Abstract
Epithelial barrier cells are proposed to be critical for host defense, and airway epithelial cell capacity for IFN signal transduction is presumed to protect against respiratory viral infection. However, it has been difficult to fully test these concepts given the absence of tools to analyze IFN signaling specific to airway epithelial cells in vivo. To address these issues, we generated a new line of transgenic mice with Cre-driver genes (Foxj1 and Scgb1a1) for a floxed-Stat1 allele (designated Foxj1-Scgb1a1-Cre-Stat1f/f mice) to target the master IFN-signal regulator STAT1 in airway epithelial cells and tested these mice for control of infection due to mouse parainfluenza (Sendai) virus (SeV) and human enterovirus (EV)-D68. Indeed, both types of infections showed increases in viral titers and severity of acute illness in Foxj1-Scgb1a1-Cre-Stat1f/f mice and conventional Stat1−/− mice compared to wild-type mice. In concert, the chronic lung disease that develops after SeV infection was also increased in Foxj1-Scgb1a1-Cre-Stat1f/f and Stat1−/− mice, marked by airway and adjacent parenchymal immune cell infiltration and mucus production for at least 7 wk after infection. Unexpectedly, relatively mild EV-D68 infection also progressed to chronic lung disease in Foxj1-Scgb1a1-Cre-Stat1f/f and Stat1−/− mice but was limited (like viral replication) to airways. The results thereby provide proof-of-concept for a critical role of barrier epithelial cells in protection from acute illness and chronic disease after viral infection and suggest a specific role for airway epithelial cells given the limitation of EV-D68 replication and acute and chronic manifestations of disease primarily to airway tissue.
Introduction
Normal function of interferon signal transduction is generally accepted as a requirement for defense against viral infections in general, and respiratory viral infections in particular (1, 2). This proposal is often based on the function of the STAT1 transcription factor that is essential for each type of IFN signaling pathway. For example, STAT1-similar to IFNR-deficiencies in transgenic mouse models results in a marked increase in susceptibility to viral infection (3–6), including respiratory viral infection (7, 8). The central role of STAT1 in anti-viral defense is further highlighted by observations that viruses often selectively target STAT1 function to subvert the immune response (9). Moreover, molecular enhancement of STAT1 function can provide increased protection against viral infection compared to the normal physiological settings of the IFN signaling system in cell and mouse models (10). In addition, susceptibility to viral infection is also markedly increased in humans with mutations that inactivate STAT1 gene function (11–13). Together, the critical nature of STAT1 function for anti-viral host defense is well established. What is less certain is the cellular site of action for STAT1 in the setting of infection and the implications for STAT1 function beyond the time of the acute infectious illness.
In that regard, another conventional concept for the immune system is the role of barrier epithelial cells in host defense against viral infection and the special role of airway epithelial cells in defense against respiratory viral infection. Here again, some of the best evidence for this concept was gained from loss-of-function mouse models. In particular, conventional Stat1−/− mice show increased susceptibility to severe infection with mouse parainfluenza virus also known as Sendai virus (SeV), and further, Stat1−/− mice that are reconstituted with wild-type bone marrow cells indicate that STAT1-expressing stromal cells rather than immune cells protect mice against acute illness, marked by weight loss and viral replication after this type of infection (7). Given that SeV infection can be readily detected in airway epithelial cells, these experiments provided initial evidence for these cells to account for the stromal cell function of STAT1 as a control point for respiratory viral infection. However, the experimental tools for this study were not sufficient to localize protection precisely to epithelial cells versus other stromal cells or even further to the airway epithelial cell subset since bone marrow chimeras do not provide epithelial cell-specific targeting and SeV infection is not likely to be restricted to airway epithelial cells. In addition, this initial study did not assess the development of chronic lung disease that might develop after clearance of infectious virus in relation to epithelial cells or any other cell type. This issue of long-term post-viral disease is vital to understanding how acute viral infections might initiate or exacerbate chronic airway disease, including the link between viral infection and asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap syndrome in humans and whether or not the connection includes host defense compromise such as deficiencies of IFN production and/or signaling function (14, 15).
