Abstract
Introduction
Baseline urodynamic characterization in patients with neurogenic lower urinary tract dysfunction (NLUTD) allows detection of unsafe storage and voiding pressures and optimization of these parameters through medical or surgical intervention. Surveillance urodynamics (sUDS) studies are performed in the ambulatory setting after baseline characterization, with the goal of monitoring bladder function. How often this study should be performed and the circumstances that should prompt repeated studies are unknown. The primary objective of this review is to evaluate the evidence supporting sUDS in the setting of NLUTD as assessed by whether the study leads to 1) change in patient management; 2) determination of new findings not suggested by imaging or symptoms; and 3) demonstration of superior outcomes compared to observation. The secondary objective is to review sUDS practice patterns among urologists in their assessment of NLUTD.
Methods
PubMed, EMBASE, and Cochrane Library databases were reviewed for English-language literature published between January 1975 and March 2018.
Results
Twenty-eight independent articles (1368 patients, 9486 patient-years of followup) were included. Given heterogeneous data, 49% of 263 subjects were asymptomatic, yet demonstrated sUDS abnormality prompting treatment. Eight cross-sectional studies (four spinal cord injury [SCI], two NLUTD, two spina bifida) surveyed urologists regarding current sUDS patterns; 53% of 498 respondents perform sUDS between one and three years.
Conclusions
Evidence supporting optimal surveillance for NLUTD is lacking. Level 2b–4 evidence suggests that sUDS is likely to modify patient treatment and often demonstrates findings that modify treatment in the absence of symptoms or imaging changes.
Introduction
Baseline urodynamic characterization (UDS) is the gold standard for the evaluation of lower urinary tract dysfunction. The prognostic value of UDS for maintenance of bladder function and protection from upper urinary tract (UUT) deterioration is mentioned in several studies in patients with neurogenic lower urinary tract dysfunction (NLUTD).1,2 Surveillance urodynamic studies (sUDS) are performed in the ambulatory setting after baseline characterization, with the goal of maintaining safe lower urinary tract parameters. Although it is well-known that clinical examination alone is not sufficient to determine individual urological management strategies in patients with NLUTD,3 data demonstrating the value sUDS in the setting of NLUTD is lacking.4 Similarly, optimal frequency of sUDS is unknown. Whether sUDS studies should be regularly scheduled or performed based on a change to patient symptoms is also undetermined.
Clinical practice guidelines suggest regular evaluation for patients at high risk of UUT deterioration, but there is a lack of consensus regarding specific risk stratification or frequency of sUDS evaluation (Table 1).5–10 Furthermore, there is no consensus if sUDS should be scheduled regularly or repeated for new patient symptoms or imaging changes. Consequently, practice patterns vary with regard to sUDS frequency11–17 and healthcare utilization data suggests low uptake of sUDS use in NLUTD within the U.S. and Canada.18,19
Table 1.
Surveillance urodynamic guideline statements
| Guideline | Population | UDS surveillance suggestion |
|---|---|---|
| European Association of Urology guidelines on neuro-urology 2013, 20165,6 | NLUTD | Urodynamic investigation is a mandatory baseline diagnostic and in high-risk patients, should be done at regular intervals |
| NICE guidelines. Urinary incontinence in neurological disease: Assessment and management, 20127 | NLUTD | Consider urodynamic investigations as part of a surveillance regimen for people at high risk of upper urinary tract complications (for example, people with spina bifida, spinal cord injury, or anorectal abnormalities) |
| Adult urodynamics: AUA/SUFU guideline, 20128 | NLUTD | Clinicians should perform a cystometrogram (CMG) during initial urological evaluation of patients with relevant neurological conditions with or without symptoms and as part of ongoing followup when appropriate |
| Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: A clinical practice guideline for healthcare providers, 200610 | SCI | Generally, a urological evaluation is done every year, although there is no consensus among doctors on the frequency this type of exam should be performed or the range of tests that should be included |
| A proposed guideline for the urological management of patients with spinal cord injury. UK guideline, 20079 | SCI | Urodynamics are recommended when: upper urinary tract safety is an issue; recent onset incontinence has occurred; previous urodynamics showed detrusor-sphincter dyssynergia with sustained raised vesicle pressure or low compliance; before and after a change in bladder management; onset of UTIs or urinary tract stones; presence of VUR; high PVR |
AUA: American Urological Association; NICE: National Institute for Health and Care Excellence; NLUTD: neurogenic lower urinary tract dysfunction; PVR: post-void residual; SCI: spinal cord injury; SUFU: Society for Urodynamics and Female Urology; UTI: urinary tract infection; VUR: vesicoureteral reflux.
The primary objective of this review was to evaluate the evidence supporting sUDS in the setting of NLUTD as assessed by whether the study leads to 1) change in patient management; 2) determination of new urodynamic findings not suggested by either physical examination, imaging change, or patient symptoms; and 3) demonstration of superior outcomes compared to surveillance without regular UDS. The secondary objective was to review current sUDS practice patterns among urologists in their assessment of NLUTD.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement20 and registered in PROSPERO bank of systematic reviews as 76662. We conducted a search of the PubMed, EMBASE, and Cochrane Library databases for English-language literature published between January 1975 and March 2018. Medical subject heading (MeSH) terms included: 1) neurogenic lower urinary tract dysfunction; 2) neurogenic bladder; and 3) urodynamic(s). Each of these terms was crossed with: 1) long-term care; 2) long-term surveillance; and 3) long-term followup (Table 2). Only studies related to NLUTD and urological followup were included in this review article. Studies were also identified by hand search of reference lists and review articles.
