Abstract
Background:
Children with autism spectrum disorder (ASD) and co-occurring attention deficit/hyperactivity disorder (ADHD) symptoms have worse functional outcomes and treatment response than those without ADHD symptoms. Here we test the hypothesis that aberrant functional connectivity of two large-scale executive brain networks implicated in ADHD — the fronto-parietal and salience/ventral attention networks – also play a role in ADHD symptoms in ASD.
Methods:
We compared resting-state functional connectivity of the two executive brain networks in children with ASD (n=77) and typically developing controls (n=82). These two executive brain networks are comprised of five sub-networks (three fronto-parietal, two salience/ventral attention). After identifying aberrant functional connections among subnetworks, we examined dimensional associations with parent-reported ADHD symptoms.
Results:
Weaker functional connectivity in ASD was present within and between fronto-parietal and salience/ventral attention sub-networks. Decreased functional connectivity within a single salience/ventral attention sub-network, as well as between two fronto-parietal sub-networks, significantly correlated with ADHD symptoms. Furthermore, follow-up linear regressions demonstrated that the salience/ventral attention and fronto-parietal sub-networks explain unique variance in ADHD symptoms. These executive brain network-ADHD symptom relationships remained significant after controlling for ASD symptoms. Finally, specificity was also demonstrated through the use of a control brain network (visual) and a control comorbid symptom domain (anxiety).
Conclusion:
The present findings provide novel evidence that both fronto-parietal and salience/ventral attention networks’ weaker connectivities are linked to ADHD symptoms in ASD. Moreover, co-occurring ADHD in the context of ASD is a source of meaningful neural heterogeneity in ASD.
Keywords: selective attention, insula, anterior cingulate, resting state, comorbidity, ADHD, ASD
For children with autism spectrum disorder (ASD), the presence of co-occurring attention deficit/hyperactivity disorder (ADHD) is associated with worse functional outcomes and treatment response (1–5). Children with ASD and co-occurring ADHD struggle with skills needed for adaptive behavior (self-care, communicating and interacting effectively with others), and experience diminished quality of life (1–3). Compared to individuals who have ADHD but not ASD, children with ASD who seek treatment for ADHD symptoms have lower response and remission rates to ADHD medications, such as stimulants or alpha2 agonists (4, 5). Defining the brain networks contributing to ADHD symptoms in ASD may provide insight into the poor treatment response of children with co-occurring ADHD and ASD. Poor treatment response likely contributes to the poor functional outcomes for children with ASD and co-occurring ADHD symptoms. Despite these highly detrimental effects, few neuroimaging studies have directly evaluated multiple brain networks that may drive co-occurring ADHD symptoms in children with ASD.
ADHD is characterized by poor cognitive control, the ability to constrain thought and action to achieve goals (6–8). The triple network model of psychopathology posits that complex cognition like cognitive control is subserved by two of the three large-scale brain networks: the fronto-parietal and salience/ventral attention networks (9, 10). The fronto-parietal network includes portions of dorsal lateral and medial prefrontal cortex (Brodmann’s Area (BA) 6/9/10/44/46), intraparietal sulcus (BA 7/39/40), precuneus (BA 7), and cingulate cortex (BA 24/31). This network is linked to cognitive control processes including behavioral response inhibition, working memory, and set-shifting. Impairments in this network in ADHD populations have long been observed in neuropsychological studies (6). Task-based functional Magnetic Resonance Imaging (fMRI) studies have demonstrated decreased fronto-parietal activation in children with ADHD (11, 12). Recovery of fronto-parietal activation is observed in stimulant medication responders with ADHD during cognitive control tasks (13, 14), alongside changes in resting state functional connectivity in dorsolateral prefrontal cortex – a key hub of the fronto-parietal network (15). Furthermore, decreased activation in the fronto-parietal network during a cognitive control fMRI task has been reported in children with ASD relative to typically developing controls, and ADHD symptoms correlated with lower functional connectivity within the fronto-parietal network during this task (Solomon et al., 2009).
The salience/ventral attention network includes the anterior cingulate cortex (BA 24/32), insula (BA 13), lateral prefrontal cortex (BA 8/9/10/44/46), precuneus/posterior cingulate cortex (BA 7/31), the supramarginal gyrus (BA 2/40) and parietal operculum (BA 40). The salience and ventral attention networks were originally studied independently (17, 18), but recent functional connectivity research on large-scale brain networks shows the two to be highly associated (19, 20). The salience/ventral attention network is linked to multiple components of attention including error monitoring, selective attention, and task switching (10, 17). Task-based fMRI studies of selective attention have shown increased activation of the salience/ventral attention network in children with ADHD compared to typically developing controls (21, 22). Functional connectivity within the salience/ventral attention network shows atypical development in children with ASD (23). The salience/ventral attention network also has atypical functional connectivity to other networks in children with ASD compared to typically developing controls (24). However, to our knowledge, no study has directly assessed whether ADHD symptoms in ASD are associated with abnormalities of functional connectivity of the salience/ventral attention network.
