Abstract
Background:
Sex-based treatment disparities occur in many diseases. Women undergo fewer procedural interventions and their care is less consistent with guideline-based therapy. There is limited research exploring sex-based differences in ulcerative colitis treatment. We hypothesized that women are less likely to be treated with strategies consistent with long-term disease remission, including surgery and maintenance medications.
Objective:
Determine if patient sex is associated with choice of treatment strategy for ulcerative colitis.
Design:
Retrospective cohort analysis.
Setting:
A large commercial insurance claims database (Truven MarketScan®) from 2007–2015.
Patients:
We identified a cohort of 38,851 newly diagnosed ulcerative colitis patients aged 12–64 with at least one year of follow-up.
Main Outcome Measures:
Differences between male and female patients in 1) rates and types of index ulcerative colitis operations, 2) rates and types of ulcerative colitis medication prescriptions, and 3) rates of opioid prescriptions.
Results:
Men were more likely to undergo surgical treatment for ulcerative colitis (2.94% vs. 1.97%, p<0.001, OR 1.51 p<0.001). The type of index operation performed did not vary by sex. Men were more likely to undergo treatment with maintenance medications, including biologic (12.4 vs. 10.2%, p<0.001, OR 1.22 p<0.001), immunomodulatory (16.3 vs. 14.9%, p<0.001, OR 1.08 p=0.006), and 5-aminosalicylate medications (67.0 vs. 63.2%, p<0.001, OR 1.18 p<0.001). Women were more likely to undergo treatment with rescue therapies and symptomatic control with corticosteroids (55.5 vs. 54.0%, p=0.002, OR 1.07 p=0.002) and opioids (50.2 vs. 45.9%, p<0.001, OR 1.17 p<0.001).
Limitations:
Claims data lack clinical characteristics acting as confounders.
Conclusions:
Men with ulcerative colitis were more likely to undergo treatment consistent with long-term remission or cure, including maintenance medications and definitive surgery. Women were more likely to undergo treatment consistent with short-term symptom management. Further studies to explore underlying mechanisms of sex-related differences in UC treatment strategies and disease trajectories are warranted. See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Gender differences, Inflammatory bowel disease, Medical therapy, Surgery, Ulcerative colitis
Introduction
An increasing body of research suggests that women experience sex-based disparities in medical treatment across a variety of diseases. In many cases, treatment patterns for women deviate from guideline-indicated treatment, leading to poorer outcomes. This pattern has been demonstrated in diabetes,1,2 cardiovascular disease,3,4 stroke,5 and some cancers, including colorectal malignancies.6 Other studies have suggested that women are less frequently offered invasive procedural interventions,7–13 although they are more likely to seek preventative care.14,15
There is limited research exploring sex-based disparities in ulcerative colitis (UC) treatment, and data that does exist comes primarily from single- or small multi-institutional studies. This limited data suggests the possibility of treatment differences by sex, leading to differences in outcomes. A previous multi-center study showed that women with inflammatory bowel disease (IBD) received fewer immunomodulatory medications than men, and that men had significantly higher remission rates. After controlling for the type of medication prescribed, sex differences in remission rates vanished16 – suggesting that when women are appropriately treated with guideline-based therapy, their outcomes are similar to men. In our prior work, we found that young male patients were more likely than older men or women of any age to undergo surgical treatment for UC.17 To date, there is very little population-level data examining the nature and magnitude of sex-related differences in UC treatment.
The objective of this study was to determine the relationship between sex and medical and surgical treatment strategies for UC on a population level. We hypothesized that women are less likely than men to undergo treatment with strategies consistent with long-term disease remission, including immunomodulatory and/or biologic medications and definitive surgical resection.
Methods
Study Design
We performed a retrospective cohort analysis of newly diagnosed UC patients using the 2007–2015 Truven MarketScan® database. This database contains de-identified patient-level information from inpatient, outpatient, and pharmaceutical claims on 40–50 million privately insured patients per year. These claims originate from >150 large employer-sponsored health plans and include patients from all 50 states. The database includes demographic characteristics (i.e., age, sex, geographic region), encounter data (i.e., hospital admissions, outpatient visits, and associated procedures), pharmaceutical data (i.e., medications, days supply, dose dispensed, strength, administration method), and financial data (i.e., total cost, copayment, deductibles). This study was exempt from full review by the Stanford University Institutional Review Board.
Participants
We identified a cohort of patients aged 12 to 64 newly diagnosed with UC between 2008 and 2014 (Figure 1). We defined a new diagnosis of UC as a patient with 1) ≥2 inpatient or outpatient encounters with a primary diagnosis of UC within one year (International Classification of Diseases (ICD)-9 codes 556.xx and ICD-10 codes K51.xx), 2) ≥1 lower endoscopy within six months of the index UC encounter, and 3) no UC-related medication prescriptions within 12 months prior to the index UC encounter. We required at least two encounters on separate dates with a primary diagnosis of UC to exclude those whose single UC encounter may represent a spurious diagnosis. As tissue or endoscopic evidence is necessary to definitively diagnose UC, we excluded patients without lower endoscopy within six months of the index UC encounter. We required patients to be continuously enrolled for at least twelve months prior to the index UC encounter and for at least twelve months afterwards to ensure adequate lead-in and follow-up time. We also excluded patients with claims for UC medications within one year prior to diagnosis, as UC-related visits or lower endoscopies in these patients may indicate disease exacerbations rather than new diagnoses. We followed patients longitudinally during their enrollment period to assess time from diagnosis to treatment with UC medications and/or index UC operation.
Figure 1.
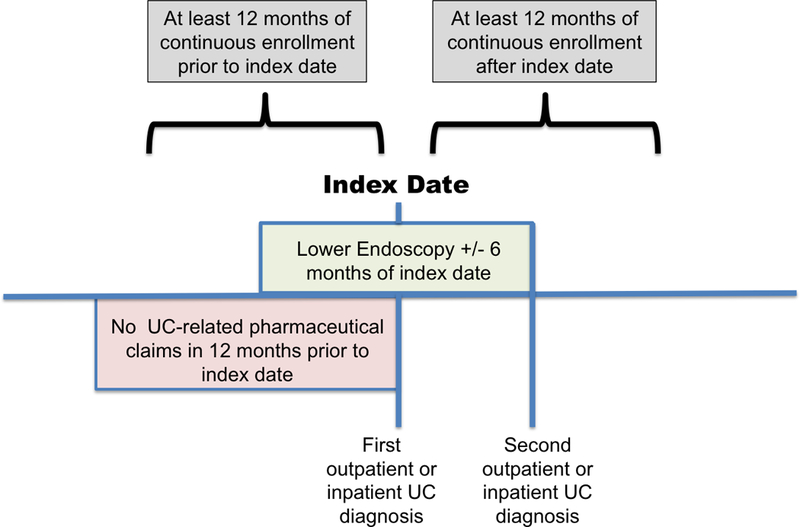
Cohort enrollment criteria for newly diagnosed ulcerative colitis patients
Variable Classification
We identified patients who underwent an index UC operation (total abdominal colectomy (TAC), total proctocolectomy (TPC) with ileostomy, or TPC with ileal pouch) using Current Procedural Terminology (CPT) codes, ICD-9, and ICD-10 procedure codes (Appendix 1). We measured UC medication use by identifying outpatient pharmaceutical claims or Healthcare Common Procedure Coding System/ J-codes for biologic medications (infliximab, adalimumab, certolizumab pegol, golimumab, natalizumab, vedolizumab, ustekinumab), corticosteroids (prednisone, prednisolone, prednisolone sodium phosphate, methylprednisolone sodium succinate, hydrocortisone sodium succinate, hydrocortisone, methylprednisolone, budesonide, hydrocortisone acetate), immunomodulatory medications (azathioprine, mercaptopurine, methotrexate, methotrexate sodium, cyclosporine, tacrolimus), and 5-aminosalicylates (mesalamine, sulfasalazine, olsalazine sodium, balsalazide sodium). We assessed opioid prescriptions using outpatient pharmaceutical claims with therapeutic class 60 (opiate-containing medications) (Appendix 6).
