Abstract
Phagocytosis is critical to tissue homeostasis, as highlighted by phagocytosis defect of retinal pigment epithelial (RPE) cells with debris accumulation, photoreceptor degeneration and blindness. Phagocytosis ligands are the key to delineating molecular mechanisms and functional roles of phagocytes, but are traditionally identified in individual cases with technical challenges. We recently developed open reading frame phage display (OPD) for phagocytosis-based functional cloning (PFC) to identify unknown ligands. One of the identified ligands was Ly-1 antibody reactive clone (Lyar) with functions poorly defined. Herein we characterized Lyar as a new ligand to stimulate RPE phagocytosis. In contrast to its reported nucleolar expression, immunohistochemistry showed that Lyar was highly expressed in photoreceptor outer segments (POSs) of the retina. Cytoplasmic Lyar was released from apoptotic cells, and selectively bound to shed POSs and apoptotic cells, but not healthy cells. POS vesicles engulfed through Lyar-dependent pathway were targeted to phagosomes and colocalized with phagosome marker Rab7. These results suggest that Lyar is a genuine RPE phagocytosis ligand, which in turn supports the validity of OPD/PFC as the only available approach for unbiased identification of phagocytosis ligands with broad applicability to various phagocytes.
Keywords: Lyar, Ly-1 antibody reactive clone, Phagocytosis, Retinal pigment epithelial cell, RPE, Phagocytosis ligand
INTRODUCTION
Phagocytosis is an important biological process to clear apoptotic cells, cellular debris and deleterious metabolic products for tissue homeostasis, injury repair and innate immune balance (Erwig and Henson, 2007; Ravichandran and Lorenz, 2007; Sierra et al., 2013). Phagocytosis dysfunction may lead to debris accumulation, autoimmune diseases and tissue degenerations. For example, phagocytosis by retinal pigment epithelial (RPE) cells is essential to maintain retinal homeostasis and photoreceptor viability (Strauss, 2005). Photoreceptor outer segments (POSs) are susceptible to photooxidation damage, which is removed by shedding membrane vesicles at the tip of POSs. RPE underneath photoreceptors engulfs shed POS vesicles and recycles nutrients for POS regeneration. The importance of RPE phagocytosis is highlighted by MerTK receptor, whose mutation or deletion in humans, rats and mice causes RPE phagocytosis defect with accumulation of unphagocytosed POS vesicles, leading to retinal degeneration and blindness (D’Cruz et al., 2000; Dowling and Sidman, 1962; Duncan et al., 2003; Gal et al., 2000). Emerging evidence suggests that phagocytosis dysfunction in aged tissues may contribute to accumulation of metabolic debris with chronic sterile inflammation and tissue degeneration, such as age-related macular degeneration (AMD) and Alzheimer’s disease (Li, 2013).
RPE phagocytosis pathways are poorly defined because their signaling molecules are traditionally identified on a case-by-case basis with technical challenges. Beyond a limited number of characterized phagocytosis ligands and receptors (Li, 2012a), we really do not know how many pathways are yet to be identified and which ones may be relatively active or disease-relevant. For instance, despite identification of five MerTK ligands (Burstyn-Cohen et al., 2012; Caberoy et al., 2012a; Caberoy et al., 2010c), we still do not know which one is disease-relevant or if such a ligand is yet to be identified. It is even more difficult to identify age-dependent phagocytosis ligands or pathways.
To tackle the challenges, we proposed a new notion that phagocytosis ligands are the key to unraveling the mystery of molecular phagocyte biology (Li, 2012a). We recently developed the new methods of open reading frame phage display (OPD) and phagocytosis-based functional cloning (PFC) for systematic identification of unknown ligands in the absence of receptor information (Caberoy et al., 2010b; Li, 2012a; Li, 2012b). The validity of OPD/PFC was demonstrated by identification and characterization of Tulp1 as a new RPE phagocytosis ligand (Caberoy et al., 2010a; Caberoy et al., 2010c). Another ligand identified was Ly-1 antibody reactive clone (Lyar) (Caberoy et al., 2010a).
Lyar was first cloned from the cDNA library of a mouse T cell leukemia line and has been implicated in the regulation of cell growth as a nucleolar oncoprotein with zinc-finger DNA-binding motifs (Su et al., 1993). Lyar is expressed at high levels in the testis, leukemia and embryonic stem cells (Cai et al., 2006; Su et al., 1993). Recent studies showed that Lyar accelerates the processing of preribosomal RNA (Miyazawa et al., 2014). Mutations in Lyar and p53 genes are synergistically lethal in female mice with neural tube defect (Wang et al., 2012). However, the functional roles of Lyar beyond RNA processing and growth regulation are poorly understood.
In this study, we independently characterized Lyar as an RPE phagocytosis ligand. Our results showed that Lyar is predominantly expressed in cytoplasmic POSs, instead of the nucleoli. Lyar was released from apoptotic cells and selectively bound to shed POS vesicles and apoptotic cells, but not healthy cells. Lyar stimulated phagocytosis of POS vesicles by D407 RPE cell line and primary RPE cells, and targeted ingested cargos to phagosomes. Characterization of Lyar as a bona fide RPE ligand in this study provides new insights into molecular mechanisms of RPE phagocytosis. These results further support the validity of our OPD/PFC, which is the only available approach for unbiased identification of phagocytosis ligands in the absence of any molecular probe. The new approach is broadly applicable to many other phagocytes.
MATERIALS AND METHODS
Materials and cell culture
D407, Neuro-2a and HEK293 cell lines were cultured as described (Caberoy et al., 2010c). Culture media and supplemental reagents were from Life Technologies (Grand Island, NY). Rabbit anti-Lyar and anti-Rab7 antibodies were purchased from Abcam (Cambridge, MA). Anti-rhodopsin 1D4 monoclonal antibody (mAb) was from Millipore (Billerica, MA). FITC-labeled or unlabeled anti-FLAG mAb was purchased from Sigma (St. Louis, MO). All secondary antibodies labeled with fluorescence or horseradish peroxidase (HRP), pHrodo™ succinimidyl ester and EGF were from Life Technologies. bFGF was from PeproTech (Suzhou, Jiangsu, China). Green fluorescent protein (GFP)-FLAG plasmid was previously described (Caberoy et al., 2010a).
