Abstract
Background:
Concomitant use of cocaine and HIV pre-exposure prophylaxis (PrEP) raises important clinical questions around adherence, retention in care, and renal toxicity.
Methods:
We assessed the associations of confirmed cocaine use with PrEP adherence (both ascertained via objective measures), care engagement, and renal function in the iPrEx open label extension. Cocaine use was measured in scalp hair samples and categorized as: light (500–3,000 pg/mg) and moderate to heavy (>3,000 pg/mg). PrEP adherence in the first three months was measured via plasma tenofovir concentrations. Disengagement from PrEP care was defined as a gap in follow-up greater than four months. Serum creatinine was assessed at baseline and quarterly visits.
Results:
Of the 400 participants included in this analysis, 90% were men who have sex with men, 10% transgender women, 74% Hispanic/Latino; 21% tested positive for cocaine use in the last three months. In adjusted analysis, light cocaine use (aOR 2.10 [95% CI 1.07–4.14]) and moderate to heavy use (aOR 2.32 [1.08–5.00]) were associated with greater odds of having plasma tenofovir concentrations below the level of quantitation. Participants with moderate to heavy use had a nearly three-fold higher rate of disengagement from PrEP care compared to non-users (aHR 2.90 [1.48–5.66]). We found no statistically or clinically significant differences in creatinine clearance and serum creatinine between participants who tested positive for cocaine and those who did not.
Conclusions:
Cocaine use decreases PrEP adherence and care engagement. Comprehensive approaches are needed to reduce cocaine use and enhance engagement along the PrEP care continuum.
Keywords: Pre-exposure prophylaxis (PrEP), cocaine, stimulants, medication adherence, objective measures, retention in care, renal insufficiency
INTRODUCTION
Uptake of HIV pre-exposure prophylaxis (PrEP) using daily oral emtricitabine-tenofovir disoproxil fumarate has increased considerably since 2012.1 However, reports from clinical implementation suggest high rates of PrEP discontinuation.2–5 The use of stimulant drugs such as cocaine has received little attention in the PrEP literature despite elevated prevalence in high priority populations.6–8 In fact, the use of stimulants is higher among men who have sex with men (MSM) and transgender women compared to the general population. 8–10
Surveillance reports from major metropolitan areas in the United States suggest that stimulant use is increasing and will continue to do so in the near term.11,12 Methamphetamine use doubled between 2011 and 2014 (from 4% to 9%) in New York City, and in some regions, rates are at their highest since 2005.6,12 San Francisco also observed a resurgence of cocaine use among MSM in recent years with as much as 23% of respondents in 2014 reporting cocaine use in the last six months.13 The prevalence of cocaine use among MSM nationwide is second only to marijuana.6 An estimated 13–22 million people globally use cocaine, and the highest prevalence of cocaine use disorders is in North America.14,15
Stimulant use is a potential barrier to achieving the optimal public health benefits of PrEP. Important clinical questions around adherence, retention in care, and renal toxicity among persons who use stimulants remain unresolved. Studies from our team and others have found some evidence that stimulant-using MSM can achieve prevention-effective adherence and rates of retention comparable to non-users.16–18 However, these studies have largely relied on self-reported drug use, which is prone to misclassification.19,20
Biological measures of stimulant use are more reliable than self-reported measures due to social desirability bias. In a prior study, we found that stimulant use, confirmed using urine immunoassays, was associated with sub-optimal PrEP adherence in the first month.21 Because urine tests have a narrow window of detection (1–3 days) and qualitative assays limited our ability to quantify stimulant drug levels, further research is needed to understand how varying degrees of stimulant drug exposure influence PrEP adherence and engagement in care.
Another important gap in the literature is that little is known about the renal safety of PrEP in persons who use stimulants such as cocaine. Although PrEP has been associated with only a modest decrease in creatinine clearance,22 it is uncertain whether concomitant use of stimulants can exacerbate renal dysfunction. Cocaine use is associated with glomerular, tubular, vascular, and interstitial damage, and can precipitate acute kidney injury.23–26 To address these gaps in the literature, we assessed the associations of biologically confirmed cocaine use with PrEP adherence (assessed objectively via a pharmacologic measure), PrEP care engagement, and renal function in the iPrEx open label extension (OLE).
METHODS
iPrEx OLE is described in detail elsewhere.27 Briefly, participants were male sex at birth, reported having anal sex with men, age 18 or older, and must have participated in a previous PrEP clinical trial. Follow-up was scheduled every month for the first three months and quarterly thereafter for up to 72 weeks. All participants provided informed consent. This study was approved by the Institutional Review Board at the University of California, San Francisco.