Table 2.
MeSH permutations used
| Search term | Concepts |
|---|---|
| Neurogenic bladder | Neurogenic and Bladder [ Keywords] |
| Neurogenic lower urinary tract dysfunction | or |
| Neurogenic and lower and urinary and tract and dysfunction | |
| and | |
| Urodynamics | Urodynamic (Urodynamics, Urodynamic study, Urodynamic evaluation) |
| and | |
| Long-term care | Long-term and care |
| Long-term surveillance | or |
| Long-term followup | Long-term and Surveillance |
| or | |
| Long-term and Followup | |
| or | |
| Hydronephrosis | Hydronephrosis |
| Vesicoureteral reflux | or |
| End-stage renal disease | Vesicoureteral and reflux |
| or | |
| Chronic kidney insufficiency | End-stage and renal and disease |
| Chronic kidney insufficiency | or |
| Chronic and kidney and insufficiency | |
| or | |
| Chronic and kidney and insufficiency |
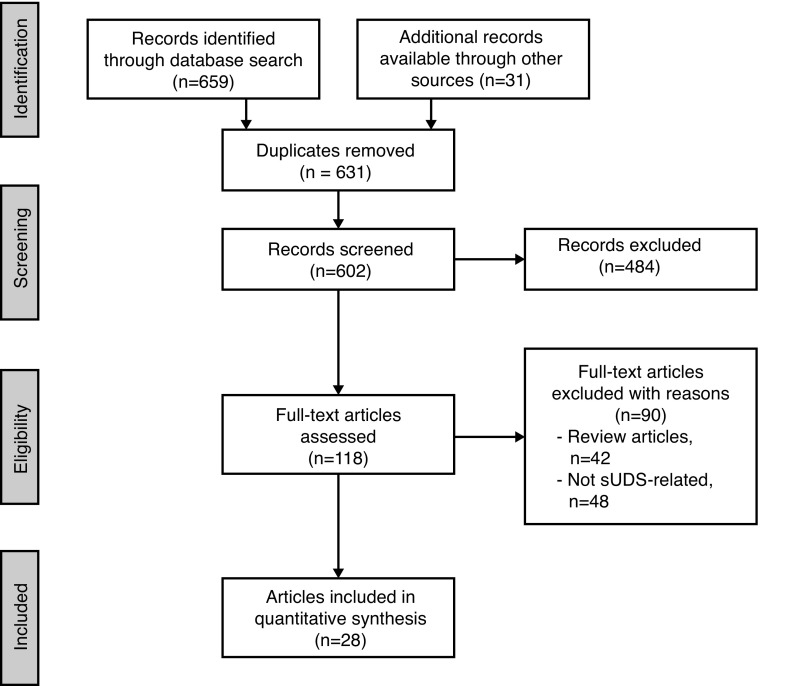
Studies were included if they presented: 1) findings related to one of the four previously mentioned inquiries; 2) pediatric or adult data relating to sUDS; 3) published since 1975; and 4) written in English. sUDS was defined as ≥2 studies performed after baseline UDS characterization. We excluded review articles and studies not available in full-text format (Fig. 1). All articles were graded according to the Oxford Centre for Evidence-based Medicine guidelines.21
Fig. 1.
Flow diagram of search strategy. sUDS: surveillance urodynamics.
Results
Initial records identified through database search included 659 articles; 31 additional records were identified through other sources. The study selection procedure is described in Fig. 1. During the data extraction process, articles were excluded if the detailed full review revealed that they did not meet the initial criteria and articles were added from the referenced bibliographies if they met the inclusion criteria. At the end of this full review, 28 of the 690 articles met our final criteria (Tables 3, 4).
Table 3.
