Abstract
Background:
The Icelandic volcano Laki erupted from June 1783 through January 1784. It produced 122 megatons of sulfur dioxide, particulate matter, and acid rain, and contributed to one of the coldest winters on record in Western Europe. Although uncontrollable volcanic eruptions continue, few studies have investigated their perinatal health implications.
Methods:
Using the Human Mortality Database, we assessed the association between the Laki event and the secondary sex ratio, infant mortality rates, and the number of births from 1751–1800 with time-series models that controlled for temporal trends.
Results:
The secondary sex ratio decreased 3% below expected levels in 1784 (95% CI: −4% to −1%). Both female and male infant mortality rates exceeded expectation in 1785, by 54% (95% CI: 25% to 83%) and 37% (−1% to 74%), respectively. We observed little change in female live births, but a reduction in male live births in 1784.
Conclusion:
Our findings are consistent with the hypothesis that the large-scale Laki volcanic eruptions of 1783–1784 resulted in adverse perinatal health outcomes in Sweden.
Keywords: Volcanic Eruptions, Infant Mortality, Sex Ratio, Pregnancy, Sulfur Dioxide, Aerosols, Acid Rain, Sweden
INTRODUCTION
The Icelandic volcano Laki erupted in June 1783, and while over 50% of the lava eruption occurred in the first 48 days, the volcano remained intermittently active through January of 1784.1 One of the largest in recorded history, the basaltic Laki eruption released 80 times the sulfuric aerosol produced in the more contemporary American Mount St. Helens eruption in 1980.2 Successive eruptions from the Laki volcano released an estimated 122 megatons of sulfur dioxide (SO2), hydrogen chloride and fluoride, and particulate matter into the atmosphere.1 From Iceland, sulfate aerosol traveled the jet stream, arrived in Western Europe in late June 1783, and resulted in months of poor air quality and acid rain that withered crops.1 Observations of the malodorous fog appeared in personal diaries, weather logs, and scientific articles. Swedes referred to the dry fog as “sol-röken,” or sun smoke.1 Europeans reported respiratory distress, headaches, and eye irritation.3,4 Laki also resulted in a severe winter in 1783–84,1,4 with an average winter temperature in Stockholm 3°C below the 250-year average.5
The Laki eruption led to increased morbidity and mortality across Europe and such an event could reoccur. Sonnek et al. predict that a similar-scale Icelandic eruption could result in average summer SO2 levels in Sweden of 60 μg/m3, with a peak >1000 μg/m3.6 Deaths in present-day Europe could total up to 140,000 based on increases in particulate matter alone.7 However, to our knowledge, just one prior study8 has examined the adverse perinatal health consequences associated with volcanic eruptions, and an eruption far less intense than the Laki event.
Prior studies have reported associations between air pollution—including SO2—and adverse birth outcomes, reduced fertility, and reduced secondary sex ratio (male-to-female ratio at birth).9–13 The fetal and early neonatal periods may be periods of heightened vulnerability to environmental stressors.14 Environmental adversity may trigger reproductive suppression via disrupted ovulation, impaired implantation or selection in utero, thereby allowing the mother to wait for more favorable conditions in which to produce offspring.15 Moreover, in times of environmental stress, once born, infants require increased parental investment to buffer against environmental insults.16 Based, in part, on these theories of reproductive suppression, we hypothesized that the Laki eruptions affected the secondary sex ratio and infant mortality, propositions we tested using birth data from 1751–1800 in Sweden.
METHODS
Birth data
We used the number of male and female births and sex-specific death rates in the first year of life from 1751 to 1800 in Sweden from the Human Mortality Database.17 Originally collected through parish registers, these historical data cover the current geographic region of the country. The Human Mortality Database follows a uniform set of procedures to ensure comparable mortality rates over time and across populations. We calculated two dependent variables of interest: annual secondary sex ratio (ratio of male to female live births); and annual infant mortality rate (sex-specific number of deaths in first year of life per 1000 live births). In a sensitivity analysis, we calculated the annual number of sex-specific live births. This analysis allowed us to examine why we observed a change in the secondary sex ratio, if such a change occurred. We transformed these variables to their natural logarithms to allow us to describe any unexpected values coincident with the Laki event as percent differences from expected.
