify patients > 18 years old with metastatic NSCLC who were treated with single-fraction SRS to the brain or WBRT between 2004 and 2014. Patients receiving brain radiation dose ranges of 12-24 Gy in one fraction were classified as having received SRS, and those who received 30 Gy in 10 fractions, 20 Gy in 5 fractions, or 37.5 Gy in 15 fractions were classified as having received WBRT. Patients who did not receive radiotherapy to the brain or did not receive treatment dose within these ranges were excluded.
Univariate comparisons were conducted to compare demographic, clinicopathologic, and health care system factors between those receiving SRS or WBRT. This was done using independent two-group t-tests and chi-square tests as appropriate. Multivariable logistic regression was performed to identify possible independent predictors of receiving SRS or WBRT. Multivariable models were selected by first including any predictor with a univariate p-value < .2 and then employing stepwise selection, with a p-value cutoff of .05 used to remain in the model. Cochran-Armitage tests were used to describe the trends in radiation use by decade of diagnosis. Data from the NCDB were filtered and all data analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Of 40,803 patients identified, 34,183 (83.8%) received WBRT and 6,620 (16.2%) received SRS. Patients were most likely to be white (83%), male (52%), and aged 55-64 years (39%). The most commonly employed WBRT doses were 30 Gy in 10 fractions (24,479 cases [60%]) and 37.5 Gy in 15 fractions (9,127 cases [22%]). The common single-fraction SRS dose range included 17-21 Gy (4,022 cases [10%]). Most patients were treated at community centers (25,465 cases [63%]), which include community cancer programs, comprehensive community cancer programs, or an integrated network cancer program, as defined by the Commission on Cancer of the American College of Surgeons. A large portion (82%) of these patients were treated in a metropolitan setting (as defined by the United States Department of Agriculture Economic Research Service).
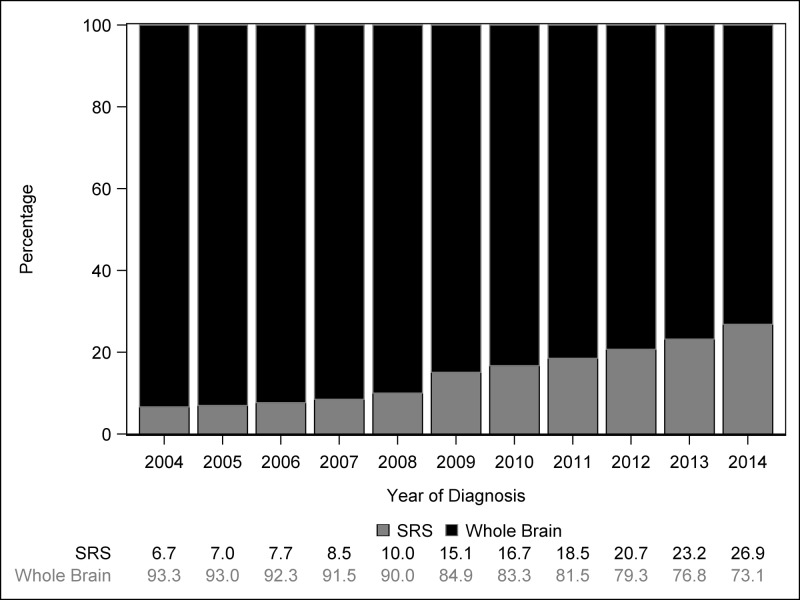
Complete patient characteristics are detailed in Table 1, which describes patient demographic, clinicopathologic, and health care system factors associated with the use of WBRT or SRS. The total number of cases treated with WBRT increased from 2,198 in 2004 to 3,662 in 2014; SRS cases increased from 157 to 1,346 over the same time period. The proportion of patients receiving SRS increased from 7% (157 cases) in 2004 to 37% (1,346 cases) in 2014 (p < .001) (Figure 1). The proportion of patients undergoing SRS delivered by LINAC versus Gamma Knife increased from 13% in 2004 to 29% in 2014 (p < .001). SRS was utilized more by academic than community facilities (22% versus 13%, p < .001).
