Abstract
We previously demonstrated that indoor house dust extracts could induce adipogenesis in pre-adipocytes, suggesting a potential role for indoor contaminant mixtures in metabolic health. Herein, we investigated the potential role of thyroid receptor beta (TRβ) antagonism in adipogenic effects (dust-induced triglyceride accumulation and pre-adipocyte proliferation) following exposure to environmental mixtures (indoor house dust extracts). Concentrations of specific flame retardants were measured in extracts, and metabolic health information was collected from residents (n=137). 90% of dust extracts exhibited significant adipogenic activity, >60% via triglyceride accumulation, and >70% via pre-adipocyte proliferation. Triglyceride accumulation was positively correlated with concentrations of each of twelve flame retardants, despite most being independently inactive; this suggests a putative role for co-exposures or mixtures. We further reported a positive correlation between dust-induced triglyceride accumulation and serum thyroid stimulating hormone concentrations, negative correlations with serum free triiodothyronine and thyroxine concentrations, and a positive and significant association between dust-induced triglyceride accumulation and residents’ body mass index (BMI). We hypothesized that inhibition of TR antagonism might counteract these effects, and both addition of a TR agonist and siRNA knock-down of TR resulted in decreased dust-induced triglyceride accumulation in a subset of samples, bolstering this as a contributory mechanism. These results highlight a contributory role of environmental TR antagonism as a putative factor in metabolic health, suggesting that further research should evaluate this mechanism and determine whether in vitro adipogenic activity could have utility as a biomarker for metabolic health in residents.
Keywords: Endocrine Disrupting Chemicals, Adipogenesis, Obesogen, Obesity, Metabolic Disruption, House Dust
Introduction
Increasing research has suggested a role for endocrine disrupting chemicals (EDCs) in the rising rates of obesity and associated metabolic diseases. EDCs include 1,000 or more synthetic or naturally occurring chemicals or mixtures that can interfere with any aspect of hormone action (1); some of these, termed “metabolic disruptors”, can increase weight gain or contribute to metabolic dysfunction in animal models and/or promote triglyceride accumulation in vitro (2,3). Confirming putative metabolic disruptors in vivo is cost and time-prohibitive. As such, utilizing appropriate in vitro models is crucial for screening individual environmental chemicals and characterizing mixtures, due to ease of assessing multiple chemicals, concentrations, and co-exposures for much lower cost and time investments. The committed 3T3-L1 mouse pre-adipocyte cell line is commonly used for this purpose; following exposure to adipogenic chemicals, these cells differentiate into adipocytes, undergo morphological changes, accumulate triglycerides, and eventually develop into a mature white fat cell (4,5). This model and others have been rigorously applied to characterize putative metabolic disruptors over the last forty years, with an increasing number of publications demonstrating effects on adipocyte commitment, differentiation, proliferation, and lipid accumulation (reviewed in (2,6). Many of these adipogenic chemicals have been further demonstrated to be active in vivo (7–10), bolstering the reliability and translational capability of this model. Moreover, the molecular mechanisms promoting adipocyte development are highly conserved across vertebrate species (11,12), substantiating the utility of this model in predicting potential human metabolic concerns. Modulation of numerous molecular pathways can regulate adipogenesis, including activation of the peroxisome proliferator-activated receptor-gamma (PPARγ), liver X receptor (LXR), glucocorticoid receptor (GR), retinoid X receptor (RXR), and/or antagonism of the thyroid hormone receptor (TR), farnesoid X receptor (FXR), androgen receptor (AR), and others (11,13,14). EDCs that can modulate these pathways are ubiquitous, being found in numerous consumer products (furniture, electronics, plastics, food packaging, etc.) and accumulating in the outdoor and indoor environment (15–18). As a result, humans are exposed to complex mixtures of EDCs throughout development, with exposure beginning during gestation (19,20) and continuing through lactation (21,22) and from the indoor environment throughout their lives (15,23–25).
Research by our laboratory and others has demonstrated that indoor house dust is consistently contaminated with numerous semi-volatile organic contaminants (SVOCs) globally, many that are suspected of being hormonally active, including phthalates, flame-retardants, pesticides, parabens, and perfluoroalkyl substances (PFASs) (24,26–31). The EPA estimates that from birth through six years of age, children ingest approximately between 60–100 mg of dust per day from indoor environments (23), contributing to chronic oral and inhalation exposures to EDCs present in the dust (15,24,25). Furthermore, our laboratory and others have previously demonstrated that levels of various classes of contaminants in household dust are bio-accessible and highly correlated with blood and urine levels in residents (16,18,32–38), suggesting a clear exposure pathway. Due to the diversity of potentially endocrine-active chemicals in house dust, several laboratories have assessed various nuclear receptor bioactivities, reporting agonist activities for PPARγ, GR, and ER, and antagonist activities for AR and TR, at concentrations between 10–40 μg dust equivalence (31,39–41). Previous research from our laboratory assessed the ligand binding and subsequent activation of PPARγ by house dust extracts; 84% exhibited significant PPARγ binding at ≥120 μg dust equivalence per well (42), and 60% exhibited significant receptor activation at ≥4 μg dust per well (43,44). More recently, we profiled the adipogenic activity (defined as the ability to stimulate triglyceride accumulation and/or pre-adipocyte proliferation) of 40+ SVOCs common in indoor house dust samples (45); more than two thirds of these chemicals exhibited significant adipogenic activity. We further assessed a small subset of 11 house dust extracts, reporting that ten of 11 exhibited significant adipogenic activity at <20 μg dust per well (45). This suggests a potential role of indoor house dust in resident’s metabolic health, as well as a need to determine molecular mechanisms promoting these effects, causative chemicals present in these mixtures, and assessment of whether the adipogenic activities in dust are associated with adverse metabolic health in residents living in these homes.
Specifically, antagonism of TR has been demonstrated to robustly modulate adipocyte differentiation, purportedly through reciprocal regulation with PPARγ (46). We previously demonstrated that antagonism of TR was one of the most active/potent adipogenic pathways, as measured via triglyceride accumulation in 3T3-L1 pre-adipocytes (14), suggesting a potential role in disruption of TR in environmental mixture-induced differentiation. Thyroid hormones have long been considered anti-obesogenic, and hypothyroid-associated adiposity can be reduced with supplementation (47). All of the TR isoforms are expressed in white and brown adipocytes, with TRα purportedly playing a larger role in regulating thermogenesis and TRβ in cholesterol metabolism and lipogenesis, as well as directly regulating (or through crosstalk with PPARγ) a number of genes and enzymes necessary for pre-adipocyte proliferation and adipocyte differentiation (48). As such, a parallel study examined the extent of TRβ antagonism in the same dust extracts used in this study (41), and these data were assessed as a potential contributory mechanism in the observed adipogenic effects. Approximately half of these extracts significantly antagonized TRβ activity, decreasing triiodothyronine (T3)-mediated efficacy by 20–67% compared to the T3 added agonist control. Further, potencies (concentrations required to inhibit 20% of T3-mediated efficacy) were negatively correlated with free serum thyroxine (FT4) concentrations in residents (more potent samples exhibited greater FT4 concentrations), suggesting a proximate impact of the environmental TR ligands on thyroid function in these individuals.
The goals of this study were to more comprehensively assess the metabolic disruption potential of indoor house dust extracts and further assess the role that TRβ plays in observed adipogenesis. Murine 3T3-L1 cells (Zenbio, Inc.) were used to screen and evaluate the dose responses of 137 indoor house dust extracts for triglyceride accumulation and pre-adipocyte proliferation. Twelve flame retardants (FRs) were previously measured in these dust extracts (49); herein, we performed correlations to determine whether FR concentrations were associated with the observed activities. Further, the observed adipogenic activities were examined for their associations with health outcomes in the adult residents of the households where dust was collected. Health measurements included body mass index (BMI), serum thyroid stimulating hormone (TSH), serum free triiodothyronine (FT3), and FT4. We hypothesized that house dust would promote triglyceride accumulation and pre-adipocyte proliferation at low concentrations, and that this would be mediated in part by inhibition of TRβ.
Materials and Methods
Chemicals.
Chemicals were purchased as follows: rosiglitazone (Sigma cat # R2408, ≥ 98%), 1–850 (Millipore cat # 609315, ≥ 98%), and triiodothyronine (T3; VWR cat # 80057–656, ≥ 98%). Stock solutions were prepared in 100% DMSO (Sigma cat # D2650) and stored at − 20 °C between uses.
Patient enrollment and clinical assessments.
House dust samples (n=137) were collected and processed as described previously (49). Briefly, dust samples were obtained from a case-control study investigating indoor exposures and thyroid cancer. Patients newly diagnosed with papillary thyroid cancer between April 2014 and January 2016 and referred to endocrinology or endocrine surgery at the Duke Cancer Institute or Duke University Hospital were approached by their physician. Willing participants were contacted by the study team and enrolled, and control participants were recruited from other Duke patients undergoing routine wellness care or care for unrelated medical issues or were recruited from by posted notices in the Durham, NC area. Inclusion was restricted to individuals living within 50 miles of Duke University (Durham, NC, USA) and to individuals who had lived in the same home for at least two years to ensure that levels of exposure were reflective of historical exposure occurring over the last several years. Control inclusion was restricted to individuals without a previous diagnosis of malignant or benign thyroid disease, and pregnant women were excluded, as their thyroid hormone levels would be expected to be different than a non-pregnant population. Upon enrollment, researchers visited each home to obtain house dust samples and collect information using a study questionnaire, including self-reported age, race/ethnicity, educational attainment, height and weight (for calculation of BMI), time lived in current residence, and other general health information. Laboratory measurements of FT3, FT4, and TSH were performed for all controls, but not for cancer patients, as their thyroid hormones are dysregulated and, in many cases, regulated with synthetic thyroid hormones administered by their medical team. Thyroid hormones were quantified by LabCorp (Burlington, NC) following standard protocols (49). All study protocols were reviewed and approved by the Duke University Health System Institutional Review Board.
Dust sample collection and processing.
