Abstract
Cancer-related fatigue is a debilitating syndrome that persists for many cancer survivors for years after treatment. Symptoms include early and persistent fatigue, functional decline, depression and cognitive difficulties. Inflammation, assessed using pro-inflammatory biomarkers, is increased in cancer survivors with fatigue and treatments for fatigue are often aimed at reducing inflammation. Additionally, cancer and its treatment lead to nutritional complications, changes in body composition and nutritional deficiencies that potentially weaken the cancer survivor and impact cancer-related fatigue. We conducted a qualitative review of clinical trials that assessed nutritional interventions for preventing and treating cancer-related fatigue. Further studies were examined that used nutritional interventions to address inflammation and fatigue, due to the dearth of nutrition research directly related to cancer-related fatigue. Dietary intake prior to, during and after cancer treatment appears to affect fatigue levels. Increased protein intake may help preserve lean mass and body composition. Dietary patterns that reduce inflammation, such as the Mediterranean diet and other plant-based diets, appear tolerable to cancer survivors and may reduce fatigue. Supplementation with ginseng, ginger or probiotics may improve cancer survivors’ energy levels. Nutritional interventions, alone or in combination with other interventions should be considered as therapy for fatigue in cancer survivors.
Keywords: cachexia; vitamin E, C, D; systemic inflammatory response; soy; dietary intervention; fat/omega-3; omega-6/fish
INTRODUCTION
Cancer-related fatigue (CRF) is a persistent and debilitating syndrome that affects the majority of cancer survivors (1, 2). For many, fatigue improves within one year after treatment completion, but may persist for years in a significant number of cancer patients (2). Clinical symptoms for cancer patients experiencing CRF include cognitive difficulties, early and persistent fatigue, hot flashes, functional decline, insomnia, depression and a significant reduction in quality of life (QOL) (3, 4). The level of fatigue and range of symptoms vary depending on cancer type and treatment as well as the patient’s pre-treatment health and nutritional status (5–7).
Currently, there are limited treatment options for CRF in cancer survivors. These options include: 1) pharmaceutical agents, which have limited efficacy for the reduction of CRF and can lead to toxicities, negative interactions with cancer therapeutics (e.g., aromatase inhibitors or tamoxifen), and dependency (8); 2) psychological interventions (e.g., cognitive behavioral therapy), which can be unintuitive and demanding for survivors and therefore, difficult to comply with, limiting adoption, compliance and maintenance; and 3) exercise, which is recommended in treatment guidelines but not widely implemented in survivorship care plans beyond the use of generalized statements in which healthcare professionals encourage survivors to be physically active and exercise (8–10). Such interventions also may be unmanageable and time-consuming for cancer survivors. Nutrition addresses fatigue and is often less cumbersome than other interventions. At the same time, there is only preliminary research regarding dietary interventions and nutritional supplements for cancer survivors experiencing fatigue.
A variety of biological mechanisms (e.g. hypothalamic-pituitary-adrenal axis dysregulation, inflammatory cytokine dysregulation, anemia) may play a role in CRF etiology (2). Among those mechanisms, dysregulation of the pro-inflammatory cytokine network (increased inflammation) is the most commonly studied. Cancer treatment and cancerous tumors can activate the pro-inflammatory cytokine network, leading to symptoms of fatigue via cytokine signaling in the central nervous system (2, 11). Furthermore, previous research shows that pro-inflammatory markers, cytokines and C-reactive protein (CRP) are elevated in cancer survivors with fatigue (12–15). Therefore, treatments for fatigue in cancer patients are often aimed at reducing inflammation.
Nutritional Complications and Fatigue in Cancer Survivors
Nutritional complications arise as a consequence of cancer and its treatment, including altered taste, anorexia, unintentional weight loss, or in some cases, increased adiposity or obesity (16–18). Chemo-radiotherapy may aggravate existing symptoms or result in side effects such as loss of taste, xerostomia, nausea and vomiting and eventual malnutrition (19). Furthermore, many cancer patients suffer nutritional deficiencies as the systemic nature of cancer promotes metabolic deregulation and increased catabolism or even cachexia. Cachexia often cannot be fully reversed by conventional nutritional support; many cancer survivors with loss of lean mass suffer long-term decreased functionality (17). Reduced lean mass is tied to fatigue and maintaining or even building muscle appears to prevent or reduce symptoms of CRF (20, 21).
Additionally, there is a combined condition termed sarcopenic obesity where a cancer patient experiences a loss of lean mass concurrently with increased adiposity (16, 22). Sarcopenic obesity may lead to greater loss of functionality, lower QOL and greater mortality risk compared to cachexia or obesity alone (22–24). Breast cancer patients experience unintentional weight gain in part due to the effects of chemotherapy, endocrine treatment and/or postmenopausal status (25–27). Increased obesity is not necessarily protective against sarcopenia and can result in sarcopenic obesity in breast cancer survivors (28–30). One recent study showed that sarcopenic obesity promoted inflammation and fatigue, appearing to further weaken breast cancer survivors (31). Furthermore, chronic low-grade inflammation, as seen in obesity, is considered a factor in the development of CRF (2). The need for nutritional interventions addressing inflammation, changes in body composition and functionality for cancer survivors is critical. Currently, evidence-based nutritional guidelines for alleviating CRF are limited despite the high prevalence of nutritional problems related to fatigue and QOL in cancer survivors (32).
METHODS
We performed a qualitative review of the literature for cancer-related fatigue and nutrition in October 2017 for studies published between 1971 and 2017. An initial scoping strategy was developed on PubMed. Once the PubMed search strategy was determined, the medical librarian (D.C.) translated this search strategy for Embase and Web of Science. The strategies used for PubMed and Embase used a combination of keywords and controlled vocabulary. Where Web of Science does not use controlled vocabulary, only keywords were used. Due to resource constraints, we did not include any grey literature or unpublished data. The search terms included a combination of the following keywords: Neoplasms, Cancer, Oncology, Tumor, Nutrition Therapy, Nutritional Status, Diet, Food, Nutrition, Nutrition Assessment, Nutritional Sciences, Fatigue, Lassitude, Tired, and Exhausted. Truncation was used to find different variations of these keywords. The database searches identified over 3,800,000 articles, however, most did not specifically address the impact of nutrition on CRF or fatigue. After further narrowing the search based on studies addressing cancer survivors, CRF, fatigue, and nutrition using the deduplication feature on EndNote, 4,672 unique articles were identified. Other limits that were used included clinical trial and randomized controlled trials (Supplementary Table 1). The primary author (J.E.I.) reviewed abstracts to determine if they met the inclusion criteria for review. Data such study population, design characteristics, primary results relevant to CRF, inflammation and cancer survivorship were recorded.
From this search, only 17 studies were identified that specifically assessed the impact of nutritional interventions on CRF and/or inflammation in cancer survivors (Table 2). Due to the dearth of nutrition studies specific to addressing CRF, further research in PubMed and Web of Science was conducted to evaluate individual nutrients and interventions, such as vitamin B12 and probiotics, for potential benefit to cancer survivors in reducing fatigue and inflammation. In this research, the term cancer survivor refers to a patient who is finished with adjuvant treatment including chemotherapy, radiation, immunotherapy and surgery in this research. The cancer survivor may be on treatment not directly targeting cancer including tamoxifen, aromatase inhibitors and other hormonal therapy.
Table 2.
Study characteristics of trials involving nutritional interventions and CRF.
| Author (year) | Nutrient/Supplement | Design and Methods | Fatigue Scale | Reported Findings | Conclusion |
|---|---|---|---|---|---|
| Stobaus et al. (2015) (30) | Dietary protein intake | A prospective cohort study of 285 cancer patients undergoing chemotherapy was conducted. Protein and energy intakes were assessed by 24-h recall and CRF was measured with the Brief Fatigue Inventory. | Brief Fatigue Inventory (BFI) | Lower recent protein intake (<1 g/kg body weight) assessed by 24 h recall, was associated with more than a twofold higher risk of CRF and greater six-month mortality. Protein intake was a stronger contributor to CRF than nausea/vomiting, insomnia or age. | Lower protein intake (<1 g/kg body weight) is associated with more than a twofold increased risk of CRF in cancer patients undergoing chemotherapy. |
| Lesinski et al. (2015) (31) | Soy isoflavone supplementation | A phase II randomized clinical trial of 32 prostate cancer patients took place over eight weeks. | N/A | Pre/Post analyses indicated a significant decrease in TH1 and myeloid-derived suppressor cell cytokines (p<0.05) | Soy bread supplementation may modulate cellular biomarkers consistent with limiting inflammation and suppression of MDSCs. |
| Gramignano et al. (2006) (32) | l-carnitine supplementation | A prospective pilot study was conducted among 12 patients with various types advanced solid tumors, CRF, and/or high levels of reactive oxygen species. Patients received 6 g/d (2 g in three doses) of l-carnitine for four weeks while receiving antineoplastic treatment. Lean body mass was measured by bioelectrical impedance analysis. | Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) | The General and Physical Scores for the MFSI-SF decreased (p=0.001) and lean mass increased (p<0.05) from baseline to week 4. | L-carnitine supplementation may decrease CRF and promote increased lean mass. |
| Marx et al. (2017) (33) | Carnitine supplementation | A meta-analysis was performed using random-effects model on carnitine and CRF and 12 studies were included in the final review. | Functional Assessment of Cancer Therapy for Fatigue (FACT-F), MFSI-SF, BFI, EQ-5D Visual Analogue Scale | Eight studies reported that carnitine improved fatigue and four reported no benefit. Studies reporting improvement were overwhelmingly single arm, non-randomized studies with significant risk of bias. | More Phase III, randomized placebo-control trials are needed to examine the effect of carnitine on CRF. |
| Zick et al. (2017) (34) | Fatigue reduction diet: diet rich in fruit, vegetables, whole grains, and omega-3 fatty acid-rich foods | A three-month randomized pilot study of 30 breast cancer survivors, stages 0-III was conducted, where 15 received the Fatigue Reduction Diet and 15 controls received general health education with no dietary intervention. | BFI | Women receiving the dietary intervention experienced a greater decrease in CRF symptoms (p<0.01) compared to the control group. | Diets rich in whole grains, omega-3 rich foods and fruits and vegetables may reduce CRF in breast cancer patients. |
| Alfano et al. (2012) (35) | Intake of omega-3 polyunsaturated fatty acids (PUFAs) | A prospective cohort of 633 breast cancer survivors completed a food frequency questionnaire and provided blood samples for CRP and serum amyloid A analysis. | Piper Fatigue Scale | Those with high CRP had a greater odds of fatigue (OR=1.8; p<0.05). A higher intake of omega-6 relative to omega-3 PUFAs was associated with greater CRP (p=0.01). | Higher intake of omega-3 PUFAs are linked to decreased inflammation and decreased fatigue in cancer survivors. |
| Guest et al. (2013) (36) | Dietary intake of fat (% kcal/day) and fiber (g/day) | A cross-sectional study was performed from data obtained from a randomized controlled trial of 42 breast cancer survivors. Data from a 3-day diet record, physical activity and fatigue was analyzed. | FACT-F | Fatigue was positively associated with % kcal/day of fat (r=0.31, p<0.05). Mean fatigue was greater for participants consuming <25 g/day of fiber compared with >25 g/day of fiber (p<0.005). | Diets high in fiber and low in fat are associated with reduced fatigue in breast cancer survivors. |