Table 1. Patient demographics, clinicopathologic and health care system factors associated with WBRT or SRS.
WBRT: whole brain radiation therapy; SRS: stereotactic surgery.
| Variable | Response | WBRT (N=34183) | SRS (N=6620) | P |
| Age (years) | < 55 | 7298 (85%) | 1306 (15%) | .0180 |
| 55-64 | 11242 (84%) | 2110 (16%) | ||
| 65-74 | 10128 (83%) | 2018 (17%) | ||
| Sex | Male | 18050 (85%) | 3243 (15%) | <0.0001 |
| Female | 16133 (83%) | 3377 (17%) | ||
| Race | White | 28171 (83%) | 5572 (17%) | .0004 |
| Black/Other | 5740 (85%) | 986 (15%) | ||
| Unknown | 272 (81%) | 62 (19%) | ||
| Median income quartiles | 7165 (87%) | 1080 (13%) | <0.0001 | |
| $38,000-$47,999 | 8469 (85%) | 1501 (15%) | ||
| $48,000-$62,999 | 8932 (84%) | 1718 (16%) | ||
| $63,000 + | 8818 (80%) | 2228 (20%) | ||
| Education quartiles | ≥21% | 6320 (87%) | 945 (13%) | <0.0001 |
| 13-20% | 9579 (85%) | 1681 (15%) | ||
| 7.0-12.9% | 11040 (83%) | 2284 (17%) | ||
| <7% | 6468 (80%) | 1619 (20%) | ||
| Urban | Metro | 26779 (83%) | 5422 (17%) | <0.0001 |
| Urban | 5248 (86%) | 859 (14%) | ||
| Rural | 790 (89%) | 95 (11%) | ||
| Histology | Large cell | 1278 (86%) | 204 (14%) | <0.0001 |
| Squamous cell | 3813 (83%) | 796 (17%) | ||
| Adenocarcinoma | 19470 (83%) | 4090 (17%) | ||
| Other | 9622 (86%) | 1530 (14%) | ||
| Charlson score | 0 | 22908 (83%) | 4610 (17%) | <0.0001 |
| 1 | 7957 (85%) | 1450 (15%) | ||
| 2 | 3318 (86%) | 560 (14%) | ||
| Facility type | Academic | 11606 (78%) | 3325 (22%) | <0.0001 |
| Community | 22239 (87%) | 3226 (13%) | ||
| Facility location | East | 14089 (81%) | 3224 (19%) | <0.0001 |
| Midwest | 9908 (85%) | 1716 (15%) | ||
| South | 5186 (89%) | 612 (11%) | ||
| West | 4662 (82%) | 999 (18%) | ||
| Insurance | Not insured | 2186 (91%) | 216 (9%) | <0.0001 |
| Private insurance | 12323 (83%) | 2604 (17%) | ||
| Medicaid | 3516 (87%) | 538 (13%) | ||
| Medicare | 14910 (83%) | 3046 (17%) | ||
| Other government | 618 (86%) | 103 (14%) | ||
| Unknown | 630 (85%) | 113 (15%) | ||
| Distance from reporting facility (miles) | < 10 | 17995 (87%) | 2799 (13%) | <0.0001 |
| 10-19 | 6727 (83%) | 1378 (17%) | ||
| 20-49 | 5992 (81%) | 1390 (19%) | ||
| 50+ | 2701 (74%) | 963 (26%) | ||
| Distance (miles) | < 30 | 27900 (85%) | 4844 (15%) | <0.0001 |
| 30+ | 5515 (77%) | 1686 (23%) | ||
| Year of diagnosis | 2004 | 2198 (93%) | 157 (7%) | <0.0001 |
| 2005 | 2413 (93%) | 182 (7%) | ||
| 2006 | 2695 (92%) | 225 (8%) | ||
| 2007 | 2904 (91%) | 271 (9%) | ||
| 2008 | 3003 (90%) | 334 (10%) | ||
| 2009 | 3124 (85%) | 557 (15%) | ||
| 2010 | 3294 (83%) | 662 (17%) | ||
| 2011 | 3435 (81%) | 782 (19%) | ||
| 2012 | 3671 (79%) | 960 (21%) | ||
| 2013 | 3784 (77%) | 1144 (23%) | ||
| 2014 | 3662 (73%) | 1346 (27%) | ||
| Year of diagnosis (grouped) | 2004-2009 | 16337 (90%) | 1726 (10%) | <0.0001 |
| 2010-2014 | 17846 (78%) | 4894 (22%) |
Figure 1. Distribution of SRS and WBRT use by year of diagnosis.