Exposure.
Cocaine use was measured by determining concentrations in scalp hair samples collected at the first quarterly visit. We focused on cocaine as this was the most commonly used stimulant in our sample, consistent with the high prevalence of cocaine use in MSM and transgender women. Hair samples were collected quarterly after PrEP initiation from a subset of participants who joined a hair substudy.28,29 Approximately 100 strands of hair were cut from the occipital region and samples were stored and shipped in aluminum foil at room temperature. Hair analysis provides up to a 3-month surveillance window and is routinely used in forensics to determine amount of exposure to various illicit substances.30,31
Hair samples from 410 participants were sent to a commercial laboratory for analysis (Quest Diagnostics, Lenexa, KS) and tested using a standard 5-panel drug test (cannabis, cocaine, amphetamines, opioids, and phencyclidine). Hair samples from 10 participants were rejected due to insufficient quantity and were, thus, excluded from this analysis. Samples were screened for cocaine analytes using enzyme-linked immunosorbent assay and positive results confirmed using gas chromatography-mass spectrometry (GC-MS).
For this analysis, we utilized a confirmatory cutoff of ≥ 500 pg/mg of cocaine analyte as proposed by the Substance Abuse and Mental Health Services Administration.32 Cocaine exposure was stratified based on levels used in prior studies.33,34 Cocaine levels between 500–3,000 pg/mg corresponded to “light use” and levels >3,000 pg/mg indicated “moderate to heavy use.”
Outcomes.
Tenofovir concentrations in blood plasma were assessed in all participants in one of the study visits during the first three months after receiving PrEP using validated assays.35 Plasma concentrations reflect dosing within the previous week. Our adherence outcome was plasma tenofovir concentrations below the level of quantitation, as levels within the detectable range have been associated with >90% protection against HIV.35 Disengagement from PrEP care was defined as a gap in follow-up visit greater than four months. We used study follow-up as an indicator of clinical care engagement as the study’s follow-up schedule closely mirrored that of current PrEP clinical guidelines.36,37 To monitor renal function, serum creatinine was measured at baseline and at each quarterly visit. Creatinine clearance was estimated by the Cockcroft-Gault equation. Renal adverse events were graded based on the Division of AIDS Adverse Events Grading Table.38
Analysis
Multivariable logistic models adjusting for age, ethnicity, and transgender identity estimated the association between level of cocaine use and plasma tenofovir concentration. Cox proportional hazard regression models estimated the association between cocaine use and time to disengagement from care. Regression models used robust variance estimators to account for clustering within study sites. Mean differences in creatinine clearance by follow-up week and cocaine exposure was estimated using linear mixed effects models with a time by cocaine interaction fit by restricted maximum likelihood. All p-values are two-sided, and analyses were conducted using Stata 14 (College Station, TX).
RESULTS
Participant Characteristics
The 400 participants included in this analysis were mostly MSM (90%; 358/400), Hispanic/Latino (74%; 297/400), and completed at least secondary education (73%; 292/400). Ten percent (42/400) were transgender women. At baseline, median age was 29 years (interquartile range [IQR] 24–36). One-in-five participants (21%; 83/400) tested positive for recent cocaine use with a median cocaine concentration of 2,513 pg/mg (IQR 963–13,693 pg/mg). Of those who tested positive for cocaine analytes, 52% (43/83) were classified as light users and 48% (40/83) were moderate to heavy users. Participant characteristics and hair drug analysis results are summarized in Table 1a.
Table 1a.
Characteristics of men who have sex with men and transgender women in the iPrEx open label extension with scalp hair samples analyzed for drug metabolites‡ (N=400)
| N |
(%) |
|
|---|---|---|
| Age at baseline, median (interquartile range) | 29 | (24 – 36) |
| Study region | ||
| Andes (Ecuador and Peru) | 285 | (71) |
| Brazil | 60 | (15) |
| United States | 32 | (8) |
| Thailand | 21 | (5) |
| South Africa | 2 | (<1) |
| Hispanic/Latino | 297 | (74) |
| Transgender | 42 | (10) |
| Education | ||
| <Secondary | 103 | (26) |
| ≥Secondary | 292 | (73) |
| Drug metabolites | ||
| Cocaine | 83 | (21) |
| Cannabis | 11 | (3) |
| Amphetamine | 8 | (2) |
| Opioids | 2 | (<1) |
| Phencyclidine | 0 | (0) |
Association of Cocaine Use with PrEP Non-Adherence
One-fourth (100/400) of participants had plasma tenofovir below the level of quantitation during the first three months. In adjusted analysis, participants with cocaine levels indicative of light use (adjusted odds ratio [aOR] 2.10 [95% CI 1.07–4.14]) and moderate to heavy use (aOR 2.32 [1.08–5.00]) had significantly greater odds of having tenofovir concentrations below the level of quantitation compared to non-users (Table 1b).