House dust samples (n=137) were collected and processed as described previously (49). Samples were collected from households between 2014 and 2016 from residents that had lived in their households for at least two years and who were instructed not to vacuum for at least two days prior to collection. During collection, the main living area of the home was vacuumed (area in which the participant reported spending the most time), and dust was collected in a cellulose thimble (37,50), wrapped in foil, and stored at −20 °C. To process, samples were sieved to <500 μm, approximately 100 mg of dust was extracted with 50:50 dichloromethane:hexane (used to extract a wide range of chemical classes) via sonication, and extracts were concentrated under nitrogen gas. Half of each extract’s volume was purified using solid-phase extraction cartridges, and used for targeted analysis of brominated and organophosphate flame retardants (BFRs and PFRs, respectively) via GC/MS as described previously (49). The second half of this fraction was evaporated to dryness and reconstituted in 100 μL DMSO for use in bioassays. Laboratory blanks were prepared using laboratory solvents and techniques in the absence of dust to ensure that lab procedures did not impart any active chemicals to our assays. None of these samples exhibited significant triglyceride accumulation or pre-adipocyte proliferation at any concentration tested (Figure S1).
3T3-L1 Cell Care and Differentiation and Outcome Measurements.
3T3-L1 cells (Zenbio cat# SP-L1-F, lot# 3T3062104; Research Triangle Park, NC) were maintained as described previously in pre-adipocyte media (Dulbecco’s Modified Eagle Medium – High Glucose; DMEM-HG; Gibco cat# 11995, supplemented with 10% bovine calf serum and 1% penicillin and streptomycin; Gibco cat# 15140) (14). Cells were utilized between passages 8 (at purchase) and 14, maintained in a sub-confluent state until differentiation. Positive and negative controls were included in each assay plate, with no significant changes in control responses observed across time.
3T3-L1 cells were induced to differentiate as described in detail previously (14,45). Briefly, cells were seeded in pre-adipocyte media into 96-well tissue culture plates (Greiner cat # 655090) at ~30,000 cells per well. Once confluent, cells were allowed 48 hours for growth arrest and to allow initiation of clonal expansion. Media was then replaced with test chemicals and/or controls using a 0.1% DMSO vehicle in differentiation media (DMEM-HG with 10% fetal bovine serum, 1% penicillin/streptomycin, 1.0 μg/mL human insulin, and 0.5 mM 3-isobutyl-1-methylxanthine, IBMX). After 48 hours of differentiation induction, media was replaced with fresh dilutions of test chemicals in adipocyte maintenance media (differentiation media without IBMX), and treatments of media and test chemicals were refreshed every 2–3 days until assay, ten days after induction.
3T3-L1 Triglyceride Accumulation, Cell Proliferation, and Cell Viability Measurements.
Plates were assayed for triglyceride accumulation, DNA content, and cell viability after ten days of differentiation as described in great detail previously (14,45). Briefly, media was removed and cells rinsed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco cat # 14040) before replacing with 200 μL of a dye mixture (19 mL DPBS, 1 drop/mL NucBlue® Live ReadyProbes® Reagent (cell proliferation/cytotoxicity measure of DNA content; Thermo cat # R37605) and 500 μL AdipoRed™ (intracellular lipid measure of triglyceride accumulation; Lonza cat # PT-7009) per plate). Plates were protected from light and incubated at room temperature for approximately forty minutes; then fluorescence was measured with excitation 485 nm/emission 572 nm for AdipoRed™, 360/460 for NucBlue®. Following the lipid and DNA readings, cell viability was assessed using the CellTiter-Glo™ (cell viability measure via ATP content; Promega cat# G7572) assay as described previously (45). Briefly, 170 μL of DPBS mixture was removed from all wells, and the remaining 30 μL was mixed with 30 μL of CellTiter-Glo™ reagent. Plates were incubated for ten minutes prior to reading luminescence. CellTiter-Glo™ viability was assessed as a percent change from differentiated DMSO controls. Inhibited cell health was assessed via deviations of ≥15% in either cell viability (ATP) and/or cytotoxicity (DNA content) assays, as we described previously in detail (14,45). Four technical (replicates within each assay plate) and three biological replicates (separate cell passages/assays) were utilized for every test chemical and concentration reported herein.
For adipogenic activity, percent activities (efficacies) across the tested dose response range were calculated relative to the maximal rosiglitazone-induced fold induction over intra-assay differentiated vehicle controls (0.1% DMSO), after correcting for background fluorescence. Rosiglitazone was utilized as the positive control herein due to selective, robust, and potent binding to PPARγ (51–53); it is often utilized as a positive control in adipogenesis assays (54–60), and allows for easier comparisons to be made in test chemical response across laboratories and studies. DNA content was calculated as percent change from vehicle controls for each chemical at each concentration and was then used to normalize total triglyceride values to obtain triglyceride content per unit DNA (as a proxy for triglyceride accumulation per cell). Both total triglyceride content and triglyceride normalized to DNA content are provided throughout to ensure comparability between studies, as many do not report DNA content/normalized triglycerides. Potencies were determined using EC10 values (concentrations of each chemical/mixture that exhibit 10% of their own maximal activity) as determined using GraphPad Prism 7.0 (La Jolla, CA, USA). As we determined that extracted dust mass could be a potential confounder in maximal bioactivity, normalized metrics were created for further assessment: efficacy of response at 500 μg/mL dust extract concentration and potency (μg/mL extract concentration) at which 10% response was observed. Full dose responses of dust extracts were plotted and used to calculate the point where dose responses crossed the 500 μg/mL and 10% efficacy lines, respectively. Values were extrapolated as necessary for efficacy and potency values for samples approaching the cut-off; potency values were not extrapolated when there was no apparent activity (samples not approaching 10% activity), as potencies cannot be calculated for inactive chemicals/samples.
Ligand Recovery and siRNA Mechanism Interrogation Experiments.
For ligand recovery experiments, nine house dust extracts were randomly selected for further interrogation of molecular mechanisms and demonstrated a range of TRβ bioactivities. Cells were differentiated as described above and cells co-exposed to dose responses of dust extracts (maximal concentrations of each and four 2-fold serial dilutions) and 10 μM T3, under the presumption that TRβ antagonism was a causative factor in the triglyceride accumulation, and thus a TRβ agonist should inhibit some of the dust-induced adipogenic activity. T3 co-treatment responses were compared to dust alone to determine the effect of TRβ agonism in “recovering” potential TRβ antagonism-mediated effects. Two concentrations are reported for each dust sample to demonstrate effects across the dose response for each sample; high-dose dust utilized the maximal dust concentration for each sample (1:1000 dilution of DMSO extract), which varied according to quantity of dust collected from homes, and low-dose dust utilized the lowest concentration in each dose response (16-fold dilutions of maximal test concentration), with comparisons made between concentrations and relative to controls (T3 without dust).
For siRNA experiments, the same nine dust extracts were selected, and the low concentration was tested for each (16-fold dilutions of maximal concentrations). Sub-confluent cells were seeded into three 96-well plates as described above. One plate was retained as a non-transfected control, one was transfected with a negative control siRNA (Santa Cruz Biotech, SCBT, cat# sc-37007), and the third with both TRα (SCBT cat# sc-36708) and TRβ (SCBT cat# sc-38891) siRNAs, using siRNA Transfection Reagent (SCBT cat# sc-29528) according to modified manufacturer’s instructions (61) and similarly to previously reported (57). Briefly, after seeding cells into 96-well plates, 8 μL siRNA and 64 μL transfection reagent were diluted in transfection media (SCBT cat# sc-36868) and incubated at room temperature for 45 minutes. Following incubation, cells were rinsed with transfection medium, and 40 μL of the transfection mixture was applied to each well of the plate. Cells were transfected for approximately six hours before adding 160 μL pre-adipocyte maintenance media to end transfection and begin recovery. Cells were confluent by the following day and were then allowed a further two days to undergo growth arrest and revert to clonal expansion before cells were differentiated as described above and assessed for triglyceride accumulation and pre-adipocyte proliferation. Comparisons were made between siRNA-TR responses and both naïve and negative siRNA controls. Full dose responses of rosiglitazone, 1–850 (selected due to potent and selective TR antagonism (62)), T3 (selected as an endogenous TR agonist), and varied cell-based controls (differentiated DMSO vehicle controls, undifferentiated controls, were examined with naïve, siRNA-null, and siRNA-TR cells to determine relative effects of these TR-modulating chemicals and their potential interplay with dust extracts and siRNA knock-downs. TR agonist and antagonist dose responses under these varying conditions were utilized to assess the degree of TR knockdown in the siRNA experiments. As reported previously with PPAR knock-down utilizing this procedure (63), our functional assessment of knock-down success revealed an approximate 67% reduction in TR antagonist (1–850) efficacy (Figure S8).
Statistical Analysis.
Data are presented as means ± SEM from four technical replicates of three or four independent biological replicates (two for thyroid mechanism interrogation assays). Results were analyzed using a one-way ANOVA and Dunnett’s post-hoc test. Differences between treatment and control groups were considered statistically significant at p < 0.05. EC10 values were estimated using curves generated from raw fluorescence data using a 4-parameter variable-slope Hill model, utilizing the “log(Agonist) vs. response – Find ECanything” feature within the nonlinear regression function, and assigning an F value of 10 (for EC10 calculation) and a bottom of 0. Relationships between measures of adipogenic activity and the concentrations of flame retardants in samples and adverse health outcomes in the residents were assessed using Spearman correlation analyses. To address potential joint effects of multiple compounds, we further conducted a principal components analysis. We identified three primary factors consisting of the summed polybrominated diphenyl ethers (other than BDE-209), the BFRs comprising Firemaster 550™ (TBB, TBPH), and the PFRs. Correlations with these factors were generally similar to individual components (Table S1) and did not provide additional insights, so we have opted to discuss only individual chemicals within the text. Associations between adipogenic activity and human health endpoints were first assessed using Spearman correlations. Robust regressions were also performed to assess relationships between adipogenic activity metrics and resident health outcomes (64,65). These models allowed for the control of potential confounding variables, including age, sex, race, and education, which we anticipate could be related to health outcomes as well as the types of contaminants in the home environment driving the measured adipogenic activity. Analyses were performed using GraphPad Prism 7.0 or SAS 9.4.
