Abstract
Objective:
The capacity to precisely predict progression to type 1 diabetes in young children over a short time span is an unmet need. We sought to develop a risk algorithm to predict progression in children with high-risk human leukocyte antigen (HLA) genes followed in The Environmental Determinants of Diabetes in the Young (TEDDY) study.
Research Design and Methods:
Logistic regression and 4-fold cross validation examined 38 candidate predictors of risk from clinical, immunologic, metabolic and genetic data. TEDDY subjects with at least one persistent, confirmed autoantibody at age 3 were analyzed with progression to type 1 diabetes by age 6 serving as the primary endpoint. The logistic regression prediction model was compared to two non-statistical predictors, multiple autoantibody status and presence of IA-2A.
Results:
A total of 363 subjects had at least one autoantibody at age 3. Twenty-one percent of subjects developed type 1 diabetes by age 6. Logistic regression modelling identified 5 significant predictors – IA-2A status, HbA1c, BMI Z-score, SNP rs1270876_G, and a combination marker of autoantibody number plus fasting insulin level. The logistic model yielded a ROC area under the curve (AUC) of 0.80, higher than the two other predictors; however, the differences in AUC, sensitivity and specificity were small across models.
Conclusions:
This study highlights the application of precision medicine techniques to predict progression to diabetes over a 3-year window in TEDDY subjects. This multifaceted model provides preliminary improvement in prediction over simpler prediction tools. Additional tools are needed to maximize the predictive value of these approaches.
Keywords: type 1 diabetes, autoantibodies, metabolic, prediction, pediatric
Introduction
As the worldwide incidence of type 1 diabetes continues to increase, there is a growing urgency to develop and test therapies aimed at slowing and stopping progression to type 1 diabetes in autoantibody positive subjects. While there are currently no effective therapies to stop the development of type 1 diabetes, subgroups within some prevention studies have shown delayed progression and additional promising therapies are currently being explored. In order to most rationally apply these therapies to at-risk children, there is a desire to develop tools to precisely predict disease progression over narrow timeframes (1,2).
As we move further into the era of precision medicine, the capacity to identify those who are most at-risk for disease development and, perhaps more importantly, those who will respond best to specific therapies continues to progress. That said, efforts to accurately predict and prevent progression to type 1 diabetes have been underway for some time. The Diabetes Prevention Trial-Type 1 (DPT-1) represents the largest single effort to prevent type 1 diabetes to date (3). Using the DPT-1 data, the DPT-1 risk score (DPTRS) was developed and validated using BMI, age, log-fasting c-peptide, and 2-hour oral glucose tolerance test (OGTT) data to predict type 1 diabetes risk in DPT-1 participants (4).
In an effort to build on the concepts of the DPTRS and similar risk score methodologies, we used multivariable logistic regression to assess candidate predictors of type 1 diabetes progression among young children with one or more positive autoantibodies. The Environmental Determinants of Diabetes in the Young (TEDDY) study is a multi-site, multi-country cooperative study aimed at determining which environmental factors are involved in the pathogenesis of type 1 diabetes (5). The TEDDY cohort represents a unique group of children with an increased genetic risk of progression to type 1 diabetes followed since birth. The cohort is of younger age than earlier studies and has unique baseline and longitudinal data that could provide specific information on clinical risk prediction in young children. Such data would be highly valuable in advising high-risk families of the likelihood of disease progression and allowing for early diagnosis. Further benefits of improved risk prediction include avoiding severe complications such as diabetic ketoacidosis (DKA) at diagnosis, which is markedly higher in younger children (6,7), and aiding in designing and enrolling subjects into prevention trials.