| Okumatsu et al. (2017) (37) | Low kcalorie diet + exercise program | A randomized controlled trial was conducted amount 32 Japanese female breast cancer survivors undergoing endocrine therapy. For 12 weeks, 21 women were in the intervention group: 1200 kcal/day diet + 90 min/week exercise, and 11 were in the control group not receiving the intervention. | N/A | The intervention group experienced greater weight loss (p<0.001). Also, CRF scores decreased by 39% among the intervention group (p<.001) while remaining the same in the control group. | Breast cancer survivors may experience a significant reduction in CRF with weight loss and a combined diet plus exercise program. |
| Barton et al. (2013) (38) | 2000 mg Wisconsin ginseng | A double-blind randomized controlled trial was conducted among 364 cancer survivors with a CRF score of 4 or greater. Subjects were randomized to receive either 2000 mg Wisconsin ginseng or a placebo twice/day over eight weeks. | MFSI-SF | Changes from baseline in the general subscale of the MFSI-SF were 14.4 in the ginseng arm vs 8.2 in placebo at 4 weeks (p=0.07). A significant difference was seen at 8 weeks with a change score of 20 for the ginseng group and 10.3 for the placebo group (p=0.003). | Wisconsin ginseng reduced CRF in cancer survivors over an eight-week period. |
| Park et al. (2015) (39) | Ginseng extract, ginsenoside-β-glucosidase | A randomized controlled trial of five-week-old female Balb/c-nu/nu mice were treated with 5-fluorouracil to simulate cancer treatment. They were evaluated for CRF by running wheel activity and a forced swimming test. Control mice were given modafinil. | N/A | Treatment with ginseng extract significantly increased running wheel activity and forced swimming time compared to controls. Ginseng extract increased muscle glycogen activity and reduced serum TNF-α and IL-6 levels. | Ginseng extract may improve CRF symptoms. |
| Yennurajalingam et al.(2017) (40) | Panax ginseng | A randomized, double-blind, placebo-controlled study was conducted among 112 patients; 56 received 400 mg Panax ginseng twice daily and 56 received the placebo twice daily for 28 days. | FACT-F | There was a significant improvement in CRF in both the Panax ginseng and the placebo groups with no significant difference between groups. | Panax ginseng was no better than the placebo at reducing CRF symptoms. |
| Barton et al. (2010) (41) | American ginseng | A randomized double-blind, placebo-controlled trial was conducted among 290 patients with cancer; participants received American ginseng at 750, 1,000, or 2,000 mg/day or a placebo twice daily over eight weeks. Each group had approximately 70 subjects. | BFI | The group receiving 750 mg/day did not see an improvement in fatigue greater than the placebo. The subjects receiving higher doses of ginseng (1,000 and 2,000 mg/day) were twice as likely to experience a reduction in fatigue. | Higher doses of 1,000–2,000 mg/day of American ginseng reduced fatigue with few toxicities. |
| de Oliveira Campos et al. (2011) (42) | Guaraná (Paullinia cupana) | A randomized controlled trial was conducted with 75 breast cancer patients with fatigue after chemotherapy. They were randomized to receive either guaraná (50 mg) twice/day or a placebo for 21 days. After a 7-day washout period, subjects were re-allocated to the opposite experimental arm and the study continued for 21 more days. | BFI, FACT-F | The group receiving the Guaraná supplement had significant improvements in fatigue at days 21 and 49 compared to the placebo (p<0.01). | Guaraná reduced CRF in breast cancer patients after chemotherapy. |
| da Costa Miranda et al. (2009)(43) | Guaraná (Paullinia cupana) | A randomized controlled trial was conducted among 36 breast cancer patients undergoing adjuvant radiation therapy. Subjects were randomized to either 75 mg guaraná daily or a placebo. | Chalder Fatigue Scale, BFI | There was no significant difference between groups in fatigue levels. | Guaraná did not show any benefit over the placebo group in reducing fatigue in breast cancer patients. |
| Sette CVM et al. (2017)(44) | Guaraná (Paullinia cupana) | A review was published of two randomized controlled trials in breast cancer patients with fatigue after adjuvant chemotherapy. In the first study one group received 37.5 mg of guaraná and the other group received a placebo twice daily. In the second study, one group received 7.5 mg of guaraná, the second group received 12.5 mg guaraná and the third group received a placebo twice daily. | Chalder Fatigue Scale, BFI | There was no significant difference in fatigue levels between groups identified in either study. The placebo treatment contained magnesium silicate, which may have comparably impacted fatigue levels. | Guaraná supplementation did not appear to improve CRF. Future studies should also examine the impact of magnesium supplementation on chemotherapy-induced fatigue. |
| Zhang et al. (2015) (45) | Astragalus membranaceus | A randomized controlled trial was conducted with Kunming mice. Mice were divided into a control group or one of three astragalus dosage treatment groups: low dose (1.0 g/kg/day), mid-dose (3.0 g/kg/day) or high dose (30 g/kg/day). Fatigue was assessed based on swimming time. | N/A | The mice in the three astragalus groups had significantly longer swimming time than the placebo group (p<0.05). In the high and top dosage groups, liver glycogen and superoxide dismutase increased. | Astragalus had an anti-fatigue effect and future studies in humans are warranted. |
| Liu et al. (2016) (46) | Astragalus membranaceus | A randomized controlled trial was conducted among 64 patients with post-stroke fatigue. Participants were randomized to either 2.8 g Astragalus three times/day or a placebo for 28 days. | BFI | The treatment group experienced a significant decrease in fatigue based on BFI scores at visits 2 and 3. | Astragalus may reduce fatigue. However, larger trials for longer periods of time are needed. |
NUTRITION AND CANCER-RELATED FATIGUE
MACRONUTRIENTS
Dietary intake changes significantly in most cancer patients during cancer treatment, including chemotherapy (33, 34). Recently, de Vries et al. (2017) found breast cancer survivors reported significantly lower intakes of total energy, fat and protein after chemotherapy than women without cancer (33). Decreased intake persists in many populations of cancer survivors for years, and patients with late-stage cancer often continue to suffer weight loss, suggesting that the diets of cancer patients and survivors are inadequate (35). Nutritional interventions during treatment have not shown to be adequate in preventing long-term nutrient deficits and changes in body composition in cancer survivors that lead to fatigue (35, 36).
Protein
Protein may help maintain or build lean mass, and therefore reduce CRF (37). In a study assessing 285 cancer patients receiving chemotherapy, protein intake emerged as a stronger predictor of CRF than other common factors of nausea/vomiting, insomnia or age (37). Cancer survivors’ protein needs increase as a result of abnormalities in protein metabolism: increased protein turnover, loss of skeletal muscle and gluconeogenesis of amino acids (38). Skeletal muscle proteins are lost through increased muscle protein breakdown and decreased protein synthesis, which can lead to CRF (38). Therefore, protein needs and possible supplementation are important to assess in cancer patients and survivors (21, 37).
Protein or amino acid supplementation may benefit cancer survivors. Soy proteins are strong inhibitors of MMP-9, a matrix metalloproteinase that is implicated in tumorigenesis and cancer-associated depression (39). Soy isoflavones have pleiotropic effects on multiple targets, including antioxidant, anti-inflammatory and insulin-like growth factor binding (IGF-IGFBP) pathway modulation (39, 40). Adding a supplement of soy bread, a wheat-based bread supplemented with soy flour and soy milk powder, to prostate cancer survivors reduced pro-inflammatory cytokines and modulated immune cell phenotypes (41, 42). Besides being anti-inflammatory, soy protein may reduce fatigue in cancer survivors by helping to maintain muscular mass and strength, particularly in those who exercise. Soy has shown to be more effective than casein both in increasing skeletal muscle mass and muscular strength, although still not as effective in building mass as whey protein (43, 44).
Whey protein is considered an excellent protein for maintaining muscle even in cases of caloric restriction (45). Whey protein contains the branched chain amino acids leucine, has high amino acid content and is digested rapidly, making it a high quality protein source (46). Branched chain amino acids such as leucine, are considered major stimulators of muscle protein synthesis (45). Recent studies show that whey protein stimulates protein synthesis to a greater extent than casein or soy protein (46, 47).Carnitine, a trimethylated amino acid pivotal for energy metabolism, may be a useful supplement in increasing lean mass, improving appetite and in reducing CRF in cancer survivors. A recent systematic review, evaluating twelve studies, found that carnitine improved measures of fatigue based on the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF), Brief Fatigue Inventory (BFI) and other physical function and QOL measures (38, 48). Carnitine, often deficient in cancer patients, is an essential cofactor for the mitochondrial production of acetyl-coenzyme A and therefore energy production (49). However, the majority of the studies in this analysis were non-randomized and appeared to contain bias, where there was not enough evidence to believe it improved fatigue (48). Furthermore, carnitine’s improvements for CRF may be limited to individuals with deficiency (50, 51).
Fatty Acids
Few studies have examined the effect of fatty acid intake on fatigue in cancer survivors. Dietary intake of omega-3 and omega-6 fatty acids as well as the omega fatty acid ratio, may be tied to inflammation and fatigue. In a recent pilot study by Zick et al. (2017), in breast cancer survivors, increased dietary intake of omega-3 fatty acid-rich foods and reduced saturated fatty acid intake significantly reduced fatigue over a three month period (52). Dietary omega-3 fatty acids both through marine sources and plant-based oils such as flaxseed may reduce chronic inflammation levels (53–55). Considering that inflammation is considered one of the main mechanisms behind fatigue in cancer survivors, increasing dietary intake of omega-3 fatty acids appears helpful (2).
Dietary intake of omega-6 fatty acids appears to have varying effects on inflammation and CRF. In one analysis, breast cancer survivors with the highest intake of omega-6 fatty acids had the highest CRP levels and more than double the apparent risk for fatigue compared to survivors taking omega-3 fatty acid supplements. Omega-3 fatty acid intake was measured based on daily fish oil or flaxseed oil supplementation, but intake of the omega-6 fatty acids for this study were assessed through a food frequency questionnaire, which might not adequately measure dietary intake (55). In contrast, recent findings suggest that omega-6 fats when taken as a soybean oil supplement have an anti-inflammatory effect, not unlike fish oil, although through different mechanisms (56). Soybean oil intake appears to promote corticosterone, an anti-inflammatory hormone, and combined with other anti-inflammatory effects, suggests that omega-6 fatty acids are not as harmful as once thought (56). Therefore, omega-6 fatty acids, particularly as provided through soybean oil, may affect CRF symptoms.
In contrast, a low-fat diet is associated with reduced CRF while higher percent kilocalories from fat is associated with fatigue (57). Increased total dietary fat may promote malignant cell growth as well as cell proliferation and angiogenesis through the IGF signaling pathway, as well as reactive oxygen species (ROS) generation (58). ROS promotes increased inflammation in cancer patients, indicating a potential role of high-fat diets in CRF (59). Increased saturated fatty acid intake has been shown to stimulate a similar inflammatory response as to what normally occurs in a diet excessively high in total fat (60). Chronic higher intake of saturated fatty acids promotes inflammation in white adipose tissue, the liver, skeletal muscle and even the brain (60–62). This effect is related to pathogenic inflammatory lipopolysaccharides, as well as gut microflora release of endotoxins (62, 63). In addition, saturated fatty acids directly stimulate inflammatory gene expression by way of TLR4 signaling in vitro (63). Chronic high levels of saturated fats, as seen in the Western diet, may play a role in CRF through inflammation and changes in gut microflora (63). A reduction in saturated fatty acids intake may reduce inflammation and even mortality in cancer survivors (57, 58, 64).