Results
Dose responses of 137 house dust extracts (1000-fold dilution into exposure media and four subsequent serial two-fold dilutions) were assessed for adipogenic activity utilizing 3T3-L1 cells. Cells were differentiated over ten days of treatment with extracts and then assessed for pre-adipocyte proliferation (DNA content, relative to vehicle control), triglyceride accumulation (total triglycerides per well and per cell, normalized to DNA content; both relative to maximal rosiglitazone response), and cell viability (ATP production).
Adipogenic Activity of Indoor House Dust Extracts.
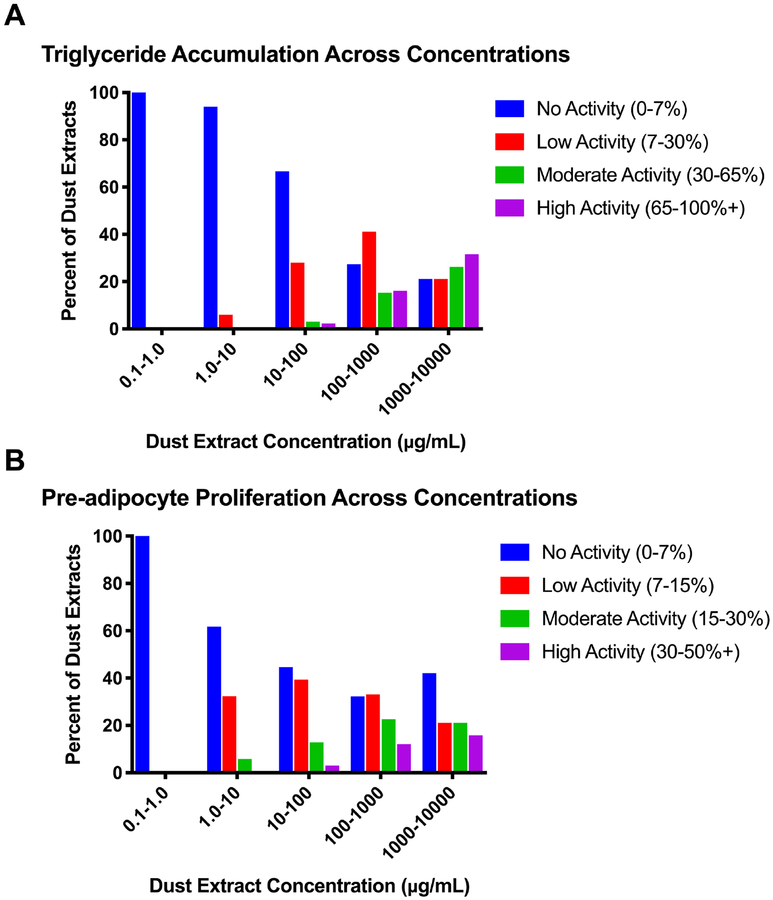
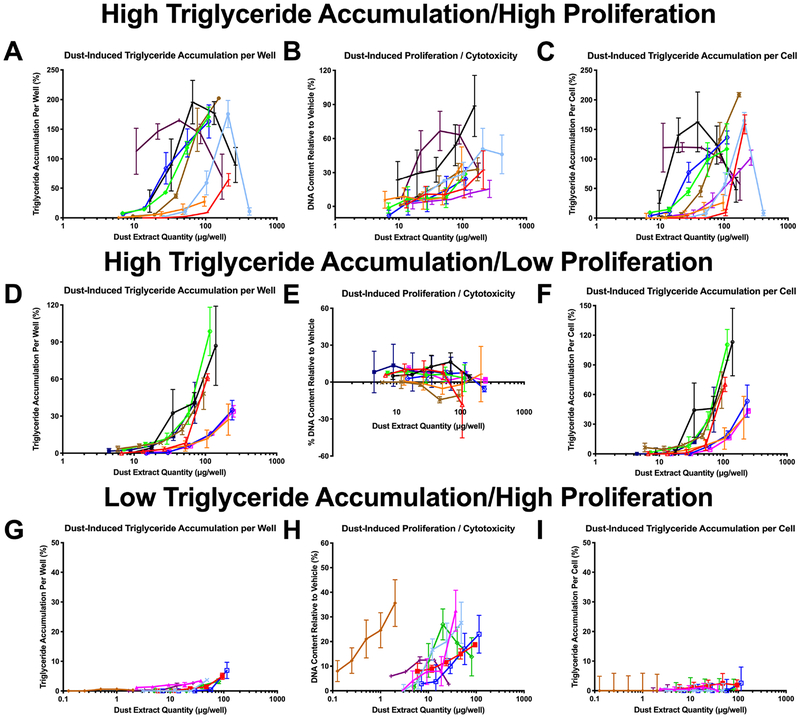
Given differing quantities of dust collected from each home, dust extract concentrations in contact with the cells varied from 10–4,449 μg/mL (dust equivalent concentration; Table 1), and each was tested at its maximal concentration and at four two-fold dilutions thereof. The majority of dust extracts exhibited adipogenic activity in our assay system, with >60% promoting significant triglyceride accumulation of up to 389% relative to the rosiglitazone-induced maximum, >70% promoting significant pre-adipocyte proliferation of up to 89% relative to the differentiated vehicle control, and ~90% exhibiting one or both activities (Table 1). Greater than 40% of extracts exhibited effects at concentrations <10 μg dust/well (50 μg/mL extract test concentration), and approximately 10% of extracts exhibited neither triglyceride accumulation nor pre-adipocyte proliferation. Approximately 6% of samples tested between 1–10 μg/mL (in diluted extracts) exhibited significant triglyceride accumulation, relative to 38% exhibiting significant pre-adipocyte proliferation (Figure 1). Adipogenic activity increased with dust extract concentration tested, with 33% of samples exhibiting significant triglyceride accumulation when diluted extracts were tested between 10–100 μg/mL, 73% when tested between 100–1000 μg/mL, and 79% when tested between 1000–10,000 μg/mL (Figure 1A); notably, some samples exhibited reduced activity at their highest concentration due to apparent cytotoxicity. The efficacies of triglyceride accumulation induced by diluted extracts also increased with increasing concentration; when diluted extracts were tested at concentrations from 10–100 μg/mL, 28% of samples exhibited 7–30% triglyceride accumulation, 3% exhibited 30–65%, and 2% exhibited 65–100%+, compared to when tested at 100–1000 μg/mL, 41% exhibited 7–30%, 15% exhibited 30–65%, and 16% exhibited 65–100%+ (Figure 1A). This was mirrored but less pronounced with pre-adipocyte proliferation, with 55.3% of diluted samples exhibiting significant proliferation when tested at concentrations between 10–100 μg/mL, 68% when tested between 100–1000 μg/mL, and 58% when tested between 1000–10,000 μg/mL (Figure 1B). Again, the efficacies of proliferation also increased with increasing concentration; when diluted extracts were tested at concentrations from 10–100 μg/mL, 39% exhibited 7–15% increased DNA content, 13% exhibited 15–30%, and 3% exhibited 30–50%+, compared to when tested at 100–1000 μg/mL, 33% exhibited 7–15% increased DNA content, 23% exhibited 15–30%, and 12% exhibited 30–50%+ (Figure 1B). Triglyceride accumulation and pre-adipocyte proliferation appeared to exhibit differing but overlapping mechanisms (Figure 2). Certain samples exhibited robust triglyceride accumulation and proliferation (Figure 2A–C), others exhibited robust triglyceride accumulation and minimal or no proliferation (Figure 2D–F), and still others exhibited minimal or no triglyceride accumulation but robust proliferation (Figure 2G–I). Classical peripheral and/or central triglyceride accumulation was observed across active dust extracts as additional visual confirmation of adipogenic effects, while dust controls exhibited no significant activity relative to the differentiated vehicle control (Figure S2).
Table 1.
Descriptive Results of Adipogenic Activity Across Dust Extracts
| Dust Sample Metrics | Range | Median | Mean |
|---|---|---|---|
| Dust extract concentrations (μg/mL) | |||
| Overall | 10.1–4449 | 494.6 | 589.2 |
| Lower tertile | 10.1–283.7 | 149.3 | 152.7 |
| Middle tertile | 290.4–626.7 | 494.6 | 469.7 |
| Upper tertile | 629.9–4449 | 990.2 | 1145 |
| Dust-induced triglyceride accumulation (%) | |||
| Overall | 0–388.9% | 14.9% | 37.4% |
| Lower tertile | 0–109.5% | 2.2% | 13.9% |
| Middle tertile | 0–388.9% | 27.2% | 44.8% |
| Upper tertile | 0–208.7% | 43.3% | 54.0% |
| Dust-induced cell proliferation (%) | |||
| Overall | 0–88.7% | 12.2% | 16.0% |
| Lower tertile | 0–60.0 % | 12.6% | 15.5% |
| Middle tertile | 0–65.5% | 10.4% | 14.9% |
| Upper tertile | 0–88.7% | 14.3% | 17.6% |
Descriptive statistics for dust extract concentrations and adipogenic activity metrics (both triglyceride accumulation and pre-adipocyte proliferation) for n=137 samples. Tertiles defined based on dust extract concentrations across groups. Dust extract concentrations provided as highest test concentration; each extract was tested at its highest concentration and four two-fold dilutions thereof.
Figure 1: Adipogenic Activity of Indoor House Dust Extracts Varies by Concentration.
3T3-L1 cells were induced to differentiate as described in Methods. Cells were assessed for degree of adipocyte differentiation after ten days of differentiation while exposed to dose responses (2-fold dilutions from maximal concentrations) of indoor house dust extracts. Dust samples exhibiting significant adipogenic activity relative to the differentiated vehicle control across each concentration range were separated according to efficacy. As such, the proportion of samples exhibiting significant activity in each concentration range can be calculated by summing the low, moderate, and high activity groups. (A) Distribution of normalized triglyceride accumulation per cell relative to maximal intra-assay response for rosiglitazone (normalized to DNA content) for each dust sample at every concentration tested. (B) Distribution of increased DNA content (pre-adipocyte proliferation) relative to a differentiated vehicle control for each dust sample at every concentration tested. Bars represent the percent of dust extracts that exhibited significant activity when tested at each concentration range. Sample number examined at each concentration range varied due to varying dust collected in each home: n= 1, 35, 132, 124, and 19 for 0.1–1.0, 1.0–10, 10–100, 100–1000, and 1000–10,000, respectively, for both (A) and (B).
