Abstract
Recent whole exome sequencing studies in humans have provided novel insight into the importance of the ephrinB2-EphB4-RASA1 signaling axis in cerebrovascular development, corroborating and extending previous work in model systems. Here, we aim to review the human cerebrovascular phenotypes associated with ephrinB2-EphB4-RASA1 mutations, including those recently discovered in Vein of Galen malformation – the most common and severe brain arteriovenous malformation in neonates. We will also discuss emerging paradigms of the molecular and cellular pathophysiology of disease-causing ephrinB2-EphB4-RASA1 mutations, including the potential role of somatic mosaicism. These observations have potential diagnostic and therapeutic implications for patients with rare congenital cerebrovascular diseases and their families.
Keywords: Ephrin signaling, cerebrovascular disorders, vasculogenesis, arterio-venous malformation, Vein of Galen malformation, neurosurgery
An Emerging Role of EphrinB2-EphB4-RASA1 Signaling Axis in Human Congenital Cerebrovascular Disorders
Normal vascular development includes de novo blood vessel formation from endothelial cells (ECs) (vasculogenesis) [1, 2], expansion of the network through vessel sprouting, splitting, and remodeling (angiogenesis) [3], and differentiation of these vessels into arteries, veins, and intervening capillaries (arterio-venous (A-V) specification) [4]. Studies in animal models have elucidated that these separate but overlapping mechanisms of vascular development are driven by a highly-regulated genetic program that involves the expression and function of multiple key signaling pathways (e.g., ephrin-Eph, Hedgehog, VEGF, TGF- β, Wnt, and Notch) and transcription factors (e.g., HEY and HES, SOX, and Forkhead factors) [4]. Among these genetic factors, animal models have demonstrated a critical role of ephrin-Eph signaling [2, 5-8].
Eph receptor tyrosine kinases (RTK) (EphA1 - EphA8, EphA10, EphB1 - EphB4, and EphB6) and their membrane tethered ligands, the ephrins (ephrinA1 - ephrinA5 and ephrinB1 - ephrinB3), play an important role in many developmental processes during embryogenesis, including axon guidance, tissue boundary formation, bone development, and vascular and lymphatic development [9, 10]. Ephrin-Eph signaling has a dizzying array of signaling modes and regulatory mechanisms (reviewed in [10, 11]), including forward, reverse, parallel, and anti-parallel signaling (see Glossary). Additionally, the strength of ephrin-Eph signaling through any one particular mode can be tuned by the abundance of the specific ephrin ligand cluster involved [12, 13], allowing for the dynamic regulation of multiple distinct molecular and cellular processes with a high precision.
Despite the high complexity of ephrin-Eph signaling, the development of the vasculature appears to be highly dependent on a specific ephrin ligand-receptor pair: ephrinB2 and EphB4 [10]. While mouse and zebrafish models have provided invaluable insights into the role of these ephrin-Eph signaling molecules, recent work in human patients employing whole exome sequencing (WES) techniques and unbiased genomic analytical strategies has corroborated and extended these findings, providing novel insights. Collectively, these efforts have identified mutations in EFNB2, EPHB4, and RASA1 in both sporadic and Mendelian congenital cerebrovascular disorders (Box 1), including capillary malformation-arteriovenous malformation (CM-AVM) and Vein of Galen malformation (VOGM), indicating a conserved role of the signaling axis through vertebrates. Importantly, CM-AVM and VOGM are traditionally thought to be two distinct diseases with overlapped phenotypes, while genetic findings in human patients suggest a shared genetic mechanism underlying these two disorders, even though more comprehensive phenotyping is required to fully answer this question. Findings in human genetics have also provided candidates for genetic testing, potential targetable nodes for therapeutic intervention, and insights into the molecular mechanisms of other more common forms of cerebrovascular disorders (see Clinician’s Corner). In this article, we will review the role of ephrinB2-EphB4-RASA1 signaling in vascular development (Figure 1, Key Figure), using data gleaned from animal models (Table 1), and more recently, WES studies in human patients with congenital cerebrovascular disease (Table 2).
Box 1:
Cerebrovascular Malformations
Cerebrovascular malformations are abnormal formations of blood vessels in the brain. They are classically subdivided into five common types: pial arteriovenous malformations (AVMs), dural arteriovenous fistulas (DAVFs), cerebral cavernous malformations (CCMs), venous malformations, and capillary telangiectasias. AVMs and DAVFs are considered to be fast-flow lesions. They are characterized by direct connections between arteries and veins within the brain and the dura mater respectively, which cause high-pressure arterial blood to flow quickly into the venous circulation without passing through an intervening capillary bed. CCMs are groups of thin-walled capillaries. Unlike AVMs and DAVFs, they are slow-flow low-pressure malformations and are predominantly associated with mutations in the genes CCM1 (KRIT1), CCM2, and CCM3 (PDCD10) [68]. Venous malformations (sometimes also called developmental venous anomalies or venous angiomas) are composed of one or more atypical enlarged veins. Finally, telangiectasias are enlarged capillaries that often occur in the brainstem. Cerebral arteriovenous malformations can occur sporadically, but they can also be observed in the context of inherited syndromes with known genetic causes, such as Capillary-Malformation Arteriovenous Malformation (CM-AVM) types 1 and 2 [31, 40] and Hereditary Hemorrhagic Telangiectasia (HHT) types 1 and 2 [69].
CM-AVM1 is classically caused by mutations in the RASA1 gene and is transmitted in an autosomal dominant fashion [40, 48]. The disorder is characterized by capillary malformations located on the head, neck, trunk, and extremities along with AVMs in the skin, muscle, and brain [30, 40, 52, 58]. CM-AVM2 is associated with mutations in EPHB4 [31]. CM-AVM2 presents with similar clinical features to CM-AVM1 including multi-focal capillary malformations but patients with CM-AVM2 display more frequent perioral and thoracic telangiectasia, a lower frequency of fast-flow lesions, and a lower frequency of central nervous system AVMs, though Vein of Galen Malformation, the most common and severe type of AVM in neonates, has been diagnosed in patients with both CM-AVM1 and CM-AVM2 [16, 30, 31, 41, 52].
HHT is an autosomal dominant disorder characterized by recurrent epistaxis, characteristic telangiectasias of the oropharynx, face, and hands, and multiple focal AVMs [69]. HHT is associated with a 10% prevalence of brain AVMs, but AVMs can also be found in the lungs, liver, gastrointestinal tract, and spinal cord. A number of subtypes of HHT have been described, each associated with a particular underlying genetic defect. Implicated genes include ENG (HHT1) [70-72]. ACVRL1/ALK1 (HHT2) [72], SMAD4 (juvenile polyposis-HHT syndrome) [73], and BMP9 (HHT5) [74].
CLINICIAN’S CORNER.
Damaging mutations in EFNB2, EPHB4, and RASA1 have been identified in multiple congenital cerebrovascular disorders, including capillary malformation-arteriovenous malformation (CM-AVM) and Vein of Galen malformation (VOGM).
The majority of the identified mutations in CM-AVM and VOGM are transmitted with incomplete penetrance and variable expressivity. Parents with mild cutaneous vascular lesions, which can be easily overlooked, are likely at higher risk for having children with a high-flow vascular lesion, including VOGM. Careful and thorough physical examination and prenatal genetic screen in pregnant women with family history of cutaneous vascular lesions and/or other vascular malformations could help the early diagnosis and treatment design of the disease.
CM-AVM and VOGM have overlapping genetic underpinnings and phenotypes, with VOGM infrequently reported as an associated AVM in CM-AVM patients, and capillary malformations (CMs) often identified in patients with VOGM. Detailed genotype-phenotype studies, especially for VOGM, with emphasis on skin findings, associated heart defects, neuropsychiatric phenotypes, and radiological anatomy of cerebrovascular lesions are needed to gain more knowledge of the phenotypical overlap of these cerebrovascular disorders, which will in turn aid the genetic study in understanding their shared mechanism.
Mutated ephrinB2-EphB4-RASA1 expression may cause excessive PI3K/mTORC1 pathway activity. Targeting PI3K-TORC1 signaling with clinically-available drugs like mTOR inhibitors might be a viable therapeutic approach for cerebrovascular diseases associated with ephrinB2-EphB4-RASA1 mutations, though much work is required to test this hypothesis.
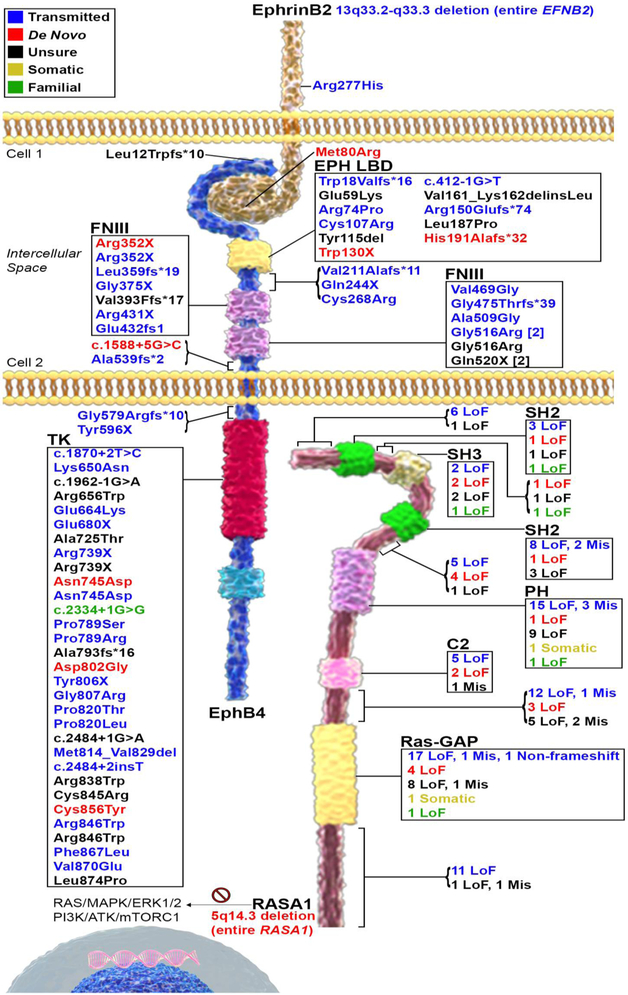
Figure 1. An emerging paradigm of ephrinB2-EphB4-RASA1 mutations in human cerebrovascular disorders.
Schematic representation of the ephrinB2-EphB4-RASA1 signaling axis in vascular development. The membrane tethered ligand, ephrinB2, binds to its receptor, EphB4, which interacts with its downstream effector and binding partner, RASA1. Mutations disrupting the signaling axis potentially lead to excessive PI3K/mTORC1 pathway activity, causing dysregulation of downstream genes. EphrinB2-EphB4-RASA1 mutations identified in human patients with vascular disorders are mapped to their protein domains. Given the space limit, only mutation type distribution is provided for RASA1. Please refer to Table 2 for detailed variant information. Mixed: a variant is reported in more than 1 inheritance models; TK = Tyrosine kinase domain.
Table 1.