Analysis
The primary outcomes of interest were UC-related medical and surgical treatments, based on pharmaceutical claims and inpatient claims for index UC operations. For surgical treatment, we assessed the type of operation performed and time between diagnosis and surgery. For medical treatment, we assessed types of prescriptions filled and time between diagnosis and medication initiation. Each medication class was assessed individually, irrespective of the other medications used by the patient. For time-to-event analyses, the first medication prescription for each event class was considered the event of interest.
To determine whether sex differences are influenced by women’s concerns over reproduction, we conducted secondary sensitivity analyses of women past usual reproductive age (defined as ≥45 years) and men ≥45 years to assess for differences in treatment strategies by age. The age of 45 years was chosen given the Centers for Disease Control and Prevention (CDC) definition of usual reproductive age.18 Given previously described risks of methotrexate on sperm counts and birth defects, we also conducted a subgroup analysis of methotrexate use in males <45 years. Finally, we conducted a post-hoc analysis comparing differences in health care utilization by men and women to determine whether treatment differences could be influenced by the rate at which care was accessed. We assessed mean number of annual outpatient visits, mean number of emergency room visits, and mean number of hospitalizations (both for UC and for any cause). We additionally assessed specialty providers accessed for care, including internal medicine physicians, gastroenterologists, surgeons, and psychiatrists.
The primary independent variable of interest was sex. Covariates adjusted for in multivariate analysis included age group, geographic region, insurance plan type, and grouped Charlson comorbidity index score (calculated using inpatient and outpatient claims from the 12-month lead-in period). These were chosen both for their clinical significance as well as their statistical significance in bivariate analysis.
We used chi-square tests to compare binary differences by sex in medical and surgical treatment, as well as frequency and type of health care utilization. We used total time at risk of undergoing surgery to calculate incidence rates of UC surgery. We used multivariate logistic regression to estimate the association between sex, surgical treatment, and treatment with UC medications while controlling for covariates. Multivariate regression was also used to determine adjusted odds ratios of seeing specialty medical providers. We used Kaplan-Meier time-to-event analyses to compare time-to-surgery and time-to-medication from index UC diagnosis between men and women. We used chi-square tests and Student’s t-tests to compare categorical and continuous demographic variables, respectively (Stata v14.2; College Station, TX).
Results
After applying inclusion criteria (Figure 2), we identified 38,851 patients with a new diagnosis of UC, 52% of whom were female (Table 1). Women were slightly older (mean age 43 vs. 42.1 years, p<0.001) and slightly more likely to have comorbidities (grouped Charlson index 0.64 vs. 0.61, p<0.001) than men. Most patients had EPO/PPO insurance. The smaller proportion of the cohort diagnosed in the study’s later years reflects the lower number of overall covered lives contained in the database during these years; the overall proportion of patients diagnosed with UC each year was constant. Patients were followed for different amounts of time after UC diagnosis relative to the availability of claims data and their continued enrollment in a participating insurance plan. The mean duration of follow-up after UC diagnosis was 3.30 years (range 1–8 years); which did not differ by sex.
Figure 2.
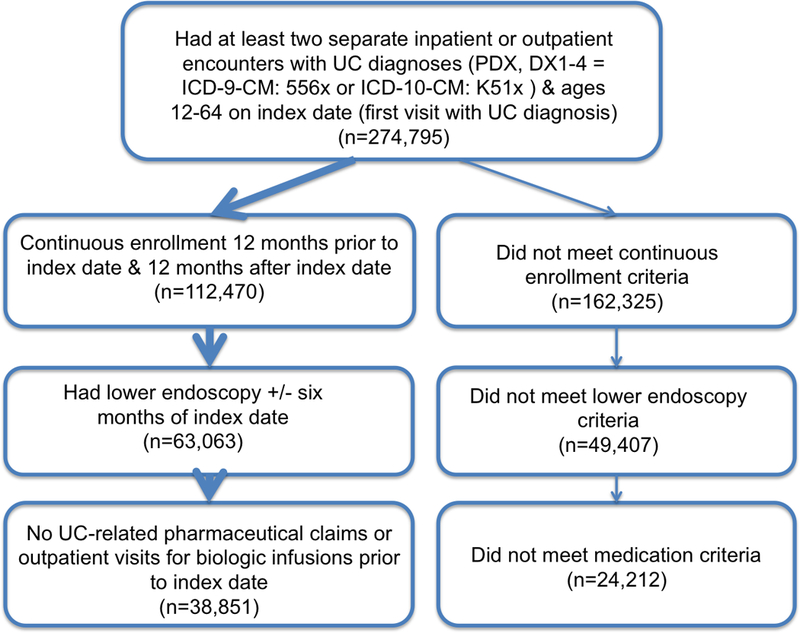
Cohort selection process
Table 1.