Figure 2: Dust Extracts Induce Heterogeneous Activity across Triglyceride Accumulation and Proliferation.
3T3-L1 cells were induced to differentiate as described in the Methods. Cells were exposed to dose-responses (2-fold dilutions from maximal concentrations) of indoor house dust extracts and assessed for degree of triglyceride accumulation and pre-adipocyte proliferation ten days post-exposure. (A, D, G) Percent raw triglyceride accumulation per well relative to maximal intra-assay response for rosiglitazone for select dust extract dose-responses. (B, E, H) Increase (pre-adipocyte proliferation) or ecrease (potential cytotoxicity) in DNA content relative to vehicle control for select dust extract dose-responses. (C, F, I) Percent normalized triglyceride accumulation per cell relative to maximal intra-assay rosiglitazone response (normalized to DNA content) for select dust extract dose-responses. Data presented as mean ± SEM from three independent experiments (n=4 per experiment). Colors represent dust samples from unique households.
Association of Adipogenic Activity with Chemical Concentrations in House Dust Extracts.
Previous work in our laboratory characterized the concentrations of 12 flame retardants in matched dust samples (49). These flame retardants (8 BFRs and 4 PFRs) were correlated with extent of maximal adipogenic activity induced by matched dust extracts in 3T3-L1 bioassays. Concentration of the FRs varied, with more than an order of magnitude difference in concentration ranges across chemicals (Table S2). PFRs were generally present at higher concentration than most BFRs, with 5–10-fold higher mean concentrations and up to 30-fold higher median concentrations (Table S2). Statistically significant correlations were reported between each individual FR and both total triglycerides per well and normalized triglycerides per cell (Table 2). None of the BFRs and PFRs were significantly correlated with dust-induced pre-adipocyte proliferation, again suggesting differing but overlapping mechanisms may mediate these two metrics.
Table 2.
Correlations of Adipogenic Activity with Chemical Concentrations in Dust Extracts
| Chemical | Total Triglyceride Accumulation (per well) | DNA Content (pre-adipocyte proliferation) | Normalized Triglyceride Accumulation (per cell/DNA) |
|---|---|---|---|
| Brominated flame retardants (BFRs) | |||
| BDE-47 | 0.268** | −0.096 | 0.279** |
| BDE-99 | 0.403** | −0.124 | 0.330** |
| BDE-100 | 0.321** | −0.043 | 0.339** |
| BDE-153 | 0.372** | −0.049 | 0.385** |
| BDE-154 | 0.394** | −0.073 | 0.394** |
| BDE-209 | 0.513** | 0.060 | 0.462** |
| TBB | 0.316** | 0.006 | 0.324** |
| TBPH | 0.335** | 0.025 | 0.341** |
| Organophosphate flame retardants (PFRs) | |||
| TCEP | 0.375** | −0.013 | 0.343** |
| TDCIPP | 0.386** | −0.099 | 0.397** |
| TCIPP | 0.330** | −0.041 | 0.290** |
| TPHP | 0.247** | −0.011 | 0.199** |
Spearman’s correlations between concentrations of select brominated and organophosphate flame retardants (BFRs and PFRs, respectively) and adipogenic activity metrics (triglyceride accumulation per well relative to maximal rosiglitazone response, DNA content/pre-adipocyte proliferation relative to differentiated vehicle control, and triglyceride accumulation per cell normalized to DNA content) as determined using the 3T3-L1 cell culture assay.
= p<0.01,
= p<0.001, using GraphPad Prism 7.0.
Association of Adipogenic Activity with Metabolic Health of Resident Adults.
In an attempt to determine whether maximal adipogenic activity was primarily driven by higher dust mass samples (i.e. homes where more dust had been able to be collected), exploratory analyses were performed between adipogenic activities and metabolic health metrics, characteristics of which were described in greater detail previously (49). Reflecting the original study design, 23% of the participants were male, and participants generally exhibited a median BMI of 25.92 (17.68–49.34), a median age of 48.4 (26–80), a mean educational attainment of 16 years (college grad; 10–23+), and were approximately 78% white, 15% black, and 7% other.
First, correlation analyses were performed between bioactivities and extracted dust masses. Total and normalized triglyceride accumulation were positively correlated with the dust mass concentration (rs > 0.45 and p<0.0001 for each; Table 1, Figure S3); as a result, normalized metrics were created for further assessment: efficacy of response at 500 μg/mL dust extract concentration and potency (μg/mL extract concentration) at which 10% response was observed (described further in Methods).
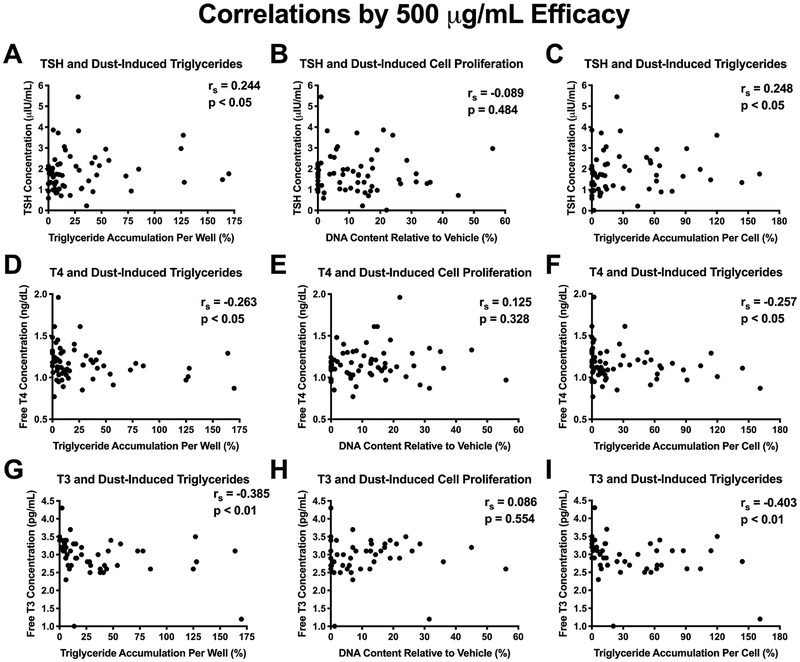
Health metrics varied across the cohort (Table S3). Increasing dust extract-induced triglyceride accumulation was correlated with circulating serum thyroid hormones measured in adult residents. Efficacies of triglyceride accumulation (per well and per cell) were positively correlated with serum TSH (rs > 0.24, p<0.05) and negatively correlated with serum FT4 (rs > −0.25, p<0.05) and serum FT3 (rs > −0.38, p<0.01; Figure 3). Results of regression analyses controlling for participant age, sex, race, and educational attainment suggest similar patterns of association, although associations were not significant in all cases (Table 3). For example, a 10% increase in triglyceride accumulation (per well) was associated with a 0.042 μIU/mL increase in serum TSH of residents (95% confidence interval (CI): −0.06, 0.89; p<0.10). Correlations and regression results were mirrored with triglyceride accumulation potencies (inverse effect, as smaller potency values represent greater potency strength): trends for negative correlations were observed with serum TSH and positive correlations with serum FT4 and FT3 (Table 3, Figure S4). Pre-adipocyte proliferation efficacies and potencies were not associated (via regressions or correlations) with any thyroid hormone measurements, again supporting the putative overlapping but distinct mechanisms mediating these two metrics.
Figure 3: Correlations of Adipogenic Activity with Thyroid Hormone Status in Resident Adults.
Spearman’s correlations comparing serum thyroid hormone measurements in adults with paired dust extract-induced adipogenic activity. (A-C) Serum thyroid stimulating hormone (TSH) concentrations,(D-F) free serum thyroxine (T4) concentrations, (G-I) and free serum triiodothyronine (T3) concentrations. The left column (A, D, G) represents raw triglyceride accumulation per well relative to maximal response for rosiglitazone), the middle column (B, E, H) represents increase (pre-adipocyte proliferation) in DNA content relative to vehicle control, and the right column (C, F, I) represents percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content). Triglyceride accumulation/cell proliferation values were normalized to responses at 500 μg/mL dust concentration and extrapolated where necessary to correct for varying dust concentrations that did not reach this concentration (n=28). Significant correlations were determined with p < 0.05 using GraphPad Prism 7.0.
Table 3.
Regressions of Resident Health Outcomes and Adipogenic Activities
| Dependent Variable | Adipogenic Metric | Measure | Estimate | 95% Confidence Limits | p Value |
|---|---|---|---|---|---|
| BMI | Total Triglycerides | Efficacy1 | 0.17 | 0.05, 0.29 | <0.01 |
| Potency2 | −0.01 | −0.05, 0.02 | 0.37 | ||
| Pre-adipocyte Proliferation | Efficacy1 | 0.23 | −0.38, 0.84 | 0.47 | |
| Potency2 | −0.02 | −0.27, 0.24 | 0.91 | ||
| Normalized Triglycerides | Efficacy1 | 0.29 | 0.11, 0.48 | <0.01 | |
| Potency2 | −0.01 | −0.06, 0.04 | 0.71 | ||
| TSH | Total Triglycerides | Efficacy1 | 0.042 | −0.01, 0.09 | 0.08 |
| Potency2 | −0.01 | −0.03, 0.01 | 0.39 | ||
| Pre-adipocyte Proliferation | Efficacy1 | 0.12 | −0.05, 0.30 | 0.17 | |
| Potency2 | 0.01 | −0.03, 0.06 | 0.60 | ||
| Normalized Triglycerides | Efficacy1 | 0.04 | −0.01, 0.09 | 0.11 | |
| Potency2 | −0.01 | −0.02, 0.01 | 0.33 | ||
| FT4 | Total Triglycerides | Efficacy1 | −0.01 | −0.02, 0.003 | 0.14 |
| Potency2 | 0.01 | 0.002, 0.01 | <0.01 | ||
| Pre-adipocyte Proliferation | Efficacy1 | 0.01 | −0.04, 0.05 | 0.80 | |
| Potency2 | −0.01 | −0.02, 0.003 | 0.22 | ||
| Normalized Triglycerides | Efficacy1 | −0.01 | −0.02, 0.003 | 0.14 | |
| Potency2 | −0.002 | −0.004, 0.001 | 0.14 | ||
| FT3 | Total Triglycerides | Efficacy1 | −0.01 | −0.04, 0.02 | 0.48 |
| Potency2 | 0.01 | −0.003, 0.02 | 0.13 | ||
| Pre-adipocyte Proliferation | Efficacy1 | −0.003 | −0.09, 0.09 | 0.96 | |
| Potency2 | −0.002 | −0.02, 0.01 | 0.83 | ||
| Normalized Triglycerides | Efficacy1 | −0.02 | −0.04, 0.01 | 0.27 | |
| Potency2 | 0.004 | −0.002, 0.01 | 0.20 |
Robust regressions (as described in (64,65)) were performed to assess relationships between adipogenic activity metrics (as determined by the 3T3-L1 cell culture assay; triglyceride accumulation per well relative to maximal rosiglitazone response, DNA content/pre-adipocyte proliferation relative to differentiated vehicle control, and triglyceride accumulation per cell normalized to DNA content) and resident health outcomes (body mass index (BMI), thyroid stimulating hormone, free triiodothyronine (T3), and free thyroxine (T4)). These models included age, sex, race, and education as potential confounding variables. Analyses were performed using SAS 9.4. N= 137 for BMI and 69 for thyroid hormone measurements.
