Abstract
Introduction
We sought to evaluate the diagnostic performance of 18F-fluorocholine positron emission tomography-computed tomography (18F-FCH PET/CT) for initial staging of patients with high-risk prostate cancer. Secondary objectives were to compare the value of 18F-FCH PET/CT to conventional imaging modalities and to evaluate its clinical impact.
Methods
We conducted a retrospective study of 76 patients who underwent 18F-FCH PET/CT for initial staging of high-risk prostate cancer. Using pre-established validation criteria, sensitivity and specificity were determined for metastatic disease. Results were compared to findings on magnetic resonance imaging (MRI), computed tomography (CT), and bone scan (BS) when available.
Results
Twenty-two (29%) PET/CT scans were positive, 49 (64%) negative, and five (7%) equivocal for nodal or metastatic disease. Of the positive scans, 17 showed regional lymph node involvement, 12 distant nodes, five bone metastases, and three lung metastases. Overall per-patient sensitivity, specificity, positive and negative predictive values for metastatic disease were 65%, 100%, 100%, and 78%, respectively. Sensitivity, specificity, and positive and negative predictive values were 64%, 100%, 100%, and 80%, respectively, for nodal involvement and 86%, 100%, 100%, and 98%, respectively, for bone and other metastases. Conventional imaging was negative for the lesion(s) found on PET/CT in five patients. PET/CT changed the clinical management in nine patients (12%).
Conclusions
Although 18F-FCH PET/CT offers some benefits over conventional imaging and demonstrates a high specificity, it remains limited by its sensitivity in the context of high-risk prostate cancer staging. PET with novel urea-based small molecule prostate-specific membrane antigen (PSMA) inhibitors may overcome some of these limitations. However, the interpretation of the study result is limited by the lack of available histological gold standard, the inclusion of several patients who received androgen-deprivation therapy (ADT) prior to PET/CT, our retrospective design, and a relatively small sample size.
Introduction
Prostate adenocarcinoma (PCa) is the most common cancer among Canadian men.1 Adequate management of newly diagnosed PCa relies primarily on proper staging and assessment of risk group stratification. While most PCa cases are of very low-, low-, and intermediate-risk category, high-risk PCa represents 16–31% of cases at time of diagnosis, depending on the classification system used.2,3 Notably, the incidence of high-risk localized and metastatic PCa seems to be increasing.4 Treatment options for high-risk localized PCa include radical prostatectomy (RP) with pelvic lymph node dissection (PLND) or external beam radiation therapy (EBRT) with the addition of androgen-deprivation therapy (ADT).5 If the PCa is metastatic at diagnosis, especially in a high-volume context, it is best managed with systemic therapies such as ADT alone or in combination with chemotherapy or abiraterone.6–8
Given the higher likelihood of metastatic disease in the high-risk patients, as well as the different available treatment options, accurate detection of nodal or skeletal disease is crucial in this group. For this reason, imaging plays a key role in PCa staging. However, the optimal imaging modality remains an ongoing debate. Most clinicians use a combination of magnetic resonance imaging (MRI), computed tomography (CT), and bone scan (BS) to perform staging despite poor reported sensitivity and specificity.9
In recent years, nuclear medicine imaging markers have been developed in an attempt to overcome this gap, such as 11C-Choline and 18F-fluorocholine (18F-FCH).10 Choline plays an essential role in the formation of phospholipid membranes and demonstrates increased uptake in PCa cells.11 The role of 18F-FCH positron emission tomography-computed tomography (PET/CT) in PCa has previously been studied for initial staging and in context of biochemical recurrence with mixed results.12–16 In studies looking at the potential role of 18F-FCH PET/CT in initial PCa staging, the benefits seemed to be optimal when investigating high-risk PCa, but the number of patients included in the studies was small and both intermediate- and high-risk patients were often analyzed together, which limited extrapolation of results.13,15,17
The primary objective of this study was to evaluate the diagnostic performance of 18F-FCH PET/CT for detection of metastatic disease in high-risk PCa patients at the time of initial staging. Secondary objectives were to compare its value to conventional imaging (MRI, CT, and BS) and to evaluate its clinical impact.