Lyar plasmids
Full-length coding region of mouse Lyar (NM_025281) was generated by reverse transcription-PCR (RT-PCR) and verified by sequencing. The coding sequence was amplified by PCR with a C-terminal FLAG tag and cloned into pEGFP-N1 plasmid (Clontech, Mountain View, CA) at Nhe I and Not I sites to replace GFP and generate Lyar-FLAG plasmid. In addition, the coding sequence was amplified by PCR, digested with BamH I and Xho I, and cloned into pMAL-c4E plasmid (New England Biolab, Ipswich, MA) at BamH I and Sal I sites to generate maltose-binding protein (MBP)-Lyar plasmid. All plasmids were verified by DNA sequencing.
Primary RPE cells
Primary RPE cells were prepared as described (Caberoy et al., 2010a) with the following modifications. The eyes were isolated from euthanized C57BL/6 mice at postnatal day 10. After removal of the cornea, lens and retina, the RPE cups were digested with 1X trypsin/EDTA for 3 min at 37°C. RPE cells were collected by pipetting, washed and cultured in Minimum Essential Medium Eagle (MEM) Alpha Modification (Sigma) supplemented with 10% FBS, 2 mM L-Glutamine, 1X nonessential amino acids, penicillin/streptomycin, bFGF (10 ng/ml), EGF (1 ng/ml), 1X N1 supplement and THT (taurine, 210 ng/ml; hydrocortisone 1.2 µg/ml; triiodothyronin, 60 ng/ml) (Sigma) (Salero et al., 2012). The medium was replaced every 2–3 days until pigmented RPE spheres grew out. The spheres were dissociated with trypsin, washed and cultured as monolayer in the same medium without bFGF and EGF for 3 days before phagocytosis assay.
All animal procedures and protocols were approved by the Institutional Animal Care and Use Committee at the University of Miami and complied with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH).
POS vesicles
POS vesicles were prepared as described (Caberoy et al., 2010a). Briefly, fresh bovine eyes within 24 h postmortem were purchased from Pel-Freez Biologicals (Rogers, AR). The POSs were detached from isolated retinas by gentle shaking at 4°C for 15 min in PBS containing 2.5% sucrose. After removal of the retinas, detached POS vesicles were collected and washed twice by centrifugation at 38,700 x g for 30 min. Purified vesicles were labeled with pHrodo, as described (Caberoy et al., 2012b). Briefly, POS vesicles (500 µg protein) were incubated with pHrodo (20 ng/ml in PBS, stock 1 mg/ml in DMSO) for 30 min at room temperature, followed by incubation with 1% BSA in PBS for 15 min. The labeled vesicles were washed twice with PBS by centrifugation at 16,000 x g for 30 min before phagocytosis assay.
Shed POS vesicles were analyzed by flow cytometry using FITC-labeled annexin V. Alternatively, shed POSs were immunostained with rabbit anti-Lyar and mouse anti-rhodopsin antibodies, followed by Alexa 594-labeled goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG antibodies. Immunostained POS vesicles were analyzed by a fluorescent microscope.
RT-PCR
Total RNA was prepared from fresh mouse retinas (C57BL/6, 6–8 weeks old) or HEK293 cells pre-transfected with Lyar-FLAG plasmid. RT-PCR was performed as described (Caberoy et al., 2010a) with the following primers: 5’-GTGCAGCGAACTTTATTGATGG-3’ and 5’-TGGTGAAGCAGGCATCTGAG-3’ for GAPDH; 5’-ATGGTATTTTTTACATGCAATG-3’ and 5’-CGGCGCTTCTTTGGCTTCTGGC-3’ for Lyar. The PCR products were analyzed on 1% agarose gel.
Western blot
Mouse retinas or HEK293 cells pre-transfected with Lyar-FLAG plasmid were homogenized in RIPA buffer (Pierce, Rockford, IL) and analyzed by Western blot using anti-Lyar antibody and HRP-conjugated secondary antibody, as described (Li and Handschumacher, 2002).
Recombinant Lyar
MBP-Lyar and pMAL-c4E control plasmids were transformed into BL21(DE3) bacteria. After induction of protein expression with IPTG, MBP-Lyar and MBP were purified with amylose columns, dialyzed against PBS and analyzed by SDS-PAGE, as described (Kim et al., 2011).
Immunohistochemistry
Mice (C57BL/6, 6–8 weeks of age) under anesthesia were perfused intracardially with 10% formalin. The eyes were nucleated and fixed with the same solution overnight at 4oC. After removal of the cornea and lens, the eye cups were incubated with sucrose gradient solutions (10% and 20% for 3 h each; 30% for overnight) at 4oC, followed by 3 rounds of freeze-thaw and OCT embedding. Frozen tissue sections in 7-µm thickness were incubated with rabbit anti-Lyar and mouse anti-rhodopsin antibodies, followed by Alexa 594-labeled goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG antibodies. The nuclei were labeled with DAPI. The fluorescence signals were analyzed by confocal microscopy.
Phagocytosis assay
D407 RPE or primary RPE cells were seeded on coverslips precoated with poly-L-lysine (Sigma) in 12-well plates and cultured overnight. pHrodo-labeled POSs (50 µg/ml) were added to RPE cells for phagocytosis in the presence of MBP-Lyar or MBP control with indicated concentrations at 37°C for 3 h. After washing, the cells were fixed for 10 min in 4% paraformaldehyde, mounted with DAPI and analyzed by confocal microscopy. Intracellular pHrodo signals were quantified by ImageJ software (NIH), normalized against the cell number in each viewing field and expressed as relative fluorescence intensity/cell.
Immunocytochemistry
D407 RPE cells with phagocytosed pHrodo-labeled POSs were fixed with 4% paraformaldehyde, permeabilized with PBS containing 1% Triton X-100, immunostained with anti-Rab7 antibody, followed by FITC-labeled secondary antibody. The nuclei were visualized by DAPI. The intracellular fluorescence signals were analyzed by confocal microscopy.