Animal Models of EphrinB2-EphB4-RASA1 Signaling Axis
| EFNB2 Animal Models | ||||
|---|---|---|---|---|
| Animal Model | Genotype | Phenotype | Description | Ref |
| Mouse | ephrinB2tlacZ | Defects in angiogenesis by both arteries and veins in the capillary networks for the head and yolk sac as well as in myocardial trabeculation | Vasculogenesis occurred normally between E8.5 and E9.0; apparent pronounced disorganization at E10; defect in yolk sac angiogenesis by E9; absence of internal carotid arterial branches; defective angiogenesis of venous capillaries in the head; capillary ingrowth into the neural tube failed to occur | [2] |
| Mouse | ephrinB2ΔC/ΔC(ephrinB2-ΔC-HA fusion protein) | Able to restore guidance of migrating cranial neural crest cells, but have defects in vasculogenesis and angiogenesis and midgestation lethality | Lethal at midgestation (E10.5-E11) with growth retardation and inflation of the pericard; normal formation of branchial arches; defects in vascular morphogenesis | [17] |
| Mouse | ephrinB2lacZ/loxp;Tie2-Cre+(endothelium and endocardium specific deletion of ephrinB2) | Angiogenic remodeling defect identical to ephrinB2 global knockout mice(Efnb2tml/tml) | Growth retardation; swollen but still beating heart with little blood flow; disorganized and less developed vasculature compared to WT; arrest in development of arteries and veins at the primary plexus stage in yolk sac; failure in anterior cardinal vein (ACV) assembly and remodeling; initial head plexus forms but remains in disorganized primitive stage | [20] |
| Mouse | CAGp-ephrin-B2 Tg (gloal overexpression of ephrinB2) | Abnormal segmental arrangement of intersomitic vessels; die of acute aortic dissecting aneurysms at neonatal stages | Thin vascular wall in the ascending aorta and aortic arch; ECs have a flat morphology with a budlike structure; SMCs and elastic bands are missing, while microvessels are seen in these spaces; abnormal vasculature in ascending and arch portion of the aorta | [25] |
| Mouse | Tie-2p-ephrin-B2 Tg (vascular ECs specific overexpression of ephrinB2) | Cranial hemorrhages with incomplete penetrance | Intracerebral bleeding | [25] |
| Mouse | ephrinB2ΔC-βgal/ΔC-βgal (ephrinB2 C-terminal truncation and βgal fusion protein) | Severe hypospadias and incomplete midline fusion of the primitive cloaca with incomplete penetrance (heterozygotes) Failure in cloacal septation with complete penetrance (homozygotes) | Severe anorectal malformations with an absence of the terminal-most hindgut and formation of a fistula that aberrantly connects the intestines to the urethra at the base of the bladder. | [21] |
| Grossly normal with no early vascular defects, but die within the first day of birth due to cardiac defects | Normal chamber formation and vascular connection; thickened cardiac valves; disrupted pathfinding of anterior commissure axons; defective maturation of cardiac valve leaflets. | [18] | ||
| Mouse | ephrinB2T/T (ephrinB2 with C-terminal truncation) | Embryonic lethal with a failure in cardiovascular development | Visibly abnormal, appearing growth-retarded and necrotic, had enlarged pericardia, displayed abnormal heart and vascular structures | [18] |
| Mouse | Efnb2ΔPC/vSMC (mural cell specific deletion of ephrinB2) | Perinatal lethality; vascular defects in skin, lung, gastrointestinal tract, and kidney glomeruli; and abnormal migration of smooth muscle cells to lymphatic capillaries | Develop to term but die shortly after birth due to respiratory failure; edema and extensive hemorrhaging in the skin; pale intestines and blood filling mesenteric lymphatics; hemorrhaging in the lung; comma- and S-shaped bodies developed normally, but the more mature glomerular tufts were poorly organized | [24] |
| Mouse | Efnb2GFP/GFP; Tg (ECs specific expression of ephrinB2 in the background of Efnb2 null) | Similar growth retardation, and heart morphogenesis defects as observed in Efnb2 null mice, but the early lethality associated with Efnb2 null mutation is partially rescued | Lethality earlier than E9.5 likely due to mixed genetic background of mice used in this study; partial rescue of the lethal phenotype of the Ephb2 null mice by E10.5; bleeding in the pericardial cavity; intersomitic vessel network appeared fused dorsally and poorly branched | [23] |
| Mouse | Efnb2CR/GFP; Tie2-CreTg/+ (vascular ECs specific expression of ephrinB2 in the background of Efnb2 null) | Perinatal lethality; normal angiogenesis; exhibit tracheoesophageal and urorectal fistulae as well as an incorrectly positioned thymus, while other bilateral structures including lungs, vena cava, and clavicles appear to be the same as controls. | Overtly normal at E9.5 without signs of angiogenic defects; normal remodeling of the primitive vascular plexus into hierarchically branched vessels at E9.5; survived to birth but died perinatally; no defects in branchial arches morphogenesis or cranial or trunk neural crest development | [22] |
| EPHB4 Animal Models | ||||
|---|---|---|---|---|
| Animal Model | Genotype | Phenotype | Description | Ref |
| Mouse | EphB4taulacZ/taulacZ | Cardiovascular defects and embryonic lethality with very high penetrance | Growth retardation, arrested heart development, and lack of blood flow by E9.5-E10; degeneration and necrosis throughout the embryo by E10.5; incomplete heart looping at E8.75-E9.0; arrest of the remodeling of the primitive dilated vessels in head; disrupted development of the ACV and both arterial and venous intersomitic vessels; heart failed to increase in size, with incomplete cardiac looping and endocardium expansion; angiogenic remodeling arrest in the yolk sac with the phenotype being more severe on the venous than the arterial sides | [6] |
| Mouse | Ephb4f/f back crossed with smMHC-Cre-IRES-eGFP tg (SMC specific EphB4 knockout) | Hypotension in male but not female knockout mice | significantly reduced systolic pressure and mean arterial pressure with normal heart rate compared to WT in male but not female knockout mice | [37] |
| RASA1 Animal Models | ||||
|---|---|---|---|---|
| Animal Model | Genotype | Phenotype | Description | Ref |
| Mouse | Rasa1−/− (RASA1 null) | Embryonic lethal by day E10.5 with blood vessel abnormalities | 16-somite stage: thinning of dorsal aorta, aberrant ventral branches resemble inter-segmental arteries; E9.5: yolk sacs visibly abnormal (rough wrinkled appearance), embryonic vasculature exhibits local ruptures | [57] |
| Mouse | Rasa1R780Q/R780Q (knock-in expressing a mutant RASA1 that lacks an arginine finger) | Mid-gestation embryonic lethal with blood vessel abnormalities | E9.5: embryos small and less developed, distended pericardial sac, wrinkled yolk sac, no organized blood vessel networks, irregularly shaped dorsal aorta, severely disrupted and irregularly shaped inter-segmental arteries | [60] |
| Mouse | RASA1fl/fl/Uber2cre (conditional rasa1 floxed allele and an inducible Cre-ERT2 transgene under the control of a ubiquitin promoter) | Lymphatic vessel abnormalities, death from chylothorax | 8 weeks post-tamoxifen administration to adult mice: hyperplasia of initial lymphatics, dilation of initial and collecting lymphatics, contractility of conducting or collecting lymphatics, chylothorax, in some cases chylous ascites | [63] |
| Complete loss of RasGAP expression | Complete loss of RasGAP expression, exon 17 spliced exclusively to exon 19 (not to other downstream exons) | [61] | ||
| Death from chylothorax, lymphatic vessel abnormalities, increased density of lymphatic vessels within and inside the chest wall | Death by chylothorax by 8 months post-tamoxifen administration, chyle found in peritoneal cavity, dye injected into the footpads/tail leaked into peritoneal cavity instead of draining into thoracic duct, dye did not follow the typical route of lymph drainage to inguinal LN (drained into an expansive lymph vessel network instead of taking a direct route to the LN), increased density of lymphatic vessels within and inside chest wall facing the pleural cavity, dilated/increased number of lymphatic vessels in skin and lungs, no pulsatile activity in hyperplastic draining lymphatics, majority of their lymph vessels not lack smooth muscle | [62] | ||
| Impaired development of LV valves, chylothorax | TM injection at 9 weeks: LVs ineffective as pumps, downstream valves re-opened inappropriately during the diastolic phase of vessel contraction and were unable to close properly in response to adverse intra-luminal pressure leading to back-leak, LV leaflets contained reduced amounts of ECM proteins; TM between E12.5-E14.5: complete absence of the LV system at E19.5 as a result of LEC apoptosis, TM at E15.5: mesenteric LVs that lack valves and appear dilated with increased # of cells | [64] | ||
| Mouse | RASA1fl/fl/Prox1er2cre (conditional floxed rasal allele and lymph vessel specific inducible CreERT2 transgene) | Lymphatic vessel abnormalities, chylothorax | TM injection at 2 months: dilated increased number of lymphatic vessels in skin, no evidence of early lethality, chylothorax, or chylous ascites; TM at E15: chylothorax, death, lymph vessel hyperplasia due to increased LEC proliferation, phenotype rescued by administration of anti-VEGF-3 antibody | [62] |
| Lymphatic valve dysnfunction and LV leaflet changes | The majority of valves dysfunctional including both mesenteric and popliteal LV valves, LV leaflets contained reduced amounts of ECM proteins | [64] | ||
| Mouse | RASA1fl/R780Q/Uber2cre(conditional floxed rasa1 knock in allele with an inducible CreERT2 transgene) | Chylothorax, LV hyperplasia | TM injection at E15.5: death by to chylothorax, mesenteric LVs lack valves, sometimes appear dilated with increased numbers of cells | [62] |
| Zebrafish | Knock down of RASA1 homologs using antisense MOs | Cell death in the head region, improper formation of the caudal vascular plexus | Widespread cell death in head region and improper formation of caudal vascular plexus resulting in enlarged caudal vascular deformity (caused arterial blood flow to abruptly return to the posterior cardinal vein), phenotype rescued by administration of PI3K-TORC1 chemical inhibitors (but not MEK1/2 inhibitor) | [8] |
Table 2.