Carbohydrates
Refined carbohydrates and diets high in sugar are also pro-inflammatory and obesogenic, especially when combined with high saturated fat intake (65–68). Weight loss that combines a lower fat diet with a lower carbohydrate diet is most effective (67, 69, 70). Reducing dietary intake of refined carbohydrates probably impacts CRF as well as weight loss (52, 65, 71).
In contrast, higher intake of dietary fiber, as found in complex carbohydrates, has been shown to be anti-inflammatory, and to even reduce fatigue (57, 72). Both soluble and insoluble fiber appear to lower CRP concentrations and systemic inflammation, possibly due to changes in intestinal microflora and decreased lipid oxidation (72, 73). In breast cancer survivors, dietary fiber was found to be inversely related to fatigue, with mean fatigue being significantly greater for participants consuming <25 g/day of fiber versus >25 g/day (57). High fiber diets also enhance satiety and appear to be as effective for weight loss as a low carbohydrate diet (74, 75). Both the anti-inflammatory effect and weight maintenance may both impact fatigue.
Recommendations for macronutrients for cancer survivors should be inclusive, whole food diets. Attention should be given to adequate protein intake for lean mass, healthy intake of omega-3 fatty acids, omega-6 fatty acids and higher fiber carbohydrates to reduce inflammation and obesity and improve CRF in the cancer survivor.
MICRONUTRIENTS
Micronutrient (vitamin and mineral) status also should be assessed in cancer survivors with CRF. Micronutrient deficiencies are common in cancer patients, both at the time of diagnosis as well as at the time of treatment (76, 77). Decreased intake of micronutrients with antioxidant properties reduces oxidative capacity, muscle quality and may increase fatigue and even promote cancer cachexia (78, 79). Cancer patients with antioxidant micronutrient deficiencies present with increased oxidative stress markers and/or inflammation, potentially increasing their risk for CRF (76, 80). The demands of cancer and its treatment potentially increase the need for intake of antioxidant nutrients such as vitamin C, due to greater numbers of free radicals (79, 81, 82). Chemotherapeutic agents, especially platinum-based agents, contribute to increased risk for micronutrient deficiencies. Chemotherapy may promote anorexia, stomatitis and gastrointestinal tract disturbances, while certain antimetabolite drugs inhibit the synthesis of essential vitamins and pyrimidines (83). Furthermore, nutritional deficiency risk is higher in cancer survivors because the population is mostly older adults (84–86).
Anemia
Anemia, particularly iron deficiency or vitamin B12 deficiency, is common in cancer patients and a contributor to fatigue and impaired physical function in cancer survivors (87, 88). Iron-deficiency anemia occurs in over 30% of cancer survivors, with high prevalence in pancreatic cancer, gastrointestinal cancer and myeloma (88, 89). Cancer survivors may develop anemia during or after treatment related to a number of stressors including nutritional deficiency, renal insufficiency, anemia of chronic disease, hemolysis, impaired erythropoiesis, inflammation, bleeding and abnormal tumor-associated vasculature (89, 90). Inflammation, often secondary to chemotherapy and radiation, further increases anemia (90). Unfortunately, anemia of cancer has proven difficult to treat, remaining pervasive in survivors. Therapy by transfusion of packed red blood cells may lead to immunosuppressive effects and infection, while a number of cancer patients, including breast cancer survivors, experience venous thromboembolism due to erythropoiesis-stimulating agents (89–91). Many cancer survivors have poor tolerance for oral iron supplementation or they do not experience meaningful improvement with oral supplements (89, 92). In recent studies in cancer patients with iron-deficiency anemia, intravenous iron supplementation appeared effective in many who had not improved with oral supplements, with significant improvements in both hemoglobin and fatigue symptoms (89, 92). Intravenous iron sucrose appears to prevent iron-deficiency anemia in cancer patients receiving chemotherapy, possibly also reducing CRF post-treatment, correcting iron deficiency more efficiently than oral supplements (93).
Patients with gastric cancer receiving total or subtotal gastrectomy often develop megaloblastic anemia due to vitamin B12 deficiency (94, 95). Symptoms include fatigue, palpitations and headache (94). Depending on the extent of gastric resection, gastric cancer patients have total cessation or severe decreases in gastric acid, pepsin and intrinsic factor production, all vital for vitamin B12 absorption (95). Vitamin B12 deficiency can occur as early as one year after surgery for gastric cancer patients and correlates with the time period they may be battling fatigue from other issues related to cancer and its treatment (95). Additionally, approximately 15% of elderly cancer patients (≥75 years) have vitamin B12 deficiency prior to surgery, while >30% of B12 deficiency is attributed to gastrectomy (94). Pancreatic cancer survivors who underwent partial gastrectomy lack sufficient intrinsic factor production from parietal cells in the stomach, putting them at further risk of vitamin B12 deficiency (84). Although patients with gastric cancer are typically treated with intramuscular injections of B12 every three to four months, greater awareness should be paid to the role of this nutrient in CRF, especially in elderly cancer survivors (96).
Fat-soluble Vitamins
Fat-soluble vitamin (A, D, E and K) deficiencies, especially vitamin D deficiency, are common in pancreatic cancer survivors due to decreased pancreatic lipase after undergoing the Whipple Procedure (97). Vitamin D deficiency is prevalent in obese breast cancer survivors as well, and is tied to reduced physical function and musculoskeletal pain (98–101). Vitamin D deficiency in cancer survivors impacts body composition, increasing the risk for both bone loss and even sarcopenia (97, 102). Vitamin D levels are inversely correlated with sarcopenia in older adults, and vitamin D supplementation is now considered a possible treatment for improving muscle function in the elderly (103–105). The mechanism by which vitamin D improves physical function may be related to anti-inflammatory properties, through local production of the hormone calcitriol, the active metabolite of vitamin D3 (101, 106). Evaluating and correcting vitamin D status may impact CRF and lead to improvements in physical function.
Currently, the benefit of micronutrient supplementation to cancer survivors with fatigue post-treatment has not been fully established, despite the evidence of deficiency in this population. Addressing micronutrient deficiency in cancer survivors may prove difficult. Research shows that cancer survivors do not respond well to nutrition supplementation, due to reduced dietary intake as well as altered metabolism and absorption (82, 107, 108),. Alternative methods for intake and/or absorption may improve nutritional status (92). Micronutrient status could play a role in CRF, but more research is warranted.
DIETARY PATTERNS
The ketogenic diet, traditionally a very low-carbohydrate and high-fat diet, was developed as an anticonvulsant therapy for epileptics (109). Increasingly, the ketogenic diet appears to promote anti-inflammatory metabolic effects, producing fewer ROS, fewer free radicals and elevated levels of the anti-inflammatory neuromodulator adenosine (110). Even so, research in cancer patients suggests the ketogenic diet might not be appropriate, as it also can promote weight loss and cachexia (111). Furthermore, long-term, a ketogenic diet might also elevate blood lipid levels, increase the risk of nonalcoholic fatty liver disease and promote insulin resistance (112).
The Mediterranean diet is anti-inflammatory and even has the potential to impact anti-inflammatory genes to improve fatigue for cancer survivors (113, 114). Recognized for preventing chronic disease, the Mediterranean diet is rich in legumes, nuts, fish and other lean sources of protein as well as fruits and vegetables. At the same time, the diet limits saturated fatty acids and trans fatty acids, and increases intake of omega-3 fatty acids, monounsaturated fatty acids and complex carbohydrates (113, 115). Dietary fiber is double (>30 g/day vs. 16 g/day) what the average American consumes daily (115, 116). The Mediterranean diet could reduce CRF through lowering inflammation and improving gut microflora. In addition, when obese individuals adopt a Mediterranean diet, they often experience weight loss (115).
The macrobiotic diet is a seasonal, low-fat, plant-based diet composed of whole grains, beans, soy, vegetables and sea vegetables, developed heavily from the traditions of Chinese medicine (117, 118). The macrobiotic food guide pyramid restricts fruits, seeds, nuts and fish to once a week or less, while other animal products such as red meat or dairy products are almost completely omitted (117, 118). Processed or refined carbohydrates and sugary foods are also heavily restricted (117, 118). While receiving attention for reducing cancer recurrence, due to anti-inflammatory and protective compounds, the macrobiotic diet also holds promise for reducing fatigue levels (57). Like the Mediterranean diet, the macrobiotic diet is considerably higher in dietary fiber than the Western diet, and is therefore anti-inflammatory (57, 119). The diet is low in pro-inflammatory fats and refined sugars and rich in phytochemicals and other nutrients that may be protective against oxidative damage from cancer and its treatment. However, the macrobiotic diet does appear somewhat too restrictive for some, and could worsen cancer cachexia in patients suffering involuntary weight loss. Macrobiotic dietary restrictions also lead to lower vitamin B12, vitamin D and calcium levels, potentially increasing the cancer survivor’s risk for bone loss, as well as other disorders (119–121). At best, this dietary pattern may be recommended in a liberalized format, giving the cancer survivor options, including more servings of dairy or fish, as well as other recommendations aimed at protecting bone health (122).
The Paleolithic dietary pattern, also called the Paleo diet or stone-age diet, is an eating pattern exemplifying the dietary intake of hunter-gatherers, or Paleolithic man (123). This diet excludes all refined sugars, breads, pasta, dairy products, processed meats, added sodium and alcohol (124). The Paleo diet also incorporates greater intakes of nuts, fruits, vegetables and fish than many Americans typically consume, being anti-inflammatory and potentially a weight loss aid (123). In a recent analysis examining middle-aged men and women, those with the highest Paleolithic diet scores (intake) had decreased CRP levels or inflammation and this reduction was comparable to the anti-inflammatory effect from a Mediterranean diet (123). Similar to the Mediterranean diet, the anti-inflammatory effect of the Paleo diet might support cancer survivors in reducing fatigue. Still, the Paleo diet has many dietary restrictions on breads, pasta and desserts, etc. which may be challenging for many individuals. Also, additional research has identified a possible iodine deficiency risk in some extreme cases due to the low sodium intake and dairy restriction (125). The Paleolithic diet may also be problematic for cancer survivors with low body weight or cachexia as the diet is lower energy and promotes satiety (125). Like the macrobiotic diet, the Paleo diet is possibly too restrictive and may require liberalization.
Recently, plant-based diets have received attention for their potential to confer protection against cancer occurrence and reoccurrence (126, 127). Such dietary patterns, including the vegan or various vegetarian diets, may also benefit cancer survivors experiencing fatigue due to their anti-inflammatory and antioxidant effects. The vegan diet promotes a more favorable fatty acid profile and higher intakes of polyphenols and certain antioxidants than non-vegetarians (128). Additionally, a primary benefit of a plant-based diet is the significant increase in dietary fiber, which not only promotes insulin sensitivity, but has been shown to decrease both inflammation and fatigue in cancer survivors (57, 126, 129). Research shows that dietary fiber intake is approximately 40% higher in men and women who consume a vegan diet, compared to those who eat meat or an omnivorous diet (130). However, more strict plant-based diets that omit dairy, such as the vegan diet, should be consumed with the awareness that they are potentially deficient in vitamin D, B12, calcium, zinc and even iodine, which could be especially concerning for elderly cancer survivors (127, 131).