SRS: stereotactic surgery; WBRT: whole brain radiation therapy.
On multivariable analysis, the strongest independent predictors of SRS use included year of diagnosis in 2010-2014 versus 2004–2009 (odds ratio [OR] 2.62, 95% CI 2.46-2.79, p < .0001), metropolitan versus rural location (OR 2.26, 95% CI 1.79-2.85, p < .0001), distance from cancer-reporting facility of ≥ 30 versus < 30 miles (OR 2.36, 95% CI 2.18-2.56, p < .0001), private insurance versus non-insured patients (OR 1.96, 95% CI 1.68-2.29, p < .0001), higher median income ($63,000 vs $38,000-$47,000, OR 1.12, 95% CI 1.02-1.23, p = .0172), and academic versus community facility type (OR 1.76, 95% CI 1.66-1.87, p < .0001) (Table 2).
Table 2. Multivariable logistic regression analysis of predictors of SRS use compared to WBRT.
SRS: stereotactic surgery; WBRT: whole brain radiation therapy.
| Characteristic | Variable | OR (95% CI) | P |
| Sex | Male vs female | 0.91 (0.86, 0.96) | .0012 |
| Education quartiles | ≥21% vs <7% | 0.75 (0.67, 0.85) | <0.0001 |
| 13-20% vs <7% | 0.83 (0.76, 0.92) | .0003 | |
| 7-12.9% vs <7% | 0.90 (0.83, 0.98) | .0123 | |
| Urban | Urban vs rural | 1.42 (1.12, 1.79) | <0.0001 |
| Metro vs rural | 2.26 (1.79, 2.85) | <0.0001 | |
| Histology | Squamous cell vs adenocarcinoma | 1.13 (1.03, 1.24) | .0074 |
| Large cell vs adenocarcinoma | 1.01 (0.86, 1.19) | .8689 | |
| Other vs adenocarcinoma | 0.93 (0.87, 1.00) | .0486 | |
| Facility | Academic vs community | 1.76 (1.66, 1.87) | <0.0001 |
| Facility location | West vs east | 1.01 (0.92, 1.09) | .9151 |
| South vs east | 0.61 (0.55, 0.67) | <0.0001 | |
| Midwest vs east | 0.75 (0.70, 0.81) | <0.0001 | |
| Insurance | Private vs not insured | 1.96 (1.68, 2.29) | <0.0001 |
| Other government vs not insured | 1.37 (1.05, 1.79) | .0223 | |
| Medicare vs not insured | 1.97 (1.69, 2.30) | <0.0001 | |
| Medicaid vs not insured | 1.36 (1.14, 1.62) | .0007 | |
| Unknown vs not insured | 1.70 (1.31, 2.20) | <0.0001 | |
| Distance (miles) | 30+ vs < 30 | 2.36 (2.18, 2.56) | <0.0001 |
| Median income (thousands) | <38 vs 38-47 | 0.93 (0.84, 1.02) | .1179 |
| 63 vs 38-47 | 1.12 (1.02, 1.23) | .0172 | |
| 48-62 vs 38-47 | 1.00 (0.92, 1.08) | .9312 | |
| Charlson score | ≥2 vs 0 | 0.88 (0.79, 0.97) | .0115 |
| 1 vs 0 | 0.95 (0.89, 1.02) | .1303 | |
| Year | 2010-2014 vs 2004-2009 | 2.62 (2.46, 2.79) | <0.0001 |
Discussion
We present a large United States hospital-registry based study analyzing the patterns of radiation delivered for patients with brain metastasis from NSCLC. This is the first study with clinical and demographic comparisons between the utilization of WBRT and SRS [11-12]. Results reveal a socioeconomic variation between the use of either modality that should be further explored, given the increasing evidence in favor of treating brain metastasis with SRS over WBRT.