The primary objective of the present study is to examine whether aberrant functional connectivity within and between the fronto-parietal and salience/ventral attention networks relates to co-occurring ADHD symptoms in children with ASD. Prior research has implicated these networks as atypical in ASD (23–28), but those studies did not test whether the functional connectivity differences were related to co-occurring ADHD symptoms. Therefore, it is not known whether differences in fronto-parietal and salience networks are strictly related to ASD, or whether they may be related to co-occurring ADHD symptoms. We used resting state fMRI to measure functional connectivity in children with ASD without an intellectual disability, as well as typically developing control (TDC) children. A dimensional index of ADHD symptoms was provided by the parent-reported ADHD rating scale (29). Based on prior literature (16), we hypothesized that a measure of the overall strength of functional connectivity within both the fronto-parietal and salience/ventral attention networks would be reduced in ASD, and that greater reductions in functional connectivity would be associated with more ADHD symptoms. To assess the specificity of our hypothesis, we also tested for group differences and correlations with a control brain network (visual sub-networks), and a control co-occurring symptom domain (anxiety). We hypothesized that ADHD symptoms would be associated with the salience/ventral attention and fronto-parietal but not visual networks, and that salience/ventral attention and fronto-parietal networks would not be associated with anxiety symptoms (30).
METHODS
Participants
A total of 214 children (111 ASD and 103 TDC) between the ages of 6 and 17 completed a resting state scan across multiple studies at the Center for Autism Research from 2010-2014. Children in the ASD group met the DSM-IV-TR criteria for autism, Asperger’s syndrome, or pervasive developmental disorder – not otherwise specified (31), informed by the Autism Diagnostic Interview – revised (32) and the Autism Diagnostic Observation Schedule (ADOS). We used the revised ADOS algorithm (33) that aligns with the second edition’s algorithm (34). DSM-IV-TR criteria were used because data collection started prior to the release of the DSM-5 and we wanted to maintain diagnostic consistency in our sample across this group of studies. Children with ASD were excluded if parents reported that their child had any known genetic, active mood or psychotic symptoms, or neurological disorder, extreme premature birth (gestational age<32 weeks), or other significant medical condition that affected functioning or completion of research procedures. TDC participants were excluded if parents reported that their child had any known genetic, language, learning, neurological, or psychiatric disorder, premature birth, any first- or second-degree relative with ASD, or if they were receiving any psychoactive medication. TDC children were also excluded if they presented scores in the clinical range on the parent-reported Child and Adolescent Symptom Inventory (35). We excluded 4 children with scores below 75 on the General Conceptual Ability (GCA – analogous to full scale IQ) as measured by the Differential Ability Scales – Second Edition (36) and excluded 48 children (ASD n=28; TDC n=20) with a mean framewise displacement>0.2mm during fMRI scanning (37). Three more children (ASD n=2) were excluded because their global functional connectivity was >4 standard deviations from their own group’s mean. Thus, our final sample included 159 children (ASD n=77; TDC n=82; ages 7-17; see Table 1 for group characteristics). Thirty-seven children with ASD were not prescribed medications at the time of the scan (48%), and seven of the 40 children prescribed medications were prescribed more than one medication. Prescribed medications included: stimulants (n=20); selective serotonin reuptake inhibitors (n=18); selective norepinephrine reuptake inhibitors (n=5); alpha 2A agonists (n=5); atypical antipsychotic (n=2); and an aminoketone anti-depressant (n=1). A subset of children prescribed stimulant medication were asked to withhold their medication on the day of scanning, to minimize the effects of these medications on brain function (n=6).
Table 1.
Participant Characteristics
| ASD n=77 |
TDC n=82 |
p-value | Hedges g | |
|---|---|---|---|---|
| Age – M(SD) | 149 mo (31 mo) | 149 mo (33mo) | 0.94 | 0.01 |
| GCA – M(SD) | 108 (18) | 112 (17) | 0.16 | 0.24 |
| Sex-Ratio (M:F) | 60:17 | 67:15 | 0.69 | -- |
| ADOS Social Affect | 8.65 (3.59) | -- | -- | |
| ADOS Repetitive Behaviors | 2.30 (1.64) | -- | -- | |
| ADOS Total Score | 10.95 (3.72) | -- | -- | |
| ADOS Calibrated Severity Score | 6.39 (2.05) | -- | -- | |
| ADHD-IV Rating Scale Total Score | 23.84 (11.56) | 4.28 (4.53) | <.001 | 2.24 |
| CASI-IV – Anxiety (20 items) | 12.49 (8.18) | 1.84 (2.33) | <.001 | 1.79 |
| In-Scanner Motion (Relative Mean Displacement) | 0.10 (0.05) | 0.11 (0.04) | 0.29 | 0.17 |
ADOS=Autism Diagnostic Observation Schedule (Revised Algorithm); ASD=Autism Spectrum Disorder; CASI=Child and Adolescent Symptom Inventory, 4th Edition; GCA=General Conceptual Ability; mo=months; TDC=Typically Developing Control
Of note, 20 children in the ASD group did not have an ADHD Rating Scale, 4th Edition for correlation analyses. The 57 children with ADHD symptom scores did not differ from the 20 missing ADHD symptom scores in age, IQ, sex-ratio, ASD or anxiety symptoms, or functional connectivity of any brain network, but did have lower in-scanner head motion (mean relative displacement: 0.09 vs. 0.13; See Table S1 for detailed comparison).
Image acquisition
Functional images were acquired on a 3T Siemens Verio scanner using a T2*-weighted gradient echo pulse sequence: 160 whole-brain volumes, 40 slices, TR/TE/Flip Angle/voxel size= 2340/25ms/60°/3.55mm isotropic. Thirty-seven children (20 ASD) received a slightly modified sequence: 172 whole-brain volumes, 36 slices, TR/TE/FOV/flip angle/voxel size=2110/25ms/60°/3.5mm isotropic (with a .35mm gap between slices). A high-resolution T1-weighted image for co-registration of the functional images was acquired with an MPRAGE sequence: TR/TE/voxel size/flip angle=300/2.46/1mm isotropic/60°. Participants were instructed to keep their eyes open and lie still while the monitor displayed a black screen with a gray cross at the center.