To address these issues, the present experiments were initiated to define the phenotype for STAT1 deficiency specifically in barrier epithelial cells that might serve as the primary host cells during viral infection. Within this cell population in the airway epithelium, specific candidates for viral host cells include ciliated cells that can be marked with Foxj1 gene expression and club cells with Scgb1a1 gene expression (16, 17). Accordingly, we generated a new line of transgenic mice with airway epithelial cell Cre-driver genes (Foxj1 and Scgb1a1) for a floxed-Stat1 allele (designated Foxj1-Scgb1a1-Cre-Stat1f/f mice) to determine whether STAT1 function in airway epithelial cells was required for controlling respiratory infection with SeV. We also extended this mouse model to studies of a human viral pathogen in the form of enterovirus D68 (EV-D68), a member of the Picornaviridae family that (unlike other enteroviruses) shares characteristics with human rhinovirus (RV) members of this family. In concert with shared viral features, EV-D68 can also cause respiratory infection, and this infection can result in severe illness in susceptible populations, particularly asthmatics (18–23) as well as acute flaccid myelitis in other patients, based possibly on neuronal receptor binding (24). The severity of EV-D68-driven illness might be based on viral capacity to subvert the IFN-based immune response (25, 26), raising the possibility that any further IFN-deficiency might no longer influence the infection. In addition, to our knowledge, EV-D68 infection is limited to an acute illness. Thus, EV-D68 unlike SeV (27–30) and influenza A virus (31) has not yet been linked to the development of chronic disease that persists after clearance of infectious virus, particularly long-term respiratory disease.
Here we also aimed to define the relationship of STAT1 function and any change in acute illness to the development of chronic lung disease as a model of viral induction, exacerbation, and/or progression of the chronic airway disease. As noted above, this issue is particularly relevant to the virus-linked airway disease found in humans with asthma, COPD, or asthma-COPD overlap syndrome (32, 33). Accordingly, we focused on the cardinal features of this type of disease, i.e., airway inflammation (marked by immune cell infiltration), mucus production, (marked by mucin Muc5ac expression), hyperreactivity (marked by baseline and methacholine-induced lung resistance), and fibrosis (marked by trichrome staining) as relevant endpoints that might develop as a consequence of viral infection. We were particularly interested in airway mucus production since it appears to be linked to the earliest decline in lung function (34) and later exacerbation, progression, and death in asthma (35–39) and COPD (40–42) and to viral reprogramming of airway progenitor epithelial cells (APECs) towards mucous cells (32). Our results indicate that barrier epithelial cell capacity for STAT1 function is critical to protect against acute illness and the subsequent chronic lung disease that develops after both SeV and EV-D68 infections. The findings thereby provide proof-of-concept for the key role of barrier epithelial cells in the short-term and long-term host responses to viral infection, linking severity of acute infection to the development of chronic disease. In addition, limitation of EV-D68 infection to airway epithelial cells suggests that STAT1 protects at the level of airway versus alveolar epithelial barrier cells in the case of disease confined to airways.
