The regenerative capacity of the liver serves as a powerful mechanism in its ability to respond to, limit, and reverse disease pathology; however, each time the liver is forced to enact regenerative programs, residual fibrosis of the tissue can occur. As injuries accumulate, hepatic cirrhosis eventually develops, thereby significantly increasing the rate of related morbidities and mortality.1 As many disparate stimuli converge onto a final pathologic mechanism of fibrosis, understanding the molecular underpinnings of tissue scarring is of critical importance in the development of therapeutics to prevent fibrosis before the manifestation of irreversible cirrhosis.
Within the hepatic microenvironment, the accumulation of myofibroblasts—derived from hepatic stellate cells (HSCs) secondary to activating stimuli—are central mediators in hepatic regeneration, but, if prolonged accumulation occurs, myofibroblasts also orchestrate progressive fibrosis and eventual cirrhosis. Accordingly, a viable therapeutic strategy lies within targeting quiescent HSCs to prevent their activation and transdifferentiation into fibrogenic myofibroblasts. Myofibroblast pathogenicity is inherently rooted in their hyperproliferation and accumulation over time, exhibiting similar metabolic demands typified by neoplastic cells. Evidence continues to support the notion of unbridled cell growth as being dependent on glutaminolysis—an anaplerotic reaction that fulfills the increased metabolic demands of proliferating cells, while simultaneously providing carbon intermediates to sustain biomass requirements.2
In this issue of Gastroenterology, Du et al3 demonstrate a necessary action of glutaminolysis in the hyperproliferation and phenotypic development of myofibroblasts from HSCs, which is under the control of hedgehog signaling and the transcriptional activator Yes-associated protein (YAP). A major concept of metabolic reprogramming in a wide range of human diseases is classically attributed to Otto Warburg’s seminal observation that neoplastic cells demonstrate a metabolic shift toward glycolysis even under normal oxygen tension, presumably to maintain biomass in daughter cells.4 This notion has been expanded by recent work, suggesting that carbon intermediates for daughter cell mass is obtained from amino acids rather than glucose.5 Coupling this knowledge with their previous work demonstrating a role for hedgehog signaling in master regulation of metabolic reprogramming to promote glycolytic predominance in HSCs, Du et al hypothesized that perhaps anaplerotic pathways like glutaminolysis are also under hedgehog-regulated signaling pathways, such as Hippo signaling and its effector YAP.6
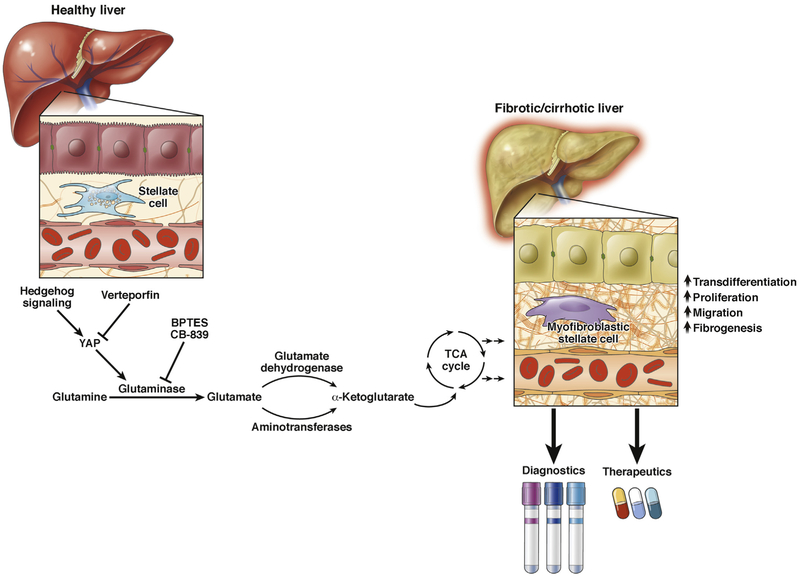
Using gene enrichment analyses in the study of primary HSCs under activating conditions, Du et al revealed preferential expression of genes involved in protein metabolism over carbohydrate metabolism, consistent with the concept of amino acids as the major energy source in proliferating populations. Combining gene expression readouts and confocal microscopy, they showed that HSCs undergoing transdifferentiation up-regulate glutaminase alongside its concomitant co-localization with markers of myofibroblastic HSCs. Glutaminase, as the rate-limiting enzyme in glutaminolysis, is known to produce glutamate from glutamine, which is then converted into a-ketoglutarate via glutamate dehydrogenase or transaminases for funneling into the tricarboxylic acid cycle (Figure 1). As such, to prove glutaminolytic dependence in HSC transdifferentiation, these investigators demonstrated that glutamine removal from media or addition of potent glutaminase inhibitors attenuates cell growth and abrogates transdifferentiation. Similarly, glutamine deprivation decreased matrix remodeling and migratory capacity, suggesting its requirement in maintaining a myofibroblastic phenotype. Using acute and chronic murine models of liver injury, they confirm glutaminase up-regulation and its colocalization with myofibroblastic markers, while demonstrating that the in vivo administration of a glutaminase inhibitor can attenuate fibrosis, presumably through reducing HSC transdifferentiation. In an attempt to define mechanistic regulation of transdifferentiation, they cultured HSCs genetically ablated for Smo—an intermediate in hedgehog signaling—to abrogate HSC transdifferentiation. They further inhibited YAP to show its necessity in the induction of glutaminase and the myofibroblastic phenotype, consistent with previous work establishing a role for YAP in glutaminase up-regulation in other disease contexts.7,8 Lastly, using transcriptomic data from non-alcoholic steatohepatitis patients, they reveal up-regulation of glutaminase in accordance with the degree of fibrosis, thereby confirming a potential role in human disease.