Methods
Patients and study design
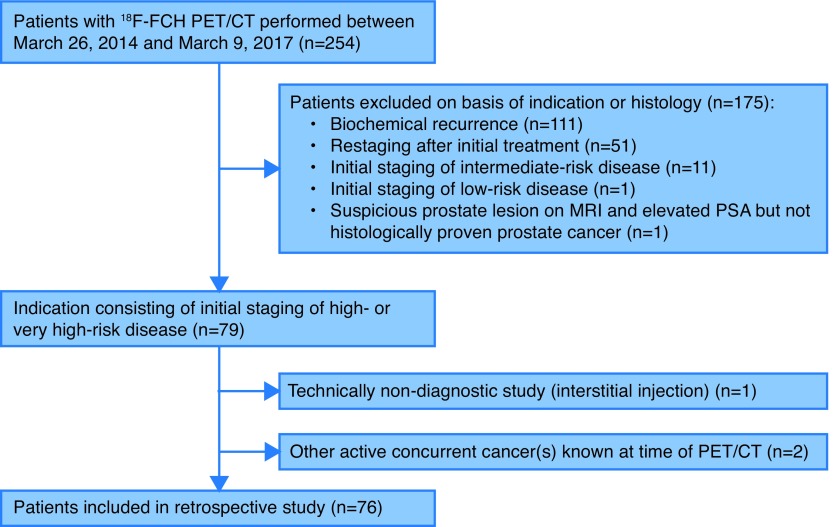
We conducted a single-centre, retrospective study including 76 patients who underwent 18F-FCH PET/CT scans performed from March 2014 to March 2017. Study protocols were approved by the hospital research ethics committee (REB #13-047 and #16-061). Health Canada approval and written informed consent from all patients was obtained. Inclusion criteria consisted of histologically proven diagnosis of PCa with high-risk features (as defined by any one of: prostate-specific antigen [PSA] level >20 ng/mL, Gleason score ≥8, or clinical T stage (cT) ≥T3a based on digital rectal examination [DRE]).18 Exclusion criteria consisted of any previously known metastasis or previous treatment for PCa (other than ADT preceding PET/CT in view of a combined treatment approach after imaging), any known concurrent active cancer(s), and technically non-diagnostic study (Fig. 1). Patient characteristics are shown in Table 1.
Fig. 1.
Flow diagram outlining inclusion and exclusion criteria and study design. 18F-FCH: 18F-fluorocholine; PET/CT: positron-emission tomography-computed tomography; MRI: magnetic resonance imaging; PSA: prostate-specific antigen.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Age, years | |
| Mean (SD) | 66.5 (8.0) |
| Median | 67.8 |
| Range | 47.1–82.8 |
| PSA at time of PET/CT, ng/mL | |
| Mean (SD) | 36.2 (86.3) |
| Median | 19.2 |
| Range | 4.9–745.7 |
| Clinical T stage, n (%) | |
| T1 | 36 (47.4) |
| T2 | 17 (22.4) |
| T3 | 22 (28.9) |
| T4 | 1 (1.3) |
| Clinical N stage, n (%) | |
| N0 | 57 (75.0) |
| N1 | 12 (15.8) |
| Nx | 7 (9.2) |
| Gleason score, n (%) | |
| 6 | 3 (3.9) |
| 7 | 11 (14.5) |
| 8 | 30 (39.5) |
| 9 | 31 (40.8) |
| 10 | 1 (1.3) |
| Proportion of positive cores on biopsy, % (NA=1) | |
| Mean (SD) | 62.1 (28.7) |
| Median | 61.9 |
| Range | 3.3–100.0 |
| ADT at time of the PET/CT, n (%) (NA=4) | |
| Yes | 9 (11.8) |
| No | 63 (82.9) |
| Treatment (+/− ADT), n (%) (NA=3) | |
| RP | 27 (35.5) |
| EBRT | 35 (46.1) |
| EBRT + chemotherapy | 5 (6.6) |
| Chemotherapy | 4 (5.3) |
| Others | 2 (2.6) |
| Pathological T stage, n (%) | |
| T2 | 6 (22.2) |
| T3 | 21 (77.8) |
ADT: androgen-deprivation therapy; EBRT: external beam radiation therapy; NA: not available; PET/CT: positron-emission tomography-computed tomography; PSA: prostate-specific antigen; RP: radical prostatectomy; SD: standard deviation.