A similarly designed study by Park et al. using NCDB data revealed increased utilization over time of LINAC-based SRS for the treatment of NSCLC brain metastasis [13]. Comparable to our analysis, Gamma Knife-based radiosurgery remained the most commonly used modality, especially in academic centers. The emergence of LINAC-based systems such as the Accuray Cyberknife (Accuray Inc., Sunnyvale, California), Varian Edge or TrueBeam (Varian Medical Systems, Palo Alto, California), or the BrainLAB Novalis (BrainLAB, Munich, Germany) have made SRS more accessible and cost-effective [14]. The total number of brain metastasis cases has increased overall as has the proportion of those treated with SRS. This pattern is concordant with the abundance of studies during this era revealing the efficacy and utility of SRS.
Our analysis revealed multiple socioeconomic factors that were more common in patients treated with SRS. Insured patients (private, Medicare, or Medicaid), higher median income, or those treated in an academic facility or metropolitan setting were more likely to receive SRS over WBRT. This pattern potentially highlights an economic disparity in the delivery of SRS to patients with brain metastases. Patients from a lower socioeconomic status may present with more advanced disease requiring WBRT. This hypothesis was validated by prior studies. In their analysis of over 400,000 patients with the 10 deadliest cancers (including breast, lung, colorectal, and head and neck cancers), Walker et al. found those with less insurance coverage were more likely to present with advanced disease and receive less radiation therapy [15]. Freedman et al. reported lower odds of receiving definitive locoregional therapy and adjuvant systemic treatments for uninsured women, Medicaid enrollees, and younger Medicare beneficiaries in patients with breast cancer [16]. Patients with private insurance are more likely to receive proper cancer screening, more prompt appointments, and necessary prescription medications [17], which may lead to presenting with earlier, more treatable stages of cancer.
Additional barriers to receiving SRS include limited access to facilities that are technically capable of safely delivering radiosurgery. This is underscored by the finding that those traveling > 30 miles were more likely to receive SRS over WBRT. Travel distance has been shown to affect treatment decisions for patients with breast, colon, rectal, lung, ovarian, and prostate cancers [18-19]. Other technically challenging and resource-intensive modalities, such as brachytherapy, have also been subject to socioeconomic disparities [20]. Our study supports the message of these earlier studies and is the first, to our knowledge, to compare utilization of radiation for brain metastasis.
Our analysis has limitations. The specific patient and tumor characteristics that factored into the treatment decision of SRS or WBRT are not known. These include number and volume of metastasis, performance status, or other treatments such as immunotherapy or targeted agents. It is also unclear how many cases later received salvage therapy with further SRS or WBRT. For simplicity, our analysis included only NSCLC histologies and SRS delivered in a single fraction. The data represented here also ended in 2014. It will be interesting to see if recent randomized data and association-based guidelines such as the American Society for Radiation Oncology’s Choosing Wisely campaign have changed practice accordingly [21]. Registry data have been shown to report variable rates of actual radiation delivered [22]. Despite these limitations, our intended purpose was to elucidate radiation treatment patterns in this subset of patients and to discover factors predictive of modality utilization.
Conclusions
The use of SRS for NSCLC brain metastases has steadily increased over time in the United States, especially in the academic setting, but WBRT remains the most common treatment modality. Wide geographic and socioeconomic variation exists in the utilization and accessibility of SRS and WBRT for this patient population.