Demographic characteristics of patients newly diagnosed with ulcerative colitis from 2008–2014 (Truven MarketScan Database®)
| Total (n=38,851) | Males (n=18,489) | Females (n=20,362) | p-value | |
|---|---|---|---|---|
| Mean Age (Median) | 42.6 (45) | 42.1 (44) | 43.0 (45) | <0.001 |
| Age Group, n (%) | ||||
| 12 to 18 | 2,377 (6.1) | 1,299 (7.0) | 1,078 (5.3) | <0.001 |
| 19 to 30 | 5,934 (15.3) | 2,957 (16.0) | 2,977 (14.6) | |
| 31 to 45 | 11,918 (30.7) | 5,566 (30.1) | 6,352 (31.2) | |
| 46 to 64 | 18,622 (47.9) | 8,667 (46.9) | 9,955 (48.9) | |
| Geographic Region, n (%) | ||||
| 1 (Northeast) | 9,228 (23.8) | 4,456 (24.1) | 4,772 (23.4) | <0.001 |
| 2 (North Central) | 8,470 (21.8) | 4,195 (22.7) | 4,275 (21.0) | |
| 3 (South) | 13,656 (35.2) | 6,261 (33.9) | 7,395 (36.3) | |
| 4 (West) | 6,721 (17.3) | 3,211 (17.4) | 3,510 (17.2) | |
| 5 (Unknown) | 776 (2.0) | 366 (2.0) | 410 (2.0) | |
| Insurance Type, n (%) | ||||
| EPO/PPO | 25,036 (64.4) | 12,019 (65.0) | 13,017 (63.9) | 0.02 |
| HMO/ Cap POS | 4,822 (12.4) | 2,223 (12.0) | 2,599 (12.8) | |
| HDHP/CDHP | 2,788 (7.2) | 1,295 (7.0) | 1,493 (7.3) | |
| POS | 3,019 (7.8) | 1,392 (7.5) | 1,627 (8.0) | |
| Comp | 748 (1.9) | 360 (2.0) | 388 (1.9) | |
| Unknown/Missing | 2,438 (6.3) | 1,200 (6.5) | 1,238 (6.1) | |
| Year of Diagnosis, n (%) | ||||
| 2008 | 6,289 (16.2) | 2,961 (16.0) | 3,328 (16.3) | 0.87 |
| 2009 | 6,911 (17.8) | 3,297 (17.8) | 3,614 (17.8) | |
| 2010 | 6,442 (16.6) | 3,083 (16.7) | 3,359 (16.5) | |
| 2011 | 6,418 (16.5) | 3,062 (16.6) | 3,356 (16.5) | |
| 2012 | 5,383 (13.9) | 2,592 (14.0) | 2,791 (13.7) | |
| 2013 | 4,588 (11.8) | 2,151 (11.6) | 2,437 (12.0) | |
| 2014 | 2,820 (7.3) | 1,343 (7.3) | 1,477 (7.3) | |
| Grouped Charlson Index | ||||
| 0 | 22,263 (57.3) | 10,902 (59.0) | 11,361 (55.8) | <.001 |
| 1 | 8,878 (22.9) | 3,982 (21.5) | 4,896 (24.0) | |
| 2+ | 7,710 (19.8) | 3,605 (19.5) | 4,105 (20.2) | |
| Years of Follow-Up After Diagnosis | ||||
| Mean (Median) | 3.30 (2.90) | 3.31 (2.93) | 3.28 (2.88) | 0.09 |
Surgical Treatment of Ulcerative Colitis
Men were 50% more likely to undergo surgical treatment for UC compared to women (2.94% vs. 1.97%, p<0.001) (Table 2). There was no difference by sex in the type of operation performed. Use of laparoscopic versus open surgery did not significantly differ by sex (men 55.4% vs. women 44.7%, p=0.17). Men had a higher incidence rate of surgery compared to women (5.2 vs. 3.5 UC surgeries per 1000 person-years, data not shown). The effect of sex persisted after adjusting for covariates, including age group, geographic region, insurance plan type, and grouped Charlson comorbidity index score (OR=1.51, 95% CI=[1.32–1.73], p<0.001) (Appendix 2). In Kaplan-Meier time-to-event analysis, men progressed to surgery more quickly than women (Figure 3, Panel A).
Table 2.
Sex differences in absolute rates of undergoing surgical resection for ulcerative colitis.
| Total (n=38,851) | Males (n=18,149) | Females (n=20,362) | p-value | |
|---|---|---|---|---|
| Total Abdominal Colectomy (N, % of surgeries) |
542 (57.4) | 312 (57.5) | 230 (57.2) | 0.35 |
| Total Proctocolectomy with Ileostomy (N, % of surgeries) |
134 (14.2) | 70 (12.9) | 64 (15.9) | |
| Total Proctocolectomy with Ileal Pouch (N, % of surgeries) |
269 (28.5) | 161 (29.7) | 108 (26.9) | |
|
Any Index UC Operation (N, % of total cohort undergoing any surgery) |
945 (2.43) | 543 (2.94) | 402 (1.97) | <0.001 |
Figure 3.
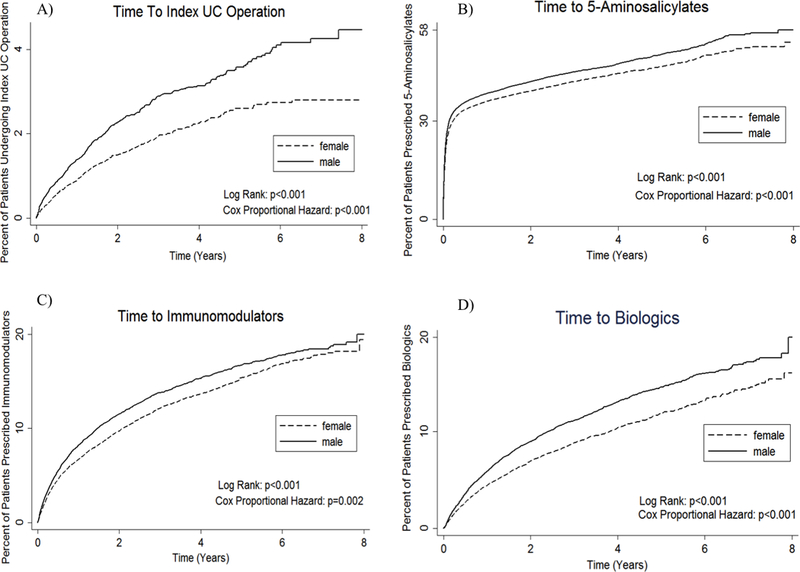
Kaplan-Meier time-to-event analysis for A) time to index ulcerative colitis operation from initial ulcerative colitis diagnosis, B) time to treatment with 5-aminosalicylate medications, C) time to treatment with immunomodulatory medications, and D) time to treatment with biologic medications
Medical Treatment of Ulcerative Colitis
Maintenance Medications
Men were significantly more likely than women to be prescribed medications used for maintenance therapy including biologic (12.43% vs. 10.19%, p<0.001), immunomodulatory (16.30% vs. 14.86%, p<0.001), and 5-aminosalicylate medications (66.96% vs. 63.21%, p<0.001) (Table 3). Men were significantly more likely than women to be treated with biologic (OR=1.22, 95% CI= [1.14–1.30], p<0.001), immunomodulatory (OR=1.08, 95% CI=[1.02– 1.14], p=0.006), and 5-aminosalicylate medications (OR=1.18, 95% CI=[1.13–1.23], p<0.001) in multivariate analysis (Appendix 3). Men started biologic, immunomodulatory, and 5-aminosalicylate medications significantly earlier than women (Figure 3, panels B-D).
Table 3.