EphrinB2-EphB4-RASA1 Mutations Identified in Human Patients With Vascular Disorders
| Gene | Type of Mutation | Coding Variant | Protein Variant | Phenotype | Ref |
|---|---|---|---|---|---|
| EFNB2 | Transmitted | c.830G>A | p.Arg277His | VOGM, hip dysplasia, neurodevelopmental delay, seizures, cutaneous capillary malformations | [16] |
| EFNB2 | Transmitted | 13q33.2-q33.3 deletion (entire EFNB2) | Proband 1: Pulmonary valve stenosis, developmental delay, pyramidal spasticity, myoclonic epilepsy heterogeneous retinal pigmentation, abnormal visually evoked response, bilateral sensorineural hearing loss, enophalmia, hyperteloerism;Proband 2: tetrology of Fallot, developmental delay, enophalmia, hypertelorism, Proband 3 (mother): learning disability, progressive bilateral hearing loss; Proband 4 (maternal grandfather): progressive bilateral sensorineural hearing loss present before age 18 | [15] | |
| EFNB2 | De novo | c.239T>G | p.Met80Arg | Anal stenosis with fisula, hypoplastic center ventricle, hypotelorism, blepharimosis, mild psychomotor delay | |
| EPHB4 | Transmitted | c.33delG | p.Leu12Trpfs*10 | Cutaneous capillary malformation | [31] |
| EPHB4 | Transmitted | c.123+3G>C; r.123_411del | p.Trp18Valfs*16 | Cutaneous capillary malformation | |
| EPHB4 | Unsure | c.175G>A | p.Glu59Lys | Cutaneous capillary malformation, Parkes Weber Syndrome(left arm) | |
| EPHB4 | Transmitted | c.221G>C | p.Arg74Pro | Cutaneous capillary malformation | |
| EPHB4 | Unsure | c.345_347delCTA | p.Tyr115del | Cutaneous capillary malformation, AVM (right face) | |
| EPHB4 | De novo | c.389G>A | p.Trp130X | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.412-1G>T | Splicing | Cutaneous capillary malformation | |
| EPHB4 | Parental DNA not sequenced | c.418_485delGTC AAinsTT | p.Val161_Lys162d elinsLeu | Cutnaeous capillary malformation | |
| EPHB4 | Transmitted | c.447_448insGAA G | p.Arg150Glufs*74 | Cutaneous capillary malformation | |
| EPHB4 | Unsure | c.560T>C | p.Leu187Pro | Cutaneous capillary malformation, Parkes Weber Syndrome(left leg) | |
| EPHB4 | Transmitted | c.632_633delTG | p.Val211Alafs*11 | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.730C>T | p.Gln244X | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.802T>C | p.Cys268Arg | Cutaneous capillary malformation, Parkes Weber Syndrome(right leg) | |
| EPHB4 | Family 1: De novo, Family 2: Transmitted | c.1054C>T | p.Arg352X | Family 1: Cutaneous capillary malformation, Family 2:Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1077_1081delCC GCT | p.Leu359fs*19 | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1123G>T | p.Gly375X | Cutaneous capillary malformation | |
| EPHB4 | Unsure | c.1177_1177delG | p.Val393Ffs*17 | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1291C>T | p.Arg431X | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1406T>G | p.Val469Gly | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1423-6G>A; r.1422_1423insAC AG | p.Gly475Thrfs*39 | Cutaneous capillary malformation | |
| EPHB4 | Family 1: transmitted, Family 2: unsure, Family 3: Transmitted | c.1546G>A | p.Gly516Arg | Family 1: cutaneous capillary malformation, AVM (left face and lip), Family 2: Cutaneous capillary malformation, AVM (left face), Family 3: Cutaneous capillary malformation | |
| EPHB4 | Family 1: Unsure, Family 2: Unsure | c.1558C>T | p.Gln520X | Family 1: Cutaneous capillary malformation, Family 2: Cutaneous capillary malformation | |
| EPHB4 | De novo | c.1588+5G>C | Splicing | Cutaneous capillary malformation, Parkes Weber Syndrome(right arm) | |
| EPHB4 | Transmitted | c.1615delG | p.Ala539fs*2 | Cutnaeous capillary malformation, Parkes Weber Lesion (right leg) | |
| EPHB4 | Transmitted | c.1733_1734insA | p.Gly579Argfs*10 | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1788t>G | p.Tyr596X | Cutaneous capillary malformation, Parkes Weber Syndrome(left arm) | |
| EPHB4 | Transmitted | c.1870+2T>C | Splicing | Cutnaeous capillary malformation, AVM (left face) | |
| EPHB4 | Parental DNA not sequenced | c.1962-1G>A | Splicing | Cutaneous capillary malformation, AVM (left face) | |
| EPHB4 | Unsure | c.1966C>T | p.Arg656Trp | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.1990G>A | p.Glu664Lys | VOGM, cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.2037_2038insT | p.Glu680X | Cutaneous capillary malformation, AVM (left arm), Parkes Weber Syndrome (right leg) | |
| EPHB4 | Parental DNA not sequenced | c.2173G>A | p.Ala725Thr | Cutaneous capillary malformation | |
| EPHB4 | Family 1: Transmitted, Family 2: unsure | c.2215C>T | p.Arg739X | Family 1: Cutaneous capillary malformation, family 2: cutaneous capillary malformation | |
| EPHB4 | Family 1: De novo, Family 2: Transmitted | c.2233A>G | p.Asn745Asp | Family 1: cutaneous capillary malformation, Family 2: Cutaneous capillary malformation, Parkes Weber Syndrome (right arm) | |
| EPHB4 | Transmitted | c.2365C>T | p.Pro789Ser | Cutaneous capillary malformation, AVM (left face) | |
| EPHB4 | Transmitted | c.2366C>G | p.Pro789Arg | Cutaenous capillary malformation | |
| EPHB4 | Unsure | c.2379delC | p.Ala793fs*16 | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.2418C>T | p.Tyr806X | Cutaneous capillary malformtion | |
| EPHB4 | Transmitted | c.2419G>A | p.Gly807Arg | Cutaneous capillary malformation, AVM (left ear) | |
| EPHB4 | Transmitted | c.2458C>A | p.Pro820Thr | Cutaneous capillary malformation | |
| EPHB4 | Transmitted | c.2459C>T | p.Pro820Leu | Cutaneous capillary malformation, AVM (lips) | |
| EPHB4 | Unsure | c.2484+1G>A | Splicing | D8-L1 permedullar AVF, cutaneous capillary malformation | |
| EPHB4 | Unsure | c.2512C>T | p.Arg838Trp | Cutaneous capillary malformation | |
| EPHB4 | Unsure | c.2533T>C | p.Cys845Arg | Cutaneous capillary malformation, AVM (face and upper lip) | |
| EPHB4 | De novo | c.2567G>A | p.Cys856Tyr | Cutaneous capillary malformation | |
| EPHB4 | Family 1: Parental DNA not sequenced, Family 2: Transmitted | c.2590C>T | p.Arg846Trp | Family 1: Cutaneous capillary malformation, Family 2: Cutaenous capillary malformation | |
| EPHB4 | Parental DNA not sequenced | c.2621T>C | p.Leu874Pro | Cutaenous capillary malformation, AVM (left face) | |
| EPHB4 | Transmitted | c.1295_1296del | p.Glu432fs1 | VOGM, neurodevelopmental delay, seizures | [16] |
| EPHB4 | Transmitted | c.1526C>G | p.Ala509Gly | VOGM, Sternum excavatum | |
| EPHB4 | Transmitted | c.1950G>T | p.Lys650Asn | VOGM, neurodevelopmental delay | |
| EPHB4 | Transmitted | c.2599T>C | p.Phe867Leu | VOGM, cryptorchidism, strabismus, cerebral palsy | |
| EPHB4 | Transmitted | c.319T>C | p.Cys107Arg | VOGM | [33] |
| EPHB4 | De novo | c.570dupG | p.Hisl91Alafs*32 | Cutaneous capillary malformations, VOGM | |
| EPHB4 | Transmitted | c.2484+1G>T | p.Met814_Val829d el | Cutaneous capillary malformations, VOGM | |
| EPHB4 | Transmitted | c.2484+2insT | Splicing | Neonatal cardiac failure, VOGM | |
| EPHB4 | Transmitted | c.2609T>A | p.Val870Glu | Prenatal hydrocephaly and leukomalacia, VOGM, pregnancy terminated | |
| EPHB4 | Familial | C.2334+ 1G>C | Splicing | Central conducting lymphatic anomaly | [27] |
| EPHB4 | De novo | c.2405A>G | p.Asp802Gly | Cutaneous capillary malformations | [32] |
| RASA1 | Transmitted | c.2603+5G>C | Splicing | Cutaneous capillary malformation | [42] |
| RASA1 | Transmitted | c.616_620del | p.Ile206fs | Cutaneous capillary malformation, lymphedema | [63] |
| RASA1 | Transmitted | c.3070A>G | p.Lysl024* | Proband: Asymmetric ears, erythema on head, neck, parotid area, ear, left forarm, and right knee, hypertrophy in epidrmins, small tufted capillary malformations in superficial dermis, abnormal networks from ECA in right auricular region and neck cutaneous tissue; Father (carrier): erythema on neck, back, chest, wasit, abdomen, right D5 dermatoma | [47] |
| RASA1 | De novo | 5q14.3 deletion (entire RASA1) | Cutaneous capillary malformation | [78] | |
| RASA1 | Familial | c.1678G>T | p.Glu560X | Mental and motor retardation, epilepsy | [55] |
| RASA1 | Familial | c.734_737delinsAA A | p.Arg245fs*8 | Cutaneous capillary malformation | [43] |
| RASA1 | Transmitted | c.2927del | p.Asn976Metfs*20 | Cutaneous capillary malformation, AVM (face) | [45] |
| RASA1 | Familial | association with 5q14-21(entire RASA1) based on linkage analysis in 13 families | Cutaneous capillary malformation; 3 families have individuals with AVM | [48] | |
| RASA1 | Familial | association with RASA1 based on 6 families | Cutaneous capillary malformation; all 6 families have individuals with AVM | [40] | |
| RASA1 | Transmitted | c.1491_1492delAG | p.L498Lfs*2 | Proband: micrognathia, bulbous nasal tip, multiple capillary malformations, patent foramen ovale, dilated right atrium, significant dilation of innominate, right carotid, and right subclavian arteries, lipoma above the left iliac crest, infratentorial AVM, complex posterior fossa AVF, mulitple torturous vascular structures in anterior thecal sac, mild delays in fine motor skiills, mild stable left ventricular dilation; Mother (carrier): mulltipel cutaneous CMs, childhood febrile seizures, frequent bifrontal headaches | [79] |
| RASA1 | Unsure | c.1279C > T | p.Arg427X | Cutaneous capillary malformation, AVM (intercranial) | [54] |
| RASA1 | Transmitted | c.2125C>T | p.Arg709X | Cutaneous capillary malformation, VOGM, hydrocephalus | |
| RASA1 | Transmitted | c.2707C > T | p.Arg903X | Cutaneous capillary malformation, AVM (intercranial) | |
| RASA1 | Transmitted | c.1583A>G | p.Tyr528Cys | Multiple blanching telangiectasias on palms, fingers, tongue, and toes, reddened nasal mucosa with daily epistaxis, family history of epistaxis | [80] |
| RASA1 | Transmitted | c.3043G>T | p.E1015X | Telangiectasias, epistaxis, family history of telangiectasias | |
| RASA1 | Familial | c.829-9G>A | Splicing | Cutaneous capillary malformation, AVM (outer ear) | [49] |
| RASA1 | Familial | c.853C>T | p.Arg285X | Cutaneous capillary malformation | |
| RASA1 | Familial | c.2252_2255dupTC AT | p.Leu751Leufs*15 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1343_1346delins CATG | p.Gln448 Valfs*44 9 | Cutaneous capillary malformation | [50] |
| RASA1 | Parental DNA not sequenced | c.2119C>T | p.Arg707Cys | VOGM | [56] |
| RASA1 | Parental DNA not sequenced | c.2912T>C | p.Leu971Ser | VOGM | |
| RASA1 | Transmitted | c.2119C>T | p.Arg707Cys | Choroidal VOGM in two consecutive pregnancies of unaffected carrier mother | [81] |
| RASA1 | Transmitted | c.1063A>T | p.Lys355X | Cutaneous capillary malformations, AVM (face) | [46] |
| RASA1 | Transmitted | c.1269_1270insTA | p.Ile423X | Cutaneous capillary malformations, AVM (maxilla digit) | |
| RASA1 | Unsure | c.1703G>A | p.Trp568X | Cutaneous capillary malformations, AVM (face), AVF (spine) | |
| RASA1 | De novo | c.2125C>T | p.Arg709X | Cutaneous capillary malformations, AVM (neck), somatic c.1534C>T p.Arg512* mutation found in cuatenous capillary malformation epithelial cells in same patient | |
