Abstract
Background:
Current pharmacologic adherence monitoring for antiretrovirals involves expensive, labor-intensive liquid-chromatography/tandem-mass-spectrometry (LC-MS/MS)-based methods. Antibody-based assays can monitor and support adherence in real-time. We developed a tenofovir (TFV)-based immunoassay and further validated it in a directly-observed-therapy (DOT) study.
Design:
Pharmacologic DOT study of TFV disoproxil fumarate (TDF)/emtricitabine (FTC) administered to HIV-noninfected volunteers
Methods:
The TARGET study provided directly-observed TDF 300mg/FTC 200mg 7 (high adherence), 4 (moderate) and 2 doses/week (low) to 30 volunteers (10/group) in Thailand, collecting a total of 637 urine samples over 6-weeks of administration and during wash-out. ELISA measured urine TFV levels by the immunoassay and LC-MS/MS-based concentrations served as the gold-standard. A mixed-effects regression model evaluated cut-offs for a point-of-care (POC) assay. Performance characteristics of the immunoassay were compared to LC-MS/MS at a chosen cut-off.
Results:
Median TFV levels were 12,000ng/mL by the immunoassay 1-day after dosing; 5000ng/mL 2-days after dosing; 1500ng/mL 3-days after dosing and below the lower-limit-of-quantification (LLOQ) thereafter (≥4 days). An immunoassay cut-off of 1500ng/mL accurately classified 98% of patients who took a dose 24 hours ago as adherent. The specificity and sensitivity of the immunoassay compared to LC-MS/MS at the 1500ng/mL cut-off were 99% and 94%; the correlation between TFV levels by the two assays was high (0.92, p<0.00001).
Conclusions:
We have developed a novel TFV immunoassay that is highly specific, sensitive and correlates strongly with LC-MS/MS measurements in a large DOT study. Adherence benchmarks from this DOT study will guide the development of a low-cost rapid POC test for PrEP and ART adherence monitoring and interventions.
Keywords: Antiretroviral treatment, PrEP, adherence, tenofovir, immunoassay, antibody, real-time, point-of-care, urine, test characteristics
INTRODUCTION
Given the limitations of self-reported adherence, pharmacologic adherence measures were critical to interpreting the results of the pre-exposure prophylaxis (PrEP) trials.1 Objective metrics of adherence are therefore being incorporated into PrEP implementation and roll-out programs. Furthermore, in the context of antiretroviral treatment (ART), there is increasing interest in objective adherence monitoring, if it can be performed quickly and economically, to avert virologic resistance and the need for second or third-line regimens.2 A major limitation of currently available pharmacologic metrics in plasma,3 hair,4,5 peripheral blood mononuclear cells (PBMCs),6 dried blood spots (DBS)7 or urine,8,9 however, is that they all require liquid chromatography/tandem mass spectrometry (LC-MS/MS), which is expensive and labor intensive. A low-cost easy-to-perform point-of-care (POC) monitoring tool to quantify tenofovir (TFV) levels would allow for real-time assessment of both PrEP and TFV-based ART adherence, allowing immediate intervention.10
Antibody-based tests for drug detection or quantification, when packaged into lateral flow immunoassays (LFA), are easily implemented by non-trained healthcare personnel at the POC. We previously reported on the development of a specific and sensitive antibody-based (immunoassay) for TFV that was tested in a small sample of volunteers provided daily TDF/FTC for 7 days.11 Appropriate cut-offs for pharmacologic measures are traditionally established in directly-observed therapy (DOT) studies where drug is administered to healthy volunteers to simulate different patterns of adherence. DOT studies have been conducted for all the other TFV-based adherence metrics, specifically for TFV levels in plasma3 and hair,4 and TFV-diphosphate (TFV-DP) levels in PBMCs3 and DBS,7 allowing other studies to interpret adherence from these metrics.12–16 Shorter-term measures (in plasma, urine) qualify adherence as yes/no at a certain cut-off17–20 whereas longer-term measures (in hair,4 DBS7) can provide adherence benchmarks to patterns of drug-taking.12,16 The objective of this study was to leverage a large, completed DOT study conducted among HIV-noninfected volunteers administered TDF/FTC under different dosing conditions to determine an appropriate interpretative cut-off for a urine-based assay for the first time, further advancing a POC adherence assay. Once deployed, a low-cost real-time adherence monitoring test will be a significant breakthrough for both HIV treatment and prevention monitoring worldwide.