Sex differences in ulcerative colitis medication prescription during follow-up period
| N=Have Ever Taken During Enrollment (%) | Overall (n=38,851) | Male (n=18,489) | Female (n=20,362) | p-value |
|---|---|---|---|---|
| Steroids | 21,289 (54.8) | 9,982 (54.0) | 11,307 (55.5) | 0.002 |
| Immunomodulators | 6,039 (15.5) | 3,013 (16.3) | 3,026 (14.9) | <0.001 |
| Biologics | 4,372(11.2) | 2,298 (12.4) | 2,074 (10.2) | <0.001 |
| 5-ASA | 25,251 (65.0) | 12,380 (67.0) | 12,871 (63.2) | <0.001 |
| Opioids | 18,707 (48.2) | 8,479 (45.9) | 10,228 (50.2) | <0.001 |
Rescue and Palliative Medications
Women were significantly more likely to be prescribed corticosteroids (55.53% vs. 53.99%, p=0.002) as well as opioids (50.23% vs. 45.86%, p<0.001) (Table 3). After adjusting for covariates, male sex remained protective against treatment with corticosteroids (OR=0.93, 95% CI=[0.90–0.98], p=0.002) and opioids (OR=0.85, 95% CI=[0.82–0.89], p<0.001) (Appendix 3). Mean number of opioid prescriptions for women was significantly higher than men (3.73 prescriptions vs. 2.73, p<0.001).
Medical and Surgical Treatment of Ulcerative Colitis in Patients Past Usual Reproductive Age
Sex differences in surgical treatment persisted among women past usual reproductive age. Compared to men ≥45 years old, women ≥45 years old were less likely to undergo surgical treatment for UC (1.82% vs. 2.44%, p=0.002). The positive effect of male sex persisted in this older age group in multivariate analysis (OR=1.29, 95% CI=[1.04–1.58], p=0.02) (Appendix 4). Women ≥45 years old progressed more slowly to surgery compared to their male peers in time-to-event analysis (Figure 4).
Figure 4.
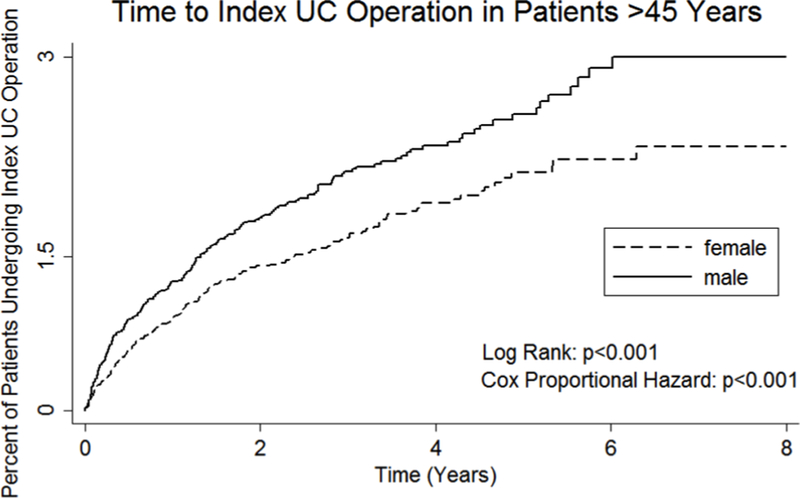
Kaplan-Meier time-to-event analysis for time to index ulcerative colitis operation from initial ulcerative colitis diagnosis for patients ≥45 years of age
Sex differences in maintenance medical treatment also persisted in an age-stratified analysis. Women past usual reproductive age underwent treatment with biologic medications less frequently than their male peers (6.19 vs. 8.12%, p<0.001) and were less likely to be treated with 5-aminosalicylates (57.40% vs. 62.12%, p<0.001). The positive effect of male sex persisted in multivariate analysis (biologics: OR=1.35, 95% CI=[1.20–1.52], p<0.001; 5-aminosalicylates: OR=1.24, 95% CI=[1.17–1.32], p<0.001) (Appendix 5). Young men <45 years old used methotrexate at a rate equivalent to their female counterparts (2.13 vs. 2.06%, p=0.72).
Finally, differences in rescue medication treatment persisted in the age-stratified cohort. Women remained more likely to undergo treatment with corticosteroids than their male peers (51.34% vs. 49.46%, p=0.01). Male sex remained protective against corticosteroid treatment in multivariate analysis (OR=0.92, 95% CI=[0.87–0.98], p=0.02).
Health Care Utilization
In a post-hoc analysis, we assessed sex differences in health care utilization. Women with UC were more likely to access the health care system for any reason compared to men. Women were more likely to visit the emergency room during their enrollment (50.2% vs. 46.8%, p<0.001, OR=1.15 [1.10–1.20], p<0.001) or to be hospitalized for any reason during their enrollment (33.2% vs. 26.8%, p<0.001, OR=1.38 [1.31–1.44], p<0.001). Women had a higher mean number of annual outpatient visits (12.2 vs. 9.1 visits, p<0.001). However, when we assessed healthcare utilization specific to UC, this pattern changed. Men had slightly higher mean annual UC-related outpatient visits (3.0 vs. 2.7, p<0.001), while women were very slightly more likely to visit the emergency room for UC (9.1% vs. 8.6%, p=0.12, OR=1.08 [1.01–1.16], p=0.04). Men and women were equally likely to be hospitalized for UC (15.4% vs. 15.1%, p=0.49, OR=1.04 [0.98–1.10], p=0.20).
Men and women sought care from specialist providers at different rates. Both men and women were very likely to seek care from internal medicine physicians and gastroenterologists. Women were very slightly more likely to see an internal medicine physician compared to men (88.7% vs. 87.4%, p<0.001, OR 1.13 [1.06–1.20, p<0.001). There were no differences in rates of gastroenterologist consultation after adjusting for covariates (80.6% vs. 79.6%, p=0.02, OR 1.05 [0.99–1.11], p=0.06). Although they underwent less surgical therapy for UC, women were more like to seek consultation with a colorectal or general surgeon (27.3% vs. 23.5%, p<0.001, OR 1.22 [1.16–1.28], p<0.001]. Women were also more likely to seek care from a psychiatrist compared to men (9.9% vs. 6.1%, p<0.001, OR 1.39 [1.28–1.50], p<0.001).
Discussion
We found persistent differences in treatment patterns between men and women with UC. Men were more likely than women to undergo treatment aligned with longer-term disease maintenance or surgical cure. Men were more likely to undergo treatment to proactively manage disease, while women were more likely to undergo treatment to reactively manage symptoms – specifically, corticosteroids and narcotic pain medication.
It is particularly interesting that women undergo less surgical therapy for UC than men, even though they are more likely to undergo surgical consultation. The underlying reasons behind this discrepancy beg further investigation – are female patients counseled by surgeons to defer or avoid surgery, or is it a patient-driven decision? Several factors may influence patient and provider choices regarding surgery. Prior studies have described a 20–30% increased risk of female infertility after restorative proctocolectomy and ileal pouch.19,20 It is possible that risk aversion towards postoperative infertility may drive female patients and providers away from proctocolectomy. However, a subgroup analysis of women ≥45 years showed that low rates of surgery persisted for women after usual reproductive age. Women may have increased concerns about postoperative body image related to stomas and scarring compared to men. A Dutch study showed that women undergoing open restorative proctocolectomy have a lower postoperative body image score than men.21
Sex-specific differences in UC medications are harder to explain, given that nearly all UC medications are considered safe even during pregnancy. In this study, the largest differences by sex included 1) biologic medications, 2) 5-aminosalicylates, and 3) opioids. Women were slower to start biologic medications compared to men, potentially indicating delay in initiation or reluctance in treatment. There is no evidence that biologic medications are teratogenic, so women are encouraged to continue biologic medications while pregnant or breast-feeding.22 For other medications considered in our analysis, 5-aminosalicylates have been shown to affect fertility in men but not women23; yet men were more likely to take these drugs than women. Methotrexate has been associated with birth defects, pregnancy loss, and azoospermia,24 yet our analysis suggests that males and females <45 years take methotrexate at equivalent rates.