| RASA1 | Unsure | c.2467dupG | p.Glu823Glyfs*6 | 20 CMs (macular and pink to brown in color with pale halo) mainly affecting face and neck | [82] |
| RASA1 | Transmitted | c.1248T>G | p.Tyr416X | Proband: cardiomegaly, LVH, center extremty lympedema and overgrowth of left thigh, inability to take food by mouth, multiple capillary malformations with hypertrophy of neckand lower face, recrrent left chylothoraces, intermittant pericardial effusions, congenital hypothyroidism, persistant compensatory severe metabolic alkalosis, GERD; Mother (carrier): multifocal capillary malformations with somatic c.2245C>T p.Arg749X in CM skin sample | [59] |
| RASA1 | Transmitted | c.613_617delCTTA T | p.Leu205Lysfs*4 | Cutaneous capillary malformation, optic glioma (before 12y), lipoma (before 10y) | [41] |
| RASA1 | Unsure | c.656C>G | p.Ser219X | Cutaneous capillary malformation | |
| RASA1 | De novo | c.806_810delTTAC | p.Leu269Profs*11 | Cutaneous capillary malformaton, PKWS, cardiac overload | |
| RASA1 | De novo | c.828+3A>T | Splicing | Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Unsure | c.951dupG | p.Met318Aspfs*10 | Cutaenous capillary malformation | |
| RASA1 | Transmitted | c.957G>A | p.Trp319X | Cutaneous capillary malformation, PKWS, cardiac overload | |
| RASA1 | Transmitted | c.1017+1G>T | Splicing | Cutaneous capillary malformation, PKWS, cardiac overload | |
| RASA1 | Transmitted | c.1192C>T | p.Arg398X | Cutaneous capillary malformation, AVM/AVF, epilepsy, hydrocephalus | |
| RASA1 | De novo | c.1208dupC | p.Thr404Asnfs*14 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1277A>G | p.Tyr426Cys | Cutaneous capillary malformation | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted | c.1279C>T | p.Arg427X | Family 1: Cutaneous capillary malformation, Family 2: Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Transmitted | c.1332+5G>A | Splicing | Cutaneous capillary malformation | |
| RASA1 | De novo | c.1336C>T | p.Gln446X | Cutaneous capillary malformation, PKWS | |
| RASA1 | Transmitted | c.1350_1351insT | p.Asn451X | Cutaneous capillary malformation, PKWS | |
| RASA1 | De novo | c.1362_1363insTC AGT | p.Asn455Serfs*30 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1480dupT | p.Tyr494Leufs*7 | Cutaneous capillary malformation, PKWS | |
| RASA1 | Unsure | c.1490T>G | p.Leu497X | Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Transmitted | c.1572_1575dup | p.Ser526Metfs*8 | Cutaneous capillary malformation, PKWS, cardiac overload, tetrology of Fallot, superficial basal cell carcinoma (33y) | |
| RASA1 | Unsure | c.1636C>T | p.Gln546X | Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Unsure | c.1682_1683dup | p.Pro562Leufs*9 | Cutaneous capillary malformation, PKWS, cardiac failure, epilepsy, hemorrhages, Atrial Septal Defect II, Patent Foramen Ovale | |
| RASA1 | Transmitted | c.1698+3_1698+4i nsT | Splicing | Cutaneous capillary malformation, PKWS, pulmonary stenosis | |
| RASA1 | Transmitted | c.1870C>T | p.Gln624X | Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Transmitted | c.2026C>T | p.Gln676X | Cutaneous capillary malformation | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted | c.2125C>T | p.Arg709X | Family 1: Cutaneous capillary malformation, AVM/AVF, PKWS, varicose veins, Family 2:Cutaneous capillary malformation, PKWS, cardiac failure | |
| RASA1 | Transmitted | c.2184+1delG | Splicing | Cutaneous capillary malformation, PKWS, Patent Ductus Arteriosus, Atrial Septal Defect, pulmonary stenosis, tricuspidvalve prolapse | |
| RASA1 | Unsure | c.2185-1G>A | Splicing | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2288A>T | p.Glu763Val | Cutaneous capillary malformation, VOGM, cardiac failure, epilepsy, died rapidly after birth | |
| RASA1 | Transmitted | c.2341G>T | p.Glu781X | Cutaneous capillary malformation, AVM/AVF, hydrocephalus | |
| RASA1 | Transmitted | c.2365C>T | p.Arg789X | Cutaneous capillary malformation, AVM/AVF, angiolipoma(1y) | |
| RASA1 | Transmitted | c.2422C>T | p.Gln808X | Cutaneous capillary malformation, PKWS | |
| RASA1 | Transmitted | c.2450_2451delCT | p.Ser817Tyrfs*12 | Cutaneous capillary malformation, AVM/AVF | |
| RASA1 | Transmitted | c.2488-2A>G | Splicing | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2488-1delGTTA | Splicing | Cutaneous capillary malformation, AVM/AVF, non-small cell lung cancer (32y), hemoptysis | |
| RASA1 | Unsure | c.2514_2515insA | p.Glu839Argfs*6 | Cutaneous capillary malformation | |
| RASA1 | De novo | c.2532_2536delTT AA | p.Leu845Thrfs*38 | Cutaneous capillary malformation, spontaneous mutation in monozygotic twins, VOGM and cardica failure in one | |
| RASA1 | Unsure | c.2579_2582delTC AT | p.Phe860Trpfs*10 | Cutaneous capillary malformation, AVM/AVF, ectopic thyroid and paraythyroid | |
| RASA1 | Transmitted | c.2603+1G>A | Splicing | Cutaneous capillary malformation, chylous ascites | |
| RASA1 | Transmitted | c.2603+2_2603+3i nsT | Splicing | Cutaneous capillary malformation, ureteral reflux, epispadias | |
| RASA1 | Transmitted | c.2603+4_2603+5i nsA | Splicing | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2603_5G>T | Splicing | Cutaneous capillary malformation, neurofibromas (53y) | |
| RASA1 | Transmitted | c.3028C>T | p.Arg1010X | Cutaneous capillary malformation, vestibular schwannoma(26y) | |
| RASA1 | Transmitted | c.3052delG | p.Ala1018Hisfs*6 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.365C>A | p.Ser122X | Cutaneous capillary malformation | [30] |
| RASA1 | Transmitted | c.409dup | p.Leu137Profs*21 | Cutaneous capillary malformation, AVM (right foot) | |
| RASA1 | Transmitted | c.442_443delinsT | p.Ala148Trpfs*26 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.463G>T | p.Glu155X | Cutaneous capillary malformation | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted | c.475_476del | p.Leu159Glyfs*20 | Family 1: Cutaneous capillary malformation, AVM (nose), renal dysplasia, left kidney ptosis, Family 2: Cutaneous capillary malformation, brain AVF (identified by chance at 12y) | |
| RASA1 | Unsure | c.492C>G | p.Tyr164X | Cutaneous capillary malformation, pial AVM (deterioration in speech at 2, 5y), mental retardation | |
| RASA1 | Transmitted | c.613_617del | p.Leu205Lysfs*4 | Cutaneous capillary malformation, retinal vascular malformation in 2 individuals | |
| RASA1 | Unsure | c.829-1G>A | Splicing | Cutaneous capillary malformation, AVM(left arm) | |
| RASA1 | Family 1: De novo, Family 2:De novo | c.853C>T | p.Arg285X | Family 1: Cutaneous capillary malformation, AVM (upper lip), epilepsy, Family 2: Cutaneous capillary malformation | |
| RASA1 | Unsure | c.899+1G>T | Splicing | AVM (ear) | |
| RASA1 | Family 1: Transmitted, Family 2: Unsure | c.1192C>T | p.Arg398X | Family 1: Cutaneous capillary malformation, pial AVM (gross motor delay at 2 y), AVM (left calfand foot), Family 2: Cutaneous capillary malformation, PKWS (left leg) | |
| RASA1 | Transmitted | c.1220_1223del | p.Asn407Serfs*3 | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.1290_1291del | p.Gln430Hisfs*3 | Cutaneous capillary malformation, PKWS (center leg) | |
| RASA1 | Unsure | c.1342C>T | p.Gln448X | Cutaneous capillary malformation, AVM (tongue) | |
| RASA1 | Transmitted | c.1384_1388del | p.Thr462Profs*3 | Cutaneous capillary malformation | |
| RASA1 | De novo | c.1386_1387insCT | p.Ile463Leufs*21 | Cutaneous capillary malformation, spinal AVM L1 (neurogenic bladder at 4y), atrial septal defect | |
| RASA1 | Unsure | c.1440T>G | p.Tyr480X | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1453+1delG | Splicing | Cutaneous capillary malformation, spinal AVF | |
| RASA1 | Unsure | c.1480dupT | p.Tyr494Leufs*7 | Cutaneous capillary malformation, AVM (right foot) | |
| RASA1 | Unsure | c.1494_1495dup | p.Gly499Argfs*22 | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.1517_1520del | p.Tyr506Leufs*13 | Cutaneous capillary malformation, PKWS (left leg) | |
| RASA1 | Transmitted | c.1534C>T | p.Arg512X | Cutaneous capillary malformation, family history of VOGM with prenatal death at 32w | |
| RASA1 | Transmitted | c.1583A>G | p.Tyr528Cys | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1589T>A | p.Val530Asp | Cutaneous capillary malformation, brainstem AVM (brain hemorrhage with facial palsy at 3w), left leg AVM, basal cell carcinoma in 2 individuals | |
| RASA1 | Transmitted | c.1596_15797del | p.Asp532Glufs*17 | Cutaneous capillary malformation, AVM (right hand) | |
| RASA1 | Transmitted | c.1636C>T | p.Gln546X | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1666_1698+15del | Splicing | Cutaneous capillary malformation, posterior fossa AVM, spinal AVF (flaccid paraplegia of lower extremities and neurogenic bladder at 6 months), family history of hydrops fetalis | |
| RASA1 | Unsure | c.1698+1G>T | Splicing | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1717C>T | p.Gln573X | Cutaneous capillary malformation, spinal AVM, PKWS (right leg) | |
| RASA1 | Transmitted | c.1726dup | p.Cys576Leufs*7 | Cutaneous capillary malformation, AVM (left arm) | |
| RASA1 | De novo | c.1870C>T | p.Gln624X | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.1877C>A | p.Ala626Glu | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.1989_1992del | p.Lys664Alafs*13 | Cutaneous capillary malformation, AVM (center arm), macular degeneration in 1 individual | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted, Family 3: Unsure, | c.2035C>T | p.Arg679X | Family 1: Cutaneous capillary malformation, AVM (right foot), Family 2: Cutaneous capillary malformation, AVM (right hand), Family 3: Cutaneous capillary malformation, Family 4: Cutaneous capillary malformation | |
| Family 4: Transmitted | |||||
| RASA1 | Transmitted | c.2081_2087del | p.Ser694Tyrfs*3 | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2125C>T | p.Arg709X | Cutaneous capillary malformation, VOGM | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted, Family 3: Transmitted, Family 4: De novo | c.2131C>T | p.Arg711X | Family 1: Cutaneous capillary malformation, AVM (lower lip), vertebral AVF (tetraplegia at 4y), Family 2: Cutaneous capillary malformation, posterior fossa AVM (engorged veins around face), family history of cerebral aneurysm, Family 3: Cutaneous capillary malformation, Family 4: Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2239C>T | p.Gln747X | Cutaneous capillary malformation, AVM (center cheek) | |
| RASA1 | Unsure | c.2245C>T | p.Arg749X | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.2288A<T | p.Glu763Val | Cutaneous capillary malformation | |
| RASA1 | De novo | c.2329G>T | p.Glu777X | Cutaneous capillary malformation, spinal artery pial fistual AVF (headaches and facial tics), right foot and calf fast flow lesion | |
| RASA1 | Transmitted | c.2344+1G>C | Splicing | Cutaneous capillary malformation, AVM (face) | |