Statistical analysis
We used Box-Jenkins transfer function models18 to account for autocorrelation including secular trends, cycles, and persistence of high and low observations to arrive at counterfactual values of the dependent variables (see eAppendix), where the counterfactual condition is that the Laki volcano did not erupt. We used these models to assess the relationship, net of autocorrelation, between the 1783–1784 Laki eruptions and the natural-logarithm of the secondary sex ratio, sex-specific infant mortality rates, and sex-specific birth rates. Because we log-transformed our dependent variables (Y), the beta coefficients from our models can be interpreted as approximating the percent change in Y associated with the Laki event. We also included the Great Famine of 1773, a known cause of unexpected birth outcomes, as a predictor in our models so that it did not inflate the estimated standard errors.
RESULTS
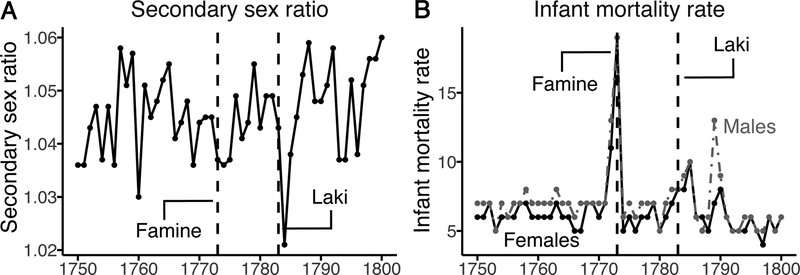
Over the 50-year study period (1751–1800), the annual secondary sex ratio in Sweden ranged from 1.02 to 1.06 (mean = 1.05, SD = 0.01), reaching its nadir in 1784 (Figure panel A). The annual male infant mortality rate ranged from 5 to 19 per 1000 (mean = 7, SD = 2) and the annual female infant mortality rate from 4 to 19 per 1000 (mean = 6, SD = 2). Annual infant mortality rates for both males and females peaked in 1773 coincident with a large famine, but annual infant mortality rates in 1784 and 1785 were all at or above the sex-specific 90th-percentile (Figure 1B).
Figure.
Secondary sex ratio (ratio of male to female live births) and infant mortality rate (deaths under 1 year per 1000 live births) in Sweden, 1751–1800. (A) The line shows sex ratio; (B) The black line shows female infant mortality rate; the grey dashed line, male infant mortality rate.
Final models showed an annual secondary sex ratio 3% lower than expected in 1784 (95% CI: −4% to −1%) (Table). Annual infant mortality rates also exceeded expectation; for females in 1784 and 1785 and for males in 1785. In 1784, the annual infant mortality rate among females increased by 29% (95% CI: 1% to 58%). The rate in 1784 increased by a similar magnitude for males (i.e., by 31%, 95% CI: −6 % to 68%) but the greater variability in male than female infant mortality over the study period led to a wider confidence interval. Both female and male infant mortality rates exceeded expectation in 1785, by 54% (95% CI: 25% to 83%) and 37% (−1% to 74%), respectively. In a sensitivity analysis (Table 1), we saw no major change in the annual number of female live births overall, but consistent with selection in utero, annual male live births fell below the expected value in both 1783 (−10%, 95% CI: −17% to −2%) and 1784 (−11%, 95% CI: −21% to 0%).
Table.
95% confidence intervals in parentheses) for Box-Jenkins transfer function models predicting the natural logarithms of the secondary sex ratio, sex-specific infant mortality, and the first differences of sex-specific live births (n = 50 years beginning 1751).