The difference in rates of opioid prescription is especially concerning, as this could serve as an indicator of poorly treated disease requiring more pain control. Differences in opioid prescription rates disappear for patients ≥45 years, suggesting that sex differences in opioid prescription exist primarily in younger patients. Prior studies have investigated sex differences in pain reporting and seeking pain relief, as well as the psychogenic attributions providers may make regarding pain in female but not male patients.25 It is possible that female patients’ complaints of pain could result in an attempt by providers to ‘deal with the symptom’ instead of prompting a more in-depth exploration of potentially inadequate disease control.26
The current study has limitations related to its retrospective nature and use of a commercial claims database. Databases built on billing codes rely on the accuracy of coding by physicians to obtain the correct diagnoses and procedures. For this reason, we required stringent enrollment criteria including ≥2 encounters with a primary diagnosis of UC, lower endoscopy, and lengthy pre- and post-diagnosis periods to ensure that those captured would represent a newly diagnosed UC patient as accurately as possible. Despite this, the mean duration of follow-up is 3.3 years, with a maximum follow-up of eight years. A longer duration of follow-up would provide additional information regarding long-term treatment choices and the consequences of the treatment differences noted here. Additionally, the database used for this analysis contains no clinical or laboratory data to address questions of disease severity (such as frequency of bowel movements, bleeding, and nutritional status) that may influence patient and provider decisions regarding treatment strategy. The ability to statistically adjust for or match on clinical and laboratory markers of disease severity (including endoscopy reports, number of bloody bowel movements, hemoglobin, albumin, C-reactive protein, and fecal calprotectin) would strengthen our conclusion that sex alone is driving the treatment differences noted here. Thus, it is possible, albeit unlikely, that men described in this analysis may have more severe UC and thus undergo more medical and surgical treatment. This dataset also does not reliably capture whether a surgery was completed on an elective or emergent basis; accordingly we have refrained from commenting about sex differences in the urgency of surgical treatment.
The magnitude of treatment differences by sex noted here are small, and if present for a single medication or treatment option, would likely bear little clinical significance despite strong statistical significance. However, the remarkable consistency across a variety of treatment classes in this large population study suggests a small but important sex-based treatment bias. Similar patterns exist for female patients with other diseases – including receiving less care consistent with guideline-indicated therapy and decreased likelihood of undergoing procedural interventions7–13 – which further strengthens this conclusion. It is critical for providers caring for UC patients to recognize that sex-based treatment preferences exist, and to appropriately explore these preferences in their own patients. It is also important to remain vigilant to the possibility of misattribution of symptoms (for example to gynecological issues). Providers and patients should engage in shared decision-making to achieve satisfactory clinical outcomes using treatment methods acceptable to patients.
In summary, our study is the largest population-level study describing treatment differences by sex for UC patients. We found that men are more likely than women to receive treatment consistent with long-term disease remission or cure. Further work is necessary to understand the implications of sex-driven treatment differences on UC outcomes and to identify the underlying reasons for these treatment differences. Understanding patient- and provider-level drivers of these differences requires a qualitative or mixed-methods approach. Exploring the decision-making process of male and female UC patients choosing between different treatment options may provide additional insight. Interviewing providers who care for UC patients may reveal different methods of counseling used for male versus female patients.
Acknowledgments
Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was also supported by a National Institutes of Health National Center for Advancing Translational Science, Clinical and Translational Science Award (KL2TR001083 and UL1TR001085). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support came from The Donna and Frederick Fleugel Colorectal Surgery Fund, and from a seed grant from Women & Sex Differences in Medicine (Stanford University).
Funding/Support: Women & Sex Differences in Medicine Seed Grant (Stanford University)
Appendices
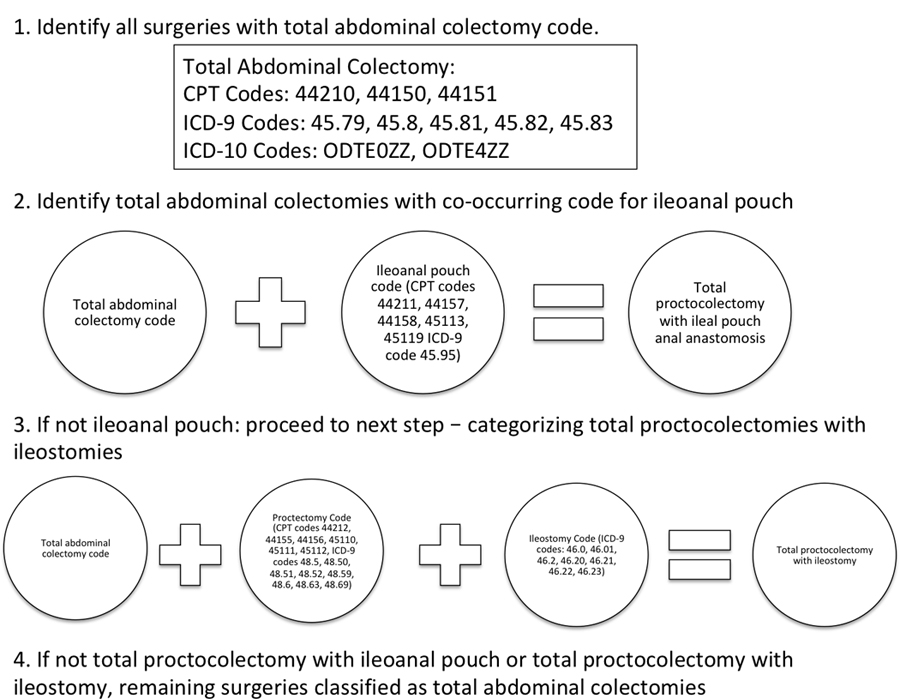
Appendix 1.
Diagram of surgical classification scheme.
Any index operation including codes specific to colectomy and creation of ileal pouch was classified as a total proctocolectomy with IPAA. Any index operation including either a code specific to proctocolectomy, or including separate codes for total colectomy and rectal resection, was classified as a total proctocolectomy with ileostomy. Any index operation remaining that included a code for total colectomy was classified as a total abdominal colectomy.
Appendix 2.