| RASA1 | Family 1: Transmitted, Family 2: Transmitted | c.2365C>T | p.Arg789X | Family 1: Cutaneous capillary malformation, congenital heart defect, Family 2:Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.2422C>T | p.Gln808X | Cutaneous capillary malformation, AVM (right foot) | |
| RASA1 | Unsure | c.2450_2451del | p.Ser817Tyrfs*12 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.2457_2460delins T | p.Leu819_lys820de linsPhe | Cutaneous capillary malformation, AVM (face, right hand) | |
| RASA1 | De novo | c.2500_2501del | p.Lys834Valfs*5 | Cutaneous capillary malformation | |
| RASA1 | Unsure | c.2532_2536del | p.Leu845Thrfs*38 | Cutaneous capillary malformation, AVM (face) | |
| RASA1 | Transmitted | c.2603+2dup | Splicing | Cutaneous capillary malformation, brain AVM, PKWS (right arm) | |
| RASA1 | Transmitted | c.2632C>T | p.Gln878X | Cutaneous capillary malformation, PKWS (right leg, ulceration), hydrops fetalis | |
| RASA1 | Transmitted | c.2707C>T | p.Arg903X | Cutaneous capillary malformation, parietal AVF (seizures at 5y) | |
| RASA1 | Transmitted | c.2925+5G>C | Splicing | Cutaneous capillary malformation, spinal AVM D12-L1 (center leg paralysis at 2y) | |
| RASA1 | Transmitted | c.2977del | p.Arg993Valfs*3 | Cutaneous capillary malformation, VOGM (stroke), PKWS (left arm) | |
| RASA1 | Transmitted | c.3024del | p.Glu1008Aspfs*16 | Cutaneous capillary malformation, VOGM (stroke at 3y), developmental delay | |
| RASA1 | Transmitted | c.3028C>T and c.3064G>T | p.Arg1010X and p.Gly22Cys | Cutaneous capillary malformation, pial AVM, family history of TOP at 7 months for VOGM | |
| RASA1 | Unsure | c.3038_3054del | p.Ser1013Thrfs*27 | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.3055C>T | p.Gln1019X | Cutaneous capillary malformation | |
| RASA1 | Transmitted | c.3109_3112del | p.Gln1037Thrfs*63 | Cutaneous capillary malformation, AVM (left leg), Naevus anemicus | |
| RASA1 | Unsure | c.2125C>T | p.Arg709X | Heuchan: VOGM, CM-AVM rash, Revencu: cutaneous capillary malformation | [30, 56] |
| RASA1 | De novo | c.1386_1387insCT | p.Ile463Leufs*21 | 2 cutaneous capillary malformations at birth, more CMs apperaed later, lower extremity weakness (non-ambulator), neurogenic bladder at 4y, AVM at conus medularis (L1) | [52] |
| RASA1 | Transmitted | c.1453+1delG | Splicing | Multifocal CMs at birth, AVF supplied by R vertebral artery and thyocervical trunk | |
| RASA1 | Transmitted | c.1666_1698+15del | Splicing | Multifocal CMs at birth, flaccid paraplegia of lower extremities and neurogenic bladder at 16 months, AVM at conus medularis(L2) | |
| RASA1 | Transmitted | c.1717C>T | p.Gln573X | Multifocal CMs at birth, acute sensorimotor deficits at 23y, AVM at L5-S1 | |
| RASA1 | De novo | c.2329G>T | p.Glu777X | CM on R plantar foot at birth, macular lesions appeared with time along with motor tics, AVF at level of C7-T1 | |
| RASA1 | Transmitted | c.1310T>G | p.Leu437Arg | Mulitfocal CMs on right palm, chest, and back, history of nosebleeds | [44] |
| RASA1 | Transmitted | c.1401_1402delAA | p.Thr467fs | Multifocal CMs, reddish-brown patch on head/face, extremities, AVF (high flow lesions underneath shins), bright red macule with telangiectasias on extremities | |
| RASA1 | Transmitted | c.1491_1492delAG | p.Leu497fs | Large brain AVM, multifocal cutaneous vascular malformation on face, nape of neck, and occipital region | |
| RASA1 | De novo | c.1617dupA | p.Asn539fs | AVM on right side of face and back, multifocal CMs | |
| RASA1 | Transmitted | c.1771_2insC | p.Asn591fs | Brain AVM near carotid, multiple CMs near eye | |
| RASA1 | De novo | c.1870C>T | p.Gln624X | Multifocal CMs on head, face, and chest, nose hypertropy and telangiectasia | |
| RASA1 | Transmitted | c.2026C>T | p.Gln676X | Multifocal CMs, macrocephaly | |
| RASA1 | Unsure | c.2084A>T | p.His695Leu | Brain AVF, brain AVM, multifocal CMs on head/face | |
| RASA1 | Transmitted | c.2225C>A | p.Ser724X | Brain AVM, multifocal CMs on head/face, trunk, and extremities, tumors (center nasal bridge and maxillary areas, center occipital lesion, multiple spots on face), macrocephaly, seizures | |
| RASA1 | De novo | c.2239C>T | p.Gln747X | Facial AVM, mulitifocal CMs |
EphrinB2
Human Diseases
Human ephrinB2 is a transmembrane protein encoded by the EFNB2 gene. EphrinB2 has one transmembrane domain, a receptor-binding domain (RBD) on the extracellular side, and a PDZ domain on the intracellular side.
A microarray study of 116 conotruncal heart defects patients focusing on large (> 500kb), rare (< 1% in platform and ethnicity matched control groups) copy number variations identified 1 copy number gain at 13q33.3-33.3 harboring the entire EFNB2 gene in 1 patient [14] A 610-kb deletion encompassing EFNB2 transmitted from asymptomatic mother was reported in 2 siblings with congenital heart disease (CHD) and mild developmental delay. The same group also reported a de novo missense mutation (c.239T>G, p.Met80Arg) in EFNB2 in a patient with anal stenosis, hypoplastic left ventricle, and mild developmental delay [15]. Additionally, in a WES study of 55 unrelated VOGM probands, 1 deleterious missense mutation (c.830G>A, p.Arg277His) was reported in 1 proband. Unaffected parents and 2 healthy siblings of the proband were also genotyped for the c.830G>A EFNB2 mutation. Interestingly, the mother, who is the only carrier of the risk allele among all unaffected family members, is also the only family member exhibiting cutaneous vascular abnormalities, suggesting incomplete penetrance and variable expressivity of the mutation [16]. Overall, despite several EFNB2 mutations occasionally reported in various disorders discussed above, no clear association has been established statistically between EFNB2 and human diseases.
Animal Studies
Studies in animal and cellular models have provided extensive evidence of a role for EFNB2 in congenital cerebrovascular disorders. Efnb2 knockout mouse model (ephrinB2tlacZ) has normally occurred vasculogenesis, but angiogenesis was disrupted in the yolk sac, the internal carotid arterial branches were absent, angiogenesis of venous capillaries was defective in the head, and capillary ingrowth into the neural tube failed to occur [2]. In an in vitro sprouting assay, ephrinB2 ligand showed similar efficiency as angiopoietin-1 (Ang1) and VEGF when inducing capillary sprouting, indicating its stimulatory role in remodeling the developing vascular system [5], Homozygous ephrinB2 knockin mice (ephrinB2ΔC/ΔC) with its carboxyl terminal (C-terminal), which encodes the PDZ domain needed for its function as a receptor in ephrin-Eph reverse signaling, replaced by a hemagglutinin (HA) epitope tag showed defects in vasculogenesis and angiogenesis as well as mid-gestation lethality very similar to what has been observed in ephrinB2 null animals [17]. However, surprisingly, the ephrinB2 knockin mice (ephrinB2ΔC-βgal/ΔC-βgal) with its C-terminal replaced by beta-galactosidase (βgal) instead of an HA tag were normal throughout gestation and exhibited none of the early vascular defects observed in ephrinB2ΔC/ΔC animals [18]. Further investigation comparing the gross phenotype and cell surface expression of ephrinB2 in these two ephrinB2 knockin mouse models with a third C-terminal truncating ephrinB2 mouse model (ephrinB2T/T [18]) generated by deleting the same C-terminal amino acid residues as ephrinB2ΔC-βgal/ΔC-βgal but not conjugating with any other proteins revealed that improper localization of ephrinB2 rather than the deletion of its C-terminal likely caused the vascular phenotype, indicating that it is likely not reverse signaling but rather ephrinB2 mediated forward signaling with EphB4 that is necessary for angiogenic remodeling. Human and mouse ephrinB2 proteins are overall highly homologous [19]. Understanding the function of each protein domain of ephrinB2 and the direction of the signaling they are mediating has provided invaluable guidance for accessing the pathogenicity of human mutations, it helps to (i) put identified mutations in context of the disease phenotype to evaluate whether they are disease-causing or benign and (ii) elucidate different pathways downstream of ephrinB2 reverse and forward signaling, respectively, underlying different human diseases.
In the vascular system, ephrinB2 is primarily expressed in (but not restricted to) arterial ECs. Conditional mouse knockout of ephrinB2 specifically in the endothelium and endocardium of the developing vasculature and heart (ephrinB2lacZ/loxP; Tie2-Cre+) showed angiogenic remodeling defects [20] identical to those seen in global ephrinB2 knockout mice [20, 21], indicating that sufficient expression of ephrinB2 in endothelial and endocardial cells is required for angiogenesis and that the expression of ephrinB2 in surrounding mesenchymal cells and mural cells, including pericytes (PCs) and vascular smooth muscle cells (vSMCs), cannot compensate for the loss of ephrinB2 in vascular cells [20]. Furthermore, restoration of ephrinB2 expression specifically in vascular ECs of Efnb2 null mice (Eftib2CR/GFP; Tie2-CreTg/+) was able to completely rescue defects in angiogenesis, suggesting the role of ephrinB2 for normal angiogenesis is restricted to vascular ECs [22], even though failure of rescuing cardiovascular defects in Efnb2 null mice by ECs specific ephrinB2 restoration (Efnb2GFP/GFP; Tg) possibly due to insufficient ephrinB2 level was also reported [23]. However, conditional mouse knockout of ephrinB2 in mural cells (Efnb2ΔPC/vSMC) resulted in perinatal lethality and caused edema and extensive hemorrhaging in the skin in addition to vascular defects in a range of organs [24] due to the scatter and loose attachment of PCs and vSMCs to the micro-vessels, suggesting a role of ephrinB2 in mediating interactions between mural cells as well as between PCs and the endothelium. Interestingly, while transgenic mice with a global overexpression of ephrinB2 (CAGp-ephrin-B2 Tg) exhibited abnormal segmental arrangement of intersomitic vessels, this was not observed in mice overexpressing ephrinB2 specifically in vascular ECs (Tie-2p-ephrin-B2 Tg) even though some do have intracerebral bleeding, suggesting that mediation of ECs by ephrin-Eph signaling can be altered by the ectopic expression of ephrinB2 [25]. These data collectively illustrate that precise regulation of the expression of ephrinB2 in specific time and space is absolutely essential for its proper function in vascular development. Both overexpression and loss of ephrinB2 expression break the balance of ephrin-Eph signaling and thus disrupt the biological processes it is regulating. Additionally, these mouse models provided important insights into the tissue specific functionality of ephrinB2, calling attention to its upstream regulators and downstream effectors in vascular ECs when studying cerebrovascular disorders.