Surveillance UDS in the setting of NLUTD
| Author | Pathology | No. of pts | Study type/quality | FU period (yrs) | UDS interval (yrs) | Regular or prompted by symptom | Percentage of studies that adjust treatment | Superior outcome compared to conservative management | New upper urinary tract deterioration | Percentage of studies that demonstrate sUDS change in asymptomatic pts |
|---|---|---|---|---|---|---|---|---|---|---|
| Linsenmeyer et al22 | SCI | 96 | Level 4, cross-sectional | 2 | 1 | Regular | 47.9% of studies prompt treatment change | No control group | None | 43% of patients had asymptomatic sUDS deterioration (46-5/96) |
| Nosseir et al23 | SCI | 80 | Level 4, retrospective cohort series | 5 | 1 | Regular | 96% of patients underwent treatment change | No control group | None | 69% of patients had asymptomatic sUDS deterioration |
| Schops et al40 | SCI | 246 | Level 4, retrospective cohort series | 6 | 6 | Regular | 40.6% of patients underwent treatment change | No control group | 1% hydronephrosis, 5% low-grade reflux | Symptoms not tracked |
| Edokpolol et al24 | SCI | 48 | Level 4, retrospective cohort series | 6.8 | Irregular* | Symptom-based | Treatment adjusted in 34%; in 10%, treatment changed for symptoms without repeating UDS | No control group | New hydronephrosis (2%) | sUDS performed only for symptomatic change |
| Chao et al41 | SCI | 28 ped | Level 4, retrospective cohort series | 3.83 | 1–2 | |||||
| Tarcan et al27 | Regular | 39% of patients underwent treatment change | No control group | None | Symptoms not tracked | |||||
| Edelstein et al29 | SB | 25 ped | Level 4, retrospective cohort series | 9.1 | 1 | Regular yearly until toilet-trained, then symptom-based | 32% of patients underwent treatment change | No control group | None | 24% of children had asymptomatic UDS deterioration (6/25) |
| Spindel et al25 | SB | 148 ped | Level 2b, retrospective cohort series | 4.5 | 1 | Regular or when imaging revealed upper urinary tract changes | 80% of patients in observation and 15% of patients in early intervention required treatment change | Less UUT deterioration in regular sUDS and intervention | UUT deterioration in 80% of patients in observation and 15% of intervention arm | Symptoms not tracked |
| Kaufman et al30 | SB | 214 ped | Level 4, retrospective cohort series | 13 | Irregular | Performed for imaging changes or incontinence at school age | 37% of patients underwent treatment change | No control group | 37% of patients had upper urinary tract deterioration | Symptoms not tracked; all 37% that required sUDS underwent this for imaging changes |
| Almodhen et al26 | SB | 37 ped | Level 4, retrospective cohort series | 5 | 1 | Regular | 35% of patients had change to voiding patter, CIC, or medication | No control group | 8%, none post-puberty | Symptoms not tracked; 10% had imaging or renal scan changes |
| Hopps et al31 | SB | 84 ped | Level 4, retrospective cohort series | 10.4 | Irregular | Based on imaging or symptom change | 56% of patients underwent treatment change | No control group | Rarely (2/84) | sUDS performed only for symptomatic change |
| Veenboer et al17 | SB | 120 | Level 4, cross-sectional | 10.4 | Irregular | Based on imaging or symptom change | 25.8% had unsafe bladder requiring treatment change | No control group | Not tracked | OR of any sUDS abnormality given patient symptoms is 0.64 |
| Ciancio et al32 | MS | 22 | Level 4, retrospective cohort series | 14 | 2.9 | Symptom-based | 55% of patients had a change to UDS pattern and all were offered treatment | No control group | None | 27% of patients had asymptomatic sUDS change |
| Wheeler et al34 | MS | 18 | Level 4, retrospective cohort series | 2.1 | Irregular | Symptom-based | 55% of patients underwent treatment change | No control group | None | Prompted by changing or persistent symptoms |
| Blaivas et al36 | MS | 41 | Level 4, retrospective cohort series | Variable | Irregular | Symptom-based | 30% had changing UDS pattern or imaging change requiring treatment | No control group | None | Bladder symptoms correlated poorly with any single urodynamic finding |
| Goldstein et al35 | MS | 9 | Level 4, retrospective cohort series | Variable | Irregular | Symptom-based | 44% had changing UDS pattern requiring treatment change | No control group | None | Prompted by changing or persistent symptoms |
| Schoenberg et al33 | MS | 33 | Level 4, retrospective cohort series | 2.5 | Irregular | Symptom-based | 36% had changing UDS pattern requiring treatment change | No control group | None | Prompted by changing or persistent symptoms |
| Bemelmans et al37 | MS | 40 | Level 4, retrospective cohort series | 2.5 | Irregular | Single point | 88% had UDS abnormality requiring treatment change | No control group | None | 50% of asymptomatic patients had UDS abnormalities requiring treatment |
Based on patients symptoms or sonographic findings (not regular intervals),
CIC: clean intermittent catheterization; DESD: detrosure external sphincter dyssynergia; FU: followup; MS: multiple sclerosis; NLUTD: neurogenic lower urinary tract dysfunction; OR: odds ratio; ped: pediatric; SB: spina bifida; SCI: spinal cord injury; sUDS: surveillance urodynamics; UDS: urodynamic study; UUT: upper urinary tract; yrs: years.
Table 4.