In recent years, dietary patterns such as the Mediterranean Diet have shown potential to reduce chronic disease and improve conditions such as CRF, being tolerable for most patients in the Western world. More restrictive diets such as the Macrobiotic, Paleo or vegan diet are not only more difficult to maintain, but may even lead to nutritional inadequacy in certain populations. Although the impact on CRF is not fully established, cancer survivors who choose these dietary patterns may benefit by taking a more liberalized approach, so that they do not exclude as many foods as these diets traditionally call for.
HERBAL SUPPLEMENTS
Cancer survivors often use dietary or herbal supplements to treat their symptoms, with or without clinical guidance (132, 133). Although there are limited studies on nutritional supplements and CRF management, herbal supplements are one of the three most common types of complementary and alternative medicine used by cancer patients (7). Herbal supplements are widely available to the American public, supplied by local pharmacies, chain and health food stores (133).
Ginseng
Ginseng, known as Panax ginseng (Chinese ginseng) or Panax quinquefolius (American ginseng), is widely used in the United States (133–135). Ginsenoside is the key active ingredient in ginseng and is believed to have anti-inflammatory and antioxidant properties (134–136). Park et al. (2015) found ginseng reduced inflammatory markers associated with fatigue including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), while increasing running wheel activity in mice (137). Human studies have identified ginseng as safe and well-tolerated for both advanced cancer patients and cancer survivors (138, 139). Barton et al. (2013) showed that ginseng reduced CRF in cancer survivors based on MFSI scores; other research in advanced cancer patients, however, found no benefit of Panax ginseng over placebo, although this effect may be dose-dependent (135, 138, 139).
Guaraná
Guaraná, or Paullinia cupana, is an herbal supplement from Latin America used for energy, cognition and weight loss (140–143). In a study among 75 breast cancer patients, Campos et al. (2011) found that guaraná supplementation reduced CRF symptoms as per Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scores, without toxicities (144). In other recent studies examining guaraná in breast cancer survivors, no improvement in fatigue could be identified, however, the effect may be dose-dependent (145, 146). In animal, human and in vitro studies, guaraná extract demonstrates both antioxidant and anti-inflammatory properties (147–149). Saponins are bioactive compounds in guaraná that demonstrate antioxidant properties (150). Caffeine, a xanthine found in guaraná seeds, stimulates and prolongs lipolysis due to its effects on the androgenic receptor and action as an adenosine receptor antagonist, speeding up cell activity, although an average beverage with guaraná contains only 2–3 mg of caffeine (141, 142, 151, 152). The improvement in energy from guaraná is also related to catechins, tannins (proanthocyanidins) and other alkaloids (142, 143). In addition, guaraná may benefit breast cancer survivors by reducing hot flashes (144, 153).
Reishi Mushroom
Reishi mushroom or Ganoderma lucidum is a medical mushroom with immunomodulating functions and anti-inflammatory effects (154–156). The main bioactive components are G. lucidum polysaccharides, which, although dose-dependent, appear to significantly reduce levels of IL-6 and TNF-α, inflammatory markers associated with CRF in both human and mouse models (155–158). G. lucidum polysaccharides appear to be safe to administer to cancer patients without toxicities and may even decrease ROS levels and promote anticancer effects (157, 158).
Ginger
Ginger (Zingiber officinale) contains the major bioactive compounds gingerol, shogaol and dehydrogingerdione that have anti-inflammatory and anticancer activity. These compounds appear to inhibit cancer cells and induce apoptosis through the p53 pathway in ovarian, breast, colon and prostate cancer (159–161). Abolaji et al. (2017) found 6-gingerol to be chemoprotective against secondary oxidative brain damage, and in other research gingerol has been used to treat chronic conditions including arthritis due to its anti-inflammatory effect (161–163). Although research on Z. officinale and fatigue is limited, the combined impact on oxidative damage and inflammation could indicate a potential role for cancer patients during and after cancer treatment to prevent CRF (164, 165). In a recent pilot study in cancer patients, Z. officinale decreased fatigue and improved quality of life at cycles 1 and 3 in comparison to the placebo group (165).Furthermore, ginger extract appears safe and tolerable for cancer patients with minimal side effects (165, 166).
Astragalus
Astragalus membranaceus, an herb from traditional Chinese medicine, has a wide range of therapeutic effects, including anti-inflammatory, immunomodulating and anticancer properties (167–169). The benefits to patients with fatigue may be related to reduced inflammation and oxidative stress via regulation of the NF-κB and AP-1 signaling pathways as well as antioxidant mechanisms (168, 170). Huang et al. (2016), found that astragalus polysaccharides, a major bioactive ingredient extracted from Astragalus membranaceus, reduced oxidative stress and mitochondrial dysfunction related to fatigue. The protective effect was related to scavenging ROS, increasing activity of antioxidant enzymes and reducing mitochondrial dysfunction and ultimately improving energy metabolism (171). In a recent study of patients with fatigue post-stroke, A. membranaceus improved BFI scores, cognitive functioning and QOL, further demonstrating a possible role in reducing fatigue (172).
Schisandra
Schisandra chinensis fruit is a well-known herb in Traditional Chinese Medicine used to treat fatigue, containing lignans with both antioxidant and anti-inflammatory properties (173, 174). In a recent animal study examining chronic fatigue, S. chinensis fruit altered metabolic pathways and restored energy metabolism, including the TCA cycle, alanine, aspartate and glutamate metabolic pathways (175). In other recent research, S. chinensis inhibited inflammatory cytokine expression TNF-α, IL-1β and the expression of nitric oxide synthase, with no toxicity, pointing to a potential role in treating CRF (176).
Herbal supplements have been used for thousands of years in the treatment of fatigue and inflammatory diseases (177, 178). The dosage and mechanisms by which supplements reduce fatigue are poorly understood, and there are a number of considerations, including the fact that CRF often improves with placebo treatment (138, 146). In addition, effectiveness may be dose-dependent or may differ across human vs. animal models (154, 155). Evaluating biomarkers and other factors, in addition to questionnaires such as the MFSI, might further help identify the effects if any, of herbal supplements on CRF.
MICROBIOME
In recent decades, the gastrointestinal microbiome, which resides in the mucosal surface of the human intestine, has come to be implicated with human health and immunity (179, 180). The human microbiome is a large, genetically diverse system containing more than 100 trillion bacteria with over 1000 microbial species, mostly anaerobic (179–183). Gut bacteria colonies are fairly stable, relying on genetic inheritance to select gut microbial diversity, however, microbial diversity is also impacted by living environment, medications and diet (180). Dietary factors mainly affect the two dominant bacterial divisions, Bacteroidetes (B) and Firmicutes (F). Diets that are more plant-based and higher in fiber increase the B/F ratio, whereas the B/F ratio is believed to decrease with diets that contain more processed carbohydrates, high levels of animal fats and higher sucrose (180, 184). A change in gut bacteria such as when Firmicutes prevail over Bacteroidetes (low B/F ratio), may lead to chronic intestinal and systemic inflammation; lower Bifidobacteria and higher numbers of aerobic bacteria such as Enterococcus and Streptococcus have been documented in chronic fatigue syndrome patients (184, 185). Recently, a relationship has been identified between perturbations in the microbiota and the development of colon tumorigenesis, where gut microbiota can create genotoxic stress in the intestinal environment, facilitating genetic changes that lead to cancer (179).
Increasing evidence ties comorbidities that the elderly experience to changes in diet and therefore to changes in gut bacteria that promote chronic inflammation and disease (183, 186). Bacteria species and their metabolites are partially determined by the human diet, including dietary fiber intake (179, 186). This may further explain how increased dietary fiber is tied to both reduced chronic inflammation and reduced CRF (57).
Probiotic and Prebiotic Supplementation
The use of probiotics to correct an imbalanced gut microbiome is associated with reduced pro-inflammatory markers CRP and TNF-α (187). Groeger D. et al. (2013) demonstrated that oral supplementation with Bifidobacteria decreased IL-6, CRP and TNF-α (187). In addition, the administration of probiotics may improve mucosal barrier function while decreasing pro-inflammatory cytokines (182). Probiotics also show promise as an intervention for several cancer-related symptoms and treatment toxicities. The most studied area is in prevention of radiation toxicity among cancer patients undergoing treatment for pelvic malignancies such as prostate, rectal, cervical, uterine or endometrial cancer (188, 189). Pelvic radiation has direct effects on the intestinal tract through cell death as well as indirect effects on inflammatory signaling, atrophy, and fibrosis; symptoms include diarrhea, nausea, abdominal pain and fatigue (190). Additional side effects include altered intestinal transit and nutrient malabsorption, which long-term may contribute to malnutrition and fatigue (191). These symptoms may be related to disruption of the normal intestinal microbiome during a course of pelvic or abdominal radiotherapy, leading investigators to hypothesize that probiotic and/or prebiotic supplementation could prevent or mitigate the disruptive effects of radiation and reduce the rate and/or severity of gastrointestinal toxicity (191). Delia et al.(2007), found that patients randomized to daily use of probiotics during radiotherapy experienced significantly less radiation induced enteritis and colitis compared with placebo (31.6% vs. 51.8%) (192). Recent research found an overall benefit of probiotic use on the incidence of radiation-induced and chemotherapy- induced diarrhea (189, 193)., Similar to radiotherapy, chemotherapy disrupts the intestinal microbiome, leading to similar effects such as malabsorption, imbalanced bacterial growth and disruption of the mucosa, which can in turn lead to systemic effects such as fatigue (194, 195).
Lee et al. (2014), examined the effect of probiotics on fatigue in a randomized trial of colorectal cancer survivors who had completed treatment up to two years prior to study enrollment (196). Survivors randomized to the probiotic arm had a small but significant improvement in fatigue over baseline, as assessed using the FACIT-F. More research is also needed examining the effect of probiotics in cancer patients when used prior to or during treatment as well as which prebiotics, fibers or dietary patterns best promote anti-inflammatory gut bacteria populations in cancer survivors and cancer survivors’ tolerability to probiotic and prebiotic treatment.
Discussion
It seems plausible that a cancer survivor’s struggle with fatigue is partly related to dietary intake prior to, during and after treatment. Cancer survivors experience altered metabolism, altered taste and changes in body composition. Dietary intake is often compromised in this population, as well as absorption of nutrients. Cancer survivors are particularly at risk for sarcopenia, sarcopenic obesity and/or nutritional inadequacy that could lead to further weakness and dysfunction, particularly in older adults. Dietary patterns rich in fruits, vegetables, whole grains and anti-inflammatory fatty acids could improve CRF in patients with cancer and cancer survivors. Some research suggests that nutritional supplements could improve CRF outcomes. However, there is very limited research on this topic, and few resources are available for cancer survivors with fatigue at this time. Perhaps in the future, more research combining nutritional interventions with more established treatments for CRF including yoga, aerobic and resistance exercise would prove most effective (197–199). More studies, particularly clinical trials, examining dietary interventions for CRF are needed, to establish CRF-specific dietary recommendations for cancer patients and survivors.
Limitations:
The area of nutritional interventions for cancer survivors with CRF is a new area of research and there is limited established research regarding the experience of cancer survivors and nutritional interventions and treatments. The interventions reviewed in this paper often did not directly examine CRF and nutritional interventions. The paper examined mechanisms that also were tied to CRF in cancer survivors including inflammation and lean mass maintenance. Another potential limitation is that different studies assessing CRF used different survey instruments, including the Brief Fatigue Inventory, Multidimensional Fatigue Symptom Inventory- Short Form and Functional assessment of Cancer Therapy for Fatigue. These survey instruments for fatigue have been shown to be valid and reliable; accurate in assessing fatigue symptoms in cancer patients. This was a preliminary and qualitative review and some results in cancer survivors varied by study in how they measured fatigue.