Materials and Methods
Mice
Wild-type C57BL/6J mice (000664) and Stat1−/− mice (012606) mice were obtained from The Jackson Laboratory. To generate mice with Stat1-flox gene expression (Stat1f/f), C57BL/6J genomic tail DNA was used to generate a 1.6-kb genomic DNA fragment containing Stat1 exons 10–12, a 1.6-kb fragment upstream of exon 10 containing a loxP sequence as the left arm, and a 2.5 kb fragment downstream of exon 12 as the right arm using PCR-based amplification with primers derived from MGI:103063 and defined in Supplemental Table I. The Stat1 targeting vector was assembled in a pSVloxfrtneofrt plasmid containing one loxP site and a Frt-flanked PGK promoter-driven neo gene. The homologous regions of the final vector consisted of a 1.6 kb genomic fragment, one loxP, exon 10, 11 and 12, loxP-frt-neo-frt and 2.5 kb genomic DNA fragment containing exon 13. The vector was transfected into C57BL/6-LacZ embryonic stem cells (B6/BLU, ATCC SCRC-1019), and positive cell clones were microinjected into foster C57BL/6J mice to produce chimeric mice that were bred with C57BL/6J mice for germline transmission of the Stat1-flox allele based on LacZ gene expression detected with X-gal staining of RBCs as described previously (43, 44). The frt-flanked neo gene cassette was removed by breeding to Flp-deleter mice (005703), and offspring were mated with wild-type C57BL/6J mice and screened for absence of the Flp transgene and presence of two loxP sites. Heterozygotes were bred to obtain homozygous Stat1f/f mice that were used to prepare genomic DNA that was digested with restriction enzymes, size-separated on a 0.8% agarose Tris-Acetate-EDTA (pH 8.0) gel, and transferred onto a Biodyne nylon membrane (Pall Life Sciences) for Southern blot with 820- and 500-bp P32-radiolabeled fragments immediately upstream of the left arm and downstream of the right arm, respectively, to validate homologous recombinants. The Stat1f/f mice were bred to CAG-Cre mice (019099) to generate CAG-Cre-Stat1f/f mice that were used to determine STAT1 expression across mouse tissues. The Foxj1-Cre mice (44) (to target ciliated cells) and Scgb1a1-Cre mice (45) (to target primarily club cells) were crossed to ROSA26mTmG reporter mice (007576) to define Cre-mediated recombination efficiency. Finally, Foxj1-Scgb1a1-Cre mice were crossed to Stat1f/f mice to generate Foxj1-Scgb1a1-Cre-Stat1f/f mice for studies of viral infection. All mice were maintained on a C57BL/6J background and were co-housed in a barrier facility using cages fitted with micro-isolator lids. Animal husbandry and experimental procedures were approved by the Animal Studies Committees of Washington University School of Medicine in accordance with the guidelines from the National Institutes of Health.
Tissue staining and microscopy
For Cre-recombination experiments, lungs from Foxj1-Cre-ROSA26mTmG and Scgb1a1-Cre-ROSA26mTmG were fixed with 10% formalin, embedded in paraffin, cut into 5-μm sections, and adhered to charged slides. Sections were deparaffinized in Fisherbrand CitroSolv (Fisher), hydrated, and treated with heat-activated antigen unmasking solution (Vector Laboratories). Immunostaining was performed using primary chicken anti-GFP antibody (Abcam), mouse anti-Foxj1 mAb from S. Brody (Washington University) (46), or goat anti-Scgb1a1 Ab (sc-9772, Santa Cruz). Primary Ab was detected with secondary Ab labeled with Alexa Fluor 594 for Foxj1 and Scgb1a1 and with Alexa Fluor 488 for GFP. Co-staining for GFP and Sftpc was performed with lung tissue that was cryopreserved in OCT compound (Tissue-Tek, Sakura Fine Tek, Torrance, CA) and then cut into 6-μm-thick sections as described previously (44), and primary rabbit anti-Sftpc Ab (ab40879, Abcam) and Alexa Fluor 647-labeled Ab (Thermo Fisher Scientific). All sections were stained with DAPI and were imaged using an Olympus BX51 microscope with a charge-coupled device camera interfaced to MagniFire software from Olympus (Melville, NY) for conventional imaging or a Zeiss LSM 880 laser scanning microscope for confocal imaging. Staining was quantified in whole lung sections using a NanoZoomer S60 slide scanner (Hamamatsu) and Image J software (47).