Practice patterns of surveillance UDS
| Author | Population | UDS strategy |
|---|---|---|
| Elliott et al13 | Spina bifida | A survey was mailed to all 169 clinics listed by the Spina Bifida Association of America; 59% obtained routine UDS, commonly at intervals of 1–2 years |
| Veenboer et al17 | Spina bifida | A questionnaire was sent to all 365 urologists in the Netherlands regarding current assessment of adult spina bifida patients. Video UDS investigations (UDS) were performed on a regular basis (1–2 years) by 24.3%; the remainder performed the study for symptomatic changes |
| Blok et al12 | NLUTD | A questionnaire was mailed to members of the Canadian Urological Association; 75% of respondents undertook urodynamic study and 11% (n=9), video UDS; this was performed annually or every other year |
| Rikken et al16 | NLUTD | A questionnaire was mailed to 304 certified urologists of the Dutch Urological Association; 12% of respondents completed regular urodynamic studies every 1–2 years |
| Bycroft et al4 | SCI | 12 Spine Injured Units in the U.K. and Eire were sent a questionnaire addressing basic practice relating to urological outpatient followup and UDS; Six units did not perform routine UDS; in four units that perform routine sUDS, range of frequency of UDS was from 1–3 years |
| Razdan et al15 | SCI | A mailed questionnaire was sent to the 269 American members of the Society for Urodynamics and Female Urology (SUFU); 65% of respondents performed surveillance video UDS every 1–2 years; the remaining 35% did not consider routine UDS needed and completed a cystogram if the patient had recurrent UTIs or deleterious upper urinary tract changes on US or other imaging study |
| Kitahara et al14 | SCI | A Japanese version of the 14-item questionnaire survey carried out in U.S. was mailed to 770 members of the Japanese Neurogenic Bladder Society (JNBS); cystometry was performed yearly by 174 (52.3%) respondents for the evaluation of vesicourethral function |
| Al Taweel et al11 | SCI | Questionnaire distributed to urologists working in Saudi Arabia and registered at the Saudi Medical Association; 62% repeat the study every year; the remaining 20% will do it every two years, and 12% will do it whenever the patients’ symptoms deteriorate |
| Cameron et al18 | SCI | Used a 5% Medicare sample to review data from over 7000 SCI patients. During the two-year period, 35.7% of patients saw a urologist and 6.7% had UDS |
| Welk et al19 | SCI | 1551 SCI patients were followed for a median of five years after discharge from a rehabilitation hospital; the proportion of patients who had regular UDS at least once every two years was 10% |
NLUTD: neurogenic lower urinary tract dysfunction; SCI: spinal cord injury; UDS: urodynamic study; UTI: urinary tract infection.
All reviewed articles focused on NLUTD secondary to either spinal cord injury (SCI), multiple sclerosis (MS), or spina bifida. Results could not be combined due to heterogeneity of underlying pathology. sUDS was performed on a regular, specific interval (1–2 years) in nine studies and based on altered symptoms or imaging findings (recurrent urinary tract infection [UTI], increased incontinence between catheterization, or alarming features on ultrasound) in nine articles (predominantly MS). Individual findings for SCI, spina bifida, and MS patients are provided in the following sections.
SCI
Five articles meeting level 4 evidence addressed sUDS in the SCI population (Table 3). Studies included 470 adults and 28 pediatric patients with 2393.4 and 107.3 patient-years of followup, respectively. Four of five articles performed sUDS based on regularly timed studies defined on a specific interval (1–2 years) while one article performed surveillance based on altered symptoms or imaging findings (recurrent UTI, increased incontinence between catheterization, or alarming features on ultrasound).
The impact of annual sUDS on adjustment of patient treatment was addressed by Linsemeyer et al.22 The authors performed a cross-sectional review of 96 individuals with stable traumatic SCI undergoing annual UDS evaluations. Changes in the urodynamic parameters and autonomic dysreflexia were determined by comparing the current study with the prior year. The main outcome measure was whether or not there was a need for intervention based on the UDS results. Overall, 47.9% of individuals required at least one type of intervention based on annual UDS: 82.6% were urological interventions (medication changes were most common, comprising 54.3% of urological interventions); 13.0% were non-urological interventions; and 4.3% were a combination of non-urological and urological interventions. The need for intervention was not influenced by the type of bladder management, the length of time post-injury, or level of injury. Only 5.2% of patients reported new-onset urological symptoms since their prior annual evaluation.
Nosseir et al23 also advised that reliance on clinical symptoms to prompt sUDS leads to failure to detect a large number of treatment failures in the SCI population. The authors reviewed 80 SCI patients with at least one followup visit per year for a minimum of five consecutive years. The focus was to determine how frequently the treatment regimen had to be modified due to annual sUDS results. Over a mean followup of 67.3 months, the treatment strategy had to be modified in almost all patients. If authors had relied solely on clinical symptoms or imaging findings, 68.75% of treatment failures would not have been detected.
Conversely, Edokpolol and colleagues24 established a safe lower urinary tract with baseline UDS, and subsequently performed annual renal ultrasonography for surveillance. sUDS was repeated only when patients presented with new symptoms or alarming radiological changes. Subjects were followed for a mean duration of 6.8 years. sUDS was repeated in 40% of subjects during the study period. After repeat sUDS for new onset of symptoms, bladder management was not changed in 64% cases. The dose or type of anticholinergic was increased or changed in 32% cases, and one subject received bladder augmentation. In four other subjects, the regimen was modified based on symptoms without repeating sUDS. Two new cases of pelvicaliectasis were present at the time of final ultrasound. One case was secondary to an obstructing stone and the second was due to refractory bladder pressures in a non-compliant patient. The authors concluded that an ultrasound-based surveillance approach is efficacious in SCI patients and suggest that annual sUDS may be unnecessary.