EphB4
Human Diseases
Human EphB4 is a transmembrane RTK encoded by the EPHB4 gene. On the extracellular side, EphB4 has a ligand-binding domain (LBD) that binds to the RBD of its ephrin ligand, a Cys-rich domain composed of a sushi domain and an epidermal growth factor (EGF)-like domain, and two fibronectin domains. Connected to the extracellular side by the transmembrane domain, the intracellular side of EphB4 encompasses a Tyrosine kinase domain, a sterile alpha motif, and a PDZ site.
Lymphatic angiogenesis starts from a certain subset of venous ECs that become responsive to lymphatic-inducing signals, which subsequently differentiate into to the lymphatic lineage and form embryonic lymph sacs [3]. In two extended families with a history of in utero and neonatal death associated with hydrops fetalis resulting from a lymphatic abnormality, WES identified two distinct rare heterozygous missense mutations in the tyrosine kinase domain of EPHB4 [26]. Both mutations caused the loss of phosphorylation activity of EphB4 in vitro and disrupted ephrinB2- dependent EphB4 activation in lymphatic ECs. All mutation carriers in these two families were either affected or presented with severe in utero swelling and/or atrial septal defect, suggesting highly variable expressivity of these mutations. Conditional knockout of Ephb4 in mouse lymphatic vasculature (Ephb4fl/fl Prox1-CreERT2 R26-mTmG) resulted in a high proportion of embryos with subcutaneous edema and/or blood filling in lymphatic vessels, recapitulating the phenotype of human patients [26]. Additionally, a splice site variant (c.2334 + 1G>C) in EPHB4 was identified by WES in a four-generation family with central conducting lymphatic anomaly (CCLA) [27]. Knockdown of the EPHB4 orthologue (ephb4a) with morpholino recapitulates human lymphatic phenotypes [27]. Of note, over-activation of mTORCl was observed in both morpholino-treated zebrafish lysates and HEK293T cells with knock-in of the EPHB4 mutation, and the zebrafish phenotype can be rescued by drugs inhibiting mTOR or RAS-MAPK signaling [27], suggesting PI3K/mTORC1 pathway downstream of ephrin-Eph signaling in vascular development. The identified human mutations accompanied by evidence from animal models strongly suggested a role of EPHB4, especially its downstream signaling through the PI3K/mTORC1 pathway mediated by its protein kinase domain, in human angiogenesis.
Like EFNB2, EPHB4 is often selected as a candidate gene for CHD based on results from mouse models [6, 28], but to date, no clear association has been established from genetic studies in human patients. In a WES study of 182 individuals from 51 families with multiple members affected by CHD, one likely pathogenic missense mutation in EPHB4 was found to co-segregate with the disease in 1 family with severe pulmonary stenosis, Tetralogy of Fallot, and ventricular septal defects [29].
CM-AVM is a congenital disorder characterized by atypical CM associated or not with AVM, arteriovenous fistula (AVF), or Parkes Weber syndrome [30]. CM-AVM in over 50% of the patients can be explained by mutations in RASA1 (CM-AVM1, caused by RASA1 mutation), a gene encoding p120RASGAP, which is a direct downstream effector of EphB4 [31]. Using genome wide linkage analysis in a large extended RASA1 mutation negative CM-AVM family, a peak on chromosome 7 harboring EPHB4 was identified. Subsequent WES screen in 11 selected patients verified EPHB4 as a disease associated gene and following targeted re-sequencing of EPHB4 in 365 index individuals with either isolated multifocal CMs or CM-AVM identified 47 distinct predicted damaging mutations in 54 patients. Further investigation of clinical data of index cases and their relatives carrying the same EPHB4 mutations revealed that EPHB4 mutations are highly penetrant but have variable expressivity, with 93% of the carriers (n = 102) affected [31]. Of note, among 54 families carrying damaging mutations in EPHB4, 2 VOGM patients from the same family were reported, of whom 2 additional family members, 1 unaffected and 1 with isolated CMs, were also identified as carriers of the EPHB4 mutation, further emphasizing the variable expressivity of EHPB4 mutation. Additionally, 1 de novo mutation in EPHB4 was reported in a 8-year-old boy affected by isolated multifocal CMs in a separate study [32]. These studies, even though mainly using targeted sequencing approaches, established EPHB4 as a risk gene for CM-AVM (CM-AVM2, caused by EPHB4 mutation).
Multiple recent studies have associated EPHB4 with VOGM. WES in 19 unrelated VOGM patients identified EPHB4, with 1 de novo and 2 transmitted variants, as a candidate disease-causing gene. Targeted re-sequencing of EPHB4 in 32 additional unrelated VOGM patients identified 2 additional predicted pathogenic missense mutations [33]. All 5 mutations identified were at different loci. Morpholino knockdown of ephb4a in zebrafish caused anomalies of the dorsal longitudinal vein, which is the orthologue of the median prosencephalic vein and the embryonic precursor of the vein of Galen, which was not observed in wild type nor ‘mismatch’ controls [33]. Additionally, using an unbiased burden test considering all genes captured in human genome (n = 18,989), an independent WES study of 55 unrelated VOGM probands identified EPHB4, with 4 distinct variants, as the only loss of function (LoF) intolerant gene (pLI≥0.9) with genome-wide significant burden of rare damaging heterozygous mutations. Moreover, the Ephrin receptor signaling pathway was identified as the only over-represented pathway in cases but not controls using hypothesis-free pathway analysis, and genes (EPHB4, RASA1, EPHA4, EPHA6, ITGB1, ITSN1, and NGEF) contributing to the significance of the pathway map to a single experimentally supported interactome [16]. It is worth noting that in both studies, uncommon cutaneous CMs were reported in multiple VOGM probands as well as in EPHB4 mutation carriers not diagnosed with VOGM, but not in non-carriers from the same family. These studies not only firmly established EPHB4 as a bona fide risk gene for VOGM, but also highlighted the incomplete penetrance and variable expressivity of disease-associated mutations in families (see below).
Animal Studies
Among the 5 receptors (EphB1 - EphB4, and EphA4) of ephrinB2, EphB3 and EphB4 are the only 2 that are expressed in vascular ECs [5]. Of these, EphB4 is the only receptor that uniquely binds to ephrinB2 [34, 35]. While mouse knockout of EphB3 did not result in a cardiovascular phenotype [5], EphB4 knockout mice (EphB4taulacZ/taulacZ [6]) lacking the signal peptide-encoding exon phenocopied ephrinB2 knockout mice (ephrinB2taulacZ/taulacZ [2]) with defects in vascular development and embryonic lethality with very high penetrance [6] suggesting EphB4 as a specific functional partner for ephrinB2 in the vascular system and also the redundant functionality of Eph receptors. Although mouse knockout of EphB2 did not result in a cardiovascular phenotype, double knockout of EphB2 and EphB3 led to embryonic lethality as a result of defects in embryonic vascular remodeling with 30% penetrance [5], indicating a potentially redundant role of EphB3 shared with EphB4 even though its expression cannot compensate for the loss of EphB4 in vascular cells.
Compared to its ligand ephrinB2, which is expressed in not only ECs but also the surrounding mesenchymal and mural cells, expression of EphB4 is usually considered to be highly specific to vascular cells [20, 36]. Recently, male (but not female) knockout mice with a SMC-specific deletion of EphB4 were shown to have hypotension [37]. In vivo cellular molecular studies in vSMCs showed that small artery contractility seems to be regulated by both ephrinB2:EphB4 forward and reverse signaling. Even though it is not yet clear how sex hormones in concert with EphB4 are able to modulate vSMCs contractility, this is a starting point in understanding the role of ephrinB2:EphB4 signaling in blood pressure regulation. This study also brings attention to roles of EPHB4 outside vasculogenesis, and could provide guidance for variant prioritization in human male patients with hypotension.
RASA1
Human Diseases
RASA1, also known as RAS p21 protein activator 1, is a GTPase activating protein (GAP) encoded by the RASA1 gene. RASA1 interacts with RAS via its arginine finger motif in the GAP domain, thereby enhancing the intrinsic GTPase activity of RAS proteins and allowing for RAS inactivation [38, 39].
Since Eerola et al first characterized CM-AVM in 2003 [40], extensive reports have associated RASA1 mutations with CM-AVM1 [30, 41-47], as well as with isolated CM [48-51] and AVM [52-54]. In 2 large scale cohort studies of CM-AVM, RASA1 germline mutations were identified in ∼70% of probands (44 and 68 out of 56 [41] and 100 [30] probands screened, respectively) with a high occurrence of de novo mutations (31.8% [41] and 26.5% [30], respectively), highlighting the high prevalence of RASA1 mutation in CM-AVM cases as well as the importance of using a trio-family design to identify mutations with various inheritance models. Among all CM-AVM1 cases reported, 8 RASA1 mutations were identified in VOGM, including two stop-gain mutations [16, 53, 55, 56] three missense variants [41, 56], and three frameshift deletions [30, 41]. However, when ascertaining for VOGM alone, only 1 RASA1 mutation was identified in a total of 55 unrelated probands [16], suggesting a shared but distinct molecular mechanism underlying CM-AVM and VOGM.
Somatic second hits in affected tissues have long been proposed as a potential explanation for phenotypic variability and multifocality of the lesion associated with CM-AVM1. Importantly, mouse embryos mosaic for Rasal−/− cells exhibited localized vascular defects [57], suggesting paradominant inheritance in human CM-AVM [58]. A mosaic loss of chromosome 5q (including the entire RASA1 gene) identified in DNA from a neurofibroma developed in the congenital Parkes Weber lesion was reported in a patient carrying heterozygous germline splice site RASA1 mutation (c.2603+5G>T), verified the somatic second hit model in CM-AVM for the first time [30]. More recently, a screen of RASA1 mutation in endothelial cells derived from the biopsied CM lesions in 4 CM-AVM patients carrying germline RASA1 mutation identified 1 somatically acquired LoF RASA1 mutation in trans with the germline mutation in 1 of the patients [46], providing clear evidence of somatic mutation in relevant cell type contributing to the disease pathogenesis. One other somatic RASA1 mutation in a CM-AVM1 patient was independently reported, even though whether the somatic hit is in trans or cis with the germline RASA1 mutation was not tested [59]. These studies provide strong evidence of somatic second hit being a mechanism through which RASA1 causes CM-AVM.
Animal Studies
RASA1 is a direct downstream effector of EphB4. Mutations disrupting the Tyrosine kinase domain of EphB4 identified in human VOGM patients abrogate the binding of RASA1 in HEK293T cells [16]. Knockdown of ephb4a and rasala in zebrafish shared a similar phenotype of vascular deformities and a failure of caudal vascular plexus formation [8]. Interestingly, similar to the ephb4a knockdown zebrafish mentioned above, phenotypes of efrib2a-ephb4a and rasala knockdown zebrafish lines could also be rescued by chemical inhibitors of PI3K/mTORC1 [8], further emphasizing a role of the PI3K/mTORC1 pathway downstream of the ephrinB2-EphB4-RASA1 signaling axis in vascular development. Consistent with the zebrafish model, the RASA1 null mouse model (Gap−/−) is embryonic lethal by E10.5 and exhibited severe blood vessel abnormalities [57], further emphasizing a crucial role of RASA1 in vasculogenesis.