METHODS:
Study population:
This study leverages samples from TARGET, a DOT randomized, open-label, clinical pharmacokinetic study of TDF/FTC in Thailand.21 In TARGET, healthy participants were randomized (1:1:1) to one of three groups (10 participants each, total n=30) to receive directly-observed doses of TDF 300mg/FTC 200mg for 6 weeks: Participants in Group 1 received TDF/FTC once daily (“high adherence”); Group 2 received TDF/FTC 4 times/week (“moderate adherence”); and Group 3 received TDF/FTC 2 times/week (“low adherence”). Participants underwent direct observation of dosing Monday through Friday; drug ingestion on weekends was monitored by video/picture calls. Urine samples were collected and stored during 6 weeks of treatment administration and over 4 weeks of wash-out.
The study was approved by Ethics Committees at the Institute for the Development of Human Research Protections at the Medical Sciences Department, Thai Ministry of Public Health; Sanpatong Hospital; and the University of Washington. The study was registered with ClinicalTrials.gov (#NCT0301260).
Laboratory methods:
Urine samples collected in TARGET were aliquoted for measurement by both LC-MS/MS and the immunoassay. Since TFV concentrates in urine,22,23 and to compare TFV levels with those in the literature,8 we diluted the urine samples 1:1000 prior to analysis. For the LC-MS/MS-based method, TFV was separated via reverse-phase high-performance liquid chromatography (LC) and quantified by tandem-mass-spectrometry (MS/MS) using electrospray positive ionization in multiple reaction monitoring mode (TFV, 287.9/175.9 (Q1/Q3)). The lower limit of quantification (LLOQ) of the LC-MS/MS-based assay was 500 nanograms (ng)/milliliter (mL). For the ELISA-based immunoassay, working solutions of TFV of known concentrations were prepared. Calibrators or different concentrations of TFV were incubated on a microtiter plate with the hapten to generate a dose response curve. An ELISA plate reader extrapolated the concentration of TFV in the unknown specimen based on the calibration curve. The LLOQ for the ELISA-based immunoassay was 1,000 ng/mL.
Statistical analysis:
To predict probabilities of being below different cut-offs of urine TFV levels for the POC assay, a mixed-effects interval regression model was used with log urine-immunoassay concentration as the dependent variable and days since the last dose as the independent variable. Our analysis was restricted to spot urine samples obtained after one week of administration, to simulate urine collection at a clinic visit after TDF/FTC-based PrEP or ART has been started. Since food effects on TDF pharmacokinetics are minimal, food intake was not considered in the models.24 The probabilities of being below a given cut-off at any time since the last dose were calculated from the model using the estimated mean, person-to-person variation, and residual variation. Based on participant feedback from prior studies that poor specificity tests were distressing,25,26 we focused on finding a cut-off with high specificity for dosing within 24 hours that still permitted adequate sensitivity for non-adherence. Because any dichotomization of time since last dose would gloss over some important distinctions, and because there were repeated measurements on the same individuals, we did not examine a simple ROC curve.
Once we determined an appropriate cut-off, we calculated the sensitivity and specificity of the immunoassay compared to LC-MS/MS by cross-tabulating TFV levels above this cut-off vs below this cut-off by the two different assays. We also calculated the Spearman correlation between TFV levels generated by the two assays, using results from all urine samples in TARGET and then restricting the calculation to urine samples with detectable drug by both assays. Finally, agreement between urine TFV levels positive both by the immunoassay and LC-MS/MS was calculated using Bland-Altman methods.27
RESULTS:
Study sample and TFV concentrations in urine after dosing:
The total number of urine samples collected in TARGET among 30 participants was 637, averaging 21 samples per participant. All 637 samples were split and TFV concentrations in each were quantified by the ELISA-immunoassay and via LC-MS/MS.