Supplementary Material
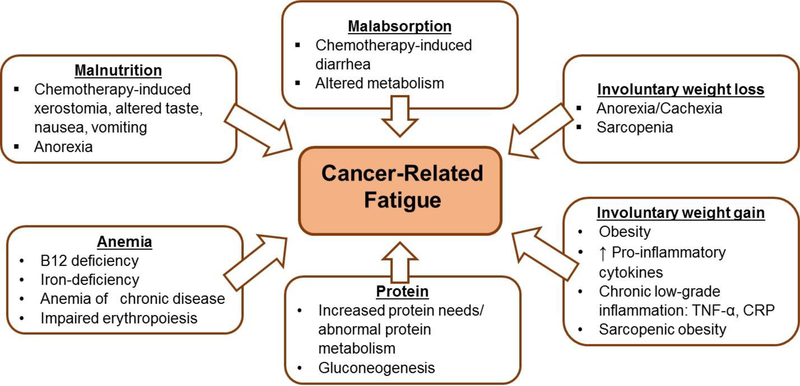
Figure 1.
Nutritional factors that contribute to cancer-related fatigue. TNF-α, tumor necrosis factor-alpha; CRP, C-reactive protein.
Acknowledgments
Funding: was supported in part by UG1 CA189961, R25 CA1026185 and K07 CA187546 from NCI.
Footnotes
Conflicts of Interest: No potential conflict of interest was reported by the authors.
References
- 1.Peoples AR, Roscoe JA, Block RC, Heckler CE, Ryan JL, et al. : Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: a multicenter NCORP study. Support Care Cancer 25, 1271–1278, 2017. doi: 10.1007/s00520-016-3520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower JE: Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11, 597–609, 2014. doi: 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palesh O, Scheiber C, Kesler S, Mustian K, Koopman C, et al. : Management of side effects during and post-treatment in breast cancer survivors. Breast J, 2017. doi: 10.1111/tbj.12862 [DOI] [PubMed] [Google Scholar]
- 4.Borneman T: Assessment and Management of Cancer-Related Fatigue. Journal of Hospice & Palliative Nursing 15, 77–86, 2013. doi: 10.1097/NJH.0b013e318286dc19 [DOI] [Google Scholar]
- 5.Mortimer JE, Waliany S, Dieli-Conwright CM, Patel SK, Hurria A, et al. : Objective physical and mental markers of self-reported fatigue in women undergoing (neo)adjuvant chemotherapy for early-stage breast cancer. Cancer 123, 1810–1816, 2017. doi: 10.1002/cncr.30426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira ML, Fonseca FL, Costa LG, Beltrame RL, Chaves CM, et al. : Supplementation with selenium can influence nausea, fatigue, physical, renal, and liver function of children and adolescents with cancer. J Med Food 18, 109–17, 2015. doi: 10.1089/jmf.2014.0030 [DOI] [PubMed] [Google Scholar]
- 7.Chandwani KD, Ryan JL, Peppone LJ, Janelsins MM, Sprod LK, et al. : Cancer-related stress and complementary and alternative medicine: a review. Evid Based Complement Alternat Med 2012, 979213, 2012. doi: 10.1155/2012/979213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palesh O, Scheiber C, Kesler S, Mustian K, Koopman C, et al. : Management of side effects during and post-treatment in breast cancer survivors. Breast J 24, 167–175, 2018. doi: 10.1111/tbj.12862 [DOI] [PubMed] [Google Scholar]
- 9.Campbell A, Stevinson C, Crank H: The BASES Expert Statement on exercise and cancer survivorship. J Sports Sci 30, 949–52, 2012. doi: 10.1080/02640414.2012.671953 [DOI] [PubMed] [Google Scholar]
- 10.Jankowski CM, Matthews EE: Exercise guidelines for adults with cancer: a vital role in survivorship. Clin J Oncol Nurs 15, 683–6, 2011. doi: 10.1188/11.Cjon.683-686 [DOI] [PubMed] [Google Scholar]
- 11.2nd International Cancer Fatigue Symposium: Putting the Pieces Together. Current Oncology 17, 2010 [Google Scholar]
- 12.Bower JE, Ganz PA, Aziz N, Fahey JL: Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 64, 604–11, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW: T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst 95, 1165–8, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR: Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12, 2759–66, 2006. doi: 10.1158/1078-0432.ccr-05-2398 [DOI] [PubMed] [Google Scholar]
- 15.Segerstrom SC, Miller GE: Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130, 601–30, 2004. doi: 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute: Nutrition in Cancer Care (PDQ®)–Health Professional Version. National Cancer Institute, 2017 [Google Scholar]
- 17.Zietarska M, Krawczyk-Lipiec J, Kraj L, Zaucha R, Malgorzewicz S: Chemotherapy-Related Toxicity, Nutritional Status and Quality of Life in Precachectic Oncologic Patients with, or without, High Protein Nutritional Support. A Prospective, Randomized Study. Nutrients 9, 2017. doi: 10.3390/nu9101108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demark-Wahnefried W, Campbell KL, Hayes SC: Weight management and its role in breast cancer rehabilitation. Cancer 118, 2277–87, 2012. doi: 10.1002/cncr.27466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossola M: Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients 7, 265–76, 2015. doi: 10.3390/nu7010265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, et al. : Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 21, 3261–70, 2013. doi: 10.1007/s00520-013-1894-4 [DOI] [PubMed] [Google Scholar]
- 21.Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, et al. : Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle 1, 177–185, 2010. doi: 10.1007/s13539-010-0016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronha AL, Camilo ME, Ravasco P: [The relevance of body composition in cancer patients: what is the evidence?]. Acta Med Port 24 Suppl 4, 769–78, 2011 [PubMed] [Google Scholar]
- 23.Carneiro IP, Mazurak VC, Prado CM: Clinical Implications of Sarcopenic Obesity in Cancer. Curr Oncol Rep 18, 62, 2016. doi: 10.1007/s11912-016-0546-5 [DOI] [PubMed] [Google Scholar]
- 24.Prado CM, Cushen SJ, Orsso CE, Ryan AM: Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc 75, 188–98, 2016. doi: 10.1017/s0029665115004279 [DOI] [PubMed] [Google Scholar]
- 25.Nyrop KA, Deal AM, Lee JT, Muss HB, Choi SK, et al. : Weight gain in hormone receptor-positive (HR+) early-stage breast cancer: is it menopausal status or something else? Breast Cancer Res Treat, 2017. doi: 10.1007/s10549-017-4501-4 [DOI] [PubMed] [Google Scholar]
- 26.Makari-Judson G, Braun B, Jerry DJ, Mertens WC: Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J Clin Oncol 5, 272–82, 2014. doi: 10.5306/wjco.v5.i3.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheean PM, Hoskins K, Stolley M: Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat 135, 663–80, 2012. doi: 10.1007/s10549-012-2200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein EA, Chen H, Prabhu M, Trogdon JG, Corso PS: The relationship between obesity and injuries among U.S. adults. Am J Health Promot 21, 460–8, 2007 [DOI] [PubMed] [Google Scholar]
- 29.George SM, McTiernan A, Villasenor A, Alfano CM, Irwin ML, et al. : Disentangling the body weight-bone mineral density association among breast cancer survivors: an examination of the independent roles of lean mass and fat mass. BMC Cancer 13, 497, 2013. doi: 10.1186/1471-2407-13-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, et al. : Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9, 629–35, 2008. doi: 10.1016/s1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 31.Ryan AM, Power DG, Daly L, Cushen SJ, Ni Bhuachalla E, et al. : Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 75, 199–211, 2016. doi: 10.1017/s002966511500419x [DOI] [PubMed] [Google Scholar]
- 32.Baguley BJ, Bolam KA, Wright ORL, Skinner TL: The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 9, 2017. doi: 10.3390/nu9091003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries YC, van den Berg M, de Vries JHM, Boesveldt S, de Kruif J, et al. : Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support Care Cancer 25, 2581–2591, 2017. doi: 10.1007/s00520-017-3668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malihi Z, Kandiah M, Chan YM, Esfandbod M, Vakili M, et al. : The effect of dietary intake changes on nutritional status in acute leukaemia patients after first induction chemotherapy. Eur J Cancer Care (Engl) 24, 542–52, 2015. doi: 10.1111/ecc.12262 [DOI] [PubMed] [Google Scholar]
- 35.Hutton JL, Martin L, Field CJ, Wismer WV, Bruera ED, et al. : Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am J Clin Nutr 84, 1163–70, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ryan AM, Power DG, Daly L, Cushen SJ, Bhuachalla EN, et al. : Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proceedings of the Nutrition Society 75, 199–211, 2016. doi: 10.1017/s002966511500419x [DOI] [PubMed] [Google Scholar]
- 37.Stobaus N, Muller MJ, Kupferling S, Schulzke JD, Norman K: Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr Cancer 67, 818–24, 2015. doi: 10.1080/01635581.2015.1040520 [DOI] [PubMed] [Google Scholar]
- 38.Gramignano G, Lusso MR, Madeddu C, Massa E, Serpe R, et al. : Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 22, 136–45, 2006. doi: 10.1016/j.nut.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 39.Lima A, Oliveira J, Saude F, Mota J, Ferreira RB: Proteins in Soy Might Have a Higher Role in Cancer Prevention than Previously Expected: Soybean Protein Fractions Are More Effective MMP-9 Inhibitors Than Non-Protein Fractions, Even in Cooked Seeds. Nutrients 9, 2017. doi: 10.3390/nu9030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucuk O: Soy foods, isoflavones, and breast cancer. Cancer 123, 1901–1903, 2017. doi: 10.1002/cncr.30614 [DOI] [PubMed] [Google Scholar]
- 41.Lesinski GB, Reville PK, Mace TA, Young GS, Ahn-Jarvis J, et al. : Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev Res (Phila) 8, 1036–44, 2015. doi: 10.1158/1940-6207.CAPR-14-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn-Jarvis JH, Riedl KM, Schwartz SJ, Vodovotz Y: Design and selection of soy breads used for evaluating isoflavone bioavailability in clinical trials. J Agric Food Chem 61, 3111–20, 2013. doi: 10.1021/jf304699k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto R, Sakai A, Murayama M, Ochi A, Abe T, et al. : Effects of dietary soy protein on skeletal muscle volume and strength in humans with various physical activities. J Med Invest 62, 177–83, 2015. doi: 10.2152/jmi.62.177 [DOI] [PubMed] [Google Scholar]