Subject-level time series processing
All functional time series data were preprocessed using a procedure that has been validated in multiple large-scale developmental datasets, and has been shown to be highly effective at reducing the influence of motion artifact on connectivity data (38–40). Preprocessing included removal of the first four volumes to allow for signal stabilization, slice time correction, realignment to the median volume, brain extraction, spatial smoothing (7mm FWHM), and grand mean scaling. Mean white matter (WM) and cerebrospinal fluid (CSF) signals were extracted from the filtered time series data using tissue segments generated for each subject. Confound regression included 9 standard confound signals (6 motion parameters + global/WM/CSF) as well as the temporal derivative, quadratic term, and the temporal derivative of the quadratic term (36 parameters total). We band-pass filtered the functional time series and the confound regressors simultaneously to retain frequencies between 0.01 and 0.08 Hz; identical temporal filtering prevented frequency mismatch between the confound parameters and the time series data (41). A recent benchmarking paper comparing more than a dozen preprocessing pipelines for resting state fMRI data demonstrated that the 36 parameter model is a good choice for pediatric group comparisons compared to other commonly used approaches (37). We also measured in-scanner motion as mean framewise displacement of brain slices. Framewise displacement was calculated by FSL’s MCFLIRT software, defined as the relative mean displacement across all rotations and translations (42).
The T1 image was skull-stripped using FSL’s BET (43), bias corrected and segmented using FSL’s FAST algorithm (44) and registered to the Montreal Neurological Institute template using DRAMMS, a highly accurate deformable registration with attribute matching and mutual salience weighting (45). Processed subject-level echo planar images were co-registered to the T1 image using boundary-based registration with integrated distortion correction as implemented in FSL5. All registrations were visually inspected.
Brain Networks and Functional Connectivity Analysis
We extracted time series data from a 200-area parcellation scheme of the cortex, which maps to 17 functional networks (19). The functional networks were derived from an independent sample (20), which included three fronto-parietal sub-networks and two salience/ventral attention sub-networks. Per Schaefer et al. (2017), the fronto-parietal-A sub-network includes parcels located in the intraparietal sulcus, dorsal and lateral portions of prefrontal cortex, and anterior cingulate cortex (see Figure 1 and Table S2 in supplemental material for comprehensive list of parcels for all sub-networks). The fronto-parietal-B sub-network includes parcels in lateral and ventrolateral portions of prefrontal cortex, lateral temporal cortex, and intraparietal lobule. The fronto-parietal-C network includes precuneus and posterior cingulate cortex. The salience/ventral attention-A sub-network includes parcels located in insula, medial parietal (operculum), and supplementary motor area (also referred to as juxtapositional cortex and medial prefrontal cortex in the Harvard-Oxford cortical atlas). The salience/ventral attention-B sub-network includes parcels in the intraparietal lobule, lateral prefrontal cortex and supplementary motor area (or medial prefrontal cortex). The visual peripheral network includes parcels in superior extrastriate cortex (BA 18/19). The visual central network includes parcels in extrastriate cortex (BA 18/19/30/31). All parcels by definition are non-overlapping even if they lie within similarly labeled regions of cortex. We estimated functional connectivity between all parcels of interest to create an 87 × 87 functional connectivity matrix, which represents pairwise Pearson’s correlations between all pairs of parcels.
Figure 1.
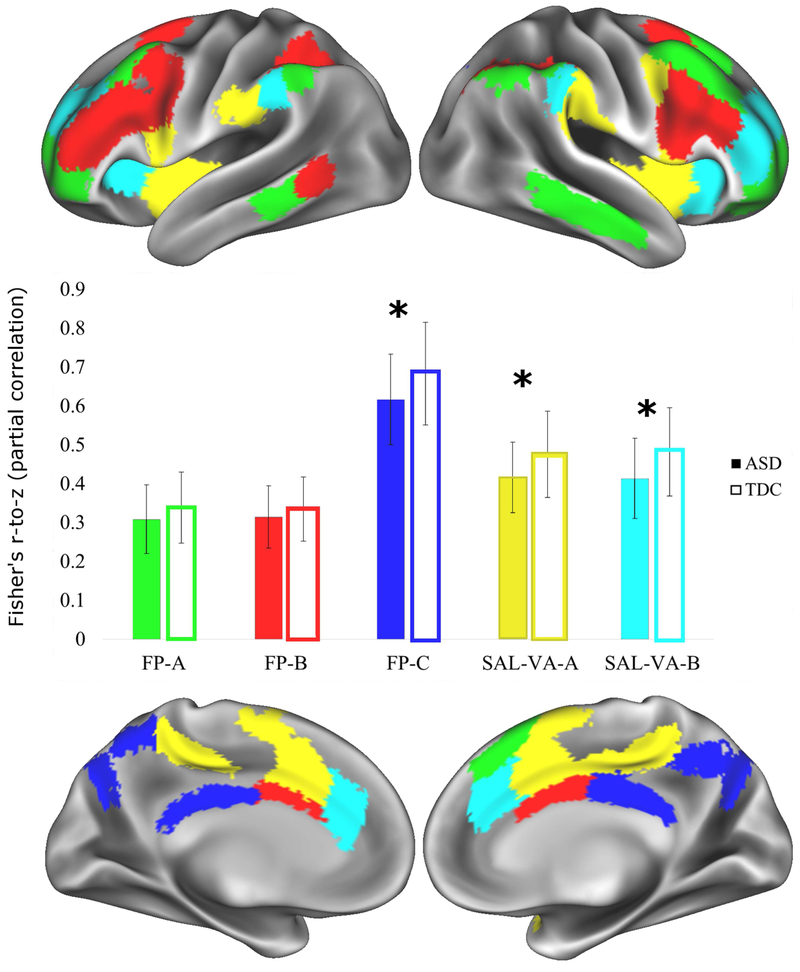
The three fronto-parietal and two salience/ventral attention sub-networks are shown here on an inflated brain on the 32,000 vertex Conte atlas available with Workbench. Green=fronto-parietal-A (FP-A); Red=fronto-parietal-B (FP-B); Dark Blue=fronto-parietal-C (FP-C); Yellow=salience/ventral attention-A (SAL-VA-A); Light Blue=salience/ventral attention-B (SAL-VA-B). The bar graph in the middle shows the means of group differences for within-sub-network functional connectivity, and the error bars represent the standard deviations. Significant group differences with Welch’s two group t-test, Bayesian analysis, and analysis of covariance surviving FDR-correction are denoted with an asterisk (*). The ASD group had significantly weaker functional connectivity within three subnetworks: fronto-parietal-C, t(156.35)=3.39, FDR-corrected p-value=0.004, Hedges g=0.53, [0.028, 0.106]; fronto-parietal-C, t(154.24)=3.65, FDR-corrected p-value=0.003, Hedges g=0.573, [0.027, 0.091]; salience/ventral attention-B, t(156.9)=3.98, FDR-corrected p-value=0.002, Hedges g=0.627, [0.034, 0.102].