Spina bifida
Seven articles meeting level 2b–4 evidence addressed sUDS in the spina bifida population (Table 3). Studies included 120 adult and 587 pediatric patients with 1248 and 5208 patient-years of followup, respectively. Five of seven articles performed sUDS based on regularly timed studies defined on a specific interval (1–2 years) while two articles performed surveillance based on altered symptoms or imaging findings (recurrent UTI, increased incontinence between catheterization, or alarming features on ultrasound).
NLUTD management in pediatric spina bifida differs from adult pathology in the magnitude of UDS evolution in the early years of life. Spindel et al25 performed a retrospective review of 79 pediatric patients that underwent annual sUDS with synergic outlets and biannual sUDS for dyssynergic outlets; 37% of patients had demonstrable changes in external urethral sphincter function over time. There was a 32% chance of having a change in external sphincter function during the first 12 months of life, a 6% chance during the second 12 months, and a 2% chance during the third 12 months. Furthermore, Almodhen et al26 demonstrated that total cystometric bladder capacity, maximum detrusor pressure, and detrusor leak point pressure increase significantly in patients with myelomeningocele following puberty on annual sUDS.
Although several pediatric studies demonstrate benefit of regular surveillance26,27 compared to expectant management,28 Edelstein et al29 provided the only prospective controlled study. Authors compared urological outcomes of a cohort of children who were at risk for urological deterioration on the basis of bladder-sphincter dyssynergia and/or high filling or voiding pressures. Those at risk were either observed until radiological deterioration occurred, or were placed on prophylactic intermittent catheterization with or without anticholinergic medication based on annual sUDS. During the followup period, 80% of children in the observation group developed radiological evidence of UUT deterioration (inadequate bladder emptying, reflux, and/or hydronephrosis). In contrast, only 15% of children in the intervention group demonstrated deterioration.
Controversy exists regarding the use of regularly scheduled sUDS compared to performing studies for symptomatic or radiological change. Kaufman et al30 reviewed 214 children presenting to a spina bifida clinic in a 13-year period. UDS were performed when UUTs deteriorated or in incontinent school-age children. On radiographic study, there was evidence of UUT deterioration in 79 children, including hydronephrosis in 34, hydronephrosis and vesicoureteral reflux (VUR) in 19, and reflux only in 26. Followup studies performed after clean intermittent catheterization and pharmacological therapy were instituted revealed resolution or improvement of UUT deterioration in 69%, while bladder compliance improved in only 42%. The results suggest that although radiological surveillance of patients with myelomeningocele allows recognition of UUT changes, the effects of elevated outlet resistance on bladder compliance are not as readily reversible as the initial radiographic findings.
Conversely, Hopps et al31 established a risk classification scheme to stratify the surveillance approach. High-risk patients underwent prompt UDS evaluation. Low-risk patients were followed closely at 2–4-month intervals with serial physical examination, UUT imaging, and urine culture. Conversion from low- to high-risk occurred with new-onset hydronephrosis, febrile UTI, urinary retention, or incidental finding of VUR at the time of evaluation for continence. After a mean followup of 10.4 years, renal deterioration occurred in only one kidney of the high-risk group and one kidney in the group that converted from low- to high-risk, representing 1.2% of all renal units.
Although controlled studies are currently lacking, use of symptom- or imaging-provoked sUDS in adult spina bifida patients may be beneficial. Veenboer et al17 performed a cross-sectional review of 120 adult spina bifida patients (median age 31.5 years) to determine characteristics associated with a hostile lower urinary tract on sUDS. In the multivariable model, unsafe bladder was significantly associated with being wheelchair-bound (odds ratio [OR] 5.36; p<0.008). Conversely, it was highly unlikely to find an unsafe bladder in asymptomatic patients that were not wheelchair-bound (negative predictive value 1.00). The authors concluded that if an adult patient with spinal dysraphism is not wheelchair-bound, unfavourable findings at sUDS are unlikely. If these patients are asymptomatic, these findings are even more unlikely. In these patients, it is probably not necessary to perform routine UDS without symptoms or imaging prompting the study.
MS
Six articles addressed sUDS in the adult MS population (Table 3). Studies included 163 adults with 528 patient-years of followup. Five of six articles performed sUDS based on changing patient symptoms (recurrent UTI, increased incontinence between catheterization, or alarming features on ultrasound).
The changing clinical course of MS is a hallmark of the disease. Ciancio et al32 followed 22 adults with repeat UDS performed because of new or persistent LUTS. Overall, 55% of patients experienced a change in their urodynamic patterns and/or compliance during a mean followup of 42 months. In the largest retrospective series, Schoenberg and Gutrich33 performed repeated UDS evaluations on 33 symptomatic patients during a 2.5-year period and found differences in 12, all of whom changed from having detrusor hypocontractility to having detrusor hyperreflexia. Wheeler, Goldstein, and Blaivas et al34–36 also found temporal changes in the urodynamic patterns in the majority of patients.
Several authors have demonstrated poor correlation between UDS findings and patient symptoms in the MS population. Ciancio and colleagues32 found that 43% of MS patients with no new urological symptoms developed a change in the urodynamic pattern and/or compliance on followup UDS evaluation. Similarly, in a prospective study by Bemelmans et al,37 52% of patients demonstrated urodynamic abnormalities without symptoms. However, the incidence of positive urodynamic findings in patients with lower urinary tract complaints was 98%. The latter finding suggests that UDS evolution may be present without symptoms, but is highly likely if voiding symptoms exist.