Adjusted odds ratios for undergoing any index ulcerative colitis operation, adjusted for sex, age group, region, insurance type, and grouped Charlson Index score by multivariate logistic regression
| OR: Any Index UC Operation | 95% CI | p-value | |
|---|---|---|---|
| Sex | |||
| Female (referent) | - | - | - |
| Male | 1.51 | 1.32–1.73 | <0.001 |
| Age Group | |||
| 12 to 18 | 1.79 | 1.40–2.28 | <0.001 |
| 19 to 30 (referent) | - | - | - |
| 31 to 45 | 0.63 | 0.51–0.77 | <0.001 |
| 45 to 64 | 0.49 | 0.40–0.59 | <0.001 |
| Region | |||
| Northeast (Referent) | - | - | - |
| North Central | 1.95 | 1.59–2.39 | <0.001 |
| South | 1.29 | 1.06–1.58 | 0.01 |
| West | 1.28 | 1.01–1.62 | 0.04 |
| Unknown | 1.3 | 0.78–2.16 | 0.32 |
| Insurance Type | |||
| EPO/PPO (referent) | - | - | - |
| HMO/Cap POS | 0.95 | 0.77–1.18 | 0.66 |
| POS | 1.05 | 0.82–1.34 | 0.71 |
| HDHP/CDHP | 1.04 | 0.81–1.34 | 0.73 |
| Comp | 1.39 | 0.94–2.06 | 0.10 |
| Grouped Charlson Index | |||
| 0 (referent) | - | - | - |
| 1 | 1.77 | 1.48–2.11 | <0.001 |
| 2+ | 3.92 | 3.32–4.62 | <0.001 |
Appendix 3.
Adjusted odds ratios for undergoing treatment with ulcerative colitis medications, adjusted for sex, age group, region, insurance type, and grouped Charlson Index score by multivariate logistic regression
| Odds Ratio: Biologic Use (95% CI) | p-value | Odds Ratio: Immunomodulator Use (95% CI) | p-value | Odds Ratio: 5-ASA Use (95% CI) | p-value | Odds Ratio: Corticosteroid Use (95% CI) | p-value | Odds Ratio: Opioid Use (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Female (referent) | - | - | - | - | - | - | - | - | - | - |
| Male | 1.22 (1.14–1.30) | <0.001 | 1.08 (1.02–1.14) | 0.006 | 1.18 (1.13–1.23) | <0.001 | 0.93 (0.90–0.98) | 0.002 | 0.85 (0.82–0.89) | <0.001 |
| Age Group | ||||||||||
| 12 to 18 | 1.75 (1.56–1.97) | <0.001 | 1.85 (1.66–2.07) | <0.001 | 1.10 (0.98–1.24) | 0.09 | 1.33 (1.20–1.47) | <0.001 | 0.98 (0.89–1.08) | 0.71 |
| 19 to 30 (referent) | - | - | - | - | - | - | - | - | - | - |
| 31 to 45 | 0.60 (0.54–0.65) | <0.001 | 0.65 (0.60–0.71) | <0.001 | 0.79 (0.73–0.85) | <0.001 | 0.85 (0.80–0.91) | <0.001 | 1.09 (1.02–1.17) | 0.009 |
| 45 to 64 | 0.31 (0.28–0.34) | <0.001 | 0.50 (0.46–0.54) | <0.001 | 0.59 (0.55–0.63) | <0.001 | 0.62 (0.58–0.66) | <0.001 | 1.02 (0.96–1.09) | 0.55 |
| Region | ||||||||||
| Northeast (Referent) | - | - | - | - | - | - | - | - | - | - |
| North Central | 1.32 (1.19–1.46) | <0.001 | 1.58 (1.45–1.73) | <0.001 | 1.79 (1.68–1.92) | <0.001 | 1.64 (1.54–1.75) | <0.001 | 1.69 (1.59–1.81) | <0.001 |
| South | 1.31(1.19–1.44) | <0.001 | 1.28 (1.18–1.39) | <0.001 | 1.55 (1.46–1.64) | <0.001 | 1.72 (1.62–1.82) | <0.001 | 1.93 (1.82–2.04) | <0.001 |
| West | 1.21 (1.07–1.36) | 0.001 | 1.29 (1.17–1.42) | <0.001 | 1.33 (1.25–1.43) | <0.001 | 1.21 (1.13–1.30) | <0.001 | 1.48 (1.48–1.69) | <0.001 |
| Unknown | 1.28 (1.00–1.63) | 0.048 | 0.85 (0.67–1.08) | 0.18 | 0.74 (0.64–0.86) | <0.001 | 0.80 (0.69–0.93) | 0.005 | 0.91 (0.77–0.89) | 0.22 |
| Insurance Type | ||||||||||
| EPO/PPO (referent) | - | - | - | - | - | - | - | - | - | - |
| HMO/Cap POS | 1.01 (0.91–1.11) | 0.91 | 1.15 (1.06–1.25) | 0.001 | 1.58 (1.47–1.70) | <0.001 | 1.28 (1.20–1.36) | <0.001 | 1.39 (1.30–1.48) | <0.001 |
| POS | 1.09 (0.97–1.23) | 0.15 | 1.20 (1.09–1.34) | <0.001 | 1.27 (1.17–1.38) | <0.001 | 1.27 (1.17–1.37) | <0.001 | 1.23 (1.14–1.33) | <0.001 |
| HDHP/CDHP | 1.21 (1.08–1.36) | 0.001 | 0.96 (0.86–1.07) | 0.44 | 1.43 (1.31–1.56) | <0.001 | 1.39 (1.28–1.50) | <0.001 | 1.10 (1.01–1.19) | 0.02 |
| Comp | 0.82 (0.62–1.07) | 0.14 | 1.13 (0.93–1.38) | 0.23 | 1.47 (1.25–1.72) | <0.001 | 1.31 (1.13–1.53) | <0.001 | 1.49 (1.28–1.73) | <0.001 |
| Grouped Charlson Index | ||||||||||
| 0 (referent) | - | - | - | - | - | - | - | - | - | - |
| 1 | 1.49 (1.38–1.62) | <0.001 | 1.33 (1.24–1.43) | <0.001 | 0.89 (0.84–0.94) | <0.001 | 1.43 (1.36–1.51) | <0.001 | 1.47 (1.39–1.55) | <0.001 |
| 2+ | 1.88 (1.72–2.06) | <0.001 | 1.65 (1.53–1.78) | <0.001 | 0.81 (0.76–0.85) | <0.001 | 1.46 (1.38–1.55) | <0.001 | 2.19 (2.06–2.32) | <0.001 |
Appendix 4.
Adjusted odds ratios for patients ≥45 years for undergoing any index ulcerative colitis operation, adjusted for sex, region, insurance type, and grouped Charlson Index score by multivariate logistic regression
| OR: Any Index UC Operation | 95% CI | p-value | |
|---|---|---|---|
| Sex | |||
| Female (referent) | - | - | - |
| Male | 1.29 | 1.04–1.53 | 0.018 |
| Region | |||
| Northeast (Referent) | - | - | - |
| North Central | 2.27 | 1.64–3.14 | <0.001 |
| South | 1.48 | 1.07–2.03 | 0.017 |
| West | 1.36 | 0.93–1.97 | 0.109 |
| Unknown | 1.28 | 0.58–2.84 | 0.548 |
| Insurance Type | |||
| EPO/PPO (referent) | - | - | - |
| HMO/Cap POS | 1.08 | 0.78–1.49 | 0.635 |
| POS | 0.99 | 0.78–1.58 | 0.637 |
| HDHP/CDHP | 1.24 | 0.83–1.85 | 0.288 |
| Comp | 1.30 | 0.78–2.16 | 0.307 |
| Grouped Charlson Index | |||
| 0 (referent) | - | - | - |
| 1 | 1.54 | 1.11–2.13 | 0.009 |
| 2+ | 4.28 | 3.32–5.52 | <0.001 |
Appendix 5.