The number of participants providing urine samples 1, 2, 3, 4, 5, 6 and 7 days after last dose was 30, 58, 28, 55, 28, 22 and 28, respectively. Among participants in all three adherence groups, median TFV levels in urine by the immunoassay were 12,000 ng/mL (IQR 7500–25,000) one day after dosing; 5000 ng/mL (IQR 2500–8000) two days after dosing; 1500 ng/mL (IQR 500–2750) three days after dosing and below the immunoassay’s LLOQ thereafter (≥4 days).
Modeling potential interpretive cut-offs for the POC immunoassay
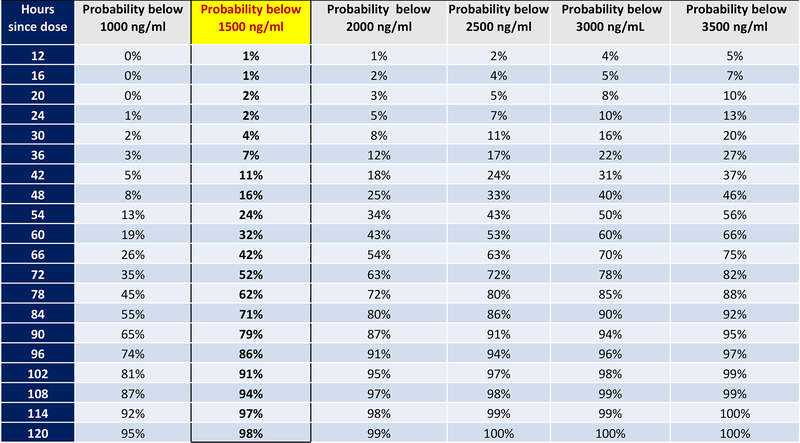
Figure 1 shows the estimated probability of the urine TFV level via the immunoassay to be below an array of different candidate cut-off values based on hours since the last witnessed TDF/FTC dose. Based on these probabilities, we chose 1500ng/ml as the cut-off for the POC assay in order to optimize specificity (i.e. to avoid incorrectly classifying individuals who had taken a dose within 24 hours), while also allowing high sensitivity for non-adherence at 96 hours. Specifically, 86% of individuals who took their last dose exactly 96 hours (4 days) ago would be correctly classified as “nonadherent”, with only 2% of individuals who dosed exactly 24 hours ago misclassified as nonadherent.
Figure 1:
Probability of urine TFV level by immunoassay being below different cut-offs based on hours since last witnessed dose in TARGET DOT study
Performance characteristics at the determined cut-off value:
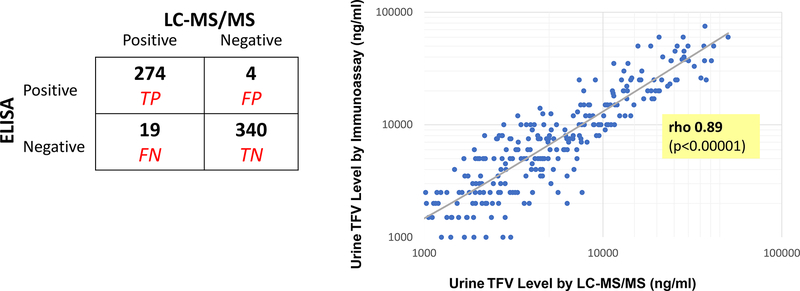
As shown in Figure 2, among the 344 TFV-negative samples by LC-MS/MS (≤1500ng/mL), 340 were also negative by the ELISA immunoassay, indicating 99% specificity of the immunoassay at this cut-off. Of the 293 TFV-positive samples by LC-MS/MS (>1500ng/ml), 274 were also positive by the immunoassay, indicating 94% sensitivity at this cut-off.