Research Design and Methods
Participants and Selection Criteria
TEDDY subjects were recruited from four countries, the United States, Germany, Sweden, and Finland, and 8676 participants with high-risk HLA genotypes were enrolled (8). Children in the TEDDY study are followed every 3 months until age 48 months for the development of islet autoantibodies, a precursor to type 1 diabetes development, then every 6 months. For those with autoantibody seroconversion, visits remain every 3 months. Those with 1 autoantibody have a hemoglobin A1c (HbA1c) measured; those with ≥ 2 autoantibodies additionally have an OGTT starting at age 3 years (2 times points only at 0 and 120 minutes obtained). The requirement for HbA1c measurement with 1 autoantibody was added to the TEDDY study four years after the study began. Thus, some subjects did not have HbA1c measurement at age 3. For these subjects, if they had an age 3 HbA1c taken outside of the TEDDY study, this measure was included in the analysis.
Subject data at age 3 was used to determine the risk of progression to type 1 diabetes by the age of 6 years. Collection of metabolic data such as HbA1c with or without OGTT begins in the TEDDY study at age 3. As the youngest children in TEDDY have reached 7 years of age, the use of type 1 diabetes status at age 6 was chosen to ensure complete data collection. All subjects were included if they had ≥ 1 autoantibody on confirmed testing (2 consecutive positive results) by the age of 3 years 5 months (to include those late for their 3-year visit) but not diagnosed with type 1 diabetes. To determine risk of developing type 1 diabetes based on predictors at a young age (3 years) and over a short time period (by age 6 years), we included both single and multiple autoantibody positive subjects. We included children at age 3 with single autoantibody status (rather than only looking at those with multiple autoantibodies) as development of additional autoantibodies is more likely in a young population and as these young single autoantibody positive children have higher risk for progression than adolescents or adults with single autoantibodies (9).
Analysis and Clinical Predictors Tested
Thirty-eight variables, determined on the basis of previous TEDDY analyses and available literature, were assessed for association with progression to type 1 diabetes by age 6 years (Supplemental Table 1). These included clinical characteristics such as gender, country, general population status versus first-degree relative (including relative specificity), weight and BMI Z-scores at 3 years of age. General laboratory immunologic and metabolic variables included autoantibody number (single vs. multiple), type (glutamic acid decarboxylase autoantibodies [GADA], insulin autoantibodies [mIAA], insulinoma-associated-2 autoantibodies [IA-2A], and zinc transporter 8 autoantibodies [ZnT8A] which was added January 2012 to the protocol), and titer, age at confirmatory autoantibody positivity, and HbA1c. OGTT with two time points (fasting and 2-hour) were performed upon confirmation of multiple autoantibodies and the results placed in clinically-relevant categories based on normal (fasting < 90 mg/dL, 2hr < 120 mg/dL), elevated but not abnormal (fasting 90–100 mg/dL, 2hr 120–140 mg/dL), or impaired/abnormal (fasting > 100 mg/dL, 2hr > 140 mg/dL) results for blood glucose. Fasting levels for C-peptide, insulin and homeostatic model assessment (HOMA) to quantify insulin resistance were included. Due to lack of blood sample, levels for C-peptide, insulin and HOMA were not available at the 2-hour time point for the large majority of subjects. Assessment of fasting and 2-hour glucose values as a continuous variable was performed for a subgroup of subjects at age 3 who had multiple autoantibodies; however, the entire group was analyzed via categorization (Supplemental Table 1) to include those subjects with 1 autoantibody who did not have an OGTT glucose result. Single nucleotide polymorphisms (SNPs) identified through genome-wide association studies and multiple Cox regression analysis and found to associate with progression to autoantibody positivity and type 1 diabetes in previous TEDDY analyses were assessed (10). The SNPs used in this analysis were: rs1004446_A, rs10517086_A, rs11711054_G, rs12708716_G, rs2292239_A, rs2476601_A, rs2816316_C, rs3184504_A, rs3825932_A, rs4948088_A, rs7111341_A, with gene names included in Supplemental Table 1.