Fortunately, the rate of UUT deterioration in MS with NLUTD is low. In a meta-analysis of 1882 patients with MS, only 1% demonstrated UUT tract abnormality.38 Fletcher et al39 investigated the prevalence of renal ultrasound abnormalities over time in MS patients with LUTS. The authors defined UUT damage as the presence of hydronephrosis, caliectasis, cortical scarring, or stone formation. Over a nine-year period, 173 patients had both UDS and renal ultrasound. Of these, 5.8% of subjects had abnormalities at initial ultrasound, whereas at followup, renal ultrasound (RUS) abnormalities were seen in 12.4% of patients. Overall, there were seven patients who developed new abnormalities. The authors concluded that the development of UUT abnormalities as determined by RUS overall is low, although older patients and those with abnormal compliance may merit closer supervision.
Current practice patterns
Eight cross-sectional studies (all level 3, four SCI, two NLUTD, two spina bifida) surveyed urologists regarding current practice patterns of sUDS in the setting of NLUTD (Table 4); 53% of 498 respondents and 39 specialty clinics in seven countries reported that they perform sUDS between 1–3 years using pooled estimate weighted average. The most common practice pattern was sUDS every 1–2 years.
These results were in contrast to two retrospective cohort series that demonstrated the actual use of sUDS among SCI patients was substantially less frequent than reported practice patterns suggest. Cameron et al18 observed a 6.7% use of sUDS in American SCI patients over a two-year period despite over 35% urological consultation in the same period. Similarly, Welk et al19 observed only 10% use of sUDS in Canadian SCI patients over a two-year period.
Discussion
Change in patient management based on sUDS
Table 3 demonstrates heterogeneous data (level 2b–4) with variable underlying pathology, variable stimulus for adjusting treatment, and variable conditions for prompting sUDS. Although pooled-estimate meta-analysis is not possible given heterogeneity, sUDS has a tendency to adjust patient treatment often. A weighted average of results demonstrated that surveillance adjusted treatment in 48.4% of patients.
Determination of new findings in asymptomatic patients without imaging changes
Similarly, clinical and methodologic heterogeneity of data limits the ability to perform pooled-estimate meta-analysis (Table 3) with respect to this question. Despite this, sUDS has a tendency to provide new findings that are not suggested by patient symptoms or imaging changes. A weighted average of results demonstrated that surveillance determined findings that prompted treatment in 48.9% of asymptomatic patients without imaging changes. However, after establishing a ‘safe’ lower urinary tract, prompting sUDS with imaging change or new symptoms did not appear to be associated with adverse outcomes in the short-term.23
Does sUDS demonstrate superior outcomes compared to long-term followup without UDS?
There are currently no high-quality studies available to support or refute this premise. Available evidence is primarily level 4 without control groups. A single level 2b study is available within the pediatric population.
What are the current sUDS practice patterns among urologists in their assessment of NLUTD?
The most common self-reported practice pattern of sUDS in the management of NLUTD is every 1–2 years. Within the U.S. and Canada, healthcare utilization data suggests that the actual rate of sUDS in the neurogenic population ranges from 6.7–10%. The difference between self-reported practice patterns and actual use highlights the need for consensus in surveillance standards.
Conclusion
Available evidence supporting optimal surveillance protocols for NLUTD is lacking. Qualitative findings from level 2b–4 evidence suggest that sUDS is likely to modify patient treatment, and often leads to new findings not suggested by physical examination, imaging findings, or new patients symptoms. Establishing a risk-benefit ratio of these findings is not possible due to lack of control groups. There is currently no evidence that demonstrates regularly scheduled sUDS has superior outcome compared to sUDS performed for symptom or imaging change.
The most common practice pattern of surveyed urologists was to repeat sUDS every 1–2 years. Review of currently available guidelines (Table 1) demonstrated two conventional approaches for UDS. The primary approach is to stratify into risk groups with baseline UDS. Low-risk groups are those that have safe storage parameters, including high capacity, high compliance, and low storage pressure. High-risk groups include parameters that place UUT at risk, including detrusor-sphincter dyssynergia with sustained raised vesicle pressure or low compliance, before and after a change in bladder management; onset of UTIs or urinary tract stones; or presence of VUR or high post-void residual. sUDS is typically reduced in the former to a lengthy interval (although no consensus exists to define this interval). The latter group is typically investigated and followed at a more closely defined and regimented schedule, such as regular sUDS every 1–2 years.
An alternative to this approach is to establish a baseline with UDS followed by on-demand sUDS if patient presentation evolves during the course of followup. Findings such as new-onset hydronephrosis, reflux, deterioration in renal function, increased infection frequency, or urinary calculi formation prompt sUDS evaluation.
The optimal sUDS strategy in surveillance of NLUTD has not yet been established and will likely require further data to establish a validated protocol. This review demonstrated that existing literature is limited by small enrollment studies with heterogeneous populations completed over a time course that is extensive. There is clearly a need for further high-quality studies to determine the optimal surveillance strategy of UDS with NLUTD.