Lyar externalization
Lyar-FLAG plasmid was transfected into HEK293 or Neuro-2a cells with JetPrime reagents (Polyplus Transfection, Illkirch, France) for 48 h. The cells were treated with or without etoposide (200 µM) to induce apoptosis for 12 h (Caberoy et al., 2010c) and detached by pipetting. After washing, the cells were labeled with anti-Lyar primary antibody and Texas red-labeled secondary antibody to detect cell surface-bound Lyar. Apoptotic cells were labeled with FITC-annexin V or propidium iodine (PI). The nuclei were visualized with DAPI. The results were analyzed by a fluorescence microscope.
POS binding assay
HEK293 cells were transfected with Lyar-FLAG or GFP-FLAG plasmid for 48 h. The cell lysates were prepared in PBS with a cocktail of protease inhibitors (Sigma) in the absence of any detergent by 3 cycles of freeze-thaw, followed by centrifugation at 16,000 x g for 30 min at 4°C. The lysates were filtered through 0.2 µm filters. POS vesicles (500 µg protein) were incubated with the cell lysates for 1 h at 4°C, washed 5 times with PBS by centrifugation. POS-bound Lyar-FLAG or GFP-Lyar was detected by anti-FLAG mAb, followed by HRP-conjugated secondary antibody. HRP signal was developed by colorimetric assay and quantified at OD490 (Caberoy et al., 2010b).
Lyar binding to apoptotic cell surface
MPB-Lyar and MBP were labeled with fluorescein succinimidyl ester (Life Technology) as described (Caberoy et al., 2010a), dialyzed against PBS and incubated with apoptotic or healthy cells. After washing, cells were analyzed by confocal microscopy to detect cell surface-bound Lyar.
Statistical analysis
All experiments were repeated independently at least 3 times. Data were expressed as means ± SEM and analyzed by paired t-test or one-way ANOVA test using GraphPad Instat software. Data in Fig. 2B were log transformed before the statistical analysis. Data were considered significant when p < 0.05.
Fig. 2.
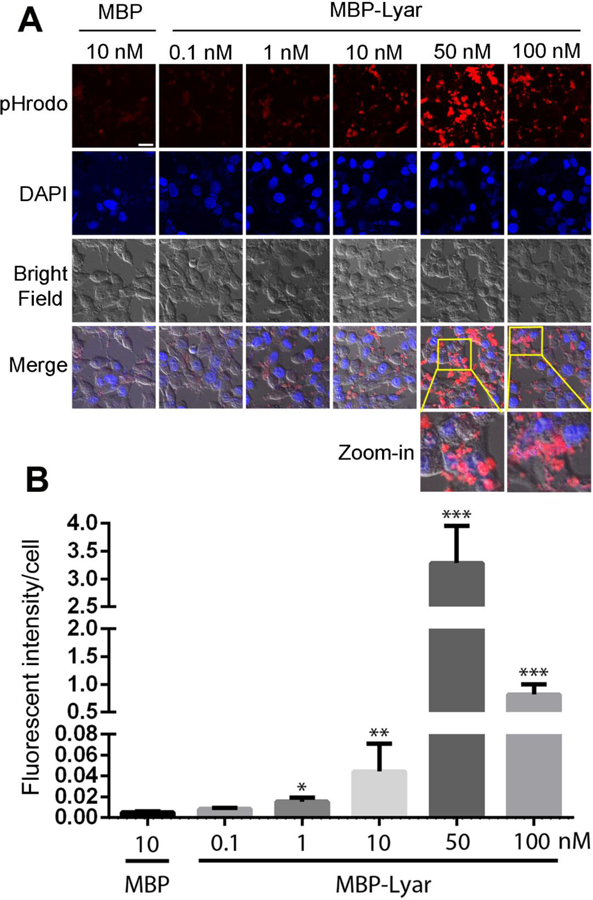
Lyar promotes RPE phagocytosis in a dose-dependent manner. (A) RPE phagocytosis was performed as in Fig. 1 with increased concentrations of MBP-Lyar. MBP control was included as a control. Bar = 20 µm. (B) Relative fluorescence intensity of D407 cells with phagocytosed cargos in (A) was quantified (± SEM, n=10, one-way ANOVA test, * P<0.05, ** P<0.01, *** P<0.001, vs. MBP).
RESULTS
Lyar stimulates POS phagocytosis by D407 RPE cells
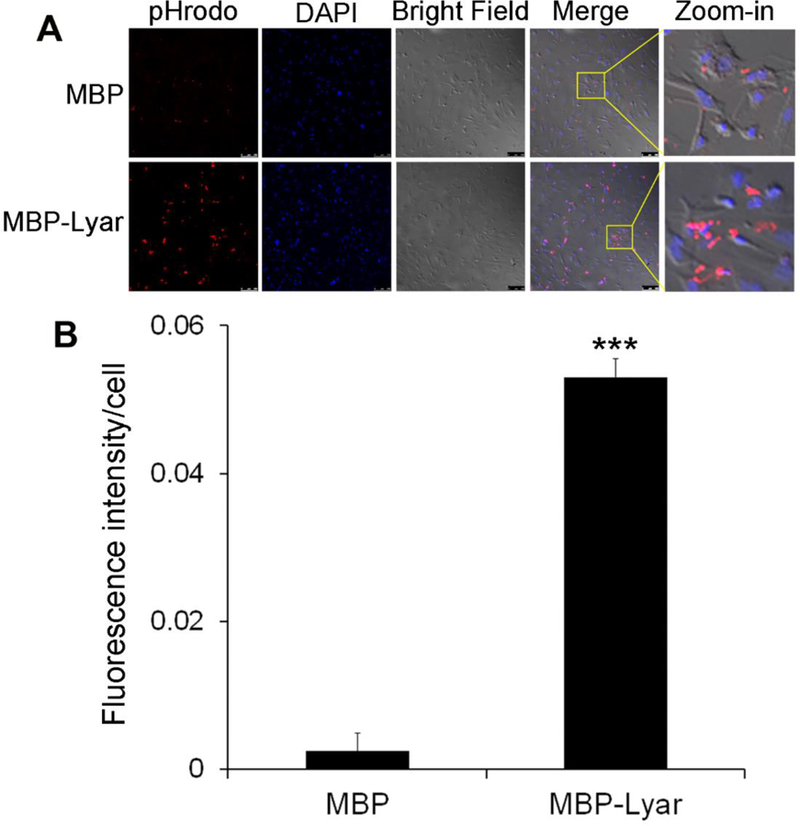
To characterize Lyar as a phagocytosis ligand, we expressed and purified MBP-Lyar and MBP (Supplementary Fig. 1). POS vesicles were prepared from fresh bovine eyes and labeled with pHrodo dye for phagocytosis assay in the presence or absence of purified MBP-Lyar fusion protein or MBP control. pHrodo is a pH-sensitive fluorogenic dye that drastically increases its fluorescence intensity after engulfment into acidic phagosomes (Caberoy et al., 2012b). Coupled with confocal microscopy, pHrodo labeling enabled reliable distinction between ingested and cell surface-bound cargos. The results showed that purified Lyar stimulated phagocytosis of POSs by human D407 RPE cells (Fig. 1A,B). Superimposed images of pHrodo signals with the cognate bright field clearly indicated that labeled POS vesicles were engulfed into D047 cells. Tubby was recently characterized as an RPE phagocytosis ligand (Caberoy et al., 2010c) and was used as a positive control for the phagocytosis assay (Supplementary Fig. 2).