We calculated the mean overall functional connectivity within and between the five a priori sub-networks after transforming the entire matrix into z-scores using Fisher’s r-to-z transformation, yielding a total of 15 variables (mean within and between sub-network functional connectivity). We compared each of those 15 variables between ASD and TDC groups with a Welch’s two-group t-test; the False Discovery Rate (FDR) was used to correct for multiple comparisons for an overall q<0.05 (46). We report Hedge’s g for effect size, and 95% confidence intervals of the difference in group means in brackets.
As a means of increasing scientific rigor, we conducted two additional analyses due to growing concerns regarding reproducibility of findings in psychology and related fields (47). We estimated the difference in group means using Bayesian Estimation Supersedes the t-test (BEST) (48) algorithm implemented in the Bayesian First Aid package (49); we report group means, difference of the means, and standard deviations with their 95% credibility intervals, as well as an associated p-value. We also conducted analyses of covariance to control for confounds that may covary with ADHD symptoms (age (linear, quadratic, and cubic effects), sex, intelligence quotient, echoplanar sequence, and relative mean displacement to capture residual effects of head motion). Initial models included all covariates, and non-significant covariates were dropped from the final models. We report partial eta-squared as the effect size for these models. These additional analyses are reported in Tables S3 and S4 of the Online Supplemental Material.
Symptom Analysis
We tested associations between ADHD symptoms and sub-networks that differed among ASD and TDC groups. For rigor, we then conducted follow-up analyses to control for potential confounds (ASD symptoms and residual motion), as well as using an alternative statistical approach (Bayesian). Finally, we had two control analyses, one using a different brain network (vision) and another using a different symptom domain (anxiety), to demonstrate the specificity of our findings. We used the Pearson’s correlation coefficient for initial analysis, and partial correlations to account for variance related to ASD symptoms or for variance related to residual motion (relative mean displacement). We utilized the raw scores from the ADHD Rating Scale, 4th Edition (0-54) and the 20 anxiety items in ASD from the Child and Adolescent Symptom Inventory (0-60) as dimensional measures of ADHD and anxiety symptoms, because both have been validated in pediatric ASD samples (50, 51). We included the ADOS Calibrated Severity Scores (52, 53) in partial correlations, because this study required the use of more than one ADOS module. Bayesian estimation was used again for the Pearson’s correlation to demonstrate robustness of findings across statistical approaches.
A secondary analysis explored whether the relationships between ADHD symptoms and fronto-parietal and salience/ventral attention sub-networks were independent or overlapping. The first step was a Pearson’s correlation between the functional connectivity strength of the salience/ventral attention-B sub-network and the functional connectivity strength between fronto-parietal-A and fronto-parietal-C sub-networks. The second step included a series of linear regressions to determine if salience/ventral attention-B and fronto-parietal-A to fronto-parietal-C explained unique variance in ADHD symptoms. All analyses were calculated using the R statistical package (54).
Results
As seen in Table 1, groups were matched on chronological age, male/female ratio, GCA, and in-scanner head motion.
Group Analyses Reveal Weaker Functional Connectivity in ASD group compared to the TDC group for Fronto-Parietal and Salience/Ventral Attention Networks
The ASD group had significantly weaker functional connectivity than the TDC group within fronto-parietal and within salience ventral-attention sub-networks (see Figure 1). Relative to the TDC group, functional connectivity was weaker for the ASD group for regions within fronto-parietal-C, t(156.35)=3.39, FDR-corrected p-value=0.004, Hedges g=0.53, [0.028, 0.106]. The ASD group had weaker functional connectivity between fronto-parietal-A and fronto-parietal-C compared to the TDC group: t(137.7)=2.78, FDR-corrected p-value=0.023, Hedges g=0.43, [0.009, 0.057]. The ASD group had weaker functional connectivity than the TDC group within the salience/ventral attention sub-networks: salience/ventral attention-A: t(154.24)=3.65, FDR-corrected p-value=0.003, Hedges g=0.573, [0.027, 0.091]. salience/ventral attention-B: t(156.9)=3.98, FDR-corrected p-value=0.002, Hedges g=0.627, [0.034, 0.102]. All other within fronto-parietal had FDR-corrected p-values >0.05 (see Table S5).