- 44.Witard OC, Wardle SL, Macnaughton LS, Hodgson AB, Tipton KD: Protein Considerations for Optimising Skeletal Muscle Mass in Healthy Young and Older Adults. Nutrients 8, 181, 2016. doi: 10.3390/nu8040181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, et al. : A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr 101, 279–86, 2015. doi: 10.3945/ajcn.114.090290 [DOI] [PubMed] [Google Scholar]
- 46.Devries MC, Phillips SM: Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci 80 Suppl 1, A8–a15, 2015. doi: 10.1111/1750-3841.12802 [DOI] [PubMed] [Google Scholar]
- 47.Witard OC, Jackman SR, Breen L, Smith K, Selby A, et al. : Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99, 86–95, 2014. doi: 10.3945/ajcn.112.055517 [DOI] [PubMed] [Google Scholar]
- 48.Marx W, Teleni L, Opie RS, Kelly J, Marshall S, et al. : Efficacy and Effectiveness of Carnitine Supplementation for Cancer-Related Fatigue: A Systematic Literature Review and Meta-Analysis. Nutrients 9, 2017. doi: 10.3390/nu9111224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, et al. : Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 15, 200–11, 2010. doi: 10.1634/theoncologist.2009-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winter SC, Szabo-Aczel S, Curry CJ, Hutchinson HT, Hogue R, et al. : Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am J Dis Child 141, 660–5, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Endo K, Tsuji A, Kondo S, Wakisaka N, Murono S, et al. : Carnitine is associated with fatigue following chemoradiotherapy for head and neck cancer. Acta Otolaryngol 135, 846–52, 2015. doi: 10.3109/00016489.2015.1030769 [DOI] [PubMed] [Google Scholar]
- 52.Zick SM, Colacino J, Cornellier M, Khabir T, Surnow K, et al. : Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat 161, 299–310, 2017. doi: 10.1007/s10549-016-4070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moura-Assis A, Afonso MS, de Oliveira V, Morari J, Dos Santos GA, et al. : Flaxseed oil rich in omega-3 protects aorta against inflammation and endoplasmic reticulum stress partially mediated by GPR120 receptor in obese, diabetic and dyslipidemic mice models. J Nutr Biochem 53, 9–19, 2017. doi: 10.1016/j.jnutbio.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Cruz M, Cruz-Guzman ODR, Almeida-Becerril T, Solis-Serna AD, Atilano-Miguel S, et al. : Potential therapeutic impact of omega-3 long chain-polyunsaturated fatty acids on inflammation markers in Duchenne muscular dystrophy: A double-blind, controlled randomized trial. Clin Nutr, 2017. doi: 10.1016/j.clnu.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 55.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, et al. : Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol 30, 1280–7, 2012. doi: 10.1200/jco.2011.36.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navarro-Xavier RA, de Barros KV, de Andrade IS, Palomino Z, Casarini DE, et al. : Protective effect of soybean oil- or fish oil-rich diets on allergic airway inflammation. J Inflamm Res 9, 79–89, 2016. doi: 10.2147/jir.s102221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guest DD, Evans EM, Rogers LQ: Diet components associated with perceived fatigue in breast cancer survivors. Eur J Cancer Care (Engl) 22, 51–9, 2013. doi: 10.1111/j.1365-2354.2012.01368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Sebastiano KM, Mourtzakis M: The role of dietary fat throughout the prostate cancer trajectory. Nutrients 6, 6095–109, 2014. doi: 10.3390/nu6126095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin S, Li Y, Zamyatnin AA Jr., Werner J, Bazhin AV: Reactive Oxygen Species and Colorectal Cancer. J Cell Physiol, 2017. doi: 10.1002/jcp.26356 [DOI] [PubMed] [Google Scholar]
- 60.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, et al. : Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9, 2124–38, 2014. doi: 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ: Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem 120, 1060–71, 2012. doi: 10.1111/j.1471-4159.2012.07660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada S, Kamada N, Amiya T, Nakamoto N, Nakaoka T, et al. : Gut microbiota-mediated generation of saturated fatty acids elicits inflammation in the liver in murine high-fat diet-induced steatohepatitis. BMC Gastroenterol 17, 136, 2017. doi: 10.1186/s12876-017-0689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fritsche KL: The science of fatty acids and inflammation. Adv Nutr 6, 293s–301s, 2015. doi: 10.3945/an.114.006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, et al. : Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med 15, 203, 2017. doi: 10.1186/s12916-017-0968-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, et al. : Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6, 897–908, 2017. doi: 10.1016/j.molmet.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nilsson J, Ericsson M, Joibari MM, Anderson F, Carlsson L, et al. : A low-carbohydrate high-fat diet decreases lean mass and impairs cardiac function in pair-fed female C57BL/6J mice. Nutr Metab (Lond) 13, 79, 2016. doi: 10.1186/s12986-016-0132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruiz-Nunez B, Dijck-Brouwer DA, Muskiet FA: The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem 36, 1–20, 2016. doi: 10.1016/j.jnutbio.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 68.Hernandez TL, Van Pelt RE, Anderson MA, Reece MS, Reynolds RM, et al. : Women With Gestational Diabetes Mellitus Randomized to a Higher-Complex Carbohydrate/Low-Fat Diet Manifest Lower Adipose Tissue Insulin Resistance, Inflammation, Glucose, and Free Fatty Acids: A Pilot Study. Diabetes Care 39, 39–42, 2016. doi: 10.2337/dc15-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alves BC, Silva TR, Spritzer PM: Sedentary Lifestyle and High-Carbohydrate Intake are Associated with Low-Grade Chronic Inflammation in Post-Menopause: A Cross-sectional Study. Rev Bras Ginecol Obstet 38, 317–24, 2016. doi: 10.1055/s-0036-1584582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu T, Yao L, Reynolds K, Whelton PK, Niu T, et al. : The Effects of a Low-Carbohydrate Diet vs. a Low-Fat Diet on Novel Cardiovascular Risk Factors: A Randomized Controlled Trial. Nutrients 7, 7978–94, 2015. doi: 10.3390/nu7095377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okumatsu K, Tsujimoto T, Seki A, Yamauchi T, Yamauchi H, et al. : Effect of a combined diet plus exercise intervention on weight loss, physical fitness, and cancer-related fatigue among Japanese women with breast cancer. Journal of Clinical Oncology 35, 2017 [Google Scholar]
- 72.Kaluza J, Harris H, Wallin A, Linden A, Wolk A: Dietary Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Epidemiology, 2017. doi: 10.1097/ede.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 73.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, et al. : Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr 83, 760–6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonstad S, Malik N, Haddad E: A high-fibre bean-rich diet versus a low-carbohydrate diet for obesity. J Hum Nutr Diet 27 Suppl 2, 109–16, 2014. doi: 10.1111/jhn.12118 [DOI] [PubMed] [Google Scholar]
- 75.Sylvetsky AC, Edelstein SL, Walford G, Boyko EJ, Horton ES, et al. : A High-Carbohydrate, High-Fiber, Low-Fat Diet Results in Weight Loss among Adults at High Risk of Type 2 Diabetes. J Nutr 147, 2060–2066, 2017. doi: 10.3945/jn.117.252395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grober U, Holzhauer P, Kisters K, Holick MF, Adamietz IA: Micronutrients in Oncological Intervention. Nutrients 8, 163, 2016. doi: 10.3390/nu8030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bilariki K, Anagnostou E, Masse V, Elie C, Grill J, et al. : Low bone mineral density and high incidences of fractures and vitamin D deficiency in 52 pediatric cancer survivors. Horm Res Paediatr 74, 319–27, 2010. doi: 10.1159/000313378 [DOI] [PubMed] [Google Scholar]
- 78.van Dijk M, Dijk FJ, Hartog A, van Norren K, Verlaan S, et al. : Reduced dietary intake of micronutrients with antioxidant properties negatively impacts muscle health in aged mice. J Cachexia Sarcopenia Muscle 9, 146–159, 2018. doi: 10.1002/jcsm.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mantovani G, Maccio A, Madeddu C, Gramignano G, Lusso MR, et al. : A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomarkers Prev 15, 1030–4, 2006. doi: 10.1158/1055-9965.epi-05-0538 [DOI] [PubMed] [Google Scholar]
- 80.Chapple IL, Griffiths HR, Milward MR, Ling MR, Grant MM: Antioxidant Micronutrients and Oxidative Stress Biomarkers. Methods Mol Biol 1537, 61–77, 2017. doi: 10.1007/978-1-4939-6685-1_4 [DOI] [PubMed] [Google Scholar]
- 81.Mantovani G, Maccio A, Madeddu C, Gramignano G, Lusso MR, et al. : A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiology Biomarkers & Prevention 15, 1030–1034, 2006. doi: 10.1158/1055-9965.Epi-05-0538 [DOI] [PubMed] [Google Scholar]
- 82.Mayland CR, Bennett MI, Allan K: Vitamin C deficiency in cancer patients. Palliat Med 19, 17–20, 2005. doi: 10.1191/0269216305pm970oa [DOI] [PubMed] [Google Scholar]
- 83.Dreizen S, McCredie KB, Keating MJ, Andersson BS: Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad Med 87, 163–7, 170, 1990 [DOI] [PubMed] [Google Scholar]
- 84.Petzel MQB, Hoffman L: Nutrition Implications for Long-Term Survivors of Pancreatic Cancer Surgery [Formula: see text]. Nutr Clin Pract 32, 588–598, 2017. doi: 10.1177/0884533617722929 [DOI] [PubMed] [Google Scholar]
- 85.Silay K, Akinci S, Silay YS, Guney T, Ulas A, et al. : Hospitalization risk according to geriatric assessment and laboratory parameters in elderly hematologic cancer patients. Asian Pac J Cancer Prev 16, 783–6, 2015 [DOI] [PubMed] [Google Scholar]
- 86.Institute NC. Percent of New Cancers by Age Group: All Cancer Sites, 2017.