RASA1’s arginine finger motif is required for its interaction with RAS but not for its RAS-independent functions [39]. RASA1 knock-in mouse lacking the arginine finger (RasalR780Q/R780Q) exhibited embryonic lethality and severe vascular abnormalities similar to homozygous Rasal null mice [60], suggesting that dysregulation of RAS and downstream signaling is likely responsible for blood vessel abnormalities associated with RASA1 mutations.
Similar to ephrinB2 and EphB4, RASA1 also plays a crucial role in lymphatic vasculogenesis. An inducible RASA1 adult mouse knockout (RASA1fl/fl/Uber2cre [61]) showed extensive lymphatic vessel hyperplasia and leakage and early lethality caused by chylothorax [62-64]. Of note, even though very limited likely due to sample availability, lymphatic abnormalities have been reported in RASA1 associated CM-AVM, particularly in patients with Parkes Weber syndrome [41, 63]. Taken together, these studies suggest that ephrinB2, EphB4, and RASA1 function in the same pathway in the context of vasculogenesis. More attention should be paid to human mutations identified in the ephrinB2-EphB4-RASA1 signaling axis as well as genes involved in ephrinB2:EphB4 forward signaling when studying cerebrovascular disorders.
Concluding Remarks and Future Perspectives
The ephrinB2-EphB4-RASA1 signaling axis is essential for development of the vascular system. Recent successful application of WES and studies of large cohort have associated mutations in EFNB2, EPHB4, and RASA1 with multiple cerebrovascular disorders, corroborating the findings in model organisms. However, many key questions of their roles in human diseases remain unanswered (see Outstanding Questions).
OUTSTANDING QUESTIONS.
What is the contribution of non-coding and regulatory regions of genes in the ephrinB2- EphB4-RASA1 signaling axis to human congenital neurovascular disorders?
What is the mechanism of the incomplete penetrance and variable expressivity of VOGM-associated ephrinB2-EphB4-RASA1 mutations?
What is the prevalence of CMs in VOGM? What are the phenotypic and genetic overlap between CM-AVM and VOGM?
Can other genes in the same pathway as EFNB2, EPHB4, and RASA1 explain some of the remaining patients with neurovascular disorders that are negative of mutation in known genes?
Can drugs targeting the PI3K/mTORC1 pathway be used to treat patients with cerebrovascular disorders associated with ephrinB2-EphB4-RASA1 mutations?
CM-AVM and VOGM are thought to be two distinct disorders, with CM-AVM characterized by atypical CMs with or without AVM in variable body parts while VOGM being a type of cerebral AVM [16]. The fact that VOGM is infrequently reported as the AVM in CM-AVM patients, and CMs are quite often identified in patients with VOGM and their unaffected family members carrying the disease-causing mutation raises the question whether there is a shared genetic mechanism underlying these two diseases. Additionally, even though it is very difficult to estimate whether there is an enrichment of ephrinB2-EphB4-RASA1 mutations in cerebrovascular diseases compared to other vascular malformations given the incompleteness of the publicly available phenotype data, that Ephrin receptor signaling pathway was identified as the only over-represented pathway in VOGM cases but not controls [16] provided strong evidence of a role for ephrinB2-EphB4-RASA1 signaling axis in cerebrovascular development. Moreover, other than cardiac defects, cerebral manifestation is the most commonly described defect in animal models of ephrinB2 [2, 20, 25], EphB4 [6], and RASA1[8]. Future study with thorough history and physical examination is needed to better understand the phenotypical overlap of different cerebrovascular disorders as well as between cerebral and other vascular defects in human, which will in turn aid the genetic study in understanding their shared mechanism and the prevalence of the risk genes.
The majority of the identified mutations in CM-AVM and VOGM are transmitted with incomplete penetrance and variable expressivity. Parents with mild cutaneous vascular lesions can have children with VOGM that is almost 100% lethal without surgical intervention.
The reason why one mutation can have such a large spectrum of manifestation is not yet completely clear. Two mechanisms can potentially explain some of the cases. First, some cases could have somatic second-hit during development, which would explain the localized nature and multifocality of the lesions, as well as the variable phenotype within families [41]. Somatic mosaicism has been verified in several vascular malformations [65, 66], including CM-AVM [30, 46]. Second mechanism would be that there are genetic modifiers that could interact and modify the expressivity of the disease-causing gene [67], or more than 1 gene with 1 defective allele (transcompound heterozygote) is needed for disease manifestation [58]. Future study with large trio based cohort and collection of tissue samples are needed to test these hypotheses.
Despite the large number of the CM-AVM cases explained by RASA1 mutations, genetic causes for many patients remain unknown. As most of the published studies performed target resequencing on candidate genes, some additional patients could be explained if the unsolved cases were screened for genes that are more recently identified, such as EPHB4 and EFNB2. Additionally, target sequencing limited the possibility of identifying novel disease-causing mutations. More unbiased genome-wide sequencing studies are in need to identify novel genes and better understand the genetic underpinnings of the disease. Furthermore, mutations in the regulatory and intronic regions of the known disease-associated genes have not yet been examined. Future study using whole genome sequencing could potentially unveil the contribution of noncoding variants in known genes and even identify novel mutations in non-coding regions.
Genetic findings in ephrinB2-EphB4-RASA1 signaling axis genes also provided insights into potential diagnostic and therapeutic strategies for cerebrovascular disorders. Given the incomplete penetrance and variable expressivity, prenatal genetic screen in pregnant women with family history of cutaneous vascular lesions and/or other vascular malformations could help the early diagnosis and treatment design of the disease. Interestingly, the phenotype in efnb2a-ephb4a and rasala knockdown zebrafish could be rescued by chemical inhibitors of PI3K-TORC1, indicating that reduced ephrinB2-EphB4-RASA1 expression may cause excessive PI3K/mTORC1 pathway activity, which has also been observed in surgical tissue samples from patients with vascular anomalies associated with RASA1 mutations [30]. This suggests therapy targeting PI3K-TORC1 signaling might be a viable therapeutic approach for cerebrovascular diseases associated with ephrinB2-EphB4-RASA1 mutations.
HIGHLIGHTS.
Genetic and functional studies in model organisms have revealed the importance of ephrinB2-EphB4-RASA1 signaling in multiple aspects of cerebrovascular development.
Recent whole exome sequencing studies in humans have identified mutations in EFNB2, EPHB4, and RASA1 in multiple congenital cerebrovascular disorders.
These diseases include capillary malformation-arteriovenous malformation (CM-AVM), Vein of Galen malformation (VOGM), and others.
The localized nature and multifocality of lesions associated with ephrinB2-EphB4-RASA1 mutations implicates somatic mosaicism in disease pathogenesis.
The ephrinB2-EphB4-RASA1 axis is a potentially targetable node for therapeutic intervention.
GLOSSARY
- CCLA:
A type of complex lymphatic anomaly characterized by dilated lymphatic channels, lymphatic channel dysmotility and distal obstruction affecting lymphatic drainage [27].
- Conotruncal heart defects
A group of congenital cardiac outflow tract anomalies that classically include tetralogy of Fallot, pulmonary atresia with ventricular septal defect, double-outlet right ventricle, double-outlet left ventricle, truncus arteriosus and transposition of the great arteries [75].
- Trio based cohort:
Cohort composed of parent-offspring trios. This kind of pedigree enables the identification of de novo, recessive, and transmitted heterozygous mutations.
- Ephrin-Eph forward, reverse, parallel, and anti-parallel signaling:
Eph receptors and ephrins function as classic receptors and ligands in ephrin:Eph forward signaling. However, the roles of Eph and ephrin proteins can be switched to ligands and receptors respectively in Eph:ephrin reverse signaling. When Eph receptors and ephrins are localized on the same cell surface, they can act as ligands for ephrins and Eph receptors respectively to activate signaling in the same direction (parallel signaling) or they can each act as receptors and ligands to activate signaling in opposite directions (anti-parallel signaling) [10, 11].
- LoF mutation:
Mutations predicted in silico to cause frameshift, premature termination codon, stoploss, startloss, and/or affect canonical splice site.
- Paradominant inheritance:
A form of non-mendelian inheritance where carriers of the heterozygous mutation are phenotypically normal until postzygotic loss of heterozygosity occurs at an early developmental stage that forms a mosaic patch affected by the disease [76].
- RAS:
The RAS gene family encodes membrane-associated guanine-nucleotide binding proteins (p21), which cycle between an active guanosine-triphosphate (GTP) bound form and an inactive guanosine-diphosphate (GDP) bound form.
- Sushi domain:
Also known as complement control protein (CCP) module. It is an evolutionarily conserved protein domain of about 60 amino acids in length that is characterized by a consensus sequence including 4 invariant cysteines, an almost invariant tryptophan and highly conserved prolines, glycines and hydrophobic residues [77].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
None.