Compared to the TDC group, the ASD group had weaker functional connectivity between the fronto-parietal-A and salience/ventral attention-B sub-networks: t(156.59)=2.43, FDR-corrected p-value=0.049, Hedges g=0.382, [0.006, 0.066]. All other group comparisons of functional connectivity among fronto-parietal and salience/ventral attention sub-network were weak effects and non-significant (FDR-corrected p-values > 0.05; see Table S5).
Our two additional analyses to enhance scientific rigor largely supported the primary analyses reported above. The Bayesian analyses replicated all group differences reported above (Table S3). The analysis of covariance replicated all group differences except the finding that the ASD group had weaker functional connectivity between the fronto-parietal-A and fronto-parietal-C sub-networks compared to the TDC group; this effect was weaker and marginally significant after applying FDR correction (Table S4 contains results and covariates that were retained in final models).
Symptom Analyses in ASD Reveal that Fronto-Parietal and Salience/Ventral Attention Differences are Related to ADHD Symptoms, but Not Each Other
For the ASD group, increased symptoms of ADHD were associated with decreased functional connectivity strength within the salience/ventral attention-B sub-network, r(55)=−0.29, p<0.05, and with the functional connectivity between fronto-parietal-A and fronto-parietal-C sub-networks, r(55)=−0.27, p<0.05 (Figure 3a and 3b). The results were largely unaffected when ASD symptoms or residual head motion were entered as covariates in partial correlations, or when a Bayesian correlational approach was used (See Table S6). Correlation of ADHD symptoms with the other three sub-networks were not significant (all r<0.15, p>0.33).
Our secondary analysis within the ASD group revealed weak and non-significant relationships between functional connectivity strength within the salience/ventral attention-B and functional connectivity strength between fronto-parietal-A and fronto-parietal-C, r(75)=−0.05, p=0.66 and Bayesian: r(n=77)=−0.05, p=0.665 [−0.28 0.18]. Furthermore, linear regressions showed that both of these functional connectivity differences in children with ASD explained significant, independent variance in ADHD symptoms, Multiple R2=.156, F(2,54)=5.00, p<0.05 (see Table 2 for details).
Table 2.
Linear Regressions of Fronto-Parietal and Salience-Ventral Attention Sub-Systems Explaining ADHD Symptoms
| Predictor | ADHD Symptoms | |||
|---|---|---|---|---|
| B | SE B | t | R2 | |
| Model 1: | ||||
| FP-A-to-FP-C | −36.61 | 17.63 | −2.08* | 0.073 |
| Model 2: | ||||
| FP-A-to-FP-C | −35.83 | 16.98 | −2.11* | |
| SAL-VA-B | −32.19 | 13.92 | −2.31* | 0.156 |
| B | SE B | t | R2 | |
|---|---|---|---|---|
| Model 1: | ||||
| SAL-VA-B | −32.78 | 14.35 | −2.29* | 0.087 |
| Model 2: | ||||
| SAL-VA-B | −32.19 | 13.92 | −2.31* | |
| FP-A-to-FP-C | −35.83 | 16.98 | −2.11* | 0.156 |
FP-A=fronto-parietal A; FP-C=fronto-parietal-C; SAL-VA-B=salience-ventral attention-B; SE=Standard Error NB: Due to rounding, R2 is slightly less than the summing the individual effect of each predictor
p<0.05
Control Analyses Reveal that Visual Networks do not Differ in Functional Connectivity Between Groups or associate with ADHD Symptoms in ASD, and that Anxiety symptoms is not associated with Functional Connectivity strength in Fronto-Parietal and Salience/Ventral-Attention Networks
Follow-up control analyses revealed no group differences in the visual networks (FDR-corrected p-values>0.05; See Table S5). Furthermore, functional connectivity within visual sub-networks were not correlated with ADHD symptoms. Finally, anxiety symptoms were not correlated with functional connectivity within the two salience-ventral attention sub-networks or the functional connectivity between fronto-parietal sub-networks A and C (all r<0.19, p>0.11).
DISCUSSION
To our knowledge, this study is the first to associate ADHD symptoms in ASD with changes in large-scale, executive brain networks. In a large, single-site sample of children with ASD that have a range of ADHD symptoms, results indicate that both fronto-parietal and salience/ventral attention sub-networks have weaker functional connectivity in children with ASD compared to children with typical development. Even after controlling for core ASD symptoms, functional connectivity in fronto-parietal and salience/ventral attention networks were significantly associated with co-occurring ADHD symptoms, but not with anxiety symptoms. Using visual sub-networks as a control, no group differences were observed in functional connectivity, and visual sub-networks were not significantly correlated with ADHD symptoms. Additional Bayesian analyses for statistical rigor supported the primary findings.
Our findings that the fronto-parietal and salience/ventral attention networks differ in the strength of their functional connectivity in children with ASD relative to typical development are consistent with an accumulating literature on atypical functional connectivity of large-scale executive networks during rest (23, 25, 26, 55) and task conditions (16, 24, 27, 28, 56).