For viral infection experiments, immunostaining was performed using primary mouse anti-Stat1 Ab (610116, BD Biosciences), chicken anti-SeV Ab (10100648, Charles River Laboratories), mouse anti-β-Tubulin IV mAb (T7941, Sigma), goat anti-Scgb1a1 Ab (sc-9772, Santa Cruz), rabbit anti-Sftpc Ab (ab40879, Abcam), rabbit anti-EV-D68 VP1 Ab (GTX132313, GeneTex), mouse anti-MUC5AC mAb (clone 45M1, MA 5–12175, Thermo Fisher Scientific), and rabbit anti-human CLCA1 Ab (aa 33–63). Primary Abs were detected with secondary Abs labeled with Alexa Fluor 488 (Thermo Fisher Scientific) or Alexa Fluor 594-labeled Ab (Thermo Fisher Scientific) followed by DAPI staining and immunofluorescence microscopy. Sections were also stained with hematoxylin and eosin or PAS and hematoxylin. PAS+ staining was quantified with settings specific for the purple-magenta-tone of mucins, Muc5ac+ immunostaining was quantified with a setting specific for brown tone of mucins, and Clca1+ and Muc5ac+ immunostaining were quantified based on fluorescence signal, each using whole lung sections and the image analysis system described above.
Virus preparation and infection
SeV was obtained from ATCC (Sendai/52 Fushimi strain, ATCC VR-105) and prepared and titered by plaque-forming assay as described previously (28). EV-D68 was identified in a clinical sample of bronchoalveolar lavage fluid with PCR and gene sequencing and was then used as a template to generate four overlapping fragments that covered the entire viral genome with RT-PCR. The fragments were cloned into a shuttle vector, and plasmid clones corresponding to the established genome sequence were placed downstream of the T7 RNA polymerase promoter and hammerhead ribozyme to produce transcripts with accurate 5’-ends (48) and poly(A) tail at the 3’end of the complete EV-D68 genome. The full-length EV-D68 genome contained identical nucleotide sequence to EV-D68 detected in the clinical sample. To generate infectious virus, the full-length genome plasmid was linearized by NsiI restriction enzyme digestion, and the RNA was transcribed in vitro using the MEGAscript kit (Invitrogen, CA). The DNA template was eliminated by treatment of the transcription mixture with TRIzol (Invitrogen, CA) and two rounds of DNase treatment and RNeasy (QIAgen) columns. HeLa cells were transfected with the transcribed RNA (3μg) by electroporation using GenePulser Xcell (Bio-Rad, CA). Electroporated cells were placed in a P100 dish and harvested at 24 h post-electroporation. Virus was purified by centrifugation through a sucrose cushion as described previously (49) and viral stocks were quantified using PCR assay for viral RNA and plaque-forming assay for adapted virus that is cytopathic in RD cells (ATCC CCL-136) to define equivalent PFU (ePFU) values as described previously (50).
Mice were infected with SeV (5 × 105 viruses based on qPCR assay for viral RNA equivalent to 1 × 105 PFU based on plaque-forming assay for infectious virus) or EV-D68 (1.5 × 108 viruses equivalent to 0.5 × 106 ePFU). Dosing was performing intranasally using SeV or EV-D68 in 30 μl of PBS or an equivalent amount of UV-inactivated virus (as a control for viral replication) or PBS alone under ketamine/xylazine anesthesia at 6–9 wk of age for SeV or 4 wk of age for EV-D68. Mouse age for SeV was based on previous work (27–30) and for EV-D68 was aimed at using younger but still adult mice to potentially increase susceptibility to infection just as done in other recent reports (51–53). Our approach thereby enables direct comparison across publications. Moreover, lung titers of EV-D68 RNA were no different for infection at 4 wk versus 6–9 wk of age for wild-type or Scgb1a1-Foxj1-Cre-Stat1f/f mice (data not shown). Results from male and female mice were pooled since no significant differences were found between sexes as reported initially for SeV (54) and confirmed for SeV and EV-D68 in preliminary studies (data not shown). Mouse lungs were frozen at −70 °C for homogenization in PBS with a cell disrupter (Mini-Beadbeater-96, Biospec Products) followed by viral plaque-forming assay to track PFU level normalized to gm of lung tissue (recognizing that one mouse lung weighs approximately 100 mg) (55). Viral titers for stock solutions and lung infections were monitored by PCR assay for EV-D68 and SeV using primers defined in Supplemental Table I.