REFERENCES
- [1].Sabin FR, Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res, 2002. 11(1): p. 5–7. [DOI] [PubMed] [Google Scholar]
- [2].Wang HU, Chen ZF, and Anderson DJ, Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph- B4. Cell, 1998. 93(5): p. 741–53. [DOI] [PubMed] [Google Scholar]
- [3].Eichmann A, et al. , Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol, 2005. 49(2–3): p. 259–67. [DOI] [PubMed] [Google Scholar]
- [4].Fish JE and Wythe JD, The molecular regulation of arteriovenous specification and maintenance. Dev Dyn, 2015. 244(3): p. 391–409. [DOI] [PubMed] [Google Scholar]
- [5].Adams RH, et al. , Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev, 1999. 13(3): p. 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gerety SS, et al. , Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell, 1999. 4(3): p. 40–314. [DOI] [PubMed] [Google Scholar]
- [7].Herbert SP, et al. , Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science, 2009. 326(5950): p. 294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kawasaki J, et al. , RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J Clin Invest, 2014. 124(6): p. 2774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuijper S, Turner CJ, and Adams RH, Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med, 2007. 17(5): p. 145–51. [DOI] [PubMed] [Google Scholar]
- [10].Kania A and Klein R, Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol, 2016. 17(4): p. 240–56. [DOI] [PubMed] [Google Scholar]
- [11].Taylor H, Campbell J, and Nobes CD, Ephs and ephrins. Curr Biol, 2017. 27(3): p. R90–R95. [DOI] [PubMed] [Google Scholar]
- [12].Egea J, et al. , Regulation of EphA 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function. Neuron, 2005. 47(4): p. 515–28. [DOI] [PubMed] [Google Scholar]
- [13].Himanen JP, et al. , Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A, 2010. 107(24): p. 10860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mak CCY, et al. , De novo large rare copy-number variations contribute to conotruncal heart disease in Chinese patients. NPJ Genom Med, 2016. 1: p. 16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levy J, et al. , EFNB2 haploinsufficiency causes a syndromic neurodevelopmental disorder. Clin Genet, 2018. 93(6): p. 1141–1147. [DOI] [PubMed] [Google Scholar]
- [16].Duran D, et al. , Mutations in Chromatin Modifier and Ephrin Signaling Genes in Vein of Galen Malformation. Neuron, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adams RH, et al. , The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell, 2001. 104(1): p. 57–69. [DOI] [PubMed] [Google Scholar]
- [18].Cowan CA, et al. , Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol, 2004. 271(2): p. 263–71. [DOI] [PubMed] [Google Scholar]
- [19].Lisle JE, et al. , Murine, but not human, ephrin-B2 can be efficiently cleaved by the serine protease kallikrein-4: implications for xenograft models of human prostate cancer. Exp Cell Res, 2015. 333(1): p. 136–46. [DOI] [PubMed] [Google Scholar]
- [20].Gerety SS and Anderson DJ, Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development, 2002. 129(6): p. 1397–410. [DOI] [PubMed] [Google Scholar]
- [21].Dravis C, et al. , Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol, 2004. 271(2): p. 272–90. [DOI] [PubMed] [Google Scholar]
- [22].Lewis AE, et al. , Neural crest defects in ephrin-B2 mutant mice are non-autonomous and originate from defects in the vasculature. Dev Biol, 2015. 406(2): p. 186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luxey M, et al. , Generation of transgenic mice overexpressing EfnB2 in endothelial cells. Genesis, 2011. 49(10): p. 811–20. [DOI] [PubMed] [Google Scholar]
- [24].Foo SS, et al. , Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell, 2006. 124(1): p. 161–73. [DOI] [PubMed] [Google Scholar]
- [25].Oike Y, et al. , Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood, 2002. 100(4): p. 1326–33. [PubMed] [Google Scholar]
- [26].Martin-Almedina S, et al. , EPHB4 kinase-inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. J Clin Invest, 2016. 126(8): p. 3080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li D, et al. , Pathogenic variant in EPHB4 results in central conducting lymphatic anomaly. Hum Mol Genet, 2018. 27(18): p. 3233–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krebs LT, et al. , Notchl activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis, 2010. 48(3): p. 146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Preuss C, et al. , Family Based Whole Exome Sequencing Reveals the Multifaceted Role of Notch Signaling in Congenital Heart Disease. PLoS Genet, 2016. 12(10): p. e1006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Revencu N, et al. , RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat, 2013. 34(12): p. 1632–41. [DOI] [PubMed] [Google Scholar]
- [31].Amyere M, et al. , Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS- MAPK Signaling. Circulation, 2017. 136(11): p. 1037–1048. [DOI] [PubMed] [Google Scholar]
- [32].Yu J, et al. , EPHB4 Mutation Implicated in Capillary Malformation-Arteriovenous Malformation Syndrome: A Case Report. Pediatr Dermatol, 2017. 34(5): p. e227–e230. [DOI] [PubMed] [Google Scholar]
- [33].Vivanti A, et al. , Loss of function mutations in EPHB4 are responsible for vein of Galen aneurysmal malformation. Brain, 2018. 141(4): p. 979–988. [DOI] [PubMed] [Google Scholar]
- [34].Brambilla R, et al. , Membrane-bound LERK2 ligand can signal through three different Eph-related receptor tyrosine kinases. EMBO J, 1995. 14(13): p. 3116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sakano S, et al. , Characterization of a ligand for receptor protein-tyrosine kinase HTK expressed in immature hematopoietic cells. Oncogene, 1996. 13(4): p. 813–22. [PubMed] [Google Scholar]
- [36].Shin D, et al. , Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol, 2001. 230(2): p. 139–50. [DOI] [PubMed] [Google Scholar]
- [37].Wang Y, et al. , EPHB4 Protein Expression in Vascular Smooth Muscle Cells Regulates Their Contractility, and EPHB4 Deletion Leads to Hypotension in Mice. J Biol Chem, 2015. 290(22): p. 14235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Trahey M, et al. , Molecular cloning of two types of GAP complementary DNA from human placenta. Science, 1988. 242(4886): p. 1697–700. [DOI] [PubMed] [Google Scholar]
- [39].Scheffzek K, et al. , The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science, 1997. 277(5324): p. 333–8. [DOI] [PubMed] [Google Scholar]
- [40].Eerola I, et al. , Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet, 2003. 73(6): p. 1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Revencu N, et al. , Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat, 2008. 29(7): p. 959–65. [DOI] [PubMed] [Google Scholar]
- [42].Buhl T, et al. , Multifocal capillary malformations due to RASA1 mutation misdiagnosed as cutaneous mastocytosis. Arch Dermatol, 2012. 148(11): p. 1334–5. [DOI] [PubMed] [Google Scholar]
- [43].de Wijn RS, et al. , Phenotypic variability in a family with capillary malformations caused by a mutation in the RASA1 gene. Eur J Med Genet, 2012. 55(3): p. 191–5. [DOI] [PubMed] [Google Scholar]
- [44].Wooderchak-Donahue W, et al. , RASA1 analysis: clinical and molecular findings in a series of consecutive cases. Eur J Med Genet, 2012. 55(2): p. 91–5. [DOI] [PubMed] [Google Scholar]
- [45].Durrington HJ, et al. ; 1;, A novel RASA1 mutation causing capillary malformation- arteriovenous malformation (CM-AVM) presenting during pregnancy. Am J Med Genet A, 2013. 161A(7): p. 1690–4. [DOI] [PubMed] [Google Scholar]
- [46].Lapinski PE, et al. ; 1;, Somatic second hit mutation of RASA1 in vascular endothelial cells in capillary malformation-arteriovenous malformation. Eur J Med Genet, 2018. 61(1): p. 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cai R, et al. ; 1;, A novel RASA1 mutation causing capillary malformation-arteriovenous malformation (CM-AVM): the first genetic clinical report in East Asia. Hereditas, 2018. 155: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eerola I, et al. ; 1;, Locus for susceptibility for familial capillary malformation (′port-wine stain′) maps to 5q. Eur J Hum Genet, 2002. 10(6): p. 375–80. [DOI] [PubMed] [Google Scholar]
- [49].Hershkovitz D, et al. ; 1;, RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol, 2008. 158(5): p. 1035–40. [DOI] [PubMed] [Google Scholar]
- [50].Hershkovitz D, Bergman R, and Sprecher E, A novel mutation in RASA1 causes capillary malformation and limb enlargement. Arch Dermatol Res, 2008. 300(7): p. 385–8. [DOI] [PubMed] [Google Scholar]
- [51].Kim C, et al. ; 1;, Histopathologic and ultrasound characteristics of cutaneous capillary malformations in a patient with capillary malformation-arteriovenous malformation syndrome. Pediatr Dermatol, 2015. 32(1): p. 128–31. [DOI] [PubMed] [Google Scholar]
- [52].Thiex R, et al. ; 1;, A novel association between RASA1 mutations and spinal arteriovenous anomalies. AJNR Am J Neuroradiol, 2010. 31(4): p. 775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Walcott BP, et al. ; 1;, Pial arteriovenous fistulae in pediatric patients: associated syndromes and treatment outcome. J Neurointerv Surg, 2013. 5(1): p. 10–4. [DOI] [PubMed] [Google Scholar]
- [54].Grillner P, et al. ; 1;, A spectrum of intracranial vascular high-flow arteriovenous shunts in RASA1 mutations. Childs Nerv Syst, 2016. 32(4): p. 709–15. [DOI] [PubMed] [Google Scholar]
- [55].Chida A, et al. ; 1;, ACVRL1 gene variant in a patient with vein of Galen aneurysmal malformation. J Pediatr Genet, 2013. 2(4): p. 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Heuchan A, et al. ; 1;, RASA1 Mutations and Vein of Galen Arterial Malformations. Arch Dis Child, 2013. 98 (Suppl 1): p. A16–A17. [Google Scholar]
- [57].Henkemeyer M, et al. , Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature, 1995. 377(6551): p. 695–701. [DOI] [PubMed] [Google Scholar]
- [58].Boon LM, Mulliken JB, and Vikkula M, RASA1: variable phenotype with capillary and arteriovenous malformations. Curr Opin Genet Dev, 2005. 15(3): p. 265–9. [DOI] [PubMed] [Google Scholar]
- [59].Macmurdo CF, et al. , RASA1 somatic mutation and variable expressivity in capillary malformation/arteriovenous malformation (CM/AVM) syndrome. Am J Med Genet A, 2016. 170(6): p. 1450–4. [DOI] [PubMed] [Google Scholar]
- [60].Lubeck BA, et al. , Blood vascular abnormalities in Rasa1(R780Q) knockin mice: implications for the pathogenesis of capillary malformation-arteriovenous malformation. Am J Pathol, 2014. 184(12): p. 3163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lapinski PE, et al. , Generation of mice with a conditional allele of the p120 Ras GTPase-activating protein. Genesis, 2007. 45(12): p. 762–7. [DOI] [PubMed] [Google Scholar]
- [62].Lapinski PE, et al. , RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest, 2012. 122(2): p. 733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Burrows PE, et al. , Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proc Natl Acad Sci U S A, 2013. 110(21): p. 8621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lapinski PE, et al. , RASA1 regulates the function of lymphatic vessel valves in mice. J Clin Invest, 2017. 127(7): p. 2569–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Brouillard P, et al. , Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet, 2002. 70(4): p. 866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pagenstecher A, et al. , A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet, 2009. 18(5): p. 911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Timberlake AT, et al. , Two locus inheritance of non-syndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stahl S, et al. , Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: in-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum Mutat, 2008. 29(5): p. 709–17. [DOI] [PubMed] [Google Scholar]
- [69].Govani FS and Shovlin CL, Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet, 2009. 17(7): p. 860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McDonald MT, et al. , A disease locus for hereditary haemorrhagic telangiectasia maps to chromosome 9q33–34. Nat Genet, 1994. 6(2): p. 197–204. [DOI] [PubMed] [Google Scholar]
- [71].Shovlin CL, et al. , A gene for hereditary haemorrhagic telangiectasia maps to chromosome 9q3. Nat Genet, 1994. 6(2): p. 205–9. [DOI] [PubMed] [Google Scholar]
- [72].Johnson DW, et al. , Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet, 1996. 13(2): p. 189–95. [DOI] [PubMed] [Google Scholar]
- [73].Gallione CJ, et al. , A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet, 2004. 363(9412): p. 852–9. [DOI] [PubMed] [Google Scholar]
- [74].Wooderchak-Donahue WL, et al. , BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet, 2013. 93(3): p. 530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Anaclerio S, et al. , Conotruncal heart defects: impact of genetic syndromes on immediate operative mortality. Ital Heart J, 2004. 5(8): p. 624–8. [PubMed] [Google Scholar]
- [76].Danarti R, Happle R, and Konig A, Paradominant inheritance may explain familial occurrence of Cutis marmorata telangiectatica congenita. Dermatology, 2001. 203(3): p. 208–11. [DOI] [PubMed] [Google Scholar]
- [77].Kirkitadze MD and Barlow PN, Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev, 2001. 180: p. 146–61. [DOI] [PubMed] [Google Scholar]
- [78].Carr CW, et al. , 5q14.3 neurocutaneous syndrome: a novel continguous gene syndrome caused by simultaneous deletion of RASA1 and MEF2C. Am J Med Genet A, 2011. 155A(7): p. 1640–5. [DOI] [PubMed] [Google Scholar]
- [79].Flore LA, et al. , RASA1 analysis guides management in a family with capillary malformation-arteriovenous malformation. J Pediatr Genet, 2012. 1(2): p. 125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hernandez F, et al. , Mutations in RASA1 and GDF2 identified in patients with clinical features of hereditary hemorrhagic telangiectasia. Hum Genome Var, 2015. 2: p. 15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kwong Y, et al. , Fetal MRI demonstrating vein of Galen malformations in two successive pregnancies--a previously unreported occurrence. Childs Nerv Syst, 2015. 31(7): p. 1033–5. [DOI] [PubMed] [Google Scholar]
- [82].Whitaker S, et al. , Multifocal capillary malformations in an older, asymptomatic child with a novel RASA1 mutation. Clin Exp Dermatol, 2016. 41(2): p. 156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]