The current study extends these findings in a number of important ways. First, this study extends the relationship of ADHD symptoms and the salience/ventral attention network into the ASD population. In particular, our study showed that both the salience and ventral attention sub-networks had weaker functional connectivity strength in the ASD group compared to controls. This finding converges with evidence that the salience/ventral-attention network plays a role in ADHD symptoms for those without ASD (22), and a critical review of animal literature implicating the network in complex forms of attention (57). Prior research on the salience/ventral attention network in ASD ranged from social and socio-cognitive processes (26, 58–60) to behavioral flexibility (24). However, the present study also expands the scope into the cooccurrence with ADHD symptoms, which was specifically related to the ventral attention sub-network. This finding is also consistent with the Research Domain Criteria initiative from the National Institute of Mental Health that ascribes all of psychopathology to five major dimensions of neurobiology and behavior that cut across diagnostic boundaries (61, 62). Findings from the present study fall in the “Cognitive Systems” Research Domain, and support the idea that differences in cognitive control associate with ADHD symptoms regardless of whether a child has a formal diagnosis of ADHD or not.
Second, this study demonstrates both reduced functional connectivity of the fronto-parietal network in resting state data and a relationship with ADHD symptoms. Our findings converged with prior task-based functional connectivity data showing weaker fronto-parietal network functional connectivity in children with ASD compared to typically developing controls. Furthermore, this diminished functional connectivity was associated with ADHD symptoms (16).
Third, our finding that the salience/ventral attention and the fronto-parietal networks explained unique variance in ADHD symptoms, suggests that that these two networks may not be affected in the same children with ASD and co-occurring ADHD symptoms. An alternative possibility is that these two networks are correlated, but these relationships may be suppressed by a subset of children with ASD whose co-occurring ADHD symptoms are adding “noise” and suppressing the relationship between the networks. Finally, our use of visual sub-networks and anxiety symptoms as negative controls for non-executive networks and co-occurring symptoms is innovative because it demonstrates that the relationship between executive brain networks and ADHD symptoms is not the result of some general feature about functional connectivity or co-occurring symptoms in ASD.
The present study has some limitations. Our sample’s IQ range had a lower bound of 75, a common problem among resting state functional connectivity studies (63); however, the IQ score threshold was scientifically motivated in the present study because the ADHD Rating Scale, 4th Edition has not yet been validated in those with ASD and an intellectual disability (51). At first glance, this study might appear to be inconsistent with our prior publication where we demonstrated an atypical topography of the ventral attention network in ASD (55). However, our prior investigation demonstrated globally weaker connectivity overall prior to examining topography. Thus, our current findings of weaker functional connectivity in salience/ventral attention and fronto-parietal networks align with our prior finding that absolute functional connectivity is weaker in ASD. The present study had a wide age range; however, the ages of participants were not equally distributed across the range which limited our ability to look at age-by-group interactions in functional connectivity. Future longitudinal investigations would be best placed to examine this question. There may be some concern that using the calibration severity score from the ADOS may not be an ideal symptom measure for correlations due to its ordinal, non-continuous nature; however, recent efforts have shown that other dimensional measures like the Social Responsiveness Scale are sensitive to ADHD symptoms in both ADHD samples (64) and ASD samples (2, 65). Thus, we elected to not use a measure that would have knowingly removed variance of interest. Finally, the present study implicates both the fronto-parietal and salience/ventral attention networks at the group-level; future work should seek to identify how one or both networks are associated with ADHD symptoms at the individual level.
The present findings open an avenue of research to further define the neural networks underlying co-occurring ADHD symptoms in ASD. Future research will need to define brain and behavioral profiles related to cognitive control and other processes affected in ADHD (e.g., working memory). Developing neurobiologically and cognitively homogeneous subgroups of children with ASD will allow us to optimize treatments – both pharmacological and behavioral – for specific subgroups of children. Doing so will reduce co-occurring ADHD symptoms and improve long-term outcomes for people with ASD.
Supplementary Material
Figure 2.
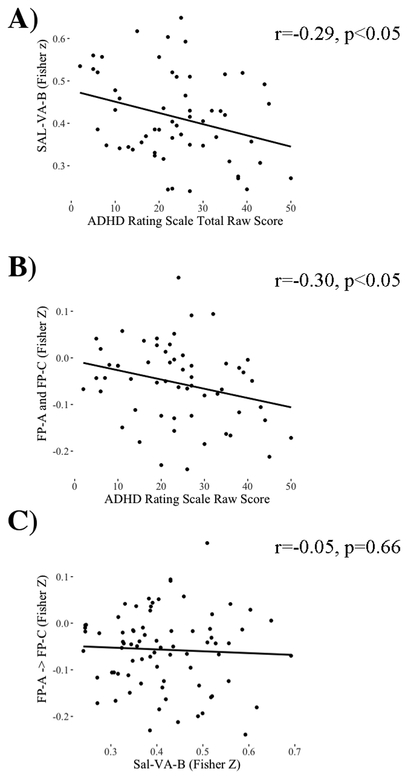
Scatterplots of the ASD group showing (a) the relationship between ADHD symptoms and functional connectivity within the salience/ventral attention network B, (b) the relationship between ADHD symptoms and functional connectivity between fronto-parietal A and frontoparietal C sub-networks (Bottom), (c) the lack of relationship between functional connectivity strength within the salience/ventral attention sub-network B and the functional connectivity strength between the fronto-parietal A and fronto-parietal C sub-networks.
ACKNOWLEDGEMENTS
The research reported here was sponsored by grants from the National Institute of Mental Health (K23MH086111; PI: B.E. Yerys, R21MH092615; PI: B.E. Yerys, RC1MH088791; R.T. Schultz), a New Program Development Award (to B.E. Yerys) through the Philadelphia Foundation, and was supported by the Clinical Translational Core of the Intellectual and Developmental Disabilities Research Center funded by the National Institute of Child and Human Development (5U54HD086984; PIs: M. Robinson & R. Schultz), a grant from the Philadelphia Foundation, a grant from the Pennsylvania Department of Health (SAP #4100042728) to R.T. Schultz, a grant from the Pennsylvania Department of Health (SAP #4100047863) to R.T. Schultz, a grant from Pfizer to R.T. Schultz, and a grant from the Robert Wood Johnson Foundation, #6672 to R.T. Schultz, and a grant to J.D. Herrington from Shire Pharmaceuticals.