Efficacy estimates and confidence intervals provided as per 10% change in triglyceride accumulation or pre-adipocyte proliferation percent.
Potency estimates and confidence intervals provided as per 100 μg/mL change in concentration at which triglyceride accumulation or pre-adipocyte proliferation reached 10% effect.
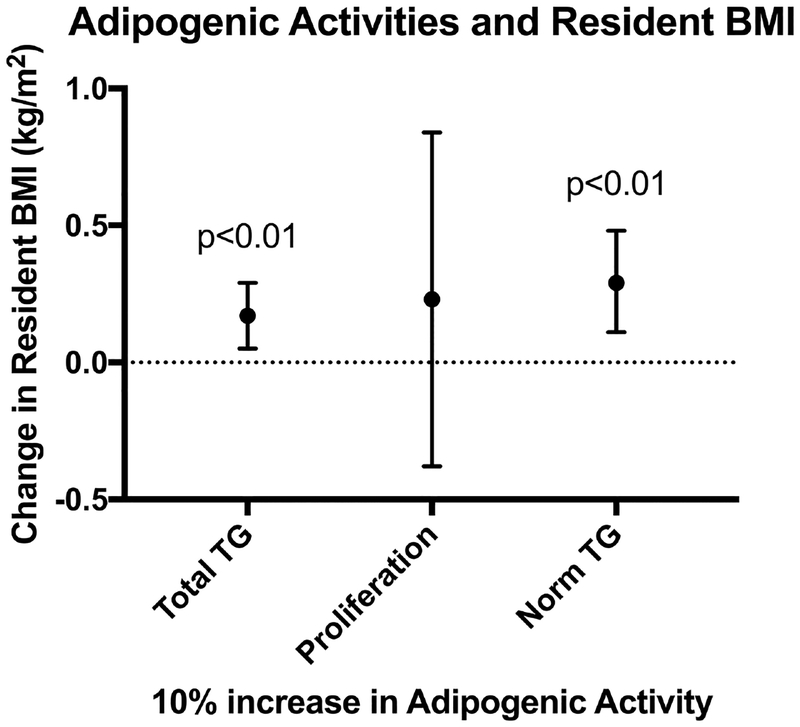
Increasing dust extract-induced triglyceride accumulation was also positively associated with the BMI of residents (each 10% increase in normalized triglyceride accumulation was associated with a 0.29 kg/m2 increase in BMI (CI 0.11, 0.48; p<0.01; Figure 4, Table 3). Potencies of triglyceride accumulation were not associated with BMI, nor were triglyceride accumulation efficacies or potencies correlated with BMI (Spearman’s correlations; Figures S5, S6). Pre-adipocyte proliferation efficacies tended to be positively correlated with BMI (rs > 0.16, p<0.10; Figure S5) and negatively correlated with pre-adipocyte proliferation potencies (rs > 0.17, p<0.10; Figure S6), although adjusted regressions did not support this association (Table 3).
Figure 4: Dust-Induced Triglycerides Associated with BMI of Adult Residents.
Robust regressions were performed to assess relationships between adipogenic activity metrics at 500 μg/mL dust extract concentrations (total triglyceride accumulation per well, pre-adipocyte proliferation, and normalized triglyceride accumulation per cell) and resident body mass index (BMI). These models controlled for potential confounding by age, sex, race, and education, which we anticipated could be related to health outcomes as well as the types of contaminants in the home environment driving the measured adipogenic activity. Analyses were performed using SAS 9.4. Adipogenic metrics were scaled such that a 10% increase in triglyceride accumulation (relative to positive control) or pre-adipocyte proliferation (relative to differentiated vehicle control) was associated with the change in BMI depicted in the figure.
Role of TRβ Antagonism in Adipogenic Activity of House Dust Extracts.
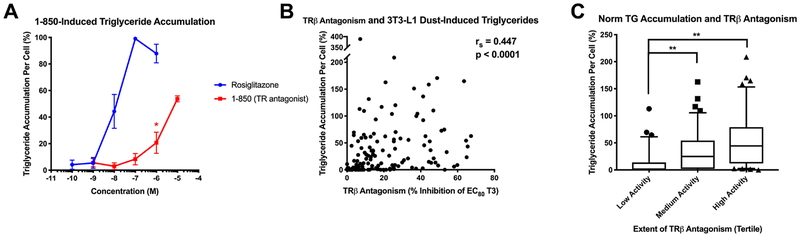
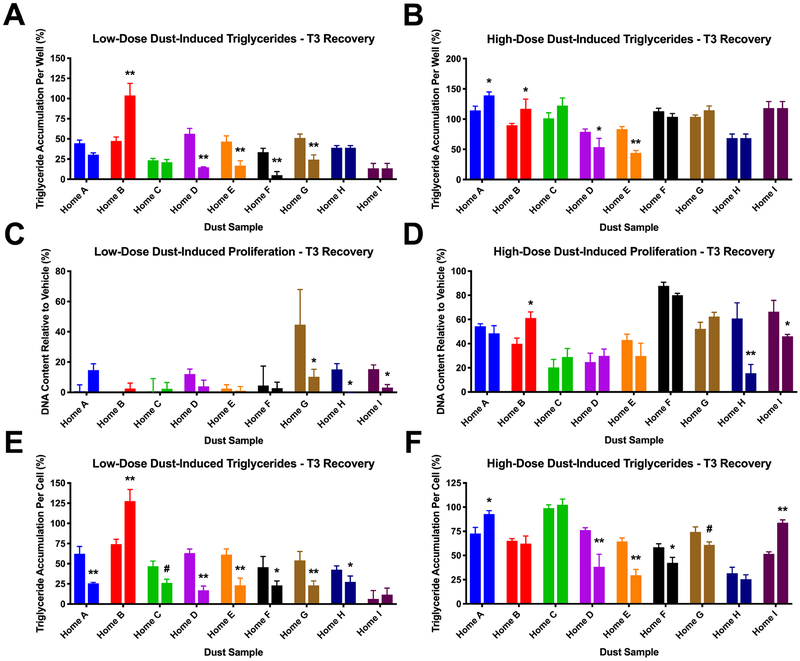
Based on adipogenic activity associations with thyroid hormone levels, TRβ antagonism was evaluated as a potential contributory factor in the observed adipogenic activities; TR has been demonstrated to be expressed in these cells by several researchers previously, and increases throughout differentiation (11,66). The TR antagonist, 1–850, stimulates a robust triglyceride accumulation response relative to rosiglitazone, a PPARγ agonist (Figure 5A) (14). Concurrent research in our laboratory evaluated the thyroid receptor agonism/antagonism by these same dust extracts using a cell-based reporter assay system (41) and the extent of TRβ antagonism was positively correlated with normalized triglyceride accumulation (rs = 0.44, p<0.0001), supporting a potential contributory role of TRβ in adipogenesis (Figure 5B). TR antagonism was not correlated with pre-adipocyte proliferation, further delineating the mechanisms mediating these adipogenic outcomes. Two targeted experiments were thus carried out to attempt to confirm this contributory mechanism to mixture-induced triglyceride accumulation. Nine dust extracts exhibiting moderate triglyceride accumulation and TRβ antagonism were selected for targeted mechanistic testing. First, these extracts were tested at low and high concentrations either alone or co-exposed with 10 μM T3; we hypothesized that if TRβ antagonism was promoting triglyceride accumulation, addition of a TR agonist would counteract this response. At low-dose extract concentrations (Figure 6A, C, E), T3 co-exposure significantly reduced dust-induced triglyceride accumulation per cell in six of nine samples (p<0.05) and tended to inhibit in one more (p<0.10; Figure 6A, E). One sample appeared to have no effect on triglyceride accumulation, and one sample increased accumulation rather than decreased it. Three samples exhibited significant decreases in pre-adipocyte proliferation following T3 co-exposure (p<0.05), while no effects were observed for the other six samples (Figure 6C). Results at high dust extract concentrations (Figure 6B, D, F) were less marked than those at lower concentrations, with only three samples exhibiting a significant decrease in triglyceride accumulation per cell (p<0.05) and tended to inhibit in one more (p<0.10; Figure 6B, F). One sample exhibited increased triglyceride accumulation per cell with T3 co-exposure, and two samples exhibited reduced pre-adipocyte proliferation (Figure 6D).
Figure 5: Putative Role of Thyroid Receptor Beta Antagonism in Dust Extract-Induced Adipogenic Activity.