Figure 1.
A Yes-associated protein (YAP)–glutaminase axis mediates hepatic stellate cell transdifferentiation and liver fibrosis. Du et al present findings demonstrating that Hedgehog signaling mediates the up-regulation of glutaminase via a downstream mediator YAP of the Hippo signaling pathway. Up-regulation of glutaminase, an anaplerotic reaction that replenishes metabolic intermediates, such as α-ketoglutarate, sustains increased bioenergetic and biomass requirements of proliferating cells. Glutaminolysis has a critical role in the transdifferentiation of hepatic stellate cells into myofibroblasts, demonstrating increased proliferative, migratory, and fibrogenic capacities in the manifestation of liver fibrosis. Novel therapeutic and diagnostic strategies could be envisioned based on these molecular findings. TCA, tricarboxylic acid.
Du et al3 further attempted to characterize the effect of glutamine deprivation on mitochondrial bioenergetics. Of striking interest, they revealed that glutamine deprivation has a profound reduction in basal respiration and adenosine triphosphate production, more so than glucose deprivation in myofibroblastic HSCs, suggesting glutamine as a preferred energy source. The observation of a proproliferative cell phenotype preferring glutamine over glucose extends the principle of the Warburg effect in hepatic scarring and offers fundamental insight into its pathogenesis.
This study offers exciting potential for guiding improved molecular strategies for the prevention of progressive and permanent liver injury. It also raises pertinent questions for future work. The study at hand exclusively examines HSC transdifferentiation, but it is unclear whether other regenerative programs or cells within the hepatic microenvironment are also affected by a glutaminolytic shift. Furthermore, prior work in pulmonary vascular biology has previously demonstrated that extracellular matrix stiffening potentiates the YAP–glutaminase axis to promote downstream vessel stiffening and matrix remodeling—sustaining a pathogenic feedback loop.8 Whether or not this mechanism is conserved within liver biology remains to be elucidated; however, previous work indicating a necessity for stiff matrix in HSC transdifferentiation suggests that widespread conservation of a mechanosensitive YAP–glutaminase axis may exist.9 Moreover, Du et al3 focused mainly on the influence of glutaminolysis in myofibroblast phenotype maintenance. It is unknown whether other anaplerotic pathways—such as that catalyzed by pyruvate carboxylase and that is known to be coordinately up-regulated with glutaminase8—also contribute to liver fibrosis.
Finally, given that the YAP inhibitor verteporfin is already approved by the US Food and Drug Administration and the glutaminase inhibitor CB-839 is currently in clinical trials, Du et al present an exciting opportunity to consider repurposing current drugs for the amelioration of fibrotic transformation long before the manifestation of irreversible cirrhosis. Identification of the importance of glutamine metabolism may also suggest new avenues to develop molecular or metabolic imaging diagnostics for use early in disease. Ultimately, understanding the full impact of this work may necessitate further study of other diseases marked by neoplastic-like characteristics. Broad molecular similarities relevant to the YAP–glutaminase axis across diseases would imply that the inhibition of glutaminolysis could serve not only as a therapeutic strategy for liver disease but also other seemingly disparate human diseases as well.
Funding
This work was supported by the NIH Medical Scientist Training Program (L.D.H.) and NIH grants R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 (S.Y.C.).
Footnotes
Conflicts of interest
The authors have made the following disclosures: Disclosures S.Y. Chan has served as a consultant for Actelion (significant), Gilead, Pfizer, and Vivus (modest). L.D. Harvey declares no conflicts of interest.
Contributor Information
Lloyd D. Harvey, Medical Scientist Training Program
Stephen Y. Chan, Center for Pulmonary Vascular Biology and Medicine and Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute and Division of Cardiology Department of Medicine University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center Pittsburgh, Pennsylvania
References
- 1.Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic Fatty liver disease: mechanisms and clinical implications. Semin Liver Dis 2015; 35:132–145. [DOI] [PubMed] [Google Scholar]
- 2.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85–95. [DOI] [PubMed] [Google Scholar]
- 3.Du K, Hyun J, Premont RT, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology 2018;154:1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburg O On the origin of cancer cells. Science 1956; 123:309–314. [DOI] [PubMed] [Google Scholar]
- 5.Hosios AM, Hecht VC, Danai LV, et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell 2016; 36:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Choi SS, Michelotti GA, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology 2012;143:1319–1329.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 2016;126:3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertero T, Cottrill KA, Lu Y, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep 2015; 13:1016–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 2011;301:G110–G118. [DOI] [PMC free article] [PubMed] [Google Scholar]