Acronyms:
- ADHD
Attention Deficit/Hyperactivity Disorder
- ASD
Autism Spectrum Disorder
- CI
Confidence Intervals
- FDR
False Discovery Rate
- fMRI
functional Magnetic Resonance Imaging
- MRI
Magnetic Resonance Imaging
- TDC
Typically Developing Controls
Footnotes
DISCLOSURES
Dr. Yerys has received research funds from Akili Interactive Labs, La Hoffman-Roche, and served as a consultant for AEVI Genomic Medicine. Dr. Yerys expects to receive additional funding for research or honoraria from National Institute of Mental Health, Akili Interactive Labs Inc., Aevi Genomic Medicine, the American Academy of Child and Adolescent Psychiatry, and Roche Pharmaceutical in the foreseeable future. Dr. Schultz has received research funding from the National Institute of Child and Human Development, the National Institute of Mental Health, National Institute of Environmental Health Sciences, Aevi Genomic Medicine, the Allerton Foundation, Simons Foundation and the NJ Governor’s Council for Medical Research over the last two years. Dr. Schultz expects to receive funding from the National Institute of Child and Human Development, the National Institute of Mental Health, and the Eagles Charitable Foundation in the foreseeable future. Dr. Schultz has served as a consultant for Akili Interactive Labs Inc., Aevi Genomic Medicine, CB Partners, Roche Pharmaceuticals in the past two years. Dr. Schultz has received honoraria from Yale University, Autism Science Foundation. All funding mentioned here is unrelated to the research in the present manuscript. Dr. Schultz also has a patent pending application for a “Biometric sensor device for quantitative phenotyping”, comprising a sensor device that collects audio, video and wearable sensor data of human behavior and human interactions, and software to analyze these data to make predictions, including diagnostic classifications and assessment of quantitative traits. This patent pending application is unrelated to the research reported here. Dr. Herrington has received research funds from Shire Pharmaceuticals and BioStream Technologies, LLC, and served as a consultant for AEVI Genomic Medicine. Dr. Herrington expects to receive additional funding for research or honoraria from National Institute of Mental Health, BioStream Technologies, and Roche Pharmaceutical in the foreseeable future.
None of the remaining authors have biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sikora DM, Vora P, Coury DL, Rosenberg D (2012): Attention-deficit/hyperactivity disorder symptoms, adaptive functioning, and quality of life in children with autism spectrum disorder. Pediatrics. 130 Suppl 2: S91–97. [DOI] [PubMed] [Google Scholar]
- 2.Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L (2009): Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2: 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yerys BE, Bertollo JR, Pandey J, Guy L, Schultz RT (In Press): ADHD symptoms associates with lower adaptive behavior skills in youth with autism J Am Acad Child Adolesc Psychiatry. . [DOI] [PubMed] [Google Scholar]
- 4.RUPP RU of PP (2005): Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 62: 1266–74. [DOI] [PubMed] [Google Scholar]
- 5.Scahill L, McCracken JT, Rockhill C, Shah B, Politte L, Sanders R, et al. (2015): Extended-Release Guanfacine for Hyperactivity in Children With Autism Spectrum Disorder. Am J Psychiatry. 172: 1197–206. [DOI] [PubMed] [Google Scholar]
- 6.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005): Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 57: 1336–46. [DOI] [PubMed] [Google Scholar]
- 7.Pennington BF, Ozonoff S (1996): Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 37: 51–87. [DOI] [PubMed] [Google Scholar]
- 8.Fair DA, Bathula D, Nikolas MA, Nigg JT (2012): Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. PNAS. 109: 6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET (1999): Hypofrontality in Attention Deficit Hyperactivity Disorder During Higher-Order Motor Control: A Study With Functional MRI. AJP. 156: 891–896. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JDE (2005): Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 162: 1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheres A, Oosterlaan J, Swanson J (2003): The Effect of Methylphenidate on Three Forms of Response Inhibition in Boys With AD/HD. Journal of Abnormal Child Psychology. 31: 105–120. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD (1998): Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 95: 14494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Kelly C, Castellanos FX, Leon T, Milham MP, Adler LA (2016): Neural Correlates of Symptom Improvement Following Stimulant Treatment in Adults with Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology. . doi: 10.1089/cap.2015.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon M, Ozonoff S, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS (2009): The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 47: 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. PNAS. 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. (2018): Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex. 28: 3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter CS, Krener P, Chaderjian M, Northcutt C, Wolfe V (1995): Abnormal processing of irrelevant information in attention deficit hyperactivity disorder. Psychiatry Research. 56: 59–70. [DOI] [PubMed] [Google Scholar]
- 22.Tegelbeckers J, Bunzeck N, Duzel E, Bonath B, Flechtner H-H, Krauel K (2015): Altered salience processing in attention deficit hyperactivity disorder: Novelty Processing in ADHD. Human Brain Mapping. 36: 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrant K, Uddin LQ (2016): Atypical developmental of dorsal and ventral attention networks in autism. Dev Sci. 19: 550–563. [DOI] [PubMed] [Google Scholar]
- 24.Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, et al. (2015): Brain State Differentiation and Behavioral Inflexibility in Autism. Cereb Cortex. 25: 4740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elton A, Di Martino A, Hazlett HC, Gao W (2016): Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biological Psychiatry. 80: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. (2013): Salience Network-Based Classification and Prediction of Symptom Severity in Children With Autism. JAMA Psychiatry. 70: 869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, et al. (2009): Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 19: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odriozola P, Uddin LQ, Lynch CJ, Kochalka J, Chen T, Menon V (2016): Insula response and connectivity during social and non-social attention in children with autism. Soc Cogn Affect Neurosci. 11: 433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998): ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. Guilford Press; New York. [Google Scholar]