Putative interrogation of thyroid receptor beta (TRβ) antagonism as a molecular mechanism promoting triglyceride accumulation. (A) Normalized triglyceride accumulation per cell relative to maximal intra-assay response for rosiglitazone (normalized to DNA content) for 1–850, a TR antagonist. 3T3-L1 cells were induced to differentiate as described in Methods and assessed for degree of adipocyte differentiation after ten days of differentiation. * denotes the lowest concentration at which a significant increase above baseline values (differentiated DMSO control) was observed. (B) Spearman’s correlation comparing normalized triglyceride accumulation per cell relative to maximal intra-assay response for rosiglitazone (normalized to DNA content) versus TRβ antagonism as measured via GeneBLAzer TRβ FRET reporter assay (Invitrogen) (Kollitz et al. 2018). Significant correlations were determined with p < 0.05 using GraphPad Prism 7.0. (C) Box and whisker plots depicting normalized triglyceride accumulation per cell (normalized to DNA content) in each tertile of maximal TRβ antagonism (low/medium/high antagonist activities). **denotes significant differences between groups as per Kruskal-Wallis test, p<0.001, using GraphPad Prism 7.0.
Figure 6: Co-Exposure to T3 Inhibits Dust-Induced Triglyceride Accumulation.
3T3-L1 cells were induced to differentiate as described in Methods. Cells were assessed for degree of adipocyte differentiation after ten days of differentiation while exposed to set concentrations of nine select indoor house dust extracts either alone or co-treated with 10 μM T3. (A, B) Raw triglyceride accumulation per well relative to maximal response for rosiglitazone, (C, D) increase (pre-adipocyte proliferation) in DNA content relative to vehicle control, and (E, F) percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content). Two concentrations were examined for each dust extract. The left column depicts low-dose concentrations that were 16-fold dilutions of the maximal concentrations for each extract (A, C, E). The right column depicts high-dose concentrations tested were the maximal concentration of each extract and varied depending on the quantity of dust collected in each household (B, D, F). The left bar in each pair depicts the response induced by the dust extract alone, while the right bar in each pair depicts the activity induced by the dust extract co-treated with T3. Data presented as mean ± SEM from two independent experiments of four biological replicates. # = p < 0.10, * = p < 0.05, ** = p < 0.01 indicate statistical differences between T3/dust co-treatment and dust samples alone as per one-way ANOVA and Dunnett’s posthoc test in GraphPad Prism 7.0.
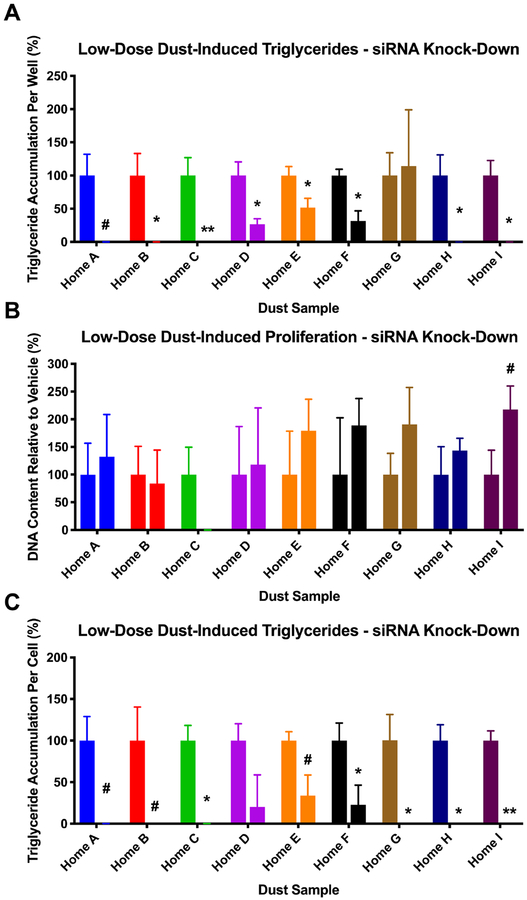
A confirmatory mechanistic test assessed this relationship via siRNA knock-down of TRα/TRβ.; we hypothesized that a decrease in available receptors (using a knock-down approach) would also reduce TR antagonism-driven triglyceride accumulation. The same nine dust extracts were assessed at the low concentration, using: dust alone, dust in cells treated with a scrambled siRNA negative control, and dust in cells treated with a TRα/TRβ siRNA knock-down protocol. To rule out potential inhibitory effects of TR knock-down on adipogenesis itself, vehicle controls and rosiglitazone responses were compared across siRNA treatments: no differences were observed between non-transfected controls and siRNA treatments (Figure S7A). To functionally determine the effect of knocking down TR before assessing the effects on dust, we examined full dose responses of 1–850 (a potent and selective TR antagonist) across these treatments. Importantly, while no differences were observed in 1–850-induced triglyceride accumulation between the naïve and scrambled siRNA cells, the TR-siRNA cells exhibited approximately a 67% reduction in triglyceride accumulation at the highest concentration of 1–850 tested (p<0.05; Figure S7B–D), similar to the magnitude of effect observed previously using this protocol to knock-down PPARγ (63). Following confirmation of vehicle and control chemical responses, we further assessed putative effects by the transfection process on dust-induced responses. We observed no significant differences between the control (dust alone) and the scrambled siRNA negative control for any dust extract (Figure S8). Finally, the TR knock-down significantly reduced dust-induced triglyceride accumulation per cell in five of nine extracts (p<0.05), tended to reduce in three more (p<0.10), and resulted in a non-significant reduction in the final extract (Figure 7A, C). No significant impacts on DNA content were observed, though one extract tended to increase DNA content following siRNA knock-down (p<0.10; Figure 7B).
Figure 7: siRNA Knock-Down of Thyroid Receptor Expression Inhibits Dust-Induced Triglyceride Accumulation.
3T3-L1 cells were transfected with either no siRNA, a negative control (scrambled) siRNA, or TRα and TRβ siRNAs and were then induced to differentiate as described in Methods. Cells were assessed after ten days of differentiation while exposed to set concentrations (16-fold dilutions of maximal concentrations) of nine select indoor house dust extracts. (A) Raw triglyceride accumulation per well relative to maximal response for rosiglitazone, (B) increase in pre-adipocyte proliferation (DNA content) relative to vehicle control, and (C) percent normalized triglyceride accumulation per cell relative to maximal rosiglitazone response (normalized to DNA content). The left bar in each pair depicts the response induced by the dust extract alone, while the right bar in each pair depicts the activity induced by the dust extract following transfection with TRα and TRβ siRNAs. Data presented as mean ± SEM from two independent experiments of four biological replicates. # = p < 0.10, * = p < 0.05, ** = p < 0.01 indicate statistical differences between TR siRNA and dust control samples as per one-way ANOVA and Dunnett’s posthoc test in GraphPad Prism 7.0. No significant differences were observed between the dust control samples and the negative control (scrambled) siRNA; see Supplemental Figures 7 and 8 for additional details on these results.
Discussion
These results suggest that complex mixtures in house dust induce adipogenic activity in vitro, at environmentally relevant concentrations, and that this activation is controlled, at least in part, by antagonism of TRβ. Furthermore, the adipogenic activity observed in a large number of indoor house dust sample extracts was associated with the BMI and serum thyroid hormone levels of adult residents living in these homes. 90% of the tested dust extracts exhibited significant adipogenic activity via either increased triglyceride accumulation and/or pre-adipocyte proliferation. Importantly, >40% of samples exhibited significant effects at concentrations <10 μg dust/well, suggesting a potent biological response by the mixtures of contaminants present in dust, particularly notable given that the EPA estimates that children ingest 60–100 mg of dust per day from indoor environments (23). While translation of oral human exposure to in vitro effects is not clear, the wide disparity in concentrations warrants further research to investigate potential in vivo effects.
The extent of triglyceride accumulation was highly positively correlated with the extracted dust masses, which is likely due to the inherently greater chemical contaminant concentrations in each assay well associated with this, and likely the causative factor in these observed effects. Interestingly, the extent of pre-adipocyte proliferation was not correlated with extracted dust masses; this may be due to differing assay sensitivities (lower dynamic range for proliferation vs. triglyceride accumulation, for example). Despite this, pre-adipocyte proliferation appeared to be a more sensitive metric than triglyceride accumulation (though notably these endpoints measure distinct but related processes), with a greater percentage of samples between 1–10 and 10–100 μg/mL exhibiting significant pre-adipocyte proliferation than triglyceride accumulation. Further research is needed to more comprehensively evaluate the apparent overlapping but distinct molecular mechanisms driving these two effects. Neither adipogenic metric was significantly different between dust samples from thyroid cancer cases relative to dust samples from control homes (the source of the dust in this study; (49)).
A notable finding of this study was that the extent of triglyceride accumulation was positively correlated with the concentrations of each of twelve BFRs and PFRs measured in the dust. Despite this, the majority of these contaminants were previously demonstrated to be inactive individually in this same cell system in our lab (45); 2,2’,4,4’,-tetrabromodiphenyl ether (BDE-47), triphenyl phosphate (TPHP), and bis (2-ethyl hexyl)-2,3,4,5-tetrabromophthalate (TBPH) demonstrated weak to moderate triglyceride accumulation and/or pre-adipocyte proliferation. However, these chemicals only account for a small fraction of the observed activity given the concentrations of these contaminants in the dust extracts. This, coupled with the significant correlations with other inactive chemicals, suggests that flame retardants are not the primary drivers of the observed activity. We hypothesize that either mixture effects or other co-occurring contaminants are primarily responsible for the effects reported herein. Numerous studies have demonstrated the “something from nothing” and “a lot from a little” effects both in vitro (67,68) and in vivo (69,70), demonstrating the potential additive or greater than additive effects of complex mixtures. Another potential concern is that the presence of multiple chemicals in a mixture likely increases the chance of inducing the expression of cytochrome P450s and other bioactivation enzymes (71–73). For example, previous work demonstrated that several PCB congeners were inactive thyroid receptor ligands when dosed individually; however, they were metabolized into bioactive compounds that promoted TR-mediated effects when present in mixtures that induced expression of CYP1A1, using a rodent pituitary cell line (74,75). House dust contains thousands of chemicals (76,77), so it is likely that there are other active constituents that have yet to be identified and measured. For example, our lab published previously on other common house dust contaminants, reporting much greater adipogenic activity for other contaminants not measured herein (45,76,78). More research is needed to further investigate the primary chemicals or effects driving this. Other chemicals as well as metals are also known to accumulate in house dust (28), and have also been demonstrated to modulate adipogenesis/adiposity (79–81), suggesting a potential multifactorial molecular mechanism. The fact that none of the quantified/identified/measured BFRs or PFRs were significantly correlated with dust-induced pre-adipocyte proliferation supports our hypothesis that overlapping but distinct mechanisms are promoting these two adipogenic metrics.