The 38 potential predictor variables were screened for inclusion in the model using a forward selection method with alpha, or significance level, set at 0.01 to enter the model. The level of 0.01 was chosen due to the known increase in false positive rate when the number of candidate predictor variables becomes large (11). The operating characteristics, or ability of the model to predict true positives and true negatives, were compared to two simpler models: 1) Does IA-2A status at age 3 predict type 1 diabetes at age 6, and 2) Does multiple autoantibody status at age 3 predict type 1 diabetes at age 6? The operating characteristics of the logistic regression model and these two models were assessed by a 4-fold cross-validation procedure (12). In the cross validation, the cohort was split into four subsets (folds) of equal size and equal number of type 1 diabetes cases. Each fold was used as the validation dataset while the remaining subjects were identified as the training dataset. The stepwise logistic regression procedure was generated four times, once for each training dataset and the operating characteristics of the resultant models were then determined on the validation datasets. The final estimates of receiver operator characteristic (ROC) area under the curve (AUC), sensitivity, specificity, positive and negative predictive values were determined for each validation dataset and summarized by weighted averages over the four validation datasets where the weight was based on the number of subjects in the validation dataset which had complete data on the chosen predictors. Sensitivity, specificity, positive and negative predictive values for the logistic regression models were based on the Youden index that maximizes the sum of the sensitivity and specificity. All analyses were performed in PC SAS Version 9.3. This study was performed with the approval of the central institutional review board overseeing the TEDDY study.
Results
Of the 8676 participants initially enrolled in TEDDY, 78 developed type 1 diabetes prior to age 3 years. Another 2235 dropped out of the study by the age of 3 years. Of the remaining subjects at age 3 years, 6000 were autoantibody negative and 363 were autoantibody positive. The subsequent analyses were completed only on the 363 subjects who were autoantibody positive at age 3 years (Figure 1). Of the 363 TEDDY participants who were autoantibody positive at age 3 with complete data, 76 were confirmed to have type 1 diabetes at age 6. Demographics of both the full cohort and the subset of subjects who progressed are shown in Table 1. The status of 11 subjects in the 363 antibody positive cohort could not be determined at age 6 and these subjects were excluded from analysis. The incidence of type 1 diabetes by age 6 years was 21.5% (76/352). Plasma glucose > 200 mg/dL (> 11.1 mmol/L) accounted for 91% of the diagnosed type 1 diabetes subjects.
Figure 1:
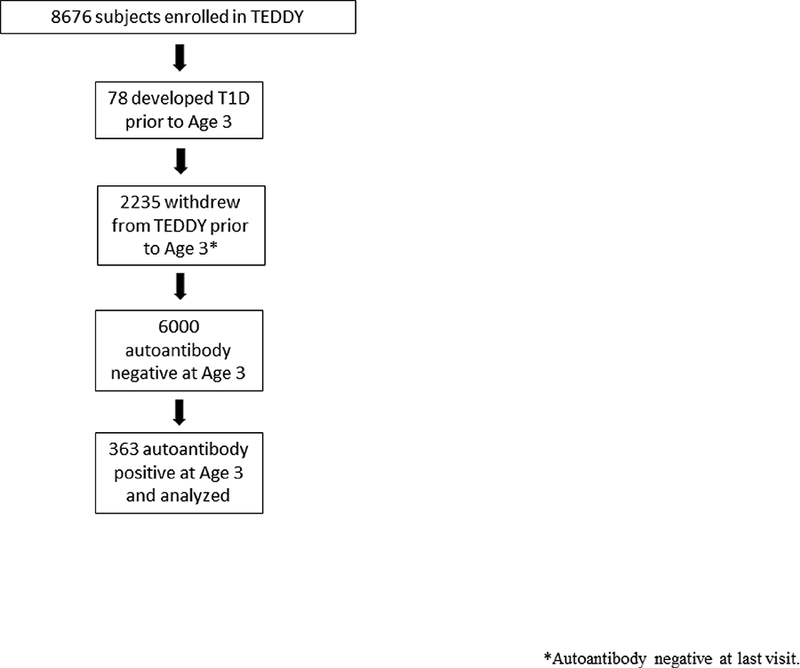
Flowchart of Children Enrolled in Cohort for Analysis.