Footnotes
To answer the multiple-choice questions associated with this article, go to: www.cuasection3credits.org/cuajapril2019. This program is an Accredited Self-Assessment Program (Section 3) as defined by the Maintenance of Certification Program of The Royal College of Physicians & Surgeons of Canada, and approved by the Canadian Urological Association. Remember to visit MAINPORT (www.mainport.org/mainport/) to record your learning and outcomes. You may claim a maximum of 1 hour of credit.
Competing interests: Dr. Kavanagh has participated in advisory board meetings for Paladin Labs and has received a research grant from Astellas. Dr. Walter is a Michael Smith Foundation for Health Research Research Trainee Award recipient, in partnership with the Rick Hansen Foundation (Grant No. 17110); has received funding from Coloplast, Pfizer, Wellspect, and the Rick Hansen Institute; and has received travel awards from the International Continence Society, American Spinal Injury Association, British Columbia Regenerative Medicine, and Faculty of Medicine at the University of British Columbia. Dr. Stothers has participated in advisory board meetings for Astellas. Dr. Boone has participated in global advisory board meetings and has been a speaker for Astellas. The remaining authors reports no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol. 1992;147:416–8. doi: 10.1016/S0022-5347(17)37254-3. [DOI] [PubMed] [Google Scholar]
- 2.McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–9. doi: 10.1016/S0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 3.Wyndaele JJ. Correlation between clinical neurological data and urodynamic function in spinal cord injured patients. Spinal Cord. 1997;35:213–6. doi: 10.1038/sj.sc.3100391. [DOI] [PubMed] [Google Scholar]
- 4.Bycroft J, Hamid R, Bywater H, et al. Variation in urological practice amongst spinal injuries units in the UK and Eire. Neurourol Urodyn. 2004;23:252–6. doi: 10.1002/nau.20005. discussion 7. [DOI] [PubMed] [Google Scholar]
- 5.Blok B, Pannek J, Castro-Diaz D, et al. EAU guidelines on neuro-urology. 2016. [Accessed Feb 12, 2019]. Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Neuro-urology-2016-1.pdf. [DOI] [PubMed]
- 6.Stohrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56:81–8. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence: Guidance. London: 2012. Urinary Incontinence in Neurological Disease: Management of Lower Urinary Tract Dysfunction in Neurological Disease. [Google Scholar]
- 8.Collins CW, Winters JC. AUA/SUFU adult urodynamics guideline: A clinical review. Urol Clin North Am. 2014;41:353–62. doi: 10.1016/j.ucl.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Abrams P, Agarwal M, Drake M, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008;101:989–94. doi: 10.1111/j.1464-410X.2008.07457.x. [DOI] [PubMed] [Google Scholar]
- 10.Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: A clinical practice guideline for health-care providers. J Spinal Cord Med. 2006;29:527–73. [PMC free article] [PubMed] [Google Scholar]
- 11.Al Taweel W, Alkhayal A. Neurogenic bladder evaluation and management after spinal cord injury: Current practice among urologists working in Saudi Arabia. Urol Ann. 2011;3:24–8. doi: 10.4103/0974-7796.75872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blok BF, Karsenty G, Corcos J. Urological surveillance and management of patients with neurogenic bladder: Results of a survey among practicing urologists in Canada. Can J Urol. 2006;13:3239–43. [PubMed] [Google Scholar]
- 13.Elliott SP, Villar R, Duncan B. Bacteriuria management and urological evaluation of patients with spina bifida and neurogenic bladder: A multicentre survey. J Urol. 2005;173:217–20. doi: 10.1097/01.ju.0000146551.87110.f4. [DOI] [PubMed] [Google Scholar]
- 14.Kitahara S, Iwatsubo E, Yasuda K, et al. Practice patterns of Japanese physicians in urologic surveillance and management of spinal cord injury patients. Spinal Cord. 2006;44:362–8. doi: 10.1038/sj.sc.3101854. [DOI] [PubMed] [Google Scholar]
- 15.Razdan S, Leboeuf L, Meinbach DS, et al. Current practice patterns in the urologic surveillance and management of patients with spinal cord injury. Urology. 2003;61:893–6. doi: 10.1016/S0090-4295(02)02518-9. [DOI] [PubMed] [Google Scholar]
- 16.Rikken B, Blok BF. Management of neurogenic bladder patients in The Netherlands: Do urologists follow guidelines? Neurourol Urodyn. 2008;27:758–62. doi: 10.1002/nau.20582. [DOI] [PubMed] [Google Scholar]
- 17.Veenboer PW, Ruud Bosch JL, de Kort LM. Assessment of bladder and kidney functioning in adult spina bifida patients by Dutch urologists: A survey. Neurourol Urodyn. 2014;33:289–95. doi: 10.1002/nau.22413. [DOI] [PubMed] [Google Scholar]