Fig. 1.
Lyar stimulates phagocytosis of POSs by D407 RPE cells. (A) POS vesicles were labeled with pHrodo and incubated with D407 cells for phagocytosis in the presence of purified MBP-Lyar (50 nM) or MBP control. After washing, phagocytosed POSs were analyzed by confocal microscopy. Scale bar = 50 µm. (B) Relative fluorescence intensity of D407 cells with phagocytosed cargos in (A) was quantified (± SEM, n=7, t-test, ***P<0.001).
Lyar facilitated RPE phagocytosis in a dose-dependent manner (Fig. 2A,B). Lyar triggered significantly higher phagocytosis of POSs at 1 nM and reached to the maximal activity at 50 nM with ~625-fold increase in engulfed signals. Interestingly, Lyar at 100 nM had a reduced capacity to stimulate RPE phagocytosis. Control MBP showed minimal activity.
Lyar expression in the retina
Unlike macrophages and microglia with mobility, RPE is a stationary phagocyte. The location of Lyar expression in the retina is highly relevant to its role as an RPE phagocytosis ligand. We verified Lyar expression in the retina by RT-PCR (Fig. 3A). Lyar was detected in both retina homogenate and shed POSs by Western blot (Fig. 3B, Supplementary Fig. 3). It is worth noting that no β-actin was detected in shed POSs, probably due to the special structure of POSs. We further characterized Lyar expression in the retina by immunohistochemistry. The results showed that Lyar is predominantly expressed in POSs and is fully colocalized with POS biomarker rhodopsin (Fig. 3C), suggesting that Lyar has a convenient access to RPE for phagocytosis. Expression of Lyar and rhodopsin in shed POSs were verified by immunostaining using anti-Lyar and anti-rhodopsin antibodies (Supplementary Fig. 4).
Fig. 3.
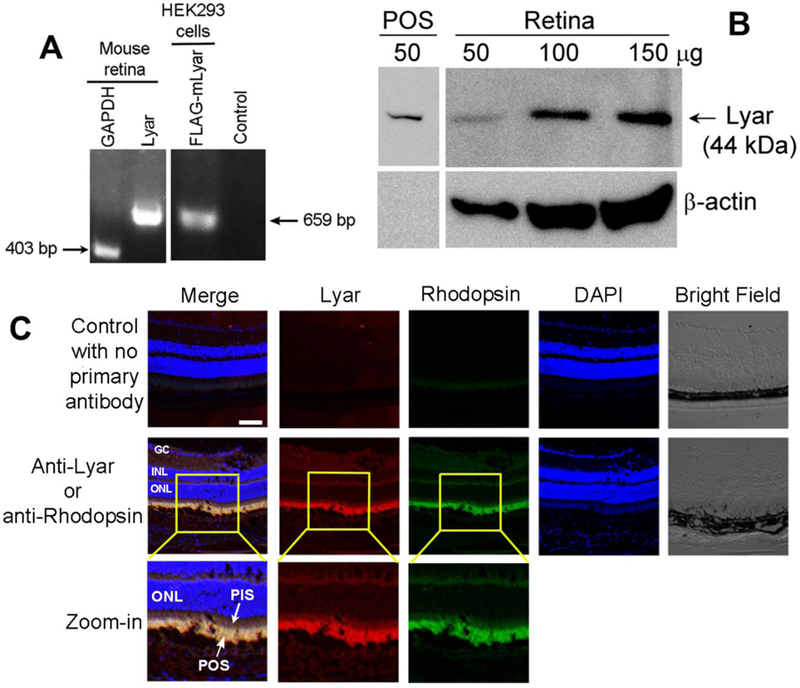
Lyar is expressed in the retina. (A) Lyar mRNA transcript in the retina was detected by RT-PCR. Housekeeping gene GAPDH was included as a positive control. HEK293 cells pre-transfected with Lyar-FLAG plasmid was used as a positive control. No template was used as a negative control. (B) Lyar expression in mouse retina or shed POSs was analyzed by Western blot with different amount of total retinal proteins. (C) Lyar expression in mouse retina was analyzed by immunohistochemistry. GC, ganglion cell; INL, inner nuclear layer; ONL, outer nuclear layer; PIS, photoreceptor inner segment.
Lyar was previously reported to be expressed predominantly in the nucleoli for promoting cell growth and oncogenic transformation (Su et al., 1993). In contrast, our results showed Lyar expression in cytoplasmic POSs, instead of the outer nuclear layer (ONL) of the photoreceptors (Fig. 3C). Moreover, Lyar expression in postmitotically differentiated photoreceptors suggests that Lyar may participate in cellular function(s) other than cell proliferation. Our finding of Lyar as a ligand to stimulate RPE phagocytosis supports this notion.