Table 1b.
PrEP non-adherence and care disengagement in men who have sex with men and transgender women with biologically-confirmed cocaine use
| PrEP Non-adherence |
Care Disengagement |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | aOR | (95% CI) | HR | (95% CI) | aHR | (95% CI) | |
| Age, baseline | 0.94 | (0.90 – 0.97) | 0.94 | (0.90 – 0.97) | 0.98 | (0.96 – 1.01) | 0.97 | (0.94 – 1.00) |
| Hispanic/Latino | 1.07 | (0.62 – 1.86) | 0.76 | (0.42 –1.38) | 0.85 | (0.47 – 1.51) | 0.67 | (0.35 – 1.27) |
| Transgender | 0.78 | (0.36 – 1.70) | 0.69 | (0.30 –1.59) | 0.78 | (0.34 – 1.75) | 0.64 | (0.28 – 1.46) |
| Cocaine exposure | ||||||||
| Light a | 1.89 | (0.95 – 3.74) | 2.10 | (1.07 – 4.14) | 1.24 | (0.48 – 3.23) | 1.47 | (0.53 – 4.08) |
| Moderate/heavyb | 1.83 | (0.91 – 3.69) | 2.32 | (1.08 – 5.00) | 2.44 | (1.28 – 4.65) | 2.90 | (1.48 – 5.66) |
OR = odds ratio; aOR = adjusted odds ratio; HR = hazard ratio; aHR = adjusted hazard ratio; CI = confidence interval
Hair analysis reflects cumulative dosing over a 3-month timeframe.
Adjusted models controlled for all other variables in the table.
cocaine levels indicative of light use: 500–3,000 pg/mg
cocaine levels indicative of moderate/heavy use: >3,000 pg/mg
Association of Cocaine use with PrEP Disengagement
Fifteen percent (60/400) of participants had a gap in their follow-up by more than four months. Participants with cocaine levels indicative of moderate to heavy use had a nearly three-fold higher rate of disengagement from care compared to non-users (adjusted hazard ratio [aHR] 2.90 [1.48–5.66]; Table 1) after adjusting for age, ethnicity, and transgender identity. Light cocaine users also had a higher rate of PrEP disengagement, but the effect was not statistically significant (aHR 1.47 [0.53–4.08]).
Renal Function and Adverse Events
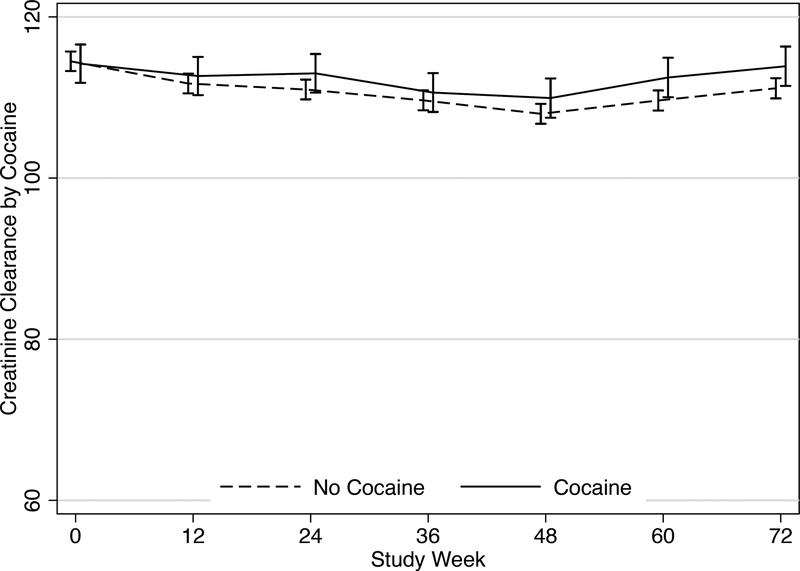
There was no statistically significant difference in creatinine clearance over time among persons who tested positive for cocaine compared to those who did not (Figure 1). The mean difference in creatinine clearance between groups ranged from 0.93 mL/min to 2.85 mL/min over the 72 study weeks (p-values >0.05). When we limited our analysis to only those with evidence of PrEP adherence during the first three months, we also found no meaningful difference in creatinine clearance between groups, ranging from differences of 0.06 mL/min to 1.48 mL/min over 72 weeks (p-values >0.05).