Figure 2:
Performance Characteristics of the Urine Immunoassay
Figure 2a: 2 × 2 table of LC-MS/MS vs immunoassay TFV levels (based on cut-off of 1500ng/mL) in urine samples collected in TARGET (n=637)
LC-MS/MS gold standard. TP = True positive; FP = false positive; FN= false negative; TN = true negative
Figure 2b: Correlation of urine immunoassay TFV levels with LC-MS/MS levels in TARGET urine samples with drug detectable via both assays (n=274)
The correlation between TFV levels generated by the two assays, using results from all 637 urine samples, was 0.92 (p <0.00001). Restricting the analysis only to samples that had detectable drug in both assays (n=274), the correlation was 0.89 (p<0.00001). The Bland-Altman analysis of the average relative difference between log-transformed values in samples positive by both assays suggests that 95% of immunoassay values would fall within 70% below and 98% above the LC-MS/MS value.27
DISCUSSION:
This study significantly advances an antibody-based test for TFV quantification by providing the appropriate adherence cut-off for the lateral flow immunoassay, which will allow for point-of-care testing. The results from this study will allow for packaging and deployment of this low-cost real-time adherence monitoring tool in the next few months, significantly advancing HIV treatment and prevention monitoring worldwide. In this study, leveraging a large DOT study where urine samples were collected from HIV-noninfected volunteers administered TDF/FTC under DOT conditions at 2, 4 and 7 doses a week, we determined an appropriate cut-off for the POC urine immunoassay (<1500ng/ml) and showed that our immunoassay had excellent performance characteristics at this cut-off (99% specificity, 94% sensitivity) when compared to LC-MS/MS.
Although other studies have employed drug level monitoring to trigger adherence interventions in PrEP9,28,29 and ART,30 no truly POC metric of adherence has yet been developed or deployed. Qualitative data, however, can inform how such a test should be designed in terms of its ability to classify patients as adherent or nonadherent. In the VOICE PrEP trial, at least 50% of women on active drug had undetectable TFV in all plasma tested,25 but over-reported adherence.31 When participants were provided their plasma TFV level data retrospectively, qualitative interviews revealed that the most important feature of a real-time test would be its specificity, e.g. minimizing the chance that one would be told they were non-adherent when taking the drug.25 Qualitative work in the dapivirine ring trial (MTN-025/HOPE) verified that accuracy of a real-time adherence test was of utmost importance and test scores that showed low adherence in the face of self-reported consistent use were particularly upsetting to participants.26 We therefore chose a cut-off for our TFV-immunoassay that would optimize specificity. Our modeling indicated that the risk of significant misclassification was low at a TFV concentration of 1500 ng/mL by the immunoassay. A patient who has taken a TDF/FTC dose 24 hours ago has a 98% probability of the test being positive at this cut-off and the probability of the test remaining positive if the last dose was taken 4 days ago is low (14%). Our group expects to initially package this POC urine assay into a rapid strip test using a 1500 ng/mL cut-off within the next few months. This test will be low cost (<$2 per test) and easy to implement in the field by non-trained personnel.
At this cut-off, our antibody-based TFV immunoassay was highly specific (99%) and sensitive (94%) compared to LC-MS/MS. The immunoassay estimates TFV levels in urine that correlate strongly with those measured via the gold standard of LC-MS/MS (r=0.92) in 637 urine samples from the TARGET study.21 To ensure that the correlation calculation would not be inflated by inclusion of specimens without drug, we repeated the correlation analysis in the subset of samples where drug was detected in both assays (n=274) and it remained high (r=0.89). This antibody-based test is therefore highly suitable for packaging into a POC assay.