Lyar extracellular trafficking and selective binding to phagocytosis cargos
One of the criteria for a protein to be qualified as a phagocytosis ligand is its extracellular trafficking for access to phagocytosis cargos and receptors. Phagocytosis ligands can be either secreted through conventional or nonconventional pathways, or passively released from apoptotic cells. Another criterion is that phagocytosis ligands should discriminatively bind to apoptotic cells or other intended cargos, but not healthy cells, so that phagocytosis will not destroy healthy cells.
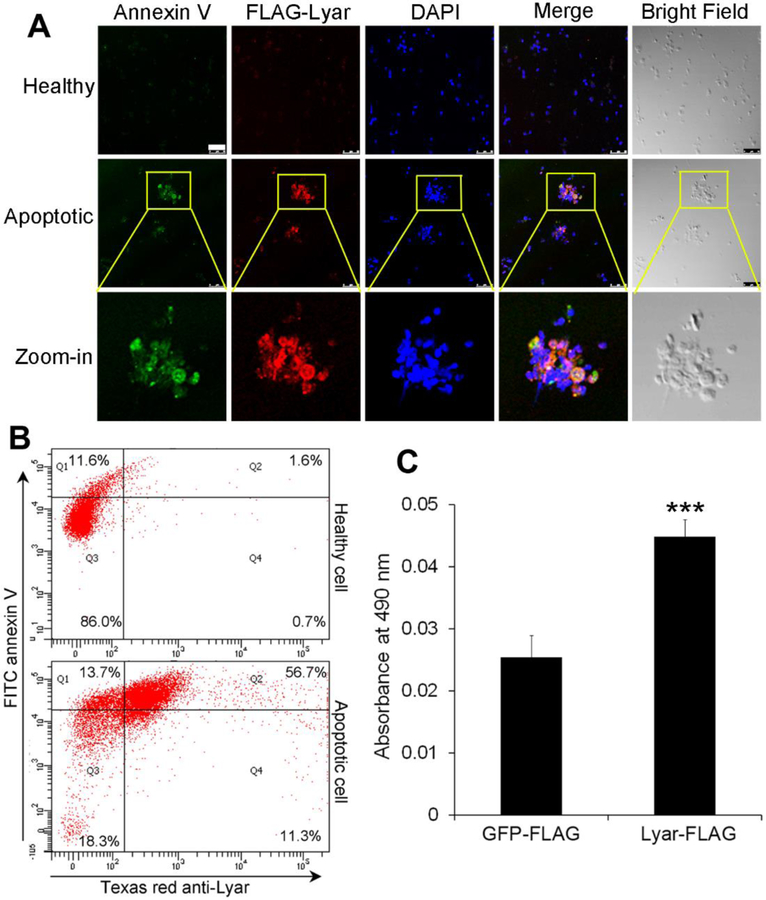
Lyar is a cytoplasmic protein with no signal peptide for conventional secretion. To demonstrate its extracellular trafficking and selective cargo recognition, we expressed Lyar-FLAG in HEK293 cells, which were treated with or without etoposide to induce apoptosis. Immunocytochemistry revealed that Lyar was detected on the surface of apoptotic cells but not healthy cells (Fig. 4A,B, Supplementary Fig. 5), suggesting that Lyar was released from and selectively bound to apoptotic cells in an autocrine manner.
Fig. 4.
Lyar selectively binds to apoptotic cells and shed POS vesicles, but not healthy cells. Lyar-FLAG was expressed in HEK293 (A) or Neuro-2a cells (B), followed by induction of apoptosis. Lyar binding to apoptotic cells was detected with anti-FLAG antibody, followed by Texas red-labeled secondary antibody. Apoptotic cells were labeled with FITC-annexin V and analyzed by fluorescence microscopy (A) or flow cytometry (B). Bar = 50 μm. (C) Lyar binds to POS vesicles. POS vesicles were incubated with Lyar-FLAG or GFP-FLAG lysate. After washing, POS-bound FLAG-tagged protein was detected by anti-FLAG mAb and HRP-labeled secondary antibody, followed by colorimetric assay (± SEM, n=5, t-test, ***P<0.001)
Our previous study showed that plasma membrane-bound GFP was detected as fluorescent rings on the edge of cells by confocal microscopy, whereas soluble GFP distributed uniformly throughout the cytoplasm (Caberoy et al., 2010a). Our results showed that FITC-MBP-Lyar, but not FITC-MBP, selectively bound to the surface of apoptotic cells (Supplementary Fig. 6). Little FITC-Lyar was detected in the cytoplasm and nuclei of apoptotic cells. These data suggest that Lyar specifically binds to the surface of apoptotic but not healthy cells.
Shed POS vesicles share many similarities to apoptotic bodies as phagocytosis cargos, including surface expression of phosphatidylserine (Fig. 4A,B, Supplementary Fig. 7). Thus, Lyar can selectively bind to the surface of apoptotic cells and shed POSs, but not healthy cells (Fig. 4A, B, Supplementary Fig. 4). Moreover, we incubated Lyar-FLAG or GFP-FLAG cell lysate with POS vesicles and demonstrated that Lyar-FLAG bound to the surface of shed POS vesicles (Fig. 4C). GFP-FLAG was a negative control.
Lyar-dependent engulfment is coupled to phagosomes
To verify that Lyar-mediated engulfment of pHrodo-labeled POSs by RPE is through the phagocytic pathway, we detected the distribution of phagosome biomarker Rab7 and demonstrated that Rab7 was colocalized with ingested POSs (Fig. 5). This result suggests that Lyar-mediated internalization is a phagocytosis process by targeting the ingested POSs to phagosomes.
Fig. 5.