Acknowledgments
We would like to thank Stephanie Stebens, MLIS, and Sarah Whitehouse, MAW, for their review and help with preparation of this manuscript.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Au K, Meng Y, Suppiah S, Nater A, Jalali R, Zadeh G. New Approaches to the Management of Primary and Secondary CNS Tumors. Rijeka, Croatia: InTech; 2017. Current management of brain metastases: overview and teaching cases; pp. 121–148. [Google Scholar]
- 2.Cancer treatment and survivorship statistics, 2016. Miller KD, Siegel RL, Lin CC, et al. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) Sperduto PW, Yang TJ, Beal K, et al. JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. Rapp SR, Case LD, Peiffer A, et al. J Clin Oncol. 2015;33:1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Mulvenna P, Nankivell M, Barton R, et al. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. Brown PD, Jaeckle K, Ballman KV, et al. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A randomized trial of surgery in the treatment of single metastases to the brain. Patchell RA, Tibbs PA, Walsh JW, et al. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 8.Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. Patchell RA, Tibbs PA, Regine WF, et al. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 9.Linac radiosurgery as a tool in neurosurgery. Deinsberger R, Tidstrand J. Neurosurg Rev. 2005;28:79–88. doi: 10.1007/s10143-005-0376-7. [DOI] [PubMed] [Google Scholar]
- 10.Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 11.Radiation utilization trends in the treatment of brain metastases from non-small cell lung cancer. Modh A, Burmeister C, Elshaikh M, Siddiqui F, Siddiqui S, Shah MM. https://www.redjournal.org/article/S0360-3016(17)31867-9/abstract Int J Radiat Oncol Biol Phys. 2017;99:0. [Google Scholar]
- 12.Radiation utilization trends in the treatment of brain metastases from non-small cell lung cancer. [Jan;2019 ];Modh A, Burmeister C, Elshaikh M, Siddiqui F, Siddiqui S, Shah M. https://www.cureus.com/abstracts/229-radiation-utilization-trends-in-the-treatment-of-brain-metastases-from-non-small-cell-lung-cancer Cureus. 2017
- 13.Changing practice patterns of Gamma Knife versus linear accelerator-based stereotactic radiosurgery for brain metastases in the US. Park HS, Wang EH, Rutter CE, Corso CD, Chiang VL, Yu JB. J Neurosurg. 2016;124:1018–1024. doi: 10.3171/2015.4.JNS1573. [DOI] [PubMed] [Google Scholar]
- 14.Cranial stereotactic radiosurgery: current status of the initial paradigm shifter. Sheehan JP, Yen CP, Lee CC, Loeffler JS. J Clin Oncol. 2014;32:2836–2846. doi: 10.1200/JCO.2013.53.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. Walker GV, Grant SR, Guadagnolo BA, et al. J Clin Oncol. 2014;32:3118–3125. doi: 10.1200/JCO.2014.55.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Freedman RA, Virgo KS, He Y, et al. Cancer. 2011;117:180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 17.Association of insurance with cancer care utilization and outcomes. Ward E, Halpern M, Schrag N, et al. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 18.Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Eur J Cancer. 2008;44:992–999. doi: 10.1016/j.ejca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. J Natl Cancer Inst. 2000;92:269–271. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 20.Disparities in standard of care treatment and associated survival decrement in patients with locally advanced cervical cancer. Robin TP, Amini A, Schefter TE, Behbakht K, Fisher CM. Gynecol Oncol. 2016;143:319–325. doi: 10.1016/j.ygyno.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 21.ASTRO releases second list of five radiation oncology treatments to question, as part of national Choosing Wisely campaign. [Dec;2017 ];http://www.choosingwisely.org/astro-releases-second-list/ 2014
- 22.Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. Walker GV, Giordano SH, Williams M, et al. Int J Radiat Oncol Biol Phys. 2013;86:686–693. doi: 10.1016/j.ijrobp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]