Table 1:
Demographics and characteristics at age 3 of complete cohort and the outcome group with type 1 diabetes at age 6 years.
| Complete Cohort (N=363) N (%) | T1D Subjects - Age 6 (N=76) N (%) | |
|---|---|---|
| Age 6 T1D | ||
| Yes | 76 (21) | 76 |
| No | 276 (76) | 0 |
| Missing | 11 (3) | 0 |
| Country | ||
| United States | 124 (34) | 20 (26) |
| Finland | 83 (23) | 24 (32) |
| Germany | 25 (7) | 6 (8) |
| Sweden | 131(36) | 26 (34) |
| Gender | ||
| Female | 163 (45) | 40 (53) |
| Male | 200 (55) | 36 (47) |
| First Degree Relative | ||
| No | 290 (80) | 62 (82) |
| Yes | 73 (20) | 14 (18) |
| HLA | ||
| DR3/DR4 | 177 (49) | 43 (57) |
| DR4/DR4 | 72 (20) | 12 (16) |
| DR4/DR8 | 56 (15) | 10 (13) |
| DR3/DR3 | 43 (12) | 7 (9) |
| Other | 15 (4) | 4 (5) |
| Number of Autoantibodies | ||
| 1 | 168 (46) | 8 (11) |
| > 1 | 195 (54) | 68 (89) |
| IA-2A Status | ||
| Positive | 134 (37) | 57 (75) |
| Negative | 229 (63) | 19 (25) |
| SNP rs12708716_G number of minor alleles | ||
| 0 | 167 (46) | 31 (41) |
| 1 | 162 (45) | 35 (46) |
| 2 | 32 (9) | 11 (13) |
| Age in Years at Persistent Autoantibody Confirmation | Complete Cohort (N=363) | T1D Subjects - Age 6 (N=76) |
| Mean | 1.86 | 1.53 |
| SD | 0.87 | 0.72 |
| Range | 0.25–3.5 | 0.36–3.3 |
| HbA1c (%) | Complete Cohort (N=292) | All T1D Subjects - Age 6 (N=58) |
| Mean | 5.14 | 5.31 |
| SD | 0.28 | 0.27 |
| Range | 4.4–6.0 | 4.7–5.8 |
| BMI (Z-score) | Complete Cohort (N=329) | All T1D Subjects – Age 6 (N=55) |
| Mean | 0.26 | 0.42 |
| SD | 0.99 | 1.06 |
| Range | −3.35–2.33 | −2.45–1.78 |
Within the TEDDY cohort, logistic regression modeling identified five predictors at age 3 years as significant markers for progression from autoantibody positivity to type 1 diabetes by age 6 years: presence of IA-2A, HbA1c, BMI Z-score, SNP rs1270876_G, and number of antibodies (multiple autoantibodies) combined with low fasting insulin level (Table 2). The largest effect in the model was seen with IA-2A status at age 3 (odds ratio 8.7). An exploratory recursive partition algorithm chose cut points of 5.2% (33 mmol/mol) for HbA1c as optimal in differentiating between high- and low-risk groups for the diagnosis of type 1 diabetes by age 6. Another metabolic factor that contributed to risk of type 1 diabetes by age 6 years was a reduced fasting insulin level (< 2.0 mcU/mL) in the setting of multiple autoantibodies. Additionally, odds for developing type 1 diabetes by age 6 increased by 1.8 for every unit increase in BMI Z-score. Finally, SNP rs1270876_G (CLEC16A), found previously to be a susceptibility locus for islet autoantibody development (10), demonstrated an odds ratio of 2.4. The Youden index was 0.16 indicating that subjects with an estimated probability of T1D greater than 0.16 are predicted to have type 1 diabetes by age 6.