Adjusted odds ratios for patients ≥45 years for undergoing treatment with ulcerative colitis medications, adjusted for sex, region, insurance type, and grouped Charlson Index score by multivariate logistic regression.
| Odds Ratio: Biologic Use (95% CI) | p-value | Odds Ratio: Immunomodulator Use (95% CI) | p-value | Odds Ratio: 5-ASA Use (95% CI) | p-value | Odds Ratio: Corticosteroid Use (95% CI) | p-value | Odds Ratio Opioid Use (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Female (referent) | - | - | - | - | - | - | - | - | - | - |
| Male | 1.35 (1.20–1.52) | <0.001 | 0.98 (0.90–1.08) | 0.72 | 1.24 (1.17–1.32) | <0.001 | 0.92 (0.87–0.98) | 0.02 | 0.97 (0.92–1.04) | 0.41 |
| Region | ||||||||||
| Northeast (Referent) | - | - | - | - | - | - | - | - | - | - |
| North Central | 1.23 (1.03–1.47) | 0.03 | 1.57 (1.37–1.80) | <0.001 | 1.84 (1.67–2.02) | <0.001 | 1.68 (1.53–1.84) | <0.001 | 0.97 (0.92–1.04) | 0.41 |
| South | 1.29 (1.10–1.52) | 0.002 | 1.27 (1.12–1.45) | <0.001 | 1.43 (1.32–1.56) | <0.001 | 1.68 (1.54–1.82) | <0.001 | 1.80 (1.64–1.98) | <0.001 |
| West | 1.01 (0.83–1.23) | 0.90 | 1.28 (1.10–1.49) | 0.001 | 1.25 (1.13–1.38) | <0.001 | 1.12 (1.01–1.23) | 0.03 | 1.90 (1.75–2.06) | <0.001 |
| Unknown | 1.11 (0.73–1.67) | 0.64 | 0.87 (0.61–1.25) | 0.45 | 0.79 (0.64–1.05) | 0.02 | 0.85 (0.69–1.05) | 0.14 | 1.06 (0.86–1.31) | 0.58 |
| Insurance Type | ||||||||||
| EPO/PPO (referent) | - | - | - | - | - | - | - | - | - | - |
| HMO/Cap POS | 0.96 (0.80–1.16) | 0.67 | 1.15 (1.01–1.32) | 0.04 | 1.52 (1.38–1.67) | <0.001 | 1.32 (1.20–1.44) | <0.001 | 1.48 (1.34–1.62) | <0.001 |
| POS | 1.11 (0.91–1.36) | 0.29 | 1.20 (1.02–1.40) | 0.02 | 1.31 (1.17–1.46) | <0.001 | 1.32 (1.19–1.48) | <0.001 | 1.22 (1.10–1.37) | <0.001 |
| HDHP/CDHP | 1.28 (1.03–1.60) | 0.03 | 1.02 (0.84–1.22) | 0.87 | 1.38 (1.21–1.57) | <0.001 | 1.34 (1.18–1.52) | <0.001 | 1.16 (1.03–1.32) | 0.02 |
| Comp | 0.81 (0.56–1.76) | 0.25 | 1.14 (0.89–1.46) | 0.30 | 1.44 (1.20–1.74) | <0.001 | 1.30 (1.09–1.55) | 0.003 | 1.59 (1.33–1.90) | <0.001 |
| Grouped Charlson Index | ||||||||||
| 0 (referent) | - | - | - | - | - | - | - | - | - | - |
| 1 | 1.56 (1.34–1.81) | <0.001 | 1.35 (1.20–1.51) | <0.001 | 0.94 (0.87–1.02) | 0.14 | 1.47 (1.36–1.58) | <0.001 | 1.50 (1.39–1.62) | <0.001 |
| 2+ | 1.87 (1.63–2.15) | <0.001 | 1.65 (1.49–1.84) | <0.001 | 0.85 (0.79–0.92) | <0.001 | 1.46 (1.46–1.57) | <0.001 | 2.14 (1.99–2.30) | <0.001 |
Appendix 6.
Complete list of opiate-containing medications included within the Truven therapeutic class 60.
Acetaminophen/Hydrocodone Bitartrate
Acetaminophen/Codeine Phosphate
Morphine Sulfate
Oxycodone Hydrochloride
Acetaminophen/Oxycodone Hydrochloride
Acetaminophen/Propoxyphene Napsylate
Hydromorphone Hydrochloride
Bupivacaine HCl/Fentanyl Citrate/NaCl
Fentanyl Citrate
Aspirin/Codeine Phosphate
Meperidine Hydrochloride
Propoxyphene Hydrochloride
Fentanyl
Methadone Hydrochloride
Hydromorphone Hydrochloride/Sodium Chloride
Hydrocodone Bitartrate/Ibuprofen
Oxymorphone Hydrochloride
Morphine Sulfate/Sodium Chloride
Aspirin/Caffeine/Propoxyphene Hydrochloride
Fentanyl Citrate/Sodium Chloride
Acetaminophen/Propoxyphene Hydrochloride
Aspirin/Butalbital/Caffeine/Codeine
Fentanyl Citrate/Ropivacaine HCl/NaCl
ASA/Oxycodone HCl/Oxycodone Terephthalate
Bupivacaine HCl/HYDROmorphone hydrochloride
Acetaminophen/Caffeine/Dihydrocodeine
Sufentanil Citrate
Tapentadol Hydrochloride
Aspirin/Caffeine/Dihydrocodeine Bitartrate
APAP/Butalbital/Caffeine/Codeine Phosphate
Codeine Sulfate
Meperidine Hydrochloride/Sodium Chloride
Propoxyphene Napsylate
Dextrose/Morphine Sulfate
Aspirin/Carisoprodol/Codeine Phosphate
Aspirin/Hydrocodone Bitartrate
Hydrocodone Bitartrate
Codeine Phosphate
Alfentanil Hydrochloride
Meperidine HCl/Promethazine HCl
Morphine Sulfate/Naltrexone Hydrochloride
Levorphanol Tartrate
Remifentanil Hydrochloride
Opium
Bupivacaine HCl/Na Cl/Sufentanil Citrate
Chlorpheniramine Maleate/Codeine Phosphate
A.P.C. W/Codeine
Apap/Caffeine/Codeine/Salicylamide
Belladonna Alkaloids/Opium Alkaloids
Ropivacaine HCl/Na Cl/Sufentanil Citrate
Apomorphine Hydrochloride
Aspirin/Oxycodone Hydrochloride
Brompheniramine Maleate/Codeine Phosphate/Phen..