RNA analysis
Total RNA was isolated from lung tissue using the RNeasy Plus Mini kit (Qiagen) and converted to cDNA using the High-Capacity cDNA Archive kit (Life Technologies) and random hexamer primers (Applied Biosystems). Levels of viral RNA were monitored using a real-time qPCR assay for EV-D68 capsid protein (VP1) gene or SeV nucleoprotein (NP) gene with forward and reverse primers and MGB probes described in Supplemental Table I. To quantify the level of viral RNA, segments of the EV-D68 genome (nt 1–2706) or SeV genome (nt 10–1715) were cloned into plasmid pCR2.1 using the TA cloning kit (ThermoFisher Scientific) and used as standards. Lung levels of mucin Muc5ac mRNA were determined using real-time quantitative PCR (qPCR) assay with probes described in Supplemental Table I. All target mRNA and viral RNA levels were normalized to Gapdh mRNA level using the TaqMan Rodent GAPDH Control Kit. All mRNA values were expressed as fold-change normalized to Gapdh mRNA.
Western blot analysis
Mouse tissues were homogenized in 1% Nonidet P-40, 0.05M Tris, pH 8.0, 250 mM NaCl, and 1 mM EDTA containing Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific). Extracted protein was subjected to western blotting with mouse anti-Stat1 Ab (BD Biosciences) and anti-Gapdh mAb (MAB374, MilliporeSigma) detected with horseradish peroxidase-conjugated horse anti-mouse IgG Ab (7076, Cell Signaling) and enhanced chemiluminescence as described previously (10).
Airway reactivity
Airway reactivity to nebulized methacholine (Sigma, St. Louis, MO) was determined by measurements of respiratory system resistance (RRS) using the FinePointe Resistance and Compliance system (DSI Buxco Research Systems, Wilmington, NC) as described previously (28, 29). Mice were anesthetized, ventilated via tracheostomy, and sequentially challenged with aerosolized PBS (baseline) followed by doubling doses of methacholine ranging from 0.6125 to 20 mg/ml. Methacholine was delivered at 3-min intervals using an in-line nebulizer (Aerogen Laboratory; 2.4–4 μm particle size). Resistance values were recorded during a 3-min period following each challenge. Data were manually verified, and spurious epochs removed from analysis.
Statistical analysis
Unless stated otherwise, all data are presented as mean ± SEM and two-tailed unpaired Student’s t-test was used to assess statistical significance between means. In all cases, the threshold for statistical significance was set at a P value less than 0.05. Airway reactivity for viral infection versus UV-inactivated virus condition was assessed using two-way analysis of variance and Bonferroni post-hoc correction.
Results
Generation of epithelial cell-specific Stat1 gene knockout mice
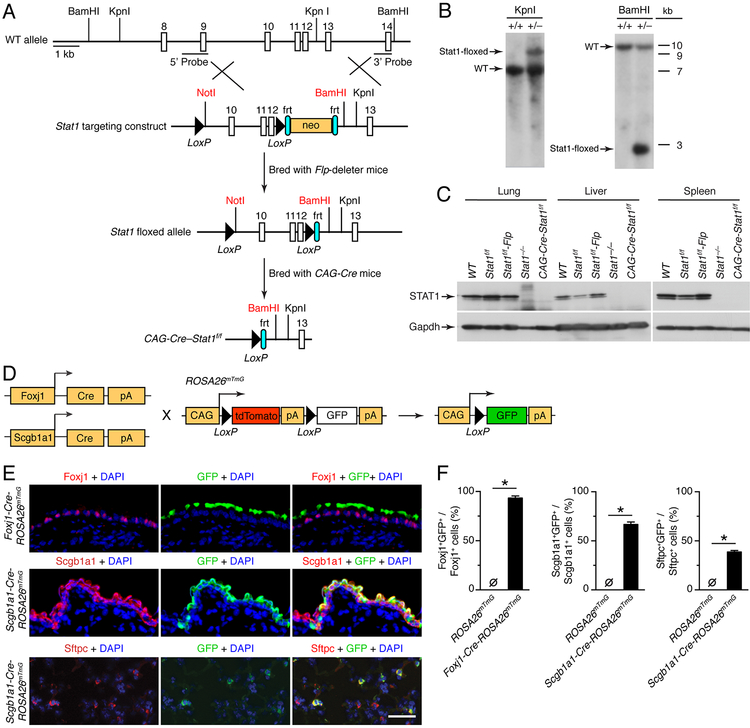