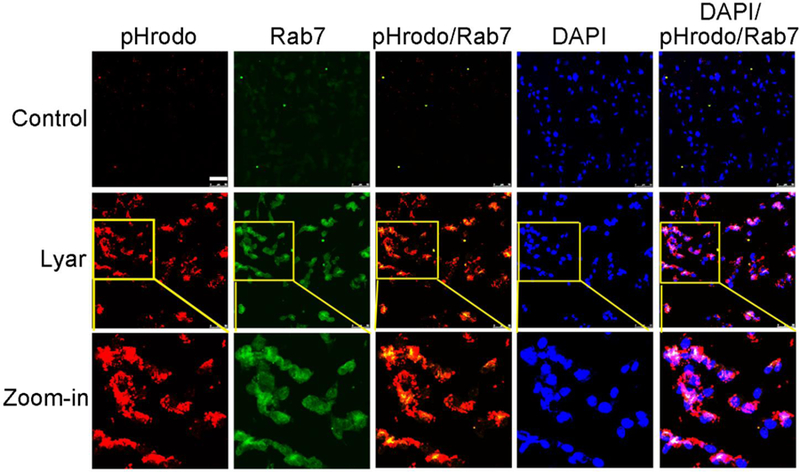
Lyar mediates RPE engulfment of POSs via phagocytosis pathway. POS phagocytosis by D407 cells was performed as in Fig. 1. Phagosome marker Rab7 was detected using anti-Rab7 antibody and FITC-labeled secondary antibody, and analyzed by confocal microscopy. pHrodo signals and FITC signals are co-localized and superimposed with DAPI signals. Bar = 50 µm.
Lyar facilitates POS phagocytosis by primary RPE cells
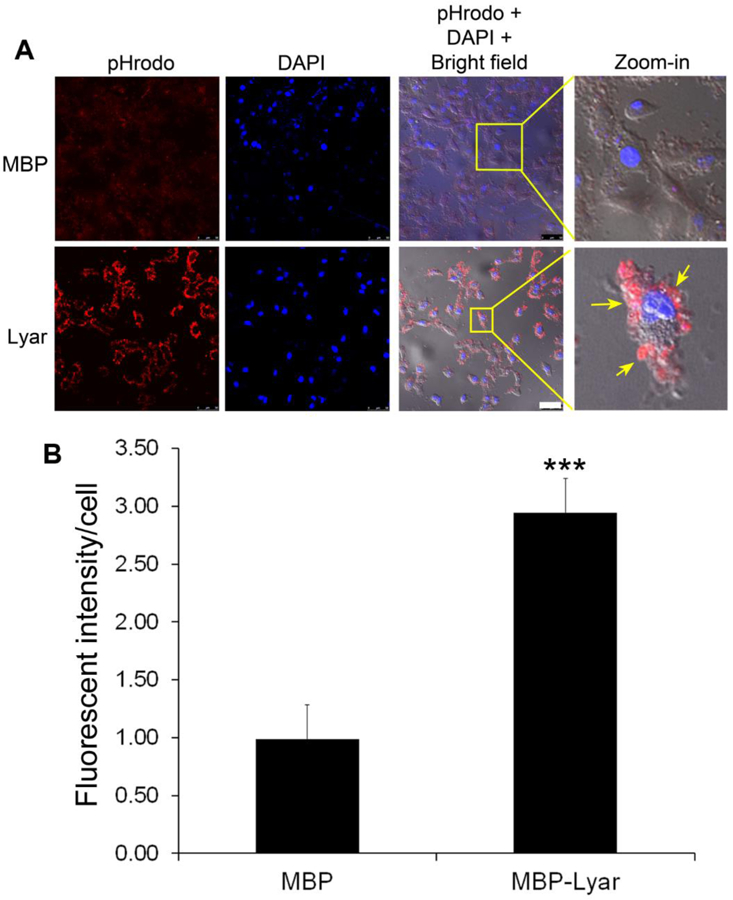
Lyar-mediated RPE phagocytosis of POS vesicles was independently verified with primary RPE cells. We prepared primary RPE cells from neonatal mouse eyes and analyzed their phagocytosis in the presence or absence of purified Lyar. The results showed that MBP-Lyar stimulated phagocytosis of POSs by primary RPE (Fig. 6). Control MBP did not induce RPE phagocytosis. These results suggest that D407 and primary RPE cells have a similar phagocytosis machinery.
Fig. 6.
Lyar stimulates phagocytosis of POSs by primary RPE cells. (A) Lyar-mediated phagocytosis was performed as in Fig. 1 but with primary RPE cells. Bar = 20 µm. (B) Relative fluorescence intensity of RPE cells with internalized pHrodo signals in (A) was quantified (± SEM, n=10, t-test, ***P<0.001).
DISCUSSION
Lyar as a genuine RPE phagocytosis ligand
RPE is one of the most active phagocytes in our body, clearing approximately 75-fold of its own volume of shed POSs each year (Li, 2012a). Defect in RPE phagocytosis causes accumulation of unphagocytosed debris, retinal degeneration and blindness (D’Cruz et al., 2000; Duncan et al., 2003; Gal et al., 2000). The molecular mechanisms of RPE phagocytosis are poorly defined. This study independently verified Lyar as a genuine phagocytosis ligand with multiple lines of evidence, including its predominant expression in POSs, release from apoptotic cells, selective binding to apoptotic cells, stimulation of RPE phagocytosis and Lyar-mediated cargo targeting to phagosomes. Characterization of Lyar as a new RPE phagocytosis ligand will help unravel the mystery of molecular phagocyte biology.
Lyar is highly expressed in undifferentiated human embryonic stem cells but significantly downregulated upon differentiation (Cai et al., 2006). Lyar was previously characterized as a nucleolar protein to regulate the growth of cancer cells and stem cells by processing preribosomal RNA (Miyazawa et al., 2014; Su et al., 1993). However, this is unlikely the only function of Lyar. For example, Lyar is highly expressed in immature spermatocytes in adult testis, but not in Sertoli cells, interstitial cells and maturing spermatozoa (Su et al., 1993). Given that immature spermatocytes lack cell proliferation capacity, Lyar may have additional cellular function(s) other than regulating cell growth. Interestingly, Lyar-deficient mice were fully fertile and showed intact spermatogenesis (Lee et al., 2013).