Technique and study interpretation
PET/CT preparation consisted of four-hour fasting. Approximately 4 MBq/kg IV of 18F-FCH were administered. Examinations were performed using a hybrid PET/CT scanner (GE Discovery ST, General Electric Medical Systems, Waukesha, WI, U.S.). Images were acquired 10–30 minutes post-injection from skull base to mid-thighs (6–7 bed positions, 4 minutes/bed). A nuclear medicine specialist interpreted the studies as positive, negative, or equivocal; a positive examination was defined as 18F-FCH uptake higher than surrounding background activity and not explained by physiological process.
Validation of results
PET/CT findings were compared to MRI, CT, BS, and histological analysis when available. Results were considered true positive if meeting any of the following validation criteria: 1) positive histological analysis; 2) treatment response on followup imaging; 3) progression on followup imaging; or 4) positive conventional imaging at initial staging. Results were considered true negative if there was: 1) negative histological analysis; or 2) negative clinical and/or imaging followup for at least six months following PET/CT. A negative clinical followup was defined as no increase in PSA value or clinical evidence of metastatic disease during the followup period. Results were considered false-positive if there was: 1) negative histological analysis; or 2) imaging followup demonstrating either stable initial findings for at least six months or improved findings any time after PET/CT without treatment. Results were considered false-negative if there was: 1) positive histological analysis; 2) treatment response on imaging any time after PET/CT; 3) positive conventional imaging at initial staging; or 4) clinical and/or imaging evidence of metastatic disease detected within six months following PET/CT. Sensitivity, specificity, and positive and negative predictive values were then calculated on a per-patient basis.
Clinical management
A summary of clinical information was provided to a senior uro-oncologist with more than 20 years of experience in PCa management who was blinded to patient-identifying information, PET/CT results, and information available afterwards. The uro-oncologist was asked to determine the theoretical management had the PET/CT not been performed. The theoretical and actual therapeutic strategies were then compared.
Statistical analysis
All statistical analyses were conducted using the software R (v.3.4.1).19 For descriptive statistics, we computed t-test for continuous variables or performed Pearson’s Chi-squared test for categorical variables. Results with p<0.05 were considered statistically significant. Finally, we evaluated inter-agreement between PET/CT and conventional imaging results using Cohen’s kappa coefficient.
Results
PET/CT results and location of disease
Seventy-six patients underwent PET/CT for initial staging of high-risk PCa with a median age of 67.8 years. Seventy-three (96%) PET/CT scans were positive, one (1%) negative, and two (3%) equivocal for prostate lesion(s). Twenty-two (29%) PET/CT scans were positive, 49 (64%) negative, and five (7%) equivocal for metastatic disease. Of the 22 positive scans, 17 (77%) demonstrated regional nodal involvement, 12 (55%) distant nodal involvement, five (23%) bone metastasis, and three (14%) lung metastasis. Examples of positive PET/CT scans are shown in Figs. 2, 3, 4.
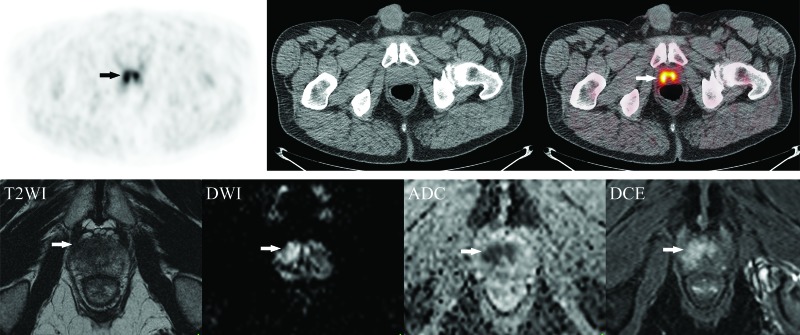
Fig. 2.