To determine if epithelial STAT1 function controls viral infection, we generated transgenic mice that expressed a floxed Stat1 allele (Stat1f/f) using the gene targeting scheme described in Materials and Methods and depicted in Fig. 1A. Mutant allele expression in heterozygous Stat1wt/f mice was confirmed using Southern blotting for tail DNA (Fig. 1B) In addition, crossing homozygous Stat1f/f mice to Flp-deleter mice and then to CAG-Cre mice resulted in Cre-mediated recombination with loss of STAT1 protein across lung, liver, and splenic tissues similar to conventional Stat1−/− mice based on western blotting (Fig. 1C). Together, these results validated the establishment of transgenic Stat1f/f mice with Flp gene deletion (hereafter referred to as Stat1f/f mice) for further study of STAT1 function in vivo. These mice exhibited normal birth frequency, development, and longevity without any histological changes in a full-organ survey that included lung tissue.
FIGURE 1.
Generation of epithelial-cell STAT1-deficient Foxj1-Scgb1a1-Cre-Stat1f/f mice. (A) Schematic of mouse wild-type (WT) Stat1 gene, Stat1 gene targeting construct, Stat1-floxed allele, and final Cre-modified Stat1flox/flox (Cre-Stat1f/f) gene locus. (B) Southern blot of tail DNA from heterozygous Stat1wt/f and control WT mice after treatment with restriction enzymes KpnI (Stat1f/f allele size of 9 kb, WT allele size of 7 kb) or BamHI (Stat1f/f allele size of 3 kb, WT allele size of 11 kb). (C) Western blot of tissue homogenates from indicated mouse strains using anti-STAT1 or anti-Gapdh Ab. (D) Breeding scheme for Foxj1-Cre or Scgb1a1-Cre mice cross to CAG-ROSA26mTmG mice to generate epithelial cell-specific CAG-GFP reporter mouse strains (Foxj1-Cre-ROSA26mTmg and Scgb1a1-Cre-ROSA26mTmG). (E) Immunostaining for Foxj1, Scgb1a1, Sfptc, and GFP and counterstain with DAPI of lung sections from Foxj1-Cre-ROSA26mTmG or Scgb1a1-ROSA26mTmG. Scale bar, 100 μm. (F) Quantitation of immunostaining for conditions in (E) along with control ROSA26mTmG mice. For (B,C,E,F), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (F), * indicates p<0.05.
To define conditions for epithelial cell deletion of the Stat1 gene, we crossed Foxj1-Cre mice (that target ciliated cells) and Scgb1a1-Cre mice (that target club cells) to ROSA26mTmG reporter mice (Fig. 1D). Lung sections from Foxj1-Cre-ROSA26mTmG mice immunostained with anti-Foxj1 and anti-GFP Abs showed co-localization (Fig. 1E) and a high level of co-staining (94.8% of Fojx1 cells were GFP+) based on image analysis (Fig. 1F). Lung sections from Scgb1a1-Cre-ROSA26mTmG mice immunostained for Scgb1a1, Sftpc, and GFP showed significant albeit lower levels of co-localization (Fig. 1E) and a correspondingly significant but lower level of co-staining (63.4% of Scgb1a1+ cells and 38.6% of Sftpc+ cells were GFP+) based on image analysis (Fig. 1F). Each of these results was consistent with our previous analysis of Foxj1-Cre mice and reports of Scgb1a1-Cre activity in club and alveolar type II (AT2) cells (56). Further, the results provided a basis for establishing combined Foxj1-Scgb1a1-Cre-Stat1f/f mice to define the role of barrier epithelial cells in STAT1 function for host defense against viral infection.