This study is the first to report an association between dust-induced triglyceride accumulation in vitro and both BMI and thyroid hormone concentrations of adult residents living in sampled homes. Triglyceride accumulation per well and per cell were positively associated with BMI using a robust regression model (β=0.29 per 10% change in triglyceride accumulation, 95% CI 0.11–0.48, p<0.01). While these results suggest that the extent of adipogenic chemicals present in the dust are associated with greater weights of residents living in those homes, these analyses are exploratory and more research with a greater sample size is needed. This association could be due to chronic exposure to chemicals in the indoor environment acting directly on the residents to alter: hormonal control of appetite and/or satiety, basal metabolic rate, adipocyte commitment and/or differentiation, the brain circuitry that controls food intake and/or energy expenditure, or by shifting energy balance to favor caloric storage, as reviewed previously (6). Future research is needed to more definitively examine this association, examine this potential association in children who have a more permanent link to their indoor environment and are in the process of developing their metabolic regulatory systems, better assess causative molecular mechanisms for the preadipocyte proliferation metric, and identify putative causative chemicals. Dust-induced triglyceride accumulation efficacies also appeared to be positively correlated with serum TSH (suggesting increased dust-induced adipogenic activity in vitro was associated with higher TSH), and negatively correlated with serum FT3 and FT4, although regression results adjusting for additional covariates were not statistically significant. However, both analyses (i.e., correlation and regression) showed the same direction of effect, bolstering a potential association, although our sample size may have limited our ability to detect statistically significant associations in the adjusted models. Notably, our previous work found that approximately half of these same extracts antagonized T3-mediated TRβ activity by 20–67% and that TRβ antagonist potencies were negatively correlated with serum FT4 (p<0.001) and serum FT3 (p<0.10) concentrations in residents, suggesting a TR-mediated impact on thyroid function (41). In addition, we’ve previously demonstrated that treatment of 3T3-L1 cells with the TR antagonist 1–850 induced a robust triglyceride accumulation response (14), providing support for this as a causative mechanism herein, supporting the associations with thyroid hormone measurements, and providing a plausible mechanism through which thyroid receptor antagonists in the dust may influence both thyroid hormone measurements and BMI of residents and the dust-induced triglyceride accumulation in vitro. As before, the degree of dust-induced pre-adipocyte proliferation was not significantly correlated with any thyroid measurements, suggesting that this may not be as important a mechanism for proliferation.
Given a significant positive correlation between the extent of triglyceride accumulation and the extent of TRβ antagonism (41), two additional targeted experiments were performed to evaluate the role of TR antagonism in the adipogenic effects: a ligand recovery experiment (co-treating cells with dust extracts and T3 to attempt to counteract TR antagonist-induced triglyceride accumulation) and an siRNA knockdown of TR. In this select set of dust extracts, both T3 and siRNA knock-down of TR isoforms succeeded in counteracting a significant amount of the dust-induced triglyceride accumulation for the majority of samples. This suggests TR antagonist binding and accompanying co-regulator recruitment may mediate these effects, given that TR knock-down did not appear to affect basal differentiation extent nor PPARγ-mediated ligand responses (rosiglitazone), yet did inhibit TR antagonist (1–850)-mediated triglyceride accumulation. These mechanistic data demonstrate TR antagonism as a contributory factor in at least some of the observed adipogenic effects in vitro as well as a likely factor in the modulated thyroid hormone concentrations in residents. Lesser effects were observed in the ligand recovery experiment with higher dust extract concentrations, suggesting a greater TR antagonism effect that would have required greater agonist addition to counteract. Further substantiation of this putative mechanism is also needed in a larger collection of dust extracts with varying degrees of TR bioactivities, though it is beyond the scope of the work reported herein. That all said, the TR antagonism does not explain the entirety of the adipogenic activity induced by these dust extracts, which is expected given the complexity of chemicals present in samples of this type. Samples are also heterogeneous (29), with different homes having varying concentrations of specific contaminants, likely due to differing consumer products and construction materials in the home (82,83). More work is needed to better characterize the chemical complexity of indoor dust samples, using both targeted and non-targeted analysis techniques to bolster our understanding of the chemicals and mixtures present in environmental matrices that may contribute to metabolic health dysfunction. Given a wider targeted chemical assessment, future research should utilize a weighted potency approach to assessing mixture effects using more sophisticated analyses. Further work should also examine the precise mechanisms of lipid accumulation by assessing gene expression for various TR endpoints, lipogenesis, lipolysis, etc.
In conclusion, we report widespread extract-induced adipogenic activity in vitro by a large collection of indoor house dust extracts collected in central NC and tested at environmentally relevant levels. We have further established a likely contributory causative molecular mechanism for the observed effects through TR antagonism, although further research is needed to establish the relative contribution of this mechanism as well as to examine this relationship in vivo. Importantly, this work establishes an important role for TR antagonism in complex environmental mixtures modulating metabolic dysfunction in vitro, a mechanism that has received limited attention previously. Further, in vitro triglyceride accumulation was positively associated with BMI and serum TSH and tended to be negatively associated with serum FT3 and FT4 levels in residents, raising concerns that these complex mixtures of indoor contaminants could affect metabolic health (via modulation of key metabolic hormones and/or adiposity). Notably, thyroid health analyses were performed only in individuals with no history of thyroid dysfunction; as such, it is possible we have removed those individuals most sensitive to environmental exposures, limiting our ability to detect potential impacts. Our study population included a relatively small sample size and a somewhat homogenous study population (reported previously in detail (49)), which might therefore limit the generalizability to the overall population; concentration of various contaminants in dust are also expected to vary geographically and temporally (84,85), so future research should attempt to reproduce these effects utilizing dust from other regions and settings. We are also limited by the cross-sectional study design, wherein we measured contaminants in the home at the same time we measured BMI, thyroid hormones, and other health metrics; however, we restricted participation to residents living in the same home for at least two years to ensure exposure measurements captured a longer-term environmental exposure (residents in our study lived in homes on average eleven years) (49). These results highlight a need for additional studies to examine further how exposure to dust extracts, and particularly exposure to mixtures of household contaminants, affects adipogenesis in vitro, in vivo, and subsequently, could affect the health of the general population.
Supplementary Material
Highlights:
Tested ability of indoor house dust extracts (HDE) to promote adipogenesis in vitro
The majority of HDE promoted triglyceride accumulation and pre-adipocyte proliferation
Adipogenic activity was positively correlated with each of twelve flame retardants
Adipogenic activity was mediated in part by thyroid receptor antagonism of HDE
Adipogenic activity of HDE was associated with metabolic health of residents
Funding:
Project supported by a grant [R01 ES016099] and a fellowship [F32 ES027320] from the National Institute of Environmental Health Sciences. Funding for the cancer cohort work was provided by Fred & Alice Stanback, the Duke Cancer Institute, and the Nicholas School of the Environment.
Competing interests declaration:
JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, supported by NovoNordisk, GlaxoSmithKline, Astra Zeneca, and Eli Lilly; CDK, EMK, KH, and HMS have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Endocrine Disruption Exchange (TEDX) (2018). TEDX List of Potential Endocrine Disruptors. Available at: https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list [accessed: 10 September 2018].
- 2.Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P. Parma consensus statement on metabolic disruptors. Environ Health. 2015;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214(5):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5(1):19–27. [DOI] [PubMed] [Google Scholar]
- 5.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3(2):127–133. [DOI] [PubMed] [Google Scholar]
- 6.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis RM, Vandenberg LN, Vom Saal FS. Metabolism disrupting chemicals and etabolic disorders. Reprod Toxicol. 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, Vom Saal FS, Taylor JA. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127(1–2):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84(2):319–327. [DOI] [PubMed] [Google Scholar]
- 11.Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. A Nuclear Receptor Atlas: 3T3-L1 adipogenesis. Mol Endocrinol. 2005;19(10):2437–2450. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Zhang K, Giesy JP, Hu J. Families of nuclear receptors in vertebrate models: characteristic and comparative toxicological perspective. Sci Rep. 2015;5:8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N. Adipose Tissue and Adipocyte Differentiation: Molecular and Cellular Aspects and Tissue Engineering Applications. Topics in Tissue Engineering. Vol 42008:1–26. [Google Scholar]
- 14.Kassotis CD, Masse L, Kim S, Schlezinger JJ, Webster TF, Stapleton HM. Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Sci Rep. 2017;7:42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol. 2012;46(2):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Perspect. 2015;123(2):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudel RA, Dodson RE, Perovich LJ, Morello-Frosch R, Camann DE, Zuniga MM, Yau AY, Just AC, Brody JG. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ Sci Technol. 2010;44(17):6583–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ Health Perspect. 2011;119(9):1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallaire F, Dewailly E, Muckle G, Ayotte P. Time trends of persistent organic pollutants and heavy metals in umbilical cord blood of Inuit infants born in Nunavik (Quebec, Canada) between 1994 and 2001. Environ Health Perspect. 2003;111(13):1660–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houlihan J, Kropp T, Wiles R, Gray S, Campbell C. BodyBurden: The Pollution in Newborns. Environmental Working Group. Available at: https://www.ewg.org/research/body-burden-pollution-newborns [accessed: 9 November 2018].