Example of local disease on 18F-fluorocholine positron emission tomography-computed tomography (18F-FCH PET/CT) with magnetic resonance imaging (MRI) correlation. (A) Axial 18F-FCH PET/CT images obtained in a 63-year-old man with prostate cancer (cT1c, Gleason score 8, prostate-specific antigen 12.6 ng/mL) showing bilateral prostate uptake (SUV 6.6) (arrows) without metastatic disease. (B) Corresponding axial MRI prostate images (from left to right: T2-weighted images [T2WI], diffusion-weighted imaging [DWI], apparent diffusion coefficient [ADC] map, and dynamic contrast-enhanced images [DCE]) demonstrating a non-circumscribed homogeneous moderately T2 hypointense lesion measuring 1.8 cm in maximal dimension located in the transition zone at the apex and mid-gland with mild extension to the right anterolateral peripheral zone (arrows). There is associated restricted diffusion on DWI/ADC and early focal enhancement on DCE (PI-RADS 5). The patient underwent radical prostatectomy with extended pelvic lymph node dissection (pT3a pN0), without evidence of biochemical recurrence after 10 months of followup. MRI images courtesy of Dr. F. Discepola, Jewish General Hospital, Montreal, QC, Canada.
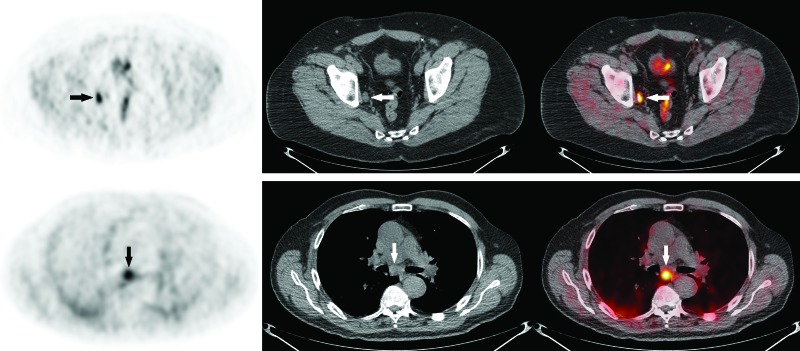
Fig. 3.
Examples of regional and distant lymph node involvement on 18F-fluorocholine positron emission tomography-computed tomography (18F-FCH PET/CT). (A) Axial 18F-FCH PET/CT images obtained in a 68-year-old man with prostate cancer (PCa) (cT1a, Gleason score 9, prostate-specific antigen [PSA] 17.2 ng/mL) showing intense 18F-FCH uptake (SUV 6.7) in a 1.1 cm right obturator lymph node (arrows). The patient underwent radical prostatectomy with pelvic lymph node dissection (pT3a pN1). (B) Axial 18F-FCH PET/CT images obtained in a 78-year-old man with metastatic PCa (cT3b, Gleason score 9, PSA 11.1 ng/mL) showing abnormal uptake in a subcarinal lymph node (arrows), proven to represent PCa metastasis on endobronchial ultrasound-guided transbronchial needle aspiration biopsy (EBUS-TBNA).
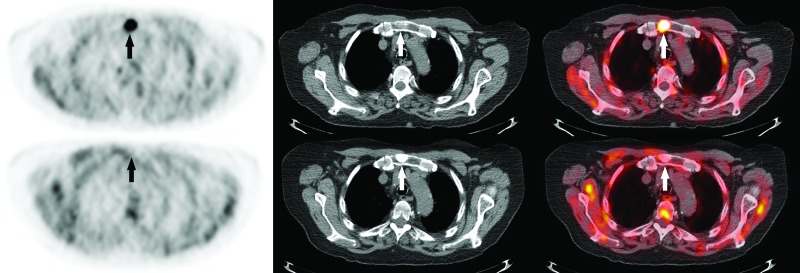
Fig. 4.
Example of bone metastasis on 18F-fluorocholine positron emission tomography-computed tomography (18F-FCH PET/CT). (A) Axial 18F-FCH PET/CT images obtained at initial staging in a 82-year-old man with prostate cancer (cT3b, Gleason score 6, prostate-specific antigen 81.0 ng/mL) showing focal intense manubrial 18F-FCH uptake (SUV 11.8) (arrows). (B) Followup PET/CT obtained four months after androgen-deprivation therapy demonstrates complete metabolic response of the manubrial lesion, which is now densely sclerotic (arrows).
Diagnostic performance and validation of PET/CT results
Excluding equivocal results (n=4) and cases where pre-established validation criteria could not be applied due to absent followup information (n=15), overall sensitivity, specificity, and positive and negative predictive values for nodal or metastatic disease were 65%, 100%, 100%, and 78%, respectively. Seventeen of 22 positive PET/CT scans for metastatic disease (77%) were validated according to treatment response on followup imaging in 12 patients (70%), histological analysis in three patients (18%), progression of lesion in one patient (6%), and correlation with conventional imaging at initial staging in one patient (6%).