In spermatogenesis, more than half of differentiating spermatogenic cells die by apoptosis before maturing into spermatozoa and are removed by Sertoli cell via phagocytosis (Nakanishi and Shiratsuchi, 2004). High-level expression of Lyar in immature spermatocytes (Su et al., 1993) implicates that the protein may be released from apoptotic cells to facilitate their phagocytic clearance.
Several phagocytosis ligands, such as MFG-E8 and Gas6, have been characterized as bridging molecules with two binding domains: one binds to eat-me signals on the surface of phagocytosis cargos and the other interacts with their cognate receptors on phagocytes (Sierra et al., 2013). Since Lyar is a soluble protein, our results imply that Lyar is a bridging molecule. Given that Lyar is released from apoptotic cells through the plasma membrane with compromised integrity (Fig. 4A), Lyar may also be released from shed POSs to facilitate RPE phagocytosis. High-level expression of Lyar in the POSs provides a geographic convenience for the ligand to regulate RPE phagocytosis.
Similar to two other bridging molecules, tubby and galectin-3 (Caberoy et al., 2012a; Caberoy et al., 2012b), Lyar at a high concentration (100 nM) had a reduced activity to stimulate RPE phagocytosis (Fig. 2). It is likely that bridging molecules at excessively high concentrations may have a reduced efficiency to bridge phagocytosis cargos to phagocytes via the same bridging molecules, similar to the reduced efficiency of plasmid ligation with excessive DNA inserts. Thus, these results support the notion that Lyar is a bridging molecule for RPE phagocytosis, even though its binding partners on RPE and shed POSs are yet to be identified. Our co-immunoprecipitation analysis showed that Lyar did not bind to Mer-Fc, suggesting that MerTK is not the receptor for Lyar (not shown).
OPD/FPC and its impact on molecular phagocyte biology
Lyar was identified by our recently developed OPD/PFC (Caberoy et al., 2010a). The validity of OPD/PFC was supported by identification and characterization of Tulp1 as a MerTK-specific phagocytosis ligand (Caberoy et al., 2010a; Caberoy et al., 2010c). Independent verification of Lyar as a genuine phagocytosis ligand in this study further corroborated the validity of OPD/PFC, which is the only available method for unbiased identification of phagocytosis ligands in the absence of receptor information. We further developed OPD-based dual functional cloning (DFC) for unbiased identification of receptor-specific phagocytosis ligands with independent validation (Caberoy et al., 2012a; Li, 2012a). We predict that these new approaches will markedly advance our knowledge of molecular regulation of various phagocytes, including RPE, macrophage and microglia.
Phagocytosis ligands, such as bridging molecules, are the key to defining the physiological and pathological roles of phagocytes by selectively recognizing deleterious cargos via “eat-me signals” and linking them to phagocytic receptors for clearance. For example, anti-amyloid β peptide (Aβ) antibodies link the deleterious metabolic products to Fc receptors on microglia for clearance (Lee and Landreth, 2010; Morgan, 2009), and galectin-3 bridges advanced glycation end products (AGEs) on aged cells to MerTK (Caberoy et al., 2012a; Vlassara et al., 1987). In other cases, phagocytosis ligands, such as phosphatidylserine and calreticulin (Gardai et al., 2005; Ravichandran and Lorenz, 2007; Ruggiero et al., 2012), are eat-me signals themselves and directly anchor on the surface of apoptotic cells. In this regard, identification of different phagocytosis ligands will help understand which deleterious cargos are selected for clearance and how phagocytes recognize them. Furthermore, these ligands control the initiation and activity of phagocytosis process by activating their cognate receptors on phagocytes and are valuable targets for developing future phagocytosis-based therapy. One example is that blockade of MFG-E8 was shown to promote neuron survival by preventing phagocytosis of live but stressed neurons in neuroinflammation (Brown and Neher, 2014; Fricker et al., 2012). Conceivably, blockade or overexpression of phagocytosis ligands may minimize tissue damage or facilitate debris clearance in various disease conditions. Systematic identification of unknown phagocytosis ligands by OPD/PFC will improve our capability to exploit ligand-based phagocytosis therapies in the future.
Phagocytosis dysfunction may arise from impairment of either a single or multiple pathways. The dysfunctional mechanisms with a single pathway should be much simpler than with multiple pathways. Mutations of MerTK or TREM2 are two examples for phagocytosis dysfunction in a single pathway. MerTK mutations cause phagocytosis dysfunction with retinal degeneration and autoimmune response (D’Cruz et al., 2000; Duncan et al., 2003; Gal et al., 2000; Scott et al., 2001), while mutations of microglial phagocytic receptor TREM2 associate with Alzheimer’s disease or Nasu-Hakola disease (Guerreiro et al., 2013; Jonsson et al., 2013; Neumann and Takahashi, 2007). Despite identification of TREM2 as a phagocytic receptor 14 years ago (Bouchon et al., 2000), its ligand(s) remains elusive. It is even more daunting to identify disease-related TREM2 ligands for understanding its pathological roles.
Multiple pathways are likely responsible for age-related phagocytosis dysfunction. Emerging evidence suggests that phagocyte dysfunction in aged tissues results in accumulation of uncleared metabolic debris, chronic sterile inflammation and tissue degeneration, including RPE dysfunction in AMD and microglial dysfunction in Alzheimer’s disease (Li, 2013). However, no single age-dependent ligand or pathway has been identified for any phagocyte. The technical challenges are two folds: how to systematically identify unknown phagocytosis ligands and how to quantify their internalization activity in young and aged phagocytes for functional activity comparison. OPD/PFC can address both challenges. In theory, thorough identification of phage clones enriched by OPD/PFC will identify all possible phagocytosis ligands. We previously used OPD/PFC as a quantification tool to determine the internalization activity of displayed ligands and successfully mapped five minimal domains in Tulp1 as MerTK-binding motifs (Caberoy et al., 2010c). These results imply that identified ligands can be quantified for their phagocytosis activity in young and aged phagocytes by OPD/PFC for quantitative comparison to delineate age-dependent ligands. Using this approach we have identified several age-dependent ligands for aged RPE and microglia (unpublished data). Characterization of these ligands and their signaling pathways will unravel the molecular mystery of phagocytosis dysfunction in age-related diseases, such as AMD and Alzheimer’s disease.