Figure 1.
Mean creatinine clearance (± standard error) by cocaine use over 72 weeks of study follow-up. There were no significant mean differences in creatinine clearance between persons who tested positive for cocaine and those who did not (p-values >0.05).
There were 26 instances of serum creatinine elevations during follow-up that were at least 1.1 times the upper limit of normal or 1.5 times above the baseline level. Of these elevations, four (15%) were in participants who tested positive for cocaine and 22 (85%) were in non-users (p=0.52). Serum creatinine levels were elevated at more than one visit in five participants, none of whom tested positive for cocaine use. Overall, 15/26 creatinine elevations were above the normal range. Of these, three (20%) were in participants who tested positive for cocaine use and 12 (80%) were in non-users (p=0.09).
DISCUSSION
We found that cocaine use at any level was associated with lower odds of achieving prevention-effective adherence in the first three months after starting PrEP. The first few months following PrEP initiation is a particularly critical period as adherence during this time is predictive of future adherence patterns.39–41 These findings underscore the importance of providing comprehensive care at the time of PrEP initiation that includes an assessment of cocaine use and other potential barriers to optimal adherence.
We found that participants who tested positive for cocaine, particularly individuals with levels indicative of moderate to heavy use, had higher rates of disengagement from PrEP care. Marcus and colleagues found a similar pattern in their study of PrEP patients in an integrated healthcare system.42 In that study, patients with a documented history of drug use were nearly twice as likely to discontinue PrEP care as those without a history of drug use. Regular clinical follow-up every three months that includes HIV testing and screening for sexually transmitted infections (STI) is recommended by current practice guidelines.36,37 Routine STI screening can facilitate early identification and treatment given the high incidence of STIs among PrEP users seen in clinical practice.43,44 Extended gaps in follow-up can also lead to interruptions in PrEP adherence and persistence, which have been associated with seroconversions.27,37,45 Expanded efforts are needed to assist persons who use cocaine with completing routine follow-up visits that allow for regular monitoring and present an opportunity to support adherence, discuss risk reduction, and address co-occurring stimulant use.
Results provided no evidence of increased renal toxicity or adverse events from the concurrent use of cocaine and PrEP. Given the high prevalence of stimulant use among MSM and transgender women, it is important for clinicians to be aware of potential renal complications. In a systematic review of nephrotoxicities related to drugs of abuse, cocaine use was associated with an elevated risk of rhabdomyolysis and ischemic nephropathy.26 Ongoing monitoring of renal function as recommended by PrEP clinical guidelines is warranted, but more intensive renal monitoring with concomitant stimulant use does not seem indicated.
The biggest strength of our analysis is the use of objective measures to assess both stimulant use and PrEP adherence. The use of biologic measures can eliminate the social desirability bias associated with self-report. However, our results have some limitations. Studies have found that the incorporation of drug analytes in hair can vary by pigmentation, potentially resulting in artificially higher concentrations in persons with darker hair.46 Plasma tenofovir concentrations reflect recent adherence and may not accurately reflect long-term adherence patterns. Measurement of renal function among the most severe cocaine users at later time points in iPrEx OLE may be limited as these individuals were more likely to miss follow-up visits. Lastly, we were not sufficiently powered to stratify our analysis by age as glomerular dysfunction may be more apparent among older individuals.37
Our findings demonstrate that biologically confirmed cocaine use is associated with lower odds of achieving prevention-effective PrEP adherence and that those with the highest levels of cocaine use are at greater risk for disengagement from PrEP care. We also found no evidence of increased risk of nephrotoxicity from concomitant use of PrEP and cocaine. Findings from this study can inform targeted efforts to simultaneously reduce cocaine use and enhance engagement along the PrEP care continuum to optimize the public health benefits of PrEP.
Acknowledgments
Parts of the data in this manuscript were presented at the HIV Research for Prevention Conference, Madrid, in October 2018.