Excluding equivocal results (n=2) and cases missing necessary information to validate results (n=14), sensitivity, specificity, and positive and negative predictive values were 64%, 100%, 100%, and 80%, respectively for detection of nodal metastases. Sixteen of 21 positive PET/CT scans for regional and/or non-regional lymph nodes (76%) were validated according to treatment response on followup imaging in 11 patients (69%), histological analysis in three patients (19%), and correlation with conventional imaging at initial staging in two patients (12%). Five PET/CT scans positive for nodal metastasis could not be validated due to lack of available information.
Looking more specifically at the subgroup of patients who underwent RP with PLND (n=26), we found sensitivity, specificity, and positive and negative predictive values of 10%, 100%, 100%, and 64%, respectively, for regional lymph node metastases based on histological analysis. Among these patients, nine had false-negative PET/CT results for regional lymph node metastases; six of these had macrometastases (>2 mm) and three had micrometastases (≤2 mm), as classified on histopathology.
Excluding equivocal results (n=2) and cases missing necessary information to validate results (n=11), sensitivity, specificity, and positive and negative predictive values for distant metastases were 86%, 100%, 100%, and 98%, respectively. All PET/CT scans positive for bone and lung metastases (n=6) were validated according to treatment response in four patients (67%) and progression of findings on followup imaging studies in two patients (33%).
Comparison with conventional imaging modalities
Among the 21 PET/CT scans showing lymph node involvement, conventional imaging was performed in 18 patients (86%). Of these, conventional imaging demonstrated the lesion(s) found on PET/CT in 11 patients (61%), was negative in five patients (28%), and was indeterminate in two patients (11%). Excluding indeterminate cases on conventional imaging, PET/CT and conventional imaging were both positive in 11 cases and both negative in 43 cases (good agreement; κ=0.68; p<0.001). Conventional imaging was falsely positive in one patient and falsely negative in 10 patients.
Among the five PET/CT scans showing bone involvement, bone scan was performed in four patients (80%). Of these, BS demonstrated the lesion(s) found on PET/CT in one patient (25%), was negative in two patients (50%), and was indeterminate in one patient (25%). Excluding indeterminate cases on BS, PET/CT and BS were both positive in one case and both negative in 42 cases (fair agreement; κ=0.29; p=0.048). BS was falsely positive in two patients and falsely negative in three patients
Association between patient characteristics and PET/CT results
When comparing positive and negative PET/CT scans for metastatic disease, there were statistically significant differences depending on clinical T and N stages, as well as in Gleason scores (Table 2). Of note, 18 of 22 PET/CT scans positive for metastatic disease were found in patients with a Gleason score of 9. Moreover, all five patients whose PET/CT demonstrated abnormal regional lymph node(s) despite negative initial conventional imaging (cN0) also had a Gleason score of 9. Patients with metastatic disease on PET/CT were more likely to have a higher proportion of positive cores on initial biopsy (75% vs. 56%; p=0.01), therefore, reflecting higher prostate volume disease. Finally, although we observed a trend for higher PSA levels in patients with positive PET/CT scans, this did not reach statistical significance (mean PSA 64.1 vs. 24.8 ng/mL; p=0.09).
Table 2.