Table 2:
Logistic regression significant predictors for type 1 diabetes development by age 6.
| Parameter | Estimate (S.E.) | p-value | Odds Ratio (95% CI) |
|---|---|---|---|
| Intercept | −22.7 (4.3) | <0.001 | |
| Age 3 IA2A status Reference = negative | 2.2 (0.5) | <0.001 | 8.7 (3.0, 25.2) |
| Age 3 HbA1c | 3.6 (0.8) | <0.001 | 1.4 (1.2, 1.6)* |
| Age 3 BMI Z Score | 0.7 (0.2) | 0.002 | 1.8 (1.2, 2.8)** |
| Number Autoantibodies & OGTT Fasting Insulin Level Reference = 1 autoantibody | 0.001 | ||
| >1 Antibody - Insulin Normal | 0.6 (0.3) | <0.001 | 11.7 (2.0, 64.3) |
| >1 Antibody - Insulin Low | 1.5 (0.4) | <0.001 | 28.5 (4.4, 182.9) |
| >1 Antibody - Insulin Missing | −0.2 (0.4) | 0.605 | 5.3 (0.8, 32.6) |
| RS12708716_G | 0.9 (0.3) | 0.008 | 2.4 (1.3, 4.5)*** |
Odds ratio for 0.1 unit increase in HbA1c
Odds ratio for 1 unit increase in Z-score
Odds ratio for 1 unit increase in number of alleles (0,1,2)
Using logistic regression, the two time point OGTT glucose levels were analyzed as continuous variables for only the subset of subjects with 2 or more autoantibodies (n=114). The 2-hour glucose (OR 1.03 [1.02, 1.05]) but not the fasting glucose (OR 0.98 [0.94, 1.02]) was found to be significantly associated with type 1 diabetes by age 6.
The estimated ROC AUC, sensitivity, specificity, positive and negative predictive values for the logistic regression algorithm and the two simpler models are shown in Table 3. In the cross validation, IA-2A status was chosen as the most important predictor in all four of the folds, followed by HbA1c in three of the four folds.
Table 3:
Operating characteristic of prediction models based on 4-fold cross validation.
| Model | ROC Area Under Curve | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| Logistic Regression | 0.80 | 0.91 | 0.59 | 0.35 | 0.96 |
| IA-2A Yes/ No | 0.78 | 0.83 | 0.73 | 0.44 | 0.95 |
| 1/ >1 Positive Autoantibody | 0.75 | 0.95 | 0.56 | 0.35 | 0.98 |
Discussion
Improved prediction of a child’s risk of progression to type 1 diabetes by age 6 has the potential to reduce poor outcomes related to DKA, which occur disproportionately in children under 5 years of age (7). In addition, time limited predictive tools are urgently needed to effectively deliver personalized medicine and provide families with actionable information when making decisions related to potential participation in prevention studies. Herein, we demonstrated that data collected from 3-year-old children participating in the TEDDY study can be used to develop a prediction model for progression to type 1 diabetes by age 6. Logistic regression modelling provided for the highest estimated AUC; however, the differences in AUC, sensitivity, and specificity between (1) logistic regression modelling, (2) IA-2A status, and (3) single versus multiple autoantibody status, were small. This may be due to the small sample size of the validation datasets, the presence of missing values for HbA1c, and/or the lack of OGTT information for the single autoantibody positive subjects. We compared our combination of factors, derived from 38 candidate predictors to two simple prediction rules—multiple autoantibody status alone or IA-2A positive status alone—to determine if there was added benefit from a model that includes not only autoantibody number, type and titer but also the addition of metabolic data (HbA1c, glucose, insulin), HLA and non-HLA genetic data, gender, country, type of first degree relative, weight, BMI, and more. Logistic regression was chosen as opposed to more algorithmic machine learning techniques because of the relatively small sample size, the relatively low rate of type 1 diabetes at age 6 and the desire for an interpretable model comparable to other, simpler models (13). It is difficult to determine whether the drop out of autoantibody negative subjects prior to age 3 is biasing the results of our analysis. With the exception of the rs1270876_G SNP, all of the other predictors selected were based on information collected at age 3 which were unavailable for the subjects who dropped out. While this is a proof-of-concept technique, due to missing data, especially metabolic data at age 3 in single autoantibody subjects, widespread use of this algorithm is not yet justified as simpler models show similarity in operating characteristics. However, additional information can be gained from analysis of the other predictors in addition to autoantibody status. This study highlights the ability to apply precision medicine techniques over a short time period in young children at increased risk of type 1 diabetes.