- 87.Ludwig H, Muldur E, Endler G, Hubl W: Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol 24, 1886–92, 2013. doi: 10.1093/annonc/mdt118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jun JH, Yoo JE, Lee JA, Kim YS, Sunwoo S, et al. : Anemia after gastrectomy in long-term survivors of gastric cancer: A retrospective cohort study. Int J Surg 28, 162–8, 2016. doi: 10.1016/j.ijsu.2016.02.084 [DOI] [PubMed] [Google Scholar]
- 89.Vadhan-Raj S, Dahl NV, Bernard K, Li Z, Strauss WE: Efficacy and safety of IV ferumoxytol for iron deficiency anemia in patients with cancer. J Blood Med 8, 199–209, 2017. doi: 10.2147/jbm.s138474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim A, Rivera S, Shprung D, Limbrick D, Gabayan V, et al. : Mouse models of anemia of cancer. PLoS One 9, e93283, 2014. doi: 10.1371/journal.pone.0093283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, et al. : Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. Jama 299, 914–24, 2008. doi: 10.1001/jama.299.8.914 [DOI] [PubMed] [Google Scholar]
- 92.Vadhan-Raj S, Strauss W, Ford D, Bernard K, Boccia R, et al. : Efficacy and safety of IV ferumoxytol for adults with iron deficiency anemia previously unresponsive to or unable to tolerate oral iron. Am J Hematol 89, 7–12, 2014. doi: 10.1002/ajh.23582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, et al. : Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol Oncol 105, 199–204, 2007. doi: 10.1016/j.ygyno.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 94.Nagao T, Hirokawa M: Diagnosis and treatment of macrocytic anemias in adults. J Gen Fam Med 18, 200–204, 2017. doi: 10.1002/jgf2.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Y, Kim HI, Hyung WJ, Song KJ, Lee JH, et al. : Vitamin B(12) deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg 258, 970–5, 2013. doi: 10.1097/sla.0000000000000214 [DOI] [PubMed] [Google Scholar]
- 96.Kim HI, Hyung WJ, Song KJ, Choi SH, Kim CB, et al. : Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol 18, 3711–7, 2011. doi: 10.1245/s10434-011-1764-6 [DOI] [PubMed] [Google Scholar]
- 97.Armstrong T, Walters E, Varshney S, Johnson CD: Deficiencies of micronutrients, altered bowel function, and quality of life during late follow-up after pancreaticoduodenectomy for malignancy. Pancreatology 2, 528–34, 2002. doi: 10.1159/000066095 [DOI] [PubMed] [Google Scholar]
- 98.Peppone LJ, Rickles AS, Janelsins MC, Insalaco MR, Skinner KA: The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann Surg Oncol 19, 2590–9, 2012. doi: 10.1245/s10434-012-2297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alco G, Igdem S, Dincer M, Ozmen V, Saglam S, et al. : Vitamin D levels in patients with breast cancer: importance of dressing style. Asian Pac J Cancer Prev 15, 1357–62, 2014 [DOI] [PubMed] [Google Scholar]
- 100.Gröber U, Kisters K, Adamietz IA: Vitamin D in oncology: Update 2015. Medizinische Monatsschrift fur Pharmazeuten 38, 512–516, 2015 [PubMed] [Google Scholar]
- 101.Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, et al. : Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res Treat 166, 491–500, 2017. doi: 10.1007/s10549-017-4429-8 [DOI] [PubMed] [Google Scholar]
- 102.Mager DR, Carroll MW, Wine E, Siminoski K, MacDonald K, et al. : Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur J Clin Nutr, 2018. doi: 10.1038/s41430-018-0105-2 [DOI] [PubMed] [Google Scholar]
- 103.Lee JH, Kim S, Kim MK, Yun BH, Cho S, et al. : Relationships between 25(OH)D concentration, sarcopenia and HOMA-IR in postmenopausal Korean women. Climacteric, 1–7, 2017. doi: 10.1080/13697137.2017.1395410 [DOI] [PubMed] [Google Scholar]
- 104.Kaneko I, Miyamoto KI, Nikawa T: [Update on recent progress in vitamin D research. Vitamin D and muscle tissues.]. Clin Calcium 27, 1571–1578, 2017. doi: CliCa171115711578 [PubMed] [Google Scholar]
- 105.Woo J: Nutritional interventions in sarcopenia: where do we stand? Curr Opin Clin Nutr Metab Care, 2017. doi: 10.1097/mco.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 106.Tukaj S, Bieber K, Witte M, Ghorbanalipoor S, Schmidt E, et al. : Calcitriol Treatment Ameliorates Inflammation and Blistering in Mouse Models of Epidermolysis Bullosa Acquisita. J Invest Dermatol 138, 301–309, 2018. doi: 10.1016/j.jid.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 107.Missel M, Hansen M, Jackson R, Siemsen M, Schonau MN: Re-embodying eating after surgery for esophageal cancer: patients’ lived experiences of participating in an education and counseling nutritional intervention. J Clin Nurs, 2018. doi: 10.1111/jocn.14297 [DOI] [PubMed] [Google Scholar]
- 108.Roemmler-Zehrer J, Geigenberger V, Stormann S, Losa M, Crippa V, et al. : Food intake regulating hormones in adult craniopharyngioma patients. Eur J Endocrinol 170, 627–35, 2014. doi: 10.1530/eje-13-0832 [DOI] [PubMed] [Google Scholar]
- 109.Ruskin DN, Kawamura M, Masino SA: Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One 4, e8349, 2009. doi: 10.1371/journal.pone.0008349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masino SA, Ruskin DN: Ketogenic diets and pain. J Child Neurol 28, 993–1001, 2013. doi: 10.1177/0883073813487595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chung HY, Park YK: Rationale, Feasibility and Acceptability of Ketogenic Diet for Cancer Treatment. J Cancer Prev 22, 127–134, 2017. doi: 10.15430/jcp.2017.22.3.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kosinski C, Jornayvaz FR: Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 9, 2017. doi: 10.3390/nu9050517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haslam A, Robb SW, Hebert JR, Huang H, Ebell MH: Greater adherence to a Mediterranean diet is associated with lower prevalence of colorectal adenomas in men of all races. Nutr Res 48, 76–84, 2017. doi: 10.1016/j.nutres.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 114.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, et al. : Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys 527, 81–9, 2012. doi: 10.1016/j.abb.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 115.Tosti V, Bertozzi B, Fontana L: The Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci, 2017. doi: 10.1093/gerona/glx227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoy MK, Goldman JD: What We Eat in America, NHANES 2009–2010.. Food Surveys Research Group Dietary Data Brief No. 12, 2014 [Google Scholar]
- 117.Lerman RH: The macrobiotic diet in chronic disease. Nutr Clin Pract 25, 621–6, 2010. doi: 10.1177/0884533610385704 [DOI] [PubMed] [Google Scholar]
- 118.Kushi LH, Cunningham JE, Hebert JR, Lerman RH, Bandera EV, et al. : The macrobiotic diet in cancer. J Nutr 131, 3056s–64s, 2001 [DOI] [PubMed] [Google Scholar]
- 119.Harmon BE, Carter M, Hurley TG, Shivappa N, Teas J, et al. : Nutrient Composition and Anti-inflammatory Potential of a Prescribed Macrobiotic Diet. Nutr Cancer 67, 933–40, 2015. doi: 10.1080/01635581.2015.1055369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swedish Council on Health Technology A: SBU Systematic Review Summaries In: Osteoporosis - Prevention, Diagnosis and Treatment: A Systematic Review. Swedish Council on Health Technology Assessment (SBU) Copyright (c) 2003 by the Swedish Council on Health Technology Assessment: Stockholm, 2003. [Google Scholar]
- 121.Dai Z, Koh WP: B-vitamins and bone health--a review of the current evidence. Nutrients 7, 3322–46, 2015. doi: 10.3390/nu7053322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maritess C, Small S, Waltz-Hill M: Alternative nutrition therapies in cancer patients. Semin Oncol Nurs 21, 173–6, 2005. doi: 10.1016/j.soncn.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 123.Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, et al. : Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with Biomarkers of Inflammation and Oxidative Balance in Adults. J Nutr 146, 1217–26, 2016. doi: 10.3945/jn.115.224048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whalen KA, Judd S, McCullough ML, Flanders WD, Hartman TJ, et al. : Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with All-Cause and Cause-Specific Mortality in Adults. J Nutr 147, 612–620, 2017. doi: 10.3945/jn.116.241919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffman R: Can the paleolithic diet meet the nutritional needs of older people? Maturitas 95, 63–64, 2017. doi: 10.1016/j.maturitas.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 126.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G: Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev 22, 286–94, 2013. doi: 10.1158/1055-9965.epi-12-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Melina V, Craig W, Levin S: Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J Acad Nutr Diet 116, 1970–1980, 2016. doi: 10.1016/j.jand.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 128.Elorinne AL, Alfthan G, Erlund I, Kivimaki H, Paju A, et al. : Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS One 11, e0148235, 2016. doi: 10.1371/journal.pone.0148235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clarys P, Deliens T, Huybrechts I, Deriemaeker P, Vanaelst B, et al. : Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 6, 1318–32, 2014. doi: 10.3390/nu6031318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, et al. : EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr 6, 259–69, 2003. doi: 10.1079/phn2002430 [DOI] [PubMed] [Google Scholar]
- 131.Le LT, Sabate J: Beyond meatless, the health effects of vegan diets: findings from the Adventist cohorts. Nutrients 6, 2131–47, 2014. doi: 10.3390/nu6062131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bours MJ, Beijer S, Winkels RM, van Duijnhoven FJ, Mols F, et al. : Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br J Nutr 114, 286–96, 2015. doi: 10.1017/s0007114515001798 [DOI] [PubMed] [Google Scholar]
- 133.Public. IoMUCotUoCaAMbtA: Dietary Supplements In: Complementary and Alternative Medicine in the United States National Academies Press (US) Washington (DC), 2005. [PubMed] [Google Scholar]
- 134.Majeed F, Malik FZ, Ahmed Z, Afreen A, Afzal MN, et al. : Ginseng phytochemicals as therapeutics in oncology: Recent perspectives. Biomed Pharmacother 100, 52–63, 2018. doi: 10.1016/j.biopha.2018.01.155 [DOI] [PubMed] [Google Scholar]
- 135.Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA, et al. : Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 105, 1230–8, 2013. doi: 10.1093/jnci/djt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Viale PH: Can ginseng alleviate cancer-related fatigue? J Adv Pract Oncol 4, 392–3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Park HJ, Shim HS, Kim JY, Kim JY, Park SK, et al. : Ginseng Purified Dry Extract, BST204, Improved Cancer Chemotherapy-Related Fatigue and Toxicity in Mice. Evid Based Complement Alternat Med 2015, 197459, 2015. doi: 10.1155/2015/197459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yennurajalingam S, Tannir NM, Williams JL, Lu Z, Hess KR, et al. : A Double-Blind, Randomized, Placebo-Controlled Trial of Panax Ginseng for Cancer-Related Fatigue in Patients With Advanced Cancer. J Natl Compr Canc Netw 15, 1111–1120, 2017. doi: 10.6004/jnccn.2017.0149 [DOI] [PubMed] [Google Scholar]
- 139.Barton DL, Soori GS, Bauer BA, Sloan JA, Johnson PA, et al. : Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer 18, 179–87, 2010. doi: 10.1007/s00520-009-0642-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lima NDS, Teixeira L, Gambero A, Ribeiro ML: Guarana (Paullinia cupana) Stimulates Mitochondrial Biogenesis in Mice Fed High-Fat Diet. Nutrients 10, 2018. doi: 10.3390/nu10020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lima NDS, Numata EP, Mesquita LMS, Dias PH, Vilegas W, et al. : Modulatory Effects of Guarana (Paullinia cupana) on Adipogenesis. Nutrients 9, 2017. doi: 10.3390/nu9060635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schimpl FC, da Silva JF, Goncalves JF, Mazzafera P: Guarana: revisiting a highly caffeinated plant from the Amazon. J Ethnopharmacol 150, 14–31, 2013. doi: 10.1016/j.jep.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 143.Moustakas D, Mezzio M, Rodriguez BR, Constable MA, Mulligan ME, et al. : Guarana provides additional stimulation over caffeine alone in the planarian model. PLoS One 10, e0123310, 2015. doi: 10.1371/journal.pone.0123310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Oliveira Campos MP, Riechelmann R, Martins LC, Hassan BJ, Casa FB, et al. : Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J Altern Complement Med 17, 505–12, 2011. doi: 10.1089/acm.2010.0571 [DOI] [PubMed] [Google Scholar]