Patient characteristics and PET/CT results for nodal or distant metastatic disease
| Characteristics | Positive (n=22) | Negative (n=49) | p |
|---|---|---|---|
| Age, years, mean (SD) | 68.9 (8.5) | 65.4 (7.8) | 0.096 |
| PSA at time of PET/CT, ng/mL, mean (SD) | 64.1 (155.8) | 24.8 (22.9) | 0.086 |
| PSA, stratified, ng/mL, n (%) | |||
| PSA ≤20 | 12 (54.5) | 28 (57.1) | 0.838 |
| PSA >20 | 10 (45.5) | 21 (42.9) | |
| cT stage, n (%) | |||
| T1 | 5 (22.7) | 29 (59.2) | 0.005 |
| T2 | 5 (22.7) | 12 (24.5) | |
| T3 | 11 (50.0) | 8 (16.3) | |
| T4 | 1 (4.5) | 0 (0.0) | |
| cN stage, n (%) | |||
| N0 | 9 (40.9) | 43 (87.8) | <0.001 |
| N1 | 9 (40.9) | 2 (4.1) | |
| Nx | 4 (18.2) | 4 (8.1) | |
| Gleason score, n (%) | |||
| 6 | 1 (4.5) | 2 (4.1) | <0.001 |
| 7 | 1 (4.5) | 9 (18.4) | |
| 8 | 1 (4.5) | 26 (53.1) | |
| 9 | 18 (81.8) | 12 (24.5) | |
| 10 | 1 (4.5) | 0 (0.0) | |
| Proportion of positive cores on biopsy, % (SD) (NA=1) | 74.6 (31.6) | 56.0 (26.2) | 0.013 |
| ADT at time of the PET/CT, n (%) (NA=4) | |||
| Yes | 5 (22.7) | 4 (8.2) | 0.110 |
| No | 17 (77.3) | 41 (83.7) |
ADT: androgen-deprivation therapy; NA: not available; PET/CT: positron-emission tomography-computed tomography; PSA: prostate-specific antigen; SD: standard deviation.
Comparison between theoretical and actual clinical management strategies
The theoretical and actual management strategies differed in nine patients (12%). In five cases, pelvic EBRT was added to ADT for treatment of locoregional disease after PET/CT excluded distant metastasis. In three cases, surgery was preferred after PET/CT clarified indeterminate results on CT and/or BS. Finally, in one case, stereotactic body radiation therapy (SBRT) was added to pelvic EBRT for treatment of oligometastatic skeletal disease.
Discussion
In this study, we report our initial Canadian, single-centre experience with 18F-FCH PET/CT in the context of initial staging of high-risk PCa.
The value of 18F-FCH PET/CT in initial staging of intermediate-to high-risk PCa is controversial, with previous studies demonstrating low sensitivity and high specificity.12,15,17,20 In a meta-analysis including 441 patients studying the role of 11C-Choline and 18F-FCH, Evangelista et al found a pooled sensitivity and specificity of 49.2% (95% confidence interval [CI] 39.9–58.4) and 95% (95% CI 92–97.1) for nodal involvement on a per-patient basis.20 In a study including 132 patients with intermediate- to high-risk PCa, Beheshti et al reported sensitivity, specificity, and positive and negative predictive values of 45%, 96%, 82%, and 83%, respectively, for malignant lymph nodes on per-patient analysis.12 Similar to previous studies, we found sensitivity, specificity, and positive and negative predictive values of 64%, 100%, 100%, and 80%, respectively, for metastatic disease to lymph nodes. Unlike other previous studies, we included only patients with high-risk PCa, which could partly explain the higher positive predictive value obtained. In the subgroup of patients who underwent RP with PLND and for whom histological analysis was available (n=26), we found that PET/CT had a low sensitivity of 10% and high specificity of 100% for regional lymph node metastases, with a high rate of false-negative results (nine patients). This is consistent with Kjölhede et al, who found that 18F-FCH PET/CT had a low sensitivity of 33% and high specificity of 92% in a study including 112 patients with extensive PLND.16 Finally, as previously reported, we also found a higher sensitivity for bone and other metastases (86%) than for nodal metastases (64%).13
Interestingly, the majority of patients with a PET/CT positive for metastatic disease (18/22 patients, 82%) had a Gleason score of 9, as did all five patients whose PET/CT demonstrated abnormal regional lymph node(s) despite negative initial conventional imaging (cN0). Therefore, we could hypothesize that perhaps the greatest utility of 18F-FCH PET/CT at detecting metastatic disease during initial staging lies in patients with Gleason scores ≥9. Nevertheless, interpretation of the results remains limited by the fact that a multivariate analysis was not performed due to our relatively small sample size. Other patient factors that are integral to the initial clinical risk stratification (clinical staging and PSA) must be considered, as they may also likely contribute to PET/CT positivity.