Birth cohort studies such as the Colorado Diabetes Autoimmunity Study in the Young (DAISY), Germany’s BABYDIAB and BABYDIET, and Finland’s Type 1 Diabetes Prediction and Prevention (DIPP) have previously demonstrated that progression to type 1 diabetes is based on age and number of autoantibodies (14–16). Young children with 2 or more antibodies have a very high lifetime risk of progression to type 1 diabetes (70% at 10 years and 84% at 15 years) (1,2). Non-birth cohorts, such as the DPT-1, have also confirmed increased risk in autoantibody positive populations (4,17,18). Similar analyses completed within TEDDY looked at participants with multiple autoantibodies and time to type 1 diabetes. Age at multiple autoantibody appearance, female sex, and non-HLA SNPs were found to be significant risk factors for time to disease (19). Our analysis, unlike others, sought to predict progression over a very narrow scope of time in very young children. While the data set from 3 years to 6 years of age was limiting, we sought to determine if data collected at age 3 could be used to accurately predict progression over a narrow 3-year time period which would increase the efficacy of clinical trials. This is an important contrast between our study and previous efforts performed on the entire TEDDY cohort (19).
Some of the risk predictors identified in our model corroborate earlier studies while others are novel. Steck et al, in previous analyses of TEDDY data, showed that elevated IAA and IA-2A titers were significant risk predictors in young children (2). In addition, IA-2A has previously been identified as the autoantibody conferring the highest risk of progression. This risk increases with autoantibody titer and epitope reactivity (2,9,20,21). While IA-2A and multiple autoantibody status were highly correlated, the strongest predictor, IA-2A status, was selected into the model. This confirmed data from previous cohort studies (22) suggesting that IA-2A positivity was a strong predictor of progression in the presence of another autoantibody. These observations are highly pertinent to young at-risk children. IAA status was not significant in the model despite the young age and known early appearance of IAA. Not surprisingly, GADA was not a predictive factor in our analysis for progression to type 1 diabetes as in DAISY (23), typically occurring in older subjects.
Excess BMI over time in children within the TrialNet Pathway to Prevention study has demonstrated increased risk of progression that was age and sex-specific (24). Here, increasing BMI Z score, even in very young children, increased the odds of type 1 diabetes. HbA1c was an important predictor in our model and has been evaluated previously as a tool for prediction within DAISY (25), TEDDY (26) and DIPP (27). Increase in HbA1c has been shown to be superior to measures of random glucose in predicting progression to type 1 diabetes (27,28). In exploratory analyses, cut-point analysis identified HbA1c at age 3 above 5.2% (33 mmol/mol) in establishing high and low risk cohorts with regards to progression at age 6 years. OGTT data based on baseline (fasting) insulin levels in conjunction with single/multiple autoantibody status were a factor in the model. In subgroup analysis of the multiple autoantibody subjects, the 2-hour glucose measurement at age 3 was the most significant OGTT parameter associated with progression by age 6.