Epithelial STAT1 function defends against acute SeV infection
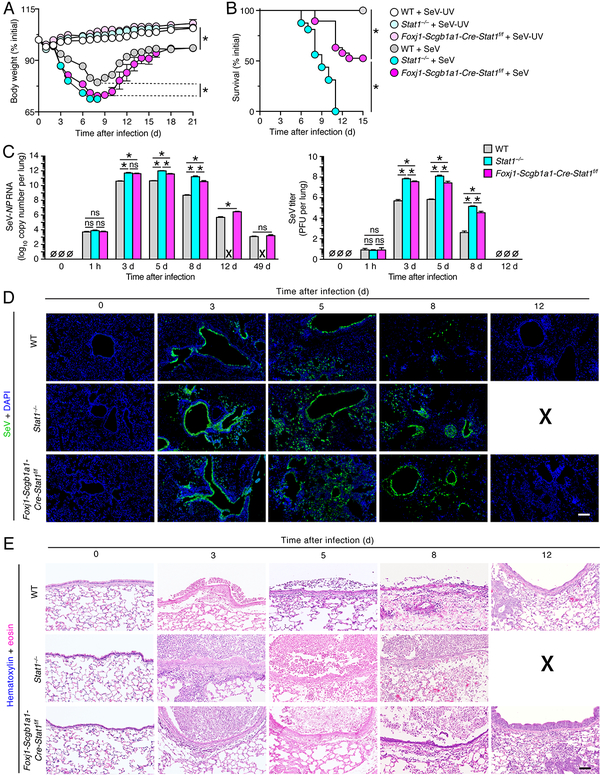
Our initial analysis of STAT1 function showed that body weight loss was significantly enhanced in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice after SeV infection (Fig. 2A). In addition, body weight was decreased slightly but significantly in Stat1−/− mice compared to Foxj1-Scgb1a1-Cre-Stat1f/f mice such that Stat1−/− mice were euthanized at 8 d after infection when body weight became <70% of the initial baseline value. Similarly, survival rates were significantly decreased in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice after SeV infection and were decreased even further in Stat1−/− mice after SeV infection (Fig. 2B). These changes were accompanied by increases in viral titers based on lung levels of SeV-NP RNA in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice, and in turn in Stat1−/− mice compared to Foxj1-Scgb1a1-Cre-Stat1f/f mice (Fig. 2C). Similar increases in viral titers were found based on infectious virus using plaque-forming assay (Fig. 2C). In concert with body weight, survival, and viral titer data, we also observed increases in the levels of viral immunostaining (Fig. 2D) and immune cell infiltration (Fig. 2E) in Foxj1-Scgb1a1-Cre-Stat1f/f mice and Stat1−/− mice compared to wild-type mice after SeV infection. Viral and immune cell infiltration was localized primarily to airway epithelial tissue at 3 d after infection but extended to alveolar sites by 5 d after infection, consistent with replication in both airway and alveolar epithelial cells.
FIGURE 2.
STAT1 deficiency in epithelial cells increases severity of SeV infection. (A) Body weights for WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at the indicated times after infection with SeV (1 × 105 PFU given intranasally) or equivalent control SeV-UV. (B) Survival rates for conditions in (A). (C) Lung levels of SeV-NP RNA using PCR assay and corresponding levels of infectious SeV titers using plaque-forming assay for WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at the indicated times after infection with SeV. (D) Immunostaining for SeV and counterstaining with DAPI in lung sections for conditions in (C). Scale bar, 400 μm. (E) Hematoxylin and eosin staining in lung sections for conditions in (C). Scale bar, 200 μm. For (A-E), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (A-B), * indicates p<0.05 by ANOVA except for survival rates by Kaplan-Meier analysis, and for (C-E), X signifies data that could not be obtained due to non-survival conditions.