Urine and plasma levels of TFV are short-term metrics of adherence (half life in plasma ~17 hours32) and the limitations of any short-term metric is the susceptibility to “white coat adherence,” where adherence improves transiently before a visit.33 Despite theoretical concern, this phenomenon has not yet been observed in PrEP. Indeed, plasma levels of TFV/FTC served as the primary adherence metric in every one of the placebo-controlled trials of PrEP.6,17,19,20,34–37 Our group found evidence against substantial white-coat adherence when examining a combination of hair and plasma adherence metrics in the VOICE trial.38 Nonetheless, combining short and long-term metrics of adherence (such as TFV levels in urine and hair) in studies may help unravel patterns of adherence. Moreover, unannounced urine assays can circumvent white-coat patterns. Finally, although this assay is expected to measure adherence in the context of tenofovir alafenamide (TAF), since the same metabolite (TFV) concentrates in the urine,8,23 further study is needed before determining appropriate cut-offs for this immunoassay with TAF.
In conclusion, we have further validated and determined an appropriate cut-off for a POC TFV-based immunoassay in urine. A TFV urine-based immunoassay will be able to monitor adherence both to PrEP and to ART. A POC metric to determine adherence in real-time has the potential to both motivate adherence by providing immediate adherence feedback to patients16,25,39–49 and trigger rapid intervention to improve outcomes.10,11,28 Further study of this tool to assess its impact on optimizing the effectiveness of HIV treatment and prevention in diverse populations is warranted.
AKNOWLEDGEMENTS:
We wish to thank the participants of the TARGET study and their families. Funding for this work and the development of the antibody at Alere Rapid Diagnostics was provided by the National Institute of Allergy and Infectious Diseases/ National Institutes of Health (NIAID/NIH) R01AI143340 (P.I. Gandhi). Three authors (WCR, GW, MV) as indicated on the title page are from Alere Rapid Diagnostics company. Further funding from this work came from NIAID/NIH 2RO1AI098472 (P.I. Gandhi); R21AI127200 and R01AI136648 (P.I. Drain). M.A.S. was supported by T32AI060530 (P.I. Havlir).
Conflicts of Interest and Sources of Funding: Dr. Monica Gandhi is currently receiving two grants from the NIH supporting this work, including the development of the antibody at Alere Rapid Diagnostics (R01AI143340 and 2RO1AI098472. Dr. Darin is receiving two grants from the NIH that helped support this work (R21AI127200 and R01AI136648). Mr. Vincent, Dr. Wang and Mr. Rodrigues are all employed by Alere Rapid Diagnostics. For the remaining authors none were declared.
REFERENCES:
- 1.van der Straten A, Brown ER, Marrazzo JM, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19(1):20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimulwo MJ, Okendo J, Aman RA, et al. Plasma nevirapine concentrations predict virological and adherence failure in Kenyan HIV-1 infected patients with extensive antiretroviral treatment exposure. PLoS One. 2017;12(2):e0172960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrix CW, Andrade A, Bumpus NN, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One. 2014;9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen EP, Thompson CG, Bokhart MT, et al. Analysis of Antiretrovirals in Single Hair Strands for Evaluation of Drug Adherence with Infrared-Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging. Anal Chem. 2016;88(2):1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England Journal of Medicine. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig HC, Mounzer K, Daughtridge GW, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017;18(6):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for Pre-exposure Prophylaxis to Prevent HIV-1 Acquisition in Young Adult Men Who Have Sex With Men and Transgender Women of Color Using a Urine Adherence Assay. J Acquir Immune Defic Syndr. 2018;79(2):173–178. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PL. What Can Urine Tell Us About Medication Adherence?. EClinical Medicine (Published by The Lancet) August-September 2018; Volumes 2–3; https://doiorg/101016/jeclinm201809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi M, Bacchetti P, Rodriguez W, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinical Medicine (Published by The Lancet) 2018; 10.1016/j.eclinm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi M, Glidden DV, Mayer K, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi M, Murnane PM, Bacchetti P, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS. 2017;31(16):2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koss CA, Bacchetti P, Hillier SL, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017;March 02. doi: 10.1089/aid.2016.0202. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis. 2018;66(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. A randomized clinical pharmacokinetic trial of Tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis. 2017;17(1):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TRUVADA® (emtricitabine and tenofovir disoproxil fumarate) tablets package insert; Approved by U.S. Food and Drug Administration 2004; Found at https://www.gilead.com/~/media/files/pdfs/medicines/hiv/truvada/truvada_pi.pdf (Accessed November 15, 2018).