The group with single autoantibodies did not have OGTT performed and this is a limitation as artificial categories were made to include the entire cohort studied. When considering the potential application of this, or any risk algorithm, we must always consider the potential logistical concerns of performing the tests required to obtain relevant data in the population of interest. In addition, the TEDDY OGTT provides only a two time point collection (fasting and 2-hour) unlike the DPT-1 or TrialNet Pathway to Prevention protocols which employ a six time point OGTT and is further limited by the fact that 3 years of age is the earliest an OGTT is performed in TEDDY. As such, the relatively limited OGTT dataset in young TEDDY subjects may inhibit our ability to detect signals associated with glucose excursions. Similarly, other variables, such as C-peptide and insulin, for example, were typically only available in the fasting state without a stimulated value.
Finally, only one SNP previously identified with risk for autoantibody positivity was confirmed as a significant predictor of progression to type 1 diabetes in our model of very young children. This demonstrates the potential of SNPs to add to risk differentiation well before symptoms or metabolic derangements are detected (10). The development of autoantibodies early in childhood is associated with HLA class II genotypes whereas those who develop autoimmunity later are less tightly linked to HLA status but non-HLA SNPs may add additional specificity even in young children. Providers caring for young children with a family history of type 1 diabetes frequently consider autoantibody testing given their well established predictive power. However, future predictive models including type of autoantibody, rising BMI, or rising HbA1c might provide incremental improvements in prediction.
In summary, this report details the performance of logistic regression modeling to predict progression to type 1 diabetes from age 3 to age 6 in TEDDY children. Given the excellent sensitivity but limited specificity of this model, data from TEDDY children at age 3 has a limited ability to predict progression to type 1 diabetes by age 6. Explanations for the low specificity and positive predictive value of the prediction model include frequently missing covariates at age 3 (i.e. HbA1c), as well as the relatively short interval (3 years) that was utilized in developing this model. Nevertheless, this model provides important proof-of-concept for developing risk scores in very young high-risk children. Improved models are urgently needed in order to stratify children for prevention studies and realize the promise of precision medicine.
Supplementary Material
Acknowledgments:
Funding sources: The TEDDY Study Group is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Centers for Disease Control and Prevention (CDC), and JDRF. This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Dr. Xiang Liu assisted in the classification tree prediction using rpart.
Abbreviations:
- HLA
human leukocyte antigen
- OGTT
oral glucose tolerance test
- SNP
single nucleotide polymorphism
- HbA1c
hemoglobin A1c
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013. June 19;309(23):2473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, She J, Simell O, Akolkar B, Krischer J, Schatz D, Rewers MJ; TEDDY Study Group. Predictors of Progression From the Appearance of Islet Autoantibodies to Early Childhood Diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care. 2015. May;38(5):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G; Diabetes Prevention Trial-Type 1 Study Group. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009. December;32(12):2269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, Cuthbertson D, Lachin JM, Sklyer JS, The Diabetes Prevention Trial- Type 1 Study Group. A risk score for Type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-Type 1. Diabetes Care. 2008; 31, 528–533. [DOI] [PubMed] [Google Scholar]
- 5.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci. 2008; 1150:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elding Larsson H, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011; 34:2347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfsdorf J, Glaser N, Sperling MA Diabetic Ketoacidosis in Infants, Children, and Adolescents – A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159. [DOI] [PubMed] [Google Scholar]
- 8.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci. 2008. December;1150:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifacio E Predicting Type 1 Diabetes Using Biomarkers. Diabetes Care 2015. June; 38(6): 989–996. [DOI] [PubMed] [Google Scholar]