18F-FCH PET/CT detected metastatic disease not otherwise identified by conventional imaging in 5/18 cases of nodal involvement (28%) and 2/4 cases of bone metastases (50%). These results are consistent with Evangelista et al, who established that 18F-FCH PET/CT had a higher sensitivity than CT (69.2% vs. 46.2%) and BS (100% vs. 90%) for detection of metastatic disease.13
In our study, we determined that the theoretical retrospective management strategy differed from the actual prospective management strategy in nine patients (12%). This result is in agreement with previous studies showing a change in the therapeutic option in 5–20% of cases using 18F-FCH PET/CT for staging of intermediate- to high-risk PCa.12,13,21,22
Since the introduction of 18F-FCH PET/CT, more sensitive and specific PET tracers have been developed to increase the yield of PET/CT at initial staging and for evaluation of biochemical recurrence. In a study including 130 patients with intermediate- to high-risk PCa who underwent RP with PLND, Maurer et al found that 68Ga-PSMA-PET/CT had a sensitivity of 65.9% and specificity of 98.9% for detection of lymph node metastases on a per-patient basis.23 Similarly, in a smaller study including 34 patients who underwent 68Ga-PSMA-PET/CT for nodal staging prior to RP with primary PLND or secondary PLND, Herlemann et al obtained a sensitivity of 84% and specificity of 82%.24 Although 18F-FCH PET/CT offers some benefits over conventional imaging and demonstrates a high specificity, it remains limited by its sensitivity in the context of high-risk PCa staging. For this reason, we suggest that future research be done with novel tracers such as 68Ga-PSMA, as it may overcome some of the limitations encountered.
There are several limitations to our study. The main limitation consists of the lack of histological confirmation available for detected metastatic lesions with PET/CT in a total of 19 patients (25%). This limitation is particularly emphasized when looking at patients who underwent non-surgical management, such as EBRT or chemotherapy, where histological confirmation of the metastatic lesions seen was not available in 17 patients (22%). This also applies to patients whose PET/CT revealed distant nodal (10 patients, 13%) and osseous metastatic lesions (six patients, 8%) for which biopsy is rarely clinically justified or performed. Therefore, we opted for a set of predefined validation criteria based on response to treatment or progression of disease on subsequent imaging and comparison with findings on conventional imaging modalities, as done in previous studies evaluating the role of 18F-FCH PET/CT in staging of PCa.12,13
Another limitation of our study consists of the inclusion of a few patients who received ADT prior to PET/CT. While the nuclear medicine specialist who interpreted the PET/CT studies was not blinded to the ADT status of patients scanned, this could have led to error in the interpretation of 18F-FCH PET/CT. Nine patients (12%) received ADT before PET/CT; among those, there were three false-negative results (one bone and two regional lymph node metastases). As ADT is known to decrease fluorocholine uptake in sites of hormone-sensitive disease, this may have contributed to the overall false-negative rate.25,26 Therefore, we suggest that 18F-FCH PET/CT done for staging of high-risk PCa should be avoided in men who previously received ADT. It is important to clarify, however, that ADT is not considered a contraindication to 18F-FCH PET/CT in the evaluation of castration-resistant prostate cancer (CRPC), as 18F-FCH PET/CT has shown some utility in mapping resistant disease and subsequently assessing treatment efficacy.27,28
Finally, our study remains limited by its retrospective design, incomplete patient followup information, and relatively small sample size. Nevertheless, to the best of our knowledge, our study represents one of the largest single cohorts of patients with high-risk PCa studied with 18F-FCH PET/CT.
Conclusion
Although 18F-FCH PET/CT offers some benefits over conventional imaging and demonstrates a high specificity, it remains limited by its sensitivity in the context of high-risk PCa staging. PET with novel urea-based small-molecule PSMA inhibitors may overcome some of these limitations.