| Sex Ratio | Male Infant Mortality | Female Infant Mortality | Male Births | Female Births | |
|---|---|---|---|---|---|
| Constant | 0.05 (0.04, 0.05) | −5.0 (−5.1, −4.9) | −5.1 (−5.2, −5.1) | None | None |
| 1773 (Famine) | −0.01 (−0.02, 0) | 0.80 (0.49, 1.1) | 1.1 (0.84, 1.4) | −0.18 (−0.23, −0.13) | −0.22 (−0.29, −0.14) |
| 1783 (Volcano) | 0 (−0.02, 0.01) | 0.20 (−0.13, 0.52) | 0.24 (−0.05, 0.53) | −0.10 (−0.17, −0.02) | −0.05 (−0.14, 0.04) |
| 1784 (Volcano) | −0.03 (−0.01, −0.04) | 0.31 (−0.06, 0.68) | 0.29 (−0.01, 0.58) | −0.11 (−0.21, 0) | 0 (−0.11, 0.12) |
| 1785 | −0.01 (−0.02, 0.01) | 0.37 (−0.01, 0.74) | 0.54 (0.25, 0.83) | −0.03 (−0.14, 0.16) | −0.01 (−0.12, 0.11) |
| 1786 | 0 (−0.02, 0.01) | −0.05 (−0.38, 0.27) | 0 (−0.29, 0.29) | 0.08 (0, 0.16) | 0.04 (−0.05, 0.13) |
| Moving Averagea | None | lag 1 −0.55 (0.14) | None | lag 3 0.62 (0.13) | None |
| Autoregressiona | None | None | None | lag 2 −0.53 (0.13) | None |
Moving average and autoregressive terms were added to remove autocorrelation from the dependent variables. The terms were identified using Box-Jenkins methods. No autocorrelation appeared in the dependent variable if no moving average or autoregressive terms were added (i.e., “None”). The eAppendix discusses model specification including moving average and autoregressive terms in greater detail.
DISCUSSION
Following the Laki eruptions in 1783–1784, a malodorous fog arrived in Western Europe, harming harvests and contributing to a colder than average winter.1 Due to development characterized by rapid cell proliferation, differentiation, and organ development, fetuses may be particularly susceptible to airborne toxins.14 Recent literature finds associations between air pollution and adverse birth outcomes,9 pregnancy loss,11 and infant mortality.10,12 The present analyses suggest that the Laki eruptions resulted in environmental change severe enough to reduce the secondary sex ratio and increase the infant mortality rate in Sweden in the following years.
Air pollution may affect perinatal health through pathways of oxidative stress and inflammation, DNA damage, and changes in endothelial function or blood flow. In the Czech Republic, Bobak and Leon reported an association between a 50 μg/m3 increase in SO2 and infant death from respiratory causes.19 Mothers also become more susceptible to respiratory problems during pregnancy, in part due to reduced functional residual capacity.20 The Laki eruptions resulted in substantially elevated levels of SO2 and particulate matter, reduced crop yields, and contributed to a frigid winter in Sweden. We observed increased infant mortality that persisted from 1784–1785. Consistent with previous studies on elevated air pollution13 and cold temperatures,21 we find a reduced secondary sex ratio the year following the Laki eruptions, driven by reduced numbers of live male births and little change in live female births. These observations provide support for the argument that the Laki eruption caused selection in utero against frail males. The elevated infant mortality in 1785 may be explained by reduced selection in utero, resulting in a weaker birth cohort confronted with persistent environmental effects from Laki.1
We observed a reduced annual secondary sex ratio in the year after the Laki eruption, which aligns with prior literature, based on evolutionary biology that finds reproductive suppression in times of environmental stress.22 After conception, environmental shocks appear to induce selection against frail fetuses, particularly small males.22,23 If born, these small male offspring also require more maternal investment during infancy and childhood compared to females.24 Therefore, when circumstances either reduce maternal capacity to invest in offspring (e.g., reduced maternal pulmonary function) or increase need for maternal investment (e.g., adverse environmental conditions),16 mechanisms conserved by natural selection may spontaneously abort small males fetuses, reducing the secondary sex ratio at birth.
Our analysis relied on high quality, historical birth data over a 50-year period from Sweden, during a time period with little out-migration or medical intervention. This should have improved the quality of our estimates. Limitations included coarse temporal (i.e., annual) resolution data, lack of individual-level data, and inability to determine which environmental conditions brought on by the eruptions–reduced air quality, crop yields, and record-low winter temperatures–may have harmed perinatal health.
Laki-like events occur in Iceland every several 100 years; the 2010 Eyjafjallajoökull and 2011 Grimsvoötn volcanic eruptions being recent, small-scale examples. A present-day Laki-type eruption could trigger peak SO2 concentrations high enough to induce respiratory symptoms and aggravate heart and lung disease in Sweden.6 Our results suggests that pregnant women would be particularly vulnerable during such an event. However, modern-day heating systems and global food trade would likely buffer pregnant women against cold temperatures and food shortages. Further assessment of the consequences of severe of volcanic eruptions for pregnant women and their gestations may assist public health professionals in disseminating health recommendations and supplying necessary medical care during future air pollution emergencies.