- 145.da Costa Miranda V, Trufelli DC, Santos J, Campos MP, Nobuo M, et al. : Effectiveness of guarana (Paullinia cupana) for postradiation fatigue and depression: results of a pilot double-blind randomized study. J Altern Complement Med 15, 431–3, 2009. doi: 10.1089/acm.2008.0324 [DOI] [PubMed] [Google Scholar]
- 146.Sette CVM, Ribas de Alcantara BB, Schoueri JHM, Cruz FM, Cubero DIG, et al. : Purified Dry Paullinia cupana (PC-18) Extract for Chemotherapy-Induced Fatigue: Results of Two Double-Blind Randomized Clinical Trials. J Diet Suppl, 1–11, 2017. doi: 10.1080/19390211.2017.1384781 [DOI] [PubMed] [Google Scholar]
- 147.Basile A, Ferrara L, Pezzo MD, Mele G, Sorbo S, et al. : Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J Ethnopharmacol 102, 32–6, 2005. doi: 10.1016/j.jep.2005.05.038 [DOI] [PubMed] [Google Scholar]
- 148.Leite RP, Wada RS, Monteiro JC, Predes FS, Dolder H: Protective effect of Guarana (Paullinia cupana var. sorbilis) pre-treatment on cadmium-induced damages in adult Wistar testis. Biol Trace Elem Res 141, 262–74, 2011. doi: 10.1007/s12011-010-8729-7 [DOI] [PubMed] [Google Scholar]
- 149.Yonekura L, Martins CA, Sampaio GR, Monteiro MP, Cesar LA, et al. : Bioavailability of catechins from guarana (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct 7, 2970–8, 2016. doi: 10.1039/c6fo00513f [DOI] [PubMed] [Google Scholar]
- 150.Mattei R, Dias RF, Espinola EB, Carlini EA, Barros SB: Guarana (Paullinia cupana): toxic behavioral effects in laboratory animals and antioxidants activity in vitro. J Ethnopharmacol 60, 111–6, 1998 [DOI] [PubMed] [Google Scholar]
- 151.Alkhatib A, Seijo M, Larumbe E, Naclerio F: Acute effectiveness of a “fat-loss” product on substrate utilization, perception of hunger, mood state and rate of perceived exertion at rest and during exercise. J Int Soc Sports Nutr 12, 44, 2015. doi: 10.1186/s12970-015-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zeidan-Chulia F, Gelain DP, Kolling EA, Rybarczyk-Filho JL, Ambrosi P, et al. : Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid Med Cell Longev 2013, 791795, 2013. doi: 10.1155/2013/791795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira SS, Del Giglio AB, Lerner TG, Zanellato RM, Tiemi L, et al. : Paullinia cupana for control of hot flashes in breast cancer patients: a pilot study. Einstein (Sao Paulo) 11, 435–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu YS, Ho SY, Nan FH, Chen SN: Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement Altern Med 16, 500, 2016. doi: 10.1186/s12906-016-1476-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan K, Jiang Q, Liu G, Miao X, Zhong D: Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol 55, 301–6, 2013. doi: 10.1016/j.ijbiomac.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 156.Chi B, Wang S, Bi S, Qin W, Wu D, et al. : Effects of ganoderic acid A on lipopolysaccharide-induced proinflammatory cytokine release from primary mouse microglia cultures. Exp Ther Med 15, 847–853, 2018. doi: 10.3892/etm.2017.5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J, Cao B, Zhao H, Feng J: Emerging Roles of Ganoderma Lucidum in Anti-Aging. Aging Dis 8, 691–707, 2017. doi: 10.14336/ad.2017.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sohretoglu D, Huang S: Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer Agents Med Chem, 2017. doi: 10.2174/1871520617666171113121246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu CM, Kao CL, Tseng YT, Lo YC, Chen CY: Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 22, 2017. doi: 10.3390/molecules22091477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pashaei-Asl R, Pashaei-Asl F, Mostafa Gharabaghi P, Khodadadi K, Ebrahimi M, et al. : The Inhibitory Effect of Ginger Extract on Ovarian Cancer Cell Line; Application of Systems Biology. Adv Pharm Bull 7, 241–249, 2017. doi: 10.15171/apb.2017.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Funk JL, Frye JB, Oyarzo JN, Chen J, Zhang H, et al. : Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 4, 123–131, 2016. doi: 10.1016/j.phanu.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bolognesi G, Belcaro G, Feragalli B, Cornelli U, Cotellese R, et al. : Movardol(R) (N-acetylglucosamine, Boswellia serrata, ginger) supplementation in the management of knee osteoarthritis: preliminary results from a 6-month registry study. Eur Rev Med Pharmacol Sci 20, 5198–5204, 2016 [PubMed] [Google Scholar]
- 163.Abolaji AO, Ojo M, Afolabi TT, Arowoogun MD, Nwawolor D, et al. : Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact 270, 15–23, 2017. doi: 10.1016/j.cbi.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 164.Lee S, Jerng UM, Liu Y, Kang JW, Nam D, et al. : The effectiveness and safety of moxibustion for treating cancer-related fatigue: a systematic review and meta-analyses. Support Care Cancer 22, 1429–40, 2014. doi: 10.1007/s00520-014-2161-z [DOI] [PubMed] [Google Scholar]
- 165.Marx W, McCarthy AL, Ried K, McKavanagh D, Vitetta L, et al. : The Effect of a Standardized Ginger Extract on Chemotherapy-Induced Nausea-Related Quality of Life in Patients Undergoing Moderately or Highly Emetogenic Chemotherapy: A Double Blind, Randomized, Placebo Controlled Trial. Nutrients 9, 2017. doi: 10.3390/nu9080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marx W, McCarthy AL, Ried K, Vitetta L, McKavanagh D, et al. : Can ginger ameliorate chemotherapy-induced nausea? Protocol of a randomized double blind, placebo-controlled trial. BMC Complement Altern Med 14, 134, 2014. doi: 10.1186/1472-6882-14-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cai Z, Liu J, Bian H, Cai J: Astragaloside IV ameliorates necrotizing enterocolitis by attenuating oxidative stress and suppressing inflammation via the vitamin D3-upregulated protein 1/NF-kappaB signaling pathway. Exp Ther Med 12, 2702–2708, 2016. doi: 10.3892/etm.2016.3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang WJ, Frei B: Astragaloside IV inhibits NF- kappa B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm 2015, 274314, 2015. doi: 10.1155/2015/274314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guo Z, Xu HY, Xu L, Wang SS, Zhang XM: IN VIVO AND IN VITRO IMMUNOMODULATORY AND ANTI-INFLAMMATORY EFFECTS OF TOTAL FLAVONOIDS OF ASTRAGALUS. Afr J Tradit Complement Altern Med 13, 60–73, 2016. doi: 10.21010/ajtcam.v13i4.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang G, Zhou SM, Zheng SJ, Liu FY, Gao YQ: Astragalus on the anti-fatigue effect in hypoxic mice. Int J Clin Exp Med 8, 14030–5, 2015 [PMC free article] [PubMed] [Google Scholar]
- 171.Huang YF, Lu L, Zhu DJ, Wang M, Yin Y, et al. : Effects of Astragalus Polysaccharides on Dysfunction of Mitochondrial Dynamics Induced by Oxidative Stress. Oxid Med Cell Longev 2016, 9573291, 2016. doi: 10.1155/2016/9573291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu CH, Tsai CH, Li TC, Yang YW, Huang WS, et al. : Effects of the traditional Chinese herb Astragalus membranaceus in patients with poststroke fatigue: A double-blind, randomized, controlled preliminary study. J Ethnopharmacol 194, 954–962, 2016. doi: 10.1016/j.jep.2016.10.058 [DOI] [PubMed] [Google Scholar]
- 173.Lin RD, Mao YW, Leu SJ, Huang CY, Lee MH: The immuno-regulatory effects of Schisandra chinensis and its constituents on human monocytic leukemia cells. Molecules 16, 4836–49, 2011. doi: 10.3390/molecules16064836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kang S, Lee KP, Park SJ, Noh DY, Kim JM, et al. : Identification of a novel anti-inflammatory compound, alpha-cubebenoate from Schisandra chinensis. J Ethnopharmacol 153, 242–9, 2014. doi: 10.1016/j.jep.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 175.Chi A, Zhang Y, Kang Y, Shen Z: Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int J Biol Macromol 93, 322–332, 2016. doi: 10.1016/j.ijbiomac.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 176.Kang YS, Han MH, Hong SH, Park C, Hwang HJ, et al. : Anti-inflammatory Effects of Schisandra chinensis (Turcz.) Baill Fruit Through the Inactivation of Nuclear Factor-kappaB and Mitogen-activated Protein Kinases Signaling Pathways in Lipopolysaccharide-stimulated Murine Macrophages. J Cancer Prev 19, 279–87, 2014. doi: 10.15430/jcp.2014.19.4.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan XX, Yang LP, Yang ZL, Xiao WL, Sun HD, et al. : Effect of nigranoic acid on Ca(2)(+) influx and its downstream signal mechanism in NGF-differentiated PC12 cells. J Ethnopharmacol 153, 725–31, 2014. doi: 10.1016/j.jep.2014.03.038 [DOI] [PubMed] [Google Scholar]
- 178.Alok S, Jain SK, Verma A, Kumar M, Mahor A, et al. : Herbal antioxidant in clinical practice: A review. Asian Pacific Journal of Tropical Biomedicine 4, 78–84, 2014. doi: 10.1016/S2221-1691(14)60213-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zou S, Fang L, Lee MH: Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf) 6, 1–12, 2018. doi: 10.1093/gastro/gox031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Inglis JE, Ilich JZ: The Microbiome and Osteosarcopenic Obesity in Older Individuals in Long-Term Care Facilities. Curr Osteoporos Rep 13, 358–62, 2015. doi: 10.1007/s11914-015-0287-7 [DOI] [PubMed] [Google Scholar]
- 181.Marx TL, Mehta AE: Polycystic ovary syndrome: Pathogenesis and treatment over the short and long term. Cleveland Clinic Journal of Medicine 70, 31–45, 2003 [DOI] [PubMed] [Google Scholar]
- 182.Lakhan SE, Kirchgessner A: Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond) 7, 79, 2010. doi: 10.1186/1743-7075-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fremont M, Coomans D, Massart S, De Meirleir K: High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 22, 50–56, 2013. doi: 10.1016/j.anaerobe.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 184.Sheedy JR, Wettenhall RE, Scanlon D, Gooley PR, Lewis DP, et al. : Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 23, 621–8, 2009 [PubMed] [Google Scholar]
- 185.Logan AC, Venket Rao A, Irani D: Chronic fatigue syndrome: lactic acid bacteria may be of therapeutic value. Med Hypotheses 60, 915–23, 2003 [DOI] [PubMed] [Google Scholar]
- 186.Dulal S, Keku TO: Gut microbiome and colorectal adenomas. Cancer J 20, 225–31, 2014. doi: 10.1097/ppo.0000000000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, et al. : Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4, 325–39, 2013. doi: 10.4161/gmic.25487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hamad A, Fragkos KC, Forbes A: A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr 32, 353–60, 2013. doi: 10.1016/j.clnu.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 189.Liu MM, Li ST, Shu Y, Zhan HQ: Probiotics for prevention of radiation-induced diarrhea: A meta-analysis of randomized controlled trials. PLoS One 12, e0178870, 2017. doi: 10.1371/journal.pone.0178870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hauer-Jensen M, Denham JW, Andreyev HJ: Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 11, 470–9, 2014. doi: 10.1038/nrgastro.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Packey CD, Ciorba MA: Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 26, 88–94, 2010. doi: 10.1097/MOG.0b013e3283361927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Delia P, Sansotta G, Donato V, Frosina P, Messina G, et al. : Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol 13, 912–5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, et al. : Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med 23, 356–62, 2015. doi: 10.1016/j.ctim.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 194.Sonis ST: The pathobiology of mucositis. Nat Rev Cancer 4, 277–84, 2004. doi: 10.1038/nrc1318 [DOI] [PubMed] [Google Scholar]
- 195.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ: The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 6, e1000879, 2010. doi: 10.1371/journal.ppat.1000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee JY, Chu SH, Jeon JY, Lee MK, Park JH, et al. : Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis 46, 1126–32, 2014. doi: 10.1016/j.dld.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 197.Kleckner IR, Dunne RF, Asare M, Cole C, Fleming F, et al. : Exercise for Toxicity Management in Cancer-A Narrative Review. Oncol Hematol Rev 14, 28–37, 2018 [PMC free article] [PubMed] [Google Scholar]
- 198.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, et al. : Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol 3, 961–968, 2017. doi: 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lin PJ, Peppone LJ, Janelsins MC, Mohile SG, Kamen CS, et al. : Yoga for the Management of Cancer Treatment-Related Toxicities. Curr Oncol Rep 20, 5, 2018. doi: 10.1007/s11912-018-0657-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.