- 21.Landrigan PJ, Sonawane B, Mattison D, McCally M, Garg A. Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environ Health Perspect. 2002;110(6):A313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol. 2015;49(17):10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Environmental Protection Agency (EPA). Exposure Factors Handbook Chapter 5 (Update): Soil and Dust Ingestion. US EPA Office of Research and Development, Washington, DC, EPA/600/R-17/384F, 2017. [Google Scholar]
- 24.Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37(20):4543–4553. [DOI] [PubMed] [Google Scholar]
- 25.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF. Associations between PBDEs in office air, dust, and surface wipes. Environ Int. 2013;59:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton HM, Harner T, Shoeib M, Keller JM, Schantz MM, Leigh SD, Wise SA. Determination of polybrominated diphenyl ethers in indoor dust standard reference materials. Anal Bioanal Chem. 2006;384(3):791–800. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen PE, Levesque C, Chenier M, Gardner HD, Jones-Otazo H, Petrovic S. Canadian House Dust Study: population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Sci Total Environ. 2013;443:520–529. [DOI] [PubMed] [Google Scholar]
- 29.Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, Zota AR. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ Sci Technol. 2016;50(19):10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kademoglou K, Xu F, Padilla-Sanchez JA, Haug LS, Covaci A, Collins CD. Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ Int. 2017;102:48–56. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki G, Tue NM, Malarvannan G, Sudaryanto A, Takahashi S, Tanabe S, Sakai S, Brouwer A, Uramaru N, Kitamura S, Takigami H. Similarities in the endocrine-disrupting potencies of indoor dust and flame retardants by using human osteosarcoma (U2OS) cell-based reporter gene assays. Environ Sci Technol. 2013;47(6):2898–2908. [DOI] [PubMed] [Google Scholar]
- 32.Fang M, Stapleton HM. Evaluating the bioaccessibility of flame retardants in house dust using an in vitro Tenax bead-assisted sorptive physiologically based method. Environ Sci Technol. 2014;48(22):13323–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int. 2018;116:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawar G, Abdallah MA, de Saa EV, Harrad S. Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. J Expo Sci Environ Epidemiol. 2016;27:100–105. [DOI] [PubMed] [Google Scholar]
- 35.Lim YW, Kim HH, Lee CS, Shin DC, Chang YS, Yang JY. Exposure assessment and health risk of poly-brominated diphenyl ether (PBDE) flame retardants in the indoor environment of elementary school students in Korea. Sci Total Environ. 2014;470–471:1376–1389. [DOI] [PubMed] [Google Scholar]
- 36.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect. 2013;121(5):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ Sci Technol. 2010;44(14):5627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou P-H, Lee C-H, Ko F-C, Lin Y-J, Kawanishi M, Yagi T, Li I-C. Detection of Hormone-Like and Genotoxic Activities in Indoor Dust from Taiwan Using a Battery of in Vitro Bioassays. Aerosol and Air Quality Research. 2015;15:1412–1421. [Google Scholar]
- 40.Suzuki G, Takigami H, Nose K, Takahashi S, Asari M, Sakai S. Dioxin-like and transthyretin-binding compounds in indoor dusts collected from Japan: average daily dose and possible implications for children. Environ Sci Technol. 2007;41(4):1487–1493. [DOI] [PubMed] [Google Scholar]
- 41.Kollitz EM, Kassotis CD, Hoffman K, Ferguson PL, Sosa JA, Stapleton HM. Chemical mixtures isolated from house dust disrupt thyroid receptor β (TRβ) signaling. Environ Sci Technol. 2018;52(20):11857–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (PPARgamma) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ Health Perspect. 2015;123(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang M, Webster TF, Stapleton HM. Activation of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) by Semi-Volatile Compounds (SVOCs) and Chemical Mixtures in Indoor Dust. Environ Sci Technol. 2015;49(16):10057–10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang M, Webster TF, Stapleton HM. Effect-Directed Analysis of Human Peroxisome Proliferator-Activated Nuclear Receptors (PPARgamma1) Ligands in Indoor Dust. Environ Sci Technol. 2015;49(16):10065–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassotis CD, Hoffman K, Stapleton HM. Characterization of Adipogenic Activity of Semi-volatile Indoor Contaminants and House Dust. Environ Sci Technol. 2017;In 51(15):8735–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu C, Cheng SY. Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors. J Mol Endocrinol. 2010;44(3):143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, Webb P, Baxter JD, Grover GJ. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol. 2008;111(3–5):262–267. [DOI] [PubMed] [Google Scholar]
- 48.Obregon M-J. Thyroid Hormone and Adipocyte Differentiation. Thyroid. 2008;18(2):185–195. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, Scheri RP, Stapleton HM, Sosa JA. Exposure to flame retardant chemicals and the occurrence and severity of papillary thyroid cancer. Environ Int. 2017;107:235–242. [DOI] [PubMed] [Google Scholar]
- 50.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor y (PPARy). J Biol Chem. 1995;270(22):12953–12956. [DOI] [PubMed] [Google Scholar]
- 52.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. [DOI] [PubMed] [Google Scholar]
- 53.Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I, Voegel JJ, Vignon F, Nicolas JC, Balaguer P. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem. 2005;344(1):8–15. [DOI] [PubMed] [Google Scholar]
- 54.Watkins AM, Wood CR, Lin MT, Abbott BD. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol. 2015;400:90–101. [DOI] [PubMed] [Google Scholar]
- 55.Chappell VA, Janesick A, Blumberg B, Fenton SE. Tetrabromobisphenol-A Promotes Early Adipogenesis and Lipogenesis in 3T3-L1 Cells. Toxicol Sci. 2018;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamorro-Garcia R, Shoucri BM, Willner S, Kach H, Janesick A, Blumberg B. Effects of Perinatal Exposure to Dibutyltin Chloride on Fat and Glucose Metabolism in Mice, and Molecular Mechanisms, in Vitro. Environ Health Perspect. 2018;126(5):057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foley B, Doheny DL, Black MB, Pendse SN, Wetmore BA, Clewell RA, Andersen ME, Deisenroth C. Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis. Toxicol Sci. 2017;155(1):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janesick AS, Dimastrogiovanni G, Vanek L, Boulos C, Chamorro-García R, Tang W, Blumberg B. On the Utility of ToxCast and ToxPi as Methods for Identifying New Obesogens. Environ Health Perspect. 2016;124(8):1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temkin AM, Bowers RR, Magaletta ME, Holshouser S, Maggi A, Ciana P, Guillette LJ, Bowden JA, Kucklick JR, Baatz JE, Spyropoulos DD. Effects of Crude Oil/Dispersant Mixture and Dispersant Components on PPARgamma Activity and: Identification of Dioctyl Sodium Sulfosuccinate (DOSS; CAS #577-11-7) as a Probable Obesogen. Environ Health Perspect. 2016;124(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012;425(1):88–90. [DOI] [PubMed] [Google Scholar]
- 61.Santa Cruz Biotechnology. (2017). siRNA Mediated Inhibition of Gene Expression. Available at: https://www.scbt.com/scbt/resources/protocols/sirna-mediated-inhibition-of-gene-expression [accessed 3 March 2018].
- 62.Schapira M, Raaka BM, Das S, Fan L, Totrov M, Zhou Z, Wilson SR, Abagyan R, Samuels HH. Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking. Proc Natl Acad Sci U S A. 2003;100(12):7354–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foley B, Clewell RA, Deisenroth C. Development of a Human Adipose-Derived Stem Cell Model for Characterization of Chemical Modulation of Adipogenesis. Applied in Vitro Toxicology. 2015;1(1):66–78. [Google Scholar]
- 64.Hampel F, Ronchetti E, Rousseeuw P, Stahel W. Robust Statistics: The Approach Based on Influence Functions. New York: Wiley; 1986. [Google Scholar]
- 65.Yohai V High breakdown-point and high efficiency robust estimates for regression. Ann Stat. 1987;15(2):642–656. [Google Scholar]
- 66.Jiang W, Miyamoto T, Kakizawa T, Sakuma T, Nishio S, Takeda T, Suzuki S, Hashizume K. Expression of thyroid hormone receptor alpha in 3T3-L1 adipocytes; triiodothyronine increases the expression of lipogenic enzyme and triglyceride accumulation. J Endocrinol. 2004;182(2):295–302. [DOI] [PubMed] [Google Scholar]
- 67.Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110(9):917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36(8):1751–1756. [DOI] [PubMed] [Google Scholar]
- 69.Runnalls TJ, Beresford N, Kugathas S, Margiotta-Casaluci L, Scholze M, Scott AP, Sumpter JP. From single chemicals to mixtures - Reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow. Aquat Toxicol. 2015;169:152–167. [DOI] [PubMed] [Google Scholar]
- 70.Thrupp TJ, Runnalls TJ, Scholze M, Kugathas S, Kortenkamp A, Sumpter JP. The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Science of the Total Environment. 2018;619–620:1482–1492. [DOI] [PubMed] [Google Scholar]
- 71.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, Schwartzman ML, Abraham NG. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96(1–4):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring). 2010;18(3):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo X, Liao K. Analysis of gene expression profile during 3T3-L1 preadipocyte differentiation. Gene. 2000;251(1):45–53. [DOI] [PubMed] [Google Scholar]
- 74.Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect. 2007;115(11):1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152(7):2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferguson PL, Vogler B, Stapleton HM. Non-targeted analysis to assess human exposure to semi-volatile organic contaminants in the indoor environment. Paper presented at: Proceedings of the 63rd ASMS Conference on Mass Spectrometry and Allied Topics; 31 May - 4 June, 2015, 2015; St. Louis, MO. [Google Scholar]
- 77.Hilton DC, Jones RS, Sjodin A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J Chromatogr A. 2010;1217(44):6851–6856. [DOI] [PubMed] [Google Scholar]
- 78.Kassotis CD, Kollitz EM, Ferguson PL, Stapleton HM. Nonionic ethoxylated surfactants induce adipogenesis in 3T3-L1 cells. Toxicol Sci. 2018;162(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee EJ, Moon JY, Yoo BS. Cadmium inhibits the differentiation of 3T3-L1 preadipocyte through the C/EBPalpha and PPARgamma pathways. Drug Chem Toxicol. 2012;35(2):225–231. [DOI] [PubMed] [Google Scholar]
- 80.Martini CN, Brandani JN, Gabrielli M, Vila Mdel C. Effect of hexavalent chromium on proliferation and differentiation to adipocytes of 3T3-L1 fibroblasts. Toxicol In Vitro. 2014;28(4):700–706. [DOI] [PubMed] [Google Scholar]
- 81.Park SS, Skaar DA, Jirtle RL, Hoyo C. Epigenetics, obesity and early-life cadmium or lead exposure. Epigenomics. 2017;9(1):57–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ Sci Technol. 2015. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett. 2017;4(3):112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL. Predictors of urinary flame retardant concentration among pregnant women. Environ Int. 2017;98:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.