- 10.Törn C, Hadley D, Lee HS, Hagopian W, Lernmark A, Simell O, et al. Role of Type 1 diabetes associated SNPs on risk of autoantibody positivity in the TEDDY Study. Diabetes. 2015. May;64(5):1818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui S Li L Positive false discover rate estimate in step-wise variable selection. Communications in Statistics – Simulation and Computation 2007: 36: 1217–1231. [Google Scholar]
- 12.Hatie T, Tibshirani R, Freedman J The Elements of Statistical Learning – Data Mining, Inference and Prediction, Section 7.3 Cross-Valdiation. pp. 214–217. 2001. Springer-Verlag: New York. [Google Scholar]
- 13.Harrell F (May 14, 2018) Road map for choosing between statistical modeling and machine learning [Blog post}. Retrieved from http://www.fharrell.com/post/stat-ml/
- 14.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M; Diabetes Autoimmunity Study in the Young. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004. August;89(8):3896–902. [DOI] [PubMed] [Google Scholar]
- 15.Kimpimäki T, Kulmala P, Savola K, Kupila A, Korhonen S, Simell T, Ilonen J, Simell O, Knip M. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2002. October;87(10):4572–9. [DOI] [PubMed] [Google Scholar]
- 16.Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr. 2011. December;94(6 Suppl):1821S–1823S [DOI] [PubMed] [Google Scholar]
- 17.Sosenko JM, Skyler JS, Mahon J, Krischer JP, Greenbaum CJ, Rafkin LE, Beam CA, Boulware DC, Matheson D, Cuthbertson D, Herold KC, Eisenbarth G, Palmer JP; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups. Use of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care. 2014. April;37(4):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, Greenbaum CJ, Rafkin LE, Cowie C, Cuthbertson D, Palmer JP, and the Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups. Validation of the Diabetes Prevention Trial–Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care. 2011. August; 34(8): 1785–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krischer J, Liu X, Lernmark A, Hagopian W, Rewers M, She J-X, Toppari J, Ziegler A, Akolkar B. The Influence of Type 1 Diabetes Genetic Susceptibility Regions, Age, Sex, and Family History to the Progression from Multiple Autoantibodies to Type 1 Diabetes: A TEDDY Study Report. Diabetes. 2017. December;66(12):3122–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler AG and Nepom GT. Prediction and Pathogenesis in Type 1 Diabetes. Immunity. 2010. April 23; 32(4): 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels A, Zhang L, Khadra A, Kushner JA, Redondo MJ, Pietropaolo M. Prediction and Prevention of Type 1 Diabetes: Update on Success of Prediction and Struggles at Prevention. Pediatr Diabetes. 2015. November; 16(7): 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decochez K, De Leeuw IH, Keymeulen B, Mathieu C, Rottiers R, Weets I, Vandemeulebroucke E, Truyen I, Kaufman L, Schuit FC, Pipeleers DG, Gorus FK; Belgian Diabetes Registry. IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia. 2002. December;45(12):1658–66. [DOI] [PubMed] [Google Scholar]
- 23.Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;6:1397–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara CT, Geyer SM, Liu YF, Evans-Molina C, Libman IM, Besser R, Becker DJ, Rodriguez H, Moran A, Gitelman SE, Redondo MJ; Type 1 Diabetes TrialNet Study Group. Excess BMI in Childhood: A Modifiable Risk Factor for Type 1 Diabetes Development? Diabetes Care. 2017. May;40(5):698–701. doi: 10.2337/dc16-2331Epub 2017 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M. Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes. 2006. October;7(5):247–53. [DOI] [PubMed] [Google Scholar]
- 26.Vehik K, Cuthbertson D, Boulware D, et al. ; TEDDY, TRIGR, Diabetes Prevention Trial–Type 1, and Type 1 Diabetes TrialNet Natural History Study Groups. Performance of HbA1c as an early diagnostic indicator of type 1 diabetes in children and youth. Diabetes Care 2012;35:1821–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helminen O, Aspholm S, Pokka T, Hautakangas MR, Haatanen N, Lempainen J, Ilonen J, Simell O, Knip M, Veijola R. HbA1c predicts time to diagnosis of type 1 diabetes in children at risk. Diabetes 2015: 64: 1719–1727. [DOI] [PubMed] [Google Scholar]
- 28.Veijola R, Koskinen M, Helminen O, Hekkala A. Dysregulation of glucose metabolism in preclinical type 1 diabetes. Pediatric Diabetes 2016: 17 (Suppl. 22): 25–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