Footnotes
Competing interests: This work was supported by the Departments of Nuclear Medicine and Urology, Jewish General Hospital, Montreal, QC, Canada. Dr. Probst has been an advisor for Bayer and has participated in a clinical trial supported by Progenics. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Cancer.ca. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. 2017. [Accessed May 19, 2018]. [updated 2017; cited 2018 May 19]. Available at: http://www.cancer.ca/
- 2.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Cowan J, Broering JM, et al. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26:211–8. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner AB, Matulewicz RS, Eggener SE, et al. Increasing incidence of metastatic prostate cancer in the United States (2004–2013) Prostate Cancer Prostatic Dis. 2016;19:395–97. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 5.Sanda MG, Chen RC, Crispino T, et al. 2017. [Accessed Nov 24, 2017]. [cited 2017 November 24]. Available at: http://www.auanet.org/guidelines/clinically-localized-prostate-cancer-new-(aua/astro/suo-guideline-2017)
- 6.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED, Stone NN, Yu EY, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83:664–9. doi: 10.1016/j.urology.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Vali R, Loidl W, Pirich C, et al. Imaging of prostate cancer with PET/CT using (18)F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96–108. [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, et al. Overexpression of choline kinase is a frequent feature in human tumour-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296:580–3. doi: 10.1016/S0006-291X(02)00920-8. [DOI] [PubMed] [Google Scholar]
- 12.Beheshti M, Imamovic L, Broinger G, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: A prospective study of 130 patients. Radiology. 2010;254:925–33. doi: 10.1148/radiol.09090413. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista L, Cimitan M, Zattoni F, et al. Comparison between conventional imaging (abdominal-pelvic computed tomography and bone scan) and [(18)F]choline positron emission tomography/computed tomography imaging for the initial staging of patients with intermediate-tohigh-risk prostate cancer: A retrospective analysis. Scand J Urol. 2015;49:345–53. doi: 10.3109/21681805.2015.1005665. [DOI] [PubMed] [Google Scholar]
- 14.Gauvin S, Cerantola Y, Haberer E, et al. Initial single-centre Canadian experience with 18F-fluoromethylcholine positron emission tomography-computed tomography (18F-FCH PET/CT) for biochemical recurrence in prostate cancer patients initially treated with curative intent. Can Urol Assoc J. 2017;11:47–52. doi: 10.5489/cuaj.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacker A, Jeschke S, Leeb K, et al. Detection of pelvic lymph node metastases in patients with clinically localized prostate cancer: comparison of [18F]fluorocholine positron emission tomography-computerized tomography and laparoscopic radioisotope guided sentinel lymph node dissection. J Urol. 2006;176:2014–8. doi: 10.1016/j.juro.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Kjölhede H, Ahlgren G, Almquist H, et al. (1)(8)F-fluorocholine PET/CT compared with extended pelvic lymph node dissection in high-risk prostate cancer. World J Urol. 2014;32:965–70. doi: 10.1007/s00345-013-1189-x. [DOI] [PubMed] [Google Scholar]
- 17.Umbehr MH, Muntener M, Hany T, et al. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: A systematic review and meta-analysis. Eur Urol. 2013;64:106–17. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 19.Team RC. Vienna, Austria: R Core Team; 2017. [Accessed Nov. 24, 2017]. [cited 2017 November 24]. Available at: https://www.R-project.org/ [Google Scholar]
- 20.Evangelista L, Guttilla A, Zattoni F, et al. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate-to high-risk prostate cancer: A systematic literature review and meta-analysis. Eur Urol. 2013;63:1040–8. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: A comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766–74. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]
- 22.Kjolhede H, Ahlgren G, Almquist H, et al. Combined 18F-fluorocholine and 18F-fluoride positron emission tomography/computed tomography imaging for staging of high-risk prostate cancer. BJU Int. 2012;110:1501–6. doi: 10.1111/j.1464-410X.2012.11123.x. [DOI] [PubMed] [Google Scholar]
- 23.Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Herlemann A, Wenter V, Kretschmer A, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–7. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 25.Evangelista L, Zattoni F, Guttilla A, et al. The effects of androgen-deprivation therapy on the 18F-choline uptake in prostate cancer patients undergoing neoadjuvant treatment. Q J Nucl Med Mol Imaging. 2016 Jul 7; doi: 10.23736/S1824-4785.16.02877-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Giovacchini G, Picchio M, Coradeschi E, et al. [(11)C]choline uptake with PET/CT for the initial diagnosis of prostate cancer: Relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging. 2008;35:1065–73. doi: 10.1007/s00259-008-0716-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Sato MM, Coel MN, et al. Prediction of PSA progression in castration-resistant prostate cancer based on treatment-associated change in tumour burden quantified by 18F-fluorocholine PET/CT. J Nucl Med. 2016;57:1058–64. doi: 10.2967/jnumed.115.169177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Giorgi U, Caroli P, Burgio SL, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448–58. doi: 10.18632/oncotarget.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]