Footnotes
Conflicts of Interest and Sources of Funding: This study was supported by the National Institute on Drug Abuse (NIDA R36 DA041906 and T32 DA007250). iPrEx OLE was funded by the National Institute of Allergy and Infectious Diseases (NIAID U01 AI064002 and R01 AI118575). Hair collection was funded by NIAID R01 AI098472. Gilead Sciences donated tenofovir disoproxil fumarate and emtricitabine to the parent study but provided no other financial support and had no role in data interpretation or manuscript development. DVG has received fees from Gilead Sciences. RMG has received research funding from ViiV Healthcare. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sullivan PS, Giler RM, Mouhanna F, et al. Trends in the use of oral emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infection, United States, 2012–2017. Ann Epidemiol. 2018. doi: 10.1016/j.annepidem.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. doi: 10.7448/IAS.19.1.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shover CL, Javanbakht M, Shoptaw S, Bolan R, Gorbach PM. High discontinuation of pre-exposure prophylaxis within six months of initiation. Presented at Conference on Retroviruses and Opportunistic Infections; March 2018; Boston, MA. [Google Scholar]
- 4.Scott H, Nordell M, Hirozawa A, et al. Racial/ethnic disparities in persistence among PrEP users in San Francisco. Presented at Conference on Retroviruses and Opportunistic Infections; February 2017; Seattle, WA. [Google Scholar]
- 5.Rolle C-PM, Onwubiko U, Jo J, Sheth AN, Kelley CF, Holland DP. PrEP implementation and persistence in a county health department in Atlanta, GA. Presented at Conference on Retroviruses and Opportunistic Infections; March 2018; Boston, MA. [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV Infection Risk, Preventing, and Testing Behaviors Among Men Who Have Sex with Men - National HIV Behavioral Surveillance, 20 U.S. Cities, 2014. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-hssr-nhbs-msm-2014.pdf. Accessed July 30, 2018.
- 7.Stall R, Paul JP, Greenwood G, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men’s Health Study. Addiction. 2001;96(11):1589–1601. doi: 10.1080/09652140120080723. [DOI] [PubMed] [Google Scholar]
- 8.Santos G-M, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev. 2014;33(3):287–295. doi: 10.1111/dar.12116. [DOI] [PubMed] [Google Scholar]
- 9.Cochran SD, Ackerman D, Mays VM, Ross MW. Prevalence of non-medical drug use and dependence among homosexually active men and women in the US population. Addiction. 2004;99(8):989–998. doi: 10.1111/j.1360-0443.2004.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein ND, Burstyn I, LeVasseur MT, Welles SL. Drug use among men by sexual behaviour, race and ethnicity: Prevalence estimates from a nationally representative US sample. Int J Drug Policy. 2016;36:148–150. doi: 10.1016/j.drugpo.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Drug Enforcement Administration. 2017. National Drug Threat Assessment. https://www.dea.gov/sites/default/files/docs/DIR-040-17_2017-NDTA.pdf. Accessed August 15, 2018.
- 12.National Drug Early Warning System. NDEWS Annual Project Report, 2015. https://ndews.umd.edu/sites/ndews.umd.edu/files/NDEWS%20Annual%20Project%20Report%202015%20Final%20Web.pdf. Accessed August 15, 2018.
- 13.San Francisco Department of Public Health Population Health Division. HIV Epidemiology Annual Report 2016. https://www.sfdph.org/dph/comupg/oprograms/HIVepiSec/HIVepiSecReports.asp. Accessed July 30, 2018.
- 14.United Nations Office on Drugs and Crime. World Drug Report 2017. https://www.unodc.org/wdr2017/field/Booklet_3_Plantbased.pdf. Accessed August 15, 2018.