- 30.Kerns CM, Maddox BB, Kendall PC, Rump K, Berry L, Schultz RT, et al. (2015): Brief measures of anxiety in non-treatment-seeking youth with autism spectrum disorder. Autism. 19: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition, 4th ed. Arlington, VA: American Psychiatric Publishing, Inc. [Google Scholar]
- 32.Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24: 659–85. [DOI] [PubMed] [Google Scholar]
- 33.Gotham K, Risi S, Pickles A, Lord C (2007): The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 37: 613–627. [DOI] [PubMed] [Google Scholar]
- 34.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S (2012): Autism Diagnostic Observation Schedule, second edition (ADOS-2) manual (Part I): Modules 1-4. Torrance, CA: Western Psychological Services. [Google Scholar]
- 35.Gadow KD, Sprafkin J (2010): Child & Adolescent Symptom Inventory - Fourth Edition Revised. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- 36.Elliott CD (2007): Differential Ability Scales-II (DAS-II). San Antonio, TX: Pearson Assessments. [Google Scholar]
- 37.Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. (2017): Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 154: 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. (2012): Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 60: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 64: 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. (2013):Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. NeuroImage. 83: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallquist MN, Hwang K, Luna B (2013): The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 82C: 208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 43.Popescu V, Battaglini M, Hoogstrate WS, Verfaillie SCJ, Sluimer IC, van Schijndel RA, et al. (2012): Optimizing parameter choice for FSL-Brain Extraction Tool (BET) on 3D T1 images in multiple sclerosis. NeuroImage. 61: 1484–1494. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 20: 45–57. [DOI] [PubMed] [Google Scholar]
- 45.Ou Y, Sotiras A, Paragios N, Davatzikos C (2011): DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Medical Image Analysis, Special section on IPMI 2009. 15: 622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate - A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 57: 289–300. [Google Scholar]
- 47.Open Science Collaboration (2015): PSYCHOLOGY. Estimating the reproducibility of psychological science. Science. 349: aac4716. [DOI] [PubMed] [Google Scholar]
- 48.Kruschke JK (2013): Bayesian estimation supersedes the t test. J Exp Psychol Gen. 142: 573–603. [DOI] [PubMed] [Google Scholar]
- 49.Bååth R (2014): Bayesian first aid. . Retrieved from http://www.sumsar.net/blog/2014/01/bayesian-first-aid/.
- 50.Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, et al. (2008): Parent-Rated Anxiety Symptoms in Children with Pervasive Developmental Disorders: Frequency and Association with Core Autism Symptoms and Cognitive Functioning. Journal of Abnormal Child Psychology. 36: 117–128. [DOI] [PubMed] [Google Scholar]
- 51.Yerys BE, Nissley-Tsiopinis J, de Marchena A, Watkins MW, Antezana L, Power TJ, Schultz RT (2017): Evaluation of the ADHD Rating Scale in Youth with Autism. Journal of Autism and Developmental Disorders. 47: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotham K, Pickles A, Lord C (2009): Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 39: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hus V, Lord C (2014): The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. J Autism Dev Disord. . doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team (2014): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/. [Google Scholar]
- 55.Yerys BE, Herrington JD, Satterthwaite TD, Guy L, Schultz RT, Bassett DS (2017): Globally weaker and topologically different: resting-state connectivity in youth with autism. Mol Autism. 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von dem Hagen EAH, Stoyanova RS, Baron-Cohen S, Calder AJ (2012): Reduced functional connectivity within and between “social” resting state networks in Autism Spectrum Conditions. Social cognitive and affective neuroscience. . doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mueller A, Hong DS, Shepard S, Moore T (2017): Linking ADHD to the Neural Circuitry of Attention. Trends in Cognitive Sciences. 21: 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burrows CA, Timpano KR, Uddin LQ (2017): Putative Brain Networks Underlying Repetitive Negative Thinking and Comorbid Internalizing Problems in Autism. Clinical Psychological Science. 5: 522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uddin LQ, Menon V (2009): The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 33: 1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogeveen J, Krug MK, Elliott MV, Solomon M (2018): Insula-Retrosplenial Cortex Overconnectivity Increases Internalizing via Reduced Insight in Autism. Biological Psychiatry. . doi: 10.1016/j.biopsych.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria (2016): Behavioral Assessment Methods for RDoC Constructs: A report by the National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria. Bethesda, MD: National Institute of Mental Health. [Google Scholar]
- 62.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010): Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 167: 748–751. [DOI] [PubMed] [Google Scholar]
- 63.Uddin LQ, Dajani DR, Voorhies W, Bednarz H, Kana RK (2017): Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Translational Psychiatry. 7: e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiersen AM, Constantino JN, Volk HE, Todd RD (2007): Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 48: 464–72. [DOI] [PubMed] [Google Scholar]
- 65.Factor RS, Ryan SM, Farley JP, Ollendick TH, Scarpa A (2017): Does the Presence of Anxiety and ADHD Symptoms Add to Social Impairment in Children with Autism Spectrum Disorder? J Autism Dev Disord. 47: 1122–1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