Carbinoxamine Maleate/Hydrocodone Bitartrate/PSE HCl
Dextrose/Fentanyl Citrate
Droperidol/Fentanyl Citrate
Morphine Sulfate Liposome
Ibuprofen/Oxycodone Hydrochloride
Methadone Hydrochloride/Sodium Chloride
Aspirin/Caffeine/Hydrocodone Bitartrate
Chlorpheniramine Maleate/Hydrocododone Bitartrate/PSE
Dihydrocodeine/Apap/Caffeine
Oxycodone
Acetaminophen/Butalbital/Codeine Phosphate
Bupivacaine HCl/Morphine Sulfate/NaCl
Prometh/Codeine/Apap
APAP/Hydrocodone Bitatrate;Medical Food
Aspirin (Buffered)/Codeine Phosphate
Codeine/Salicylamide/Apap
Codeine Phosphate/GG/PSE HCl
Fentanyl/Ropivacaine/Sodium Chloride
Hydrocodine Bitartrate/Phenylephephrine HCl/Pyril Maleate
APAP/ASA/Caffeine/Codeine/Salicylamide
Acetaminophen/Aspirin/Codeine Phosphate
Alpharodine Hydrochloride
Aspirin/Propoxyphene Hydrochloride
Aspirin/Propoxyphene Napsylate
Atropine Sulfate/Meperidine Hydrochloride
Brompheniramine Maleate/Codeine Phosphate/PSE
Brompheniramine Maleate/Codeine Phospate
Bupivacaine/Fentanyl/Sodium Chloride
Codeine Phosphate/Guaifenesin
Codeine Phosphate/Phenylephrine Hydrochloride
Dihydrocodeine/Amino Phenol/Aspirin/Caffeine
Dihydrocodeine/Apap/Salicylate
Dihydrocodeine Bitartrate/GG/Phenylephrine
HYDROmorphone hydrochloride/Ropivacaine
Levomethadyl Acetate Hydrochloride
Opium/Chlorphen/Apap/Phenylpro
Remifentanil Hydrochloride/Sodium Chloride
APAP/ASA/Caff/Codeine Phosphate/Salicylamide
APAP/Butabarbital Na/Codeine Phosphate
Acetaminophen/Aspirin/Caffeine/Codeine
Acetaminophen/Butabarbital Sodium
Acetaminophen/Butalbital/Caffeine/Hydrocodone
Acetaminophen/Meperidine Hydrochloride
Brompheniramine Tan/Hydrocodone Tannate
Bupivacaine HCl/Epi HCl/Fentanyl Citrate
Chlorpheniramine Maleate/Codeine Phosphate/PSE
Codeine Phos/Dexbrompheniramine Maleate
Codeine Phosphate/Pseudoephedrine Hydrochloride
Dextrose/HYDROmorphone hydrochloride
Dihydrocodeine Bitartrate/Phenylephrine HCl
Dihydrocodeine Bitartrate/Phenylephrine HCl
Hydrochlorides of Opium
Hydrocodone Bitartrate/Potassium Guaifenesin
Hydrocodone Tannate/Pseudoephedrine
Hydrocodone/Aspirin/Caffeine
Morphine Sulfate/Atropine Sulfate
Opium/Bismuth/Pectin/Zn.Phenol
Opium/Ipecac/Caffeine/Aspirin
Footnotes
Financial Disclaimers: None reported.
References
- 1.Rossi MC, Cristofaro MR, Gentile S, et al. ; AMD Annals Study Group. Sex disparities in the quality of diabetes care: biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the AMD Annals initiative. Diabetes Care 2013;36:3162–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 2014;23:112–119. [DOI] [PubMed] [Google Scholar]
- 3.Alabas OA, Gale CP, Hall M, et al. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon S, Goodman SG, Yan RT, et al. Bridging the gender gap: insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 2012;163:66–73. [DOI] [PubMed] [Google Scholar]
- 5.Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep 2010;12:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol 2015;21:5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathore SS, Chen J, Wang Y, Radford MJ, Vaccarino V, Krumholz HM. Sex differences in cardiac catheterization: the role of physician gender. JAMA 2001;286:2849–2856. [DOI] [PubMed] [Google Scholar]
- 8.Ayanian JZ, Epstein AM. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med 1991;325:221–225. [DOI] [PubMed] [Google Scholar]
- 9.Borkhoff CM, Hawker GA, Wright JG. Patient gender affects the referral and recommendation for total joint arthroplasty. Clin Orthop Relat Res 2011;469:1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawker GA, Wright JG, Coyte PC, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med 2000;342:1016–1022. [DOI] [PubMed] [Google Scholar]
- 11.Jüni P, Low N, Reichenbach S, Villiger PM, Williams S, Dieppe PA. Gender inequity in the provision of care for hip disease: population-based cross-sectional study. Osteoarthritis Cartilage 2010;18:640–645. [DOI] [PubMed] [Google Scholar]
- 12.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ 2007;177:1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med 2003;31:1901–1907. [DOI] [PubMed] [Google Scholar]
- 14.Pleis JR, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2007. Vital Health Stat 10 2009;(240):1–159. [PubMed] [Google Scholar]
- 15.Vaidya V, Partha G, Karmakar M. Gender differences in utilization of preventive care services in the United States. J Womens Health (Larchmt) 2012;21:140–145. [DOI] [PubMed] [Google Scholar]
- 16.Blumenstein I, Herrmann E, Filmann N, et al. Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activity: a subgroup analysis of a large multi-centre, prospective, internet-based study. J Crohn’s Colitis 2011;5:203–210. [DOI] [PubMed] [Google Scholar]
- 17.Kin C, Kate Bundorf M. As infliximab use for ulcerative colitis has increased, so has the rate of surgical resection. J Gastrointest Surg 2017;21:1159–1165. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Indicator Definitions - Reproductive Health https://www.cdc.gov/cdi/definitions/reproductive-health.html, 2018. Accessed 4 April 2018.
- 19.Waljee A, Waljee J, Morris AM, Higgins PD. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut 2006;55:1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornish JA, Tan E, Teare J, et al. The effect of restorative proctocolectomy on sexual function, urinary function, fertility, pregnancy and delivery: a systematic review. Dis Colon Rectum 2007;50:1128–1138. [DOI] [PubMed] [Google Scholar]
- 21.Polle SW, Dunker MS, Slors JF, et al. Body image, cosmesis, quality of life, and functional outcome of hand-assisted laparoscopic versus open restorative proctocolectomy: long-term results of a randomized trial. Surg Endosc 2007;21:1301–1307. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016;150:734–757 e731. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblatt E, Kane S. Sex-specific issues in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2015;11:592–601. [PMC free article] [PubMed] [Google Scholar]
- 24.Grosen A, Kelsen J, Hvas CL, Bellaguarda E, Hanauer SB. The influence of methotrexate treatment on male fertility and pregnancy outcome after paternal exposure. Inflamm Bowel Dis 2017;23:561–569. [DOI] [PubMed] [Google Scholar]
- 25.Unruh AM. Gender variations in clinical pain experience. Pain 1996;65:123–167. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein B, Kane R. Physicians’ attitudes toward female patients. Med Care 1981;19:600–608. [DOI] [PubMed] [Google Scholar]