- 15.Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 16.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV pre-exposure prophylaxis (PrEP) uptake and retention among men who have sex with men in a community-based sexual health clinic. AIDS Behav. 2018;22(4):1096–1099. doi: 10.1007/s10461-017-2009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman-Meza D, Beymer MR, Kofron RM, et al. Stimulant use and condomless sex with multiple partners: effect on PrEP adherence. Presented at Conference on Retroviruses and Opportunistic Infections; March 2018; Boston, MA. [Google Scholar]
- 18.Hoenigl M, Jain S, Moore D, et al. Substance use and adherence to HIV preexposure prophylaxis for men who have sex with men. Emerging Infect Dis. 2018;24(12). doi: 10.3201/eid2412.180400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V. The utility of drug testing in epidemiological research: results from a general population survey. Addiction. 2004;99(2):197–208. [DOI] [PubMed] [Google Scholar]
- 20.Fendrich M, Johnson TP, Sudman S, Wislar JS, Spiehler V. Validity of drug use reporting in a high-risk community sample: a comparison of cocaine and heroin survey reports with hair tests. Am J Epidemiol. 1999;149(10):955–962. [DOI] [PubMed] [Google Scholar]
- 21.Hojilla JC, Vlahov D, Glidden DV, et al. Skating on thin ice: stimulant use and sub-optimal adherence to HIV pre-exposure prophylaxis. J Int AIDS Soc. 2018;21(3):e25103. doi: 10.1002/jia2.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28(6):851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barroso-Moguel R, Mendez-Armenta M, Villeda-Hernandez J. Experimental nephropathy by chronic administration of cocaine in rats. Toxicology. 1995;98(1–3):41–46. [DOI] [PubMed] [Google Scholar]
- 24.Di Paolo N, Fineschi V, Di Paolo M, et al. Kidney vascular damage and cocaine. Clin Nephrol. 1997;47(5):298–303. [PubMed] [Google Scholar]
- 25.Buettner M, Toennes SW, Buettner S, et al. Nephropathy in illicit drug abusers: a postmortem analysis. Am J Kidney Dis. 2014;63(6):945–953. doi: 10.1053/j.ajkd.2014.01.428. [DOI] [PubMed] [Google Scholar]
- 26.Mansoor K, Kheetan M, Shahnawaz S, et al. Systematic review of nephrotoxicity of drugs of abuse, 2005–2016. BMC Nephrol. 2017;18(1):379. doi: 10.1186/s12882-017-0794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi M, Glidden DV, Liu A, et al. Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx Open Label Extension: Implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis. 2015;212(9):1402–1406. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi M, Glidden DV, Mayer K, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuPont RL, Baumgartner WA. Drug testing by urine and hair analysis: complementary features and scientific issues. Forensic Sci Int. 1995;70(1–3):63–76. [DOI] [PubMed] [Google Scholar]
- 31.Welp EAE, Bosman I, Langendam MW, Totté M, Maes RAA, van Ameijden EJC. Amount of self-reported illicit drug use compared to quantitative hair test results in community-recruited young drug users in Amsterdam. Addiction. 2003;98(7):987–994. [DOI] [PubMed] [Google Scholar]
- 32.Substance Abuse and Mental Health Services Administration. Proposed revisions to mandatory guidelines for federal workplace drug testing programs. https://www.gpo.gov/fdsys/pkg/FR-2004-04-13/pdf/04-7984.pdf. Accessed May 1, 2018.
- 33.Tassiopoulos K, Bernstein J, Heeren T, Levenson S, Hingson R, Bernstein E. Hair testing and self-report of cocaine use by heroin users. Addiction. 2004;99(5):590–597. doi: 10.1111/j.1360-0443.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 34.Gambelunghe C, Rossi R, Aroni K, et al. Norcocaine and cocaethylene distribution patterns in hair samples from light, moderate, and heavy cocaine users. Drug Test Anal. 2017;9(2):161–167. doi: 10.1002/dta.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Preexposure Prophylaxis for the of HIV Infection in the United States - 2017 Update Clinical Practice Guidelines. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed June 4, 2018.
- 37.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. doi: 10.1001/jama.2018.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Division of AIDS. DAIDS Table of Grading the Severity of Adult and Pediatric Adverse Events. https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed August 15, 2018.
- 39.Glidden DV, Buchbinder SP, Anderson PL, et al. PrEP engagement for HIV prevention: results from the iPrEx open label extension. Presented at Conference on Retroviruses and Opportunistic Infections; February 2015; Seattle, WA. [Google Scholar]
- 40.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–11. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grinsztejn B, Hoagland B, Moreira RI, et al. Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV. 2018;5(3):e136–e145. doi: 10.1016/S2352-3018(18)30008-0. [DOI] [PubMed] [Google Scholar]
- 42.Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer KH, Maloney KM, Levine K, et al. Sociodemographic and clinical factors associated with increasing bacterial sexually transmitted infection diagnoses in men who have sex with men accessing care at a Boston community health center (2005–2015). Open Forum Infect Dis. 2017;4(4):ofx214. doi: 10.1093/ofid/ofx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheidweiler KB, Cone EJ, Moolchan ET, Huestis MA. Dose-related distribution of codeine, cocaine, and metabolites into human hair following controlled oral codeine and subcutaneous cocaine administration. J Pharmacol Exp Ther. 2005;313(2):909–915. doi: 10.1124/jpet.104.082388. [DOI] [PubMed] [Google Scholar]