In summary, this study characterized Lyar as an RPE phagocytosis ligand for in-depth understanding of RPE molecular phagocyte biology. The results also support the validity of OPD/PFC, which is broadly applicable to different phagocytes and is the only available approach for unbiased identification of phagocytosis ligands in the absence of any molecular probe.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Duco Hamasaki for reviewing this manuscript and Gabriel Gaidosh for the confocal service. This work was partially supported by NIH R01GM094449 (W.L.), NIH R01EY016211-05S1 (W.L.), NIH R21HD075372 (W.L.), BrightFocus Foundation M2012026 (W.L.), Special Scholar Award from Research to Prevent Blindness (RPB) (W.L.), K99EY020865/R00EY020865 (N.B.C.), P30-EY014801, an institutional grant from RPB, and National Natural Science Foundation of China (grant #81100683; Y.Z.).
Contract grant Sponsor: NIH; Contract grant number: R01GM094449
Contract grant Sponsor: NIH; Contract grant number: R21HD075372
Contract grant Sponsor: BrightFocus Foundation; Contract grant number: M2012026
Contract grant Sponsor: NIH; Contract grant number: K99EY020865/R00EY020865
Contract grant Sponsor: National Natural Science Foundation of China: Contract grant number: 81100683
Footnotes
Conflict of interest: None.
REFERENCES
- Bouchon A, Dietrich J, and Colonna M. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164:4991–4995. [DOI] [PubMed] [Google Scholar]
- Brown GC, and Neher JJ. 2014. Microglial phagocytosis of live neurons. Nat Rev Neurosci 15:209–216. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, and Lemke G. 2012. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 76:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Alvarado G, Bigcas JL, and Li W. 2012a. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol 227:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Alvarado G, and Li W. 2012b. Tubby regulates microglial phagocytosis through MerTK. J Neuroimmunol 252:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Maiguel D, Kim Y, and Li W. 2010a. Identification of tubby and tubby-like protein 1 as eat-me signals by phage display. Exp Cell Res 316:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Jiang X, Alvarado G, and Li W. 2010b. Efficient identification of tubby-binding proteins by an improved system of T7 phage display. J Mol Recognit 23:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, and Li W. 2010c. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J 29:3898–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Chen J, Liu Y, Miura T, Luo Y, Loring JF, Freed WJ, Rao MS, and Zeng X. 2006. Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem cells 24:516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, and Vollrath D. 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651. [DOI] [PubMed] [Google Scholar]
- Dowling JE, and Sidman RL. 1962. Inherited retinal dystrophy in the rat. J Cell Biol 14:73–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, and Vollrath D. 2003. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 44:826–838. [DOI] [PubMed] [Google Scholar]
- Erwig LP, and Henson PM. 2007. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol 171:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, and Brown GC. 2012. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci 32:2657–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, and Vollrath D. 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26:270–271. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, and Henson PM. 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, and Hardy J. 2013. TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, and Stefansson K. 2013. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Caberoy NB, Alvarado G, Davis JL, Feuer WJ, and Li W. 2011. Identification of Hnrph3 as an autoantigen for acute anterior uveitis. Clin Immunol 138:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Jin S, Choi H, Kwon JT, Kim J, Jeong J, Kwon YI, and Cho C. 2013. Expression and function of the testis-predominant protein LYAR in mice. Molecules and cells 35:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, and Landreth GE. 2010. The role of microglia in amyloid clearance from the AD brain. J Neural Transm 117:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W 2012a. Eat-me signals: Keys to molecular phagocyte biology and “Appetite” control. J Cell Physiol 227:1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W 2012b. ORF phage display to identify cellular proteins with different functions. Methods 58:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W 2013. Phagocyte dysfunction, tissue aging and degeneration. Ageing research reviews (in press) [Epub ahead of print]: [DOI] [PMC free article] [PubMed]
- Li W, and Handschumacher RE. 2002. Identification of two calcineurin B-binding proteins: tubulin and heat shock protein 60. Biochim Biophys Acta 1599:72–81. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Yoshikawa H, Magae S, Ishikawa H, Izumikawa K, Terukina G, Suzuki A, Nakamura-Fujiyama S, Miura Y, Hayano T, Komatsu W, Isobe T, and Takahashi N. 2014. Human cell growth regulator Ly-1 antibody reactive homologue accelerates processing of preribosomal RNA. Genes to cells : devoted to molecular & cellular mechanisms 19:273–286. [DOI] [PubMed] [Google Scholar]
- Morgan D 2009. The role of microglia in antibody-mediated clearance of amyloid-beta from the brain. CNS & neurological disorders drug targets 8:7–15. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, and Shiratsuchi A. 2004. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biological & pharmaceutical bulletin 27:13–16. [DOI] [PubMed] [Google Scholar]
- Neumann H, and Takahashi K. 2007. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol 184:92–99. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, and Lorenz U. 2007. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7:964–974. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, and Finnemann SC. 2012. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci U S A 109:8145–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, and Temple S. 2012. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10:88–95. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, and Matsushima GK. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211. [DOI] [PubMed] [Google Scholar]
- Sierra A, Abiega O, Shahraz A, and Neumann H. 2013. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Frontiers in cellular neuroscience 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O 2005. The retinal pigment epithelium in visual function. Physiol Rev 85:845–881. [DOI] [PubMed] [Google Scholar]
- Su L, Hershberger RJ, and Weissman IL. 1993. LYAR, a novel nucleolar protein with zinc finger DNA-binding motifs, is involved in cell growth regulation. Genes Dev 7:735–748. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Valinsky J, Brownlee M, Cerami C, Nishimoto S, and Cerami A. 1987. Advanced glycosylation endproducts on erythrocyte cell surface induce receptor-mediated phagocytosis by macrophages. A model for turnover of aging cells. J Exp Med 166:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Fulkerson CM, Malek R, Ghassemifar S, Snyder PW, and Mendrysa SM. 2012. Mutations in Lyar and p53 are synergistically lethal in female mice. Birth defects research. Part A, Clinical and molecular teratology 94:729–737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.