- 23.Custodio JM, Fordyce M, Garner W, et al. Pharmacokinetics and Safety of Tenofovir Alafenamide in HIV-Uninfected Subjects with Severe Renal Impairment. Antimicrob Agents Chemother. 2016;60(9):5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) full prescribing information (package insert). 2004. Found at https://www.gilead.com/~/media/files/pdfs/medicines/hiv/truvada/truvada_pi.pdf (Accessed November 15, 2018).
- 25.van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Straten A, Katz A, Balan I, et al. A qualitative evaluation of women’s experience receiving drug feedback in MTN-025/HOPE - an HIV prevention open-label trial of the dapivirine vaginal ring. MTN-025/HOPE study group.. AIDS 2018 conference, Amsterdam, The Netherlands, 23–26 July, 2018, Abstract THPEC334 2018. [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 28.Landovitz RJ, Beymer M, Kofron R, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celum CL, Delany-Moretlwe S, Hosek S, et al. Risk Behavior, Perception and Reasons for PrEP among Young African Women in HPTN 082. Abstract #1049. 25th Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018; Boston, MA. [Google Scholar]
- 30.Castillo-Mancilla JR, Haberer JE. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep. 2018;15(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mensch BS, Brown ER, Liu K, et al. Reporting of Adherence in the VOICE Trial: Did Disclosure of Product Nonuse Increase at the Termination Visit? AIDS Behav. 2016;20(11):2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45(10):2733–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV clinical trials. 2008;9(4):238–246. [DOI] [PubMed] [Google Scholar]
- 34.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. [DOI] [PubMed] [Google Scholar]
- 35.Musinguzi N, Muganzi CD, Boum Y 2nd, et al. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. AIDS. 2016;30(7):1121–1129. [DOI] [PubMed] [Google Scholar]
- 36.Van Damme L, Corneli A. Antiretroviral preexposure prophylaxis for HIV prevention. N Engl J Med. 2013;368(1):84. [DOI] [PubMed] [Google Scholar]
- 37.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 38.Koss CA, Bacchetti P, Hillier SL, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musara P, Montgomery ET, Mgodi NM, et al. How Presentation of Drug Detection Results Changed Reports of Product Adherence in South Africa, Uganda and Zimbabwe. AIDS Behav. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312(12):1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using Artificial Intelligence to Reduce the Risk of Nonadherence in Patients on Anticoagulation Therapy. Stroke. 2017;48(5):1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain EE, Shafner L, Walling DP, et al. Use of a Novel Artificial Intelligence Platform on Mobile Devices to Assess Dosing Compliance in a Phase 2 Clinical Trial in Subjects With Schizophrenia. JMIR Mhealth Uhealth. 2017;5(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke LE, Zheng Y, Ma Q, et al. The SMARTER pilot study: Testing feasibility of real-time feedback for dietary self-monitoring. Prev Med Rep. 2017;6:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerby TJ, Asche SE, Maciosek MV, O’Connor PJ, Sperl-Hillen JM, Margolis KL. Adherence to blood pressure telemonitoring in a cluster-randomized clinical trial. J Clin Hypertens (Greenwich). 2012;14(10):668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J Med Internet Res. 2017;19(7):e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lind M, Polonsky W, Hirsch IB, et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379–387. [DOI] [PubMed] [Google Scholar]
- 48.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371–378. [DOI] [PubMed] [Google Scholar]
- 49.Givertz MM, Stevenson LW, Costanzo MR, et al. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2017;70(15):1875–1886. [DOI] [PubMed] [Google Scholar]