Supplementary Material
Acknowledgments:
This report was supported, in part, by a National Institute of Environmental Health Sciences grant K99ES027023 to J.A.C.
Footnotes
Conflict of interest: We report no conflict of interest.
Data access description: Data from the Human Mortality Database are publicly available at mortality.org.
REFERENCES
- 1.Thordarson T, Self S. Atmospheric and environmental effects of the 1783–1784 Laki eruption: A review and reassessment. J Geophys Res Atmos 2003;108(D1). [Google Scholar]
- 2.Decker R, Decker B. The eruptions of Mount St. Helens. Scientific American 1981;244(3):68–81. [Google Scholar]
- 3.Durand M, Grattan J. Extensive respiratory health effects of volcanogenic dry fog in 1783 inferred from European documentary sources. Environ Geochem Health 1999;21(4):371–376. [Google Scholar]
- 4.Witham CS, Oppenheimer C. Mortality in England during the 1783–4 Laki Craters eruption. Bull Volcanol 2004;67(1):15–26. [Google Scholar]
- 5.Bolin Centre for Climate Research. IMPROVE - Improved Understanding of Past Climatic Variability from Early Daily European Instrumental Sources. EU 4th Framework Programme. Contract ENV4-CT97–0511. 1998–1999. https://bolin.su.se/data/stockholm/homogenized_daily_mean_temperatures.php Accessed 17 Jun 2018.
- 6.Sonnek KM, Mårtensson T, Veibäck E, Tunved P, Grahn H, von Schoenberg P, Brännström N, Bucht A. The impacts of a Laki-like eruption on the present Swedish society. Nat Hazards 2017;88(3):1565–1590. [Google Scholar]
- 7.Schmidt A, Ostro B, Carslaw KS, Wilson M, Thordarson T, Mann GW, Simmons AJ. Excess mortality in Europe following a future Laki-style Icelandic eruption. Proc Natl Acad Sci U S A 2011;108(38):15710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balsa AI, Caffera M, Bloomfield J. Exposures to particulate matter from the eruptions of the Puyehue volcano and birth outcomes in Montevideo, Uruguay. Environ Health Perspect 2016;124(11):1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–11. [DOI] [PubMed] [Google Scholar]
- 10.Chay KY, Greenstone M. The impact of air pollution on infant mortality: evidence from geographic variation in pollution shocks induced by a recession. Q J Econ 2003;118(3):1121–1167. [Google Scholar]
- 11.Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health 2017;16(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sram RJ, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 2005;113(4):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenfels AJF, Gomes JB, Pieri PC, Miraglia SGEK, Hallak J, Saldiva PH. Increased levels of air pollution and a decrease in the human and mouse male-to-female ratio in São Paulo, Brazil. Fertil Steril 2007;87(1):230–232. [DOI] [PubMed] [Google Scholar]
- 14.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environmental health perspectives 2000;108(Suppl 3):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasser SK, Barash DP. Reproductive suppression among female mammals: implications for biomedicine and sexual selection theory. The Quarterly Review of Biology 1983;58(4):513–538. [DOI] [PubMed] [Google Scholar]
- 16.Quinlan RJ. Human parental effort and environmental risk. Proc R Soc Lond B Biol Sci 2007;274(1606):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Human Mortality Database, University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). www.mortality.org Accessed 10 Jul 2017.
- 18.Catalano R, Serxner S. Time series designs of potential interest to epidemiologists. Am J Epidemiol 1987;126(4):724–31. [DOI] [PubMed] [Google Scholar]
- 19.Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology 1999:666–670. [PubMed] [Google Scholar]
- 20.Bhatia P, Bhatia K. Pregnancy and the lungs. Postgrad Med J 2000;76(901):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano R, Bruckner T, Smith KR. Ambient temperature predicts sex ratios and male longevity. Proc Natl Acad Sci U S A 2008;105(6):2244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruckner TA, Catalano R. Selection in utero and population health: Theory and typology of research. SSM Popul Health 2018;5:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973;179(4068):90–92. [DOI] [PubMed] [Google Scholar]
- 24.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol 2000;202(1):65–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.