- 18.Cameron AP, Lai J, Saigal CS, et al. Project NUDiA. Urological surveillance and medical complications after spinal cord injury in the United States. Urology. 2015;86:506–10. doi: 10.1016/j.urology.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welk B, Liu K, Shariff SZ. The use of urologic investigations among patients with traumatic spinal cord injuries. Res Rep Urol. 2016;8:27–34. doi: 10.2147/RRU.S99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Oxford Centre for Evidence-based Medicine – Levels of Evidence [Internet] 2009. [Accessed Feb 12, 2019]. Available at: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- 22.Linsenmeyer TA, Linsenmeyer MA. Impact of annual urodynamic evaluations on guiding bladder management in individuals with spinal cord injuries. J Spinal Cord Med. 2013;36:420–6. doi: 10.1179/2045772313Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007;26:228–33. doi: 10.1002/nau.20319. [DOI] [PubMed] [Google Scholar]
- 24.Edokpolo LU, Foster HE., Jr Renal tract ultrasonography for routine surveillance in spinal cord injury patients. Top Spinal Cord Inj Rehabil. 2013;19:54–60. doi: 10.1310/sci1901-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spindel MR, Bauer SB, Dyro FM, et al. The changing neurourologic lesion in myelodysplasia. JAMA. 1987;258:1630–3. doi: 10.1001/jama.1987.03400120080029. [DOI] [PubMed] [Google Scholar]
- 26.Almodhen F, Capolicchio JP, Jednak R, et al. Postpubertal urodynamic and upper urinary tract changes in children with conservatively treated myelomeningocele. J Urol. 2007;178:1479–82. doi: 10.1016/j.juro.2007.05.171. [DOI] [PubMed] [Google Scholar]
- 27.Tarcan T, Bauer S, Olmedo E, et al. Long-term followup of newborns with myelodysplasia and normal urodynamic findings: Is followup necessary? J Urol. 2001;165:564–7. doi: 10.1097/00005392-200102000-00070. [DOI] [PubMed] [Google Scholar]
- 28.Bruschini H, Almeida FG, Srougi M. Upper and lower urinary tract evaluation of 104 patients with myelomeningocele without adequate urological management. World J Urol. 2006;24:224–8. doi: 10.1007/s00345-006-0087-x. [DOI] [PubMed] [Google Scholar]
- 29.Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500–4. doi: 10.1016/S0022-5347(01)66914-3. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman AM, Ritchey ML, Roberts AC, et al. Decreased bladder compliance in patients with myelomeningocele treated with radiological observation. J Urol. 1996;156:2031–3. doi: 10.1016/S0022-5347(01)65427-2. [DOI] [PubMed] [Google Scholar]
- 31.Hopps CV, Kropp KA. Preservation of renal function in children with myelomeningocele managed with basic newborn evaluation and close followup. J Urol. 2003;169:305–8. doi: 10.1016/S0022-5347(05)64112-2. [DOI] [PubMed] [Google Scholar]
- 32.Ciancio SJ, Mutchnik SE, Rivera VM, et al. Urodynamic pattern changes in multiple sclerosis. Urology. 2001;57:239–45. doi: 10.1016/S0090-4295(00)01070-0. [DOI] [PubMed] [Google Scholar]
- 33.Schoenberg HW, Gutrich JM. Management of vesical dysfunction in multiple sclerosis. Urology. 1980;16:444–7. doi: 10.1016/0090-4295(80)90162-4. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler JS, Jr, Siroky MB, Pavlakis AJ, et al. The changing neurourologic pattern of multiple sclerosis. J Urol. 1983;130:1123–6. doi: 10.1016/S0022-5347(17)51716-4. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein I, Siroky MB, Sax DS, et al. Neurourologic abnormalities in multiple sclerosis. J Urol. 1982;128:541–5. doi: 10.1016/S0022-5347(17)53037-2. [DOI] [PubMed] [Google Scholar]
- 36.Blaivas JG, Bhimani G, Labib KB. Vesicourethral dysfunction in multiple sclerosis. J Urol. 1979;122:342–7. doi: 10.1016/S0022-5347(17)56397-1. [DOI] [PubMed] [Google Scholar]
- 37.Bemelmans BL, Hommes OR, Van Kerrebroeck PE, et al. Evidence for early lower urinary tract dysfunction in clinically silent multiple sclerosis. J Urol. 1991;145:1219–24. doi: 10.1016/S0022-5347(17)38581-6. [DOI] [PubMed] [Google Scholar]
- 38.Litwiller SE, Frohman EM, Zimmern PE. Multiple sclerosis and the urologist. J Urol. 1999;161:743–57. doi: 10.1016/S0022-5347(01)61760-9. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher SG, Dillon BE, Gilchrist AS, et al. Renal deterioration in multiple sclerosis patients with neurovesical dysfunction. Mult Scler. 2013;19:1169–74. doi: 10.1177/1352458512474089. [DOI] [PubMed] [Google Scholar]
- 40.Schops TF, Schneider MP, Steffen F, et al. Neurogenic lower urinary tract dysfunction (NLUTD) in patients with spinal cord injury: Long-term urodynamic findings. BJU Int. 2015;115(Suppl6):33–8. doi: 10.1111/bju.13085. [DOI] [PubMed] [Google Scholar]
- 41.Chao R, Mayo ME. Long-term urodynamic follow up in pediatric spinal cord injury. Paraplegia. 1994;32:806–9. doi: 10.1038/sc.1994.127. [DOI] [PubMed] [Google Scholar]