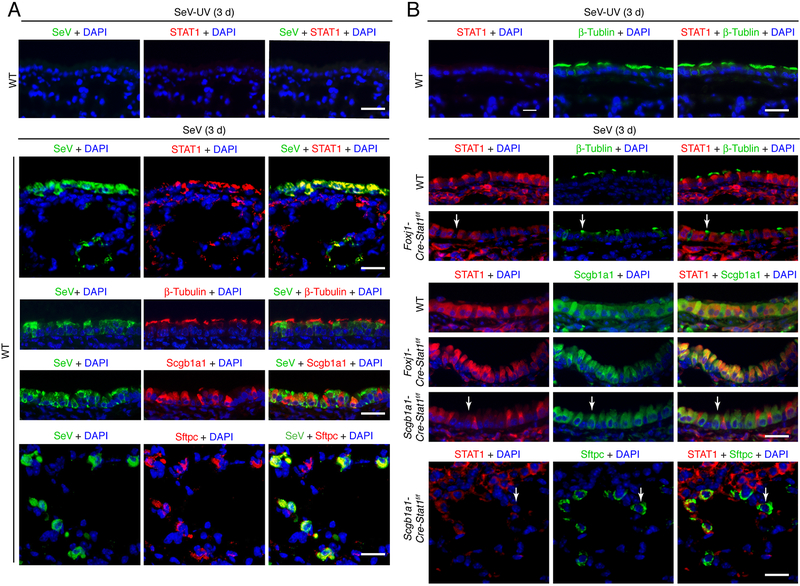
To address this issue, we also used SeV co-staining with epithelial cell markers to more precisely define the site of SeV replication. In that regard, immunostaining for SeV and STAT1 was markedly increased at 3 d after SeV infection and SeV immunostaining was co-localized with STAT1 as well as ciliated cell marker β-Tubulin IV, club cell marker of Scgb1a1, and AT2 cell marker Sftpc at 3 d after infection (Fig. 3A). These results were consistent with SeV replication in ciliated cell, club cell, and AT2 cells leading to induction of STAT1 expression at these cell sites. We also found that STAT1 immunostaining was lost in ciliated cells in Foxj1-Cre-Stat1f/f mice and in both club cells and AT2 cells in Scgb1a1-Cre-Stat1f/f mice at 3 d after SeV infection (Fig. 3B), consistent with the patterns of Cre recombination observed in Foxj1-Cre-ROSA26mTmG and Scgb1a1-Cre-ROSA26mTmG reporter mice (Fig. 1D). Moreover, the efficiencies for Cre-mediated Stat1 gene deletion in airway and alveolar epithelial cells (63% in club cells and 39% in AT2 cells) fit with the partial increase in severity of infection in Foxj1-Scgb1a1-Cre-Stat1f/f compared to the full increase in Stat1−/− mice. Nonetheless, the results provide a system for epithelial cell-specific deletion of Stat1 gene expression and consequent evidence that loss of STAT1 function in barrier epithelial cells leads to increased viral replication and severity of infection.
FIGURE 3.
SeV replication and STAT1 induction co-localizes to epithelial cells. (A) Immunostaining for SeV and STAT1, β-Tubulin IV, Scgb1a1, or Sftpc and counterstaining with DAPI in lung sections from WT mice at 3 d after infection with SeV. Scale bar, 100 μm. (B) Immunostaining for STAT1 and β-Tubulin IV, Scgb1a1, or Sftpc and counterstaining with DAPI in lung sections from Foxj1-Cre-Stat1f/f, Scgb1a1-Cre-Stat1f/f, and WT mice at 3 d after infection with SeV. Arrows indicate epithelial cells (green staining) without STAT1 (red staining) in the context of Stat1 gene deletion. Scale bar, 100 μm. For (A,B), values are representative of 3 separate experiments (n≥8 mice per condition in each experiment).
Epithelial STAT1 function protects against SeV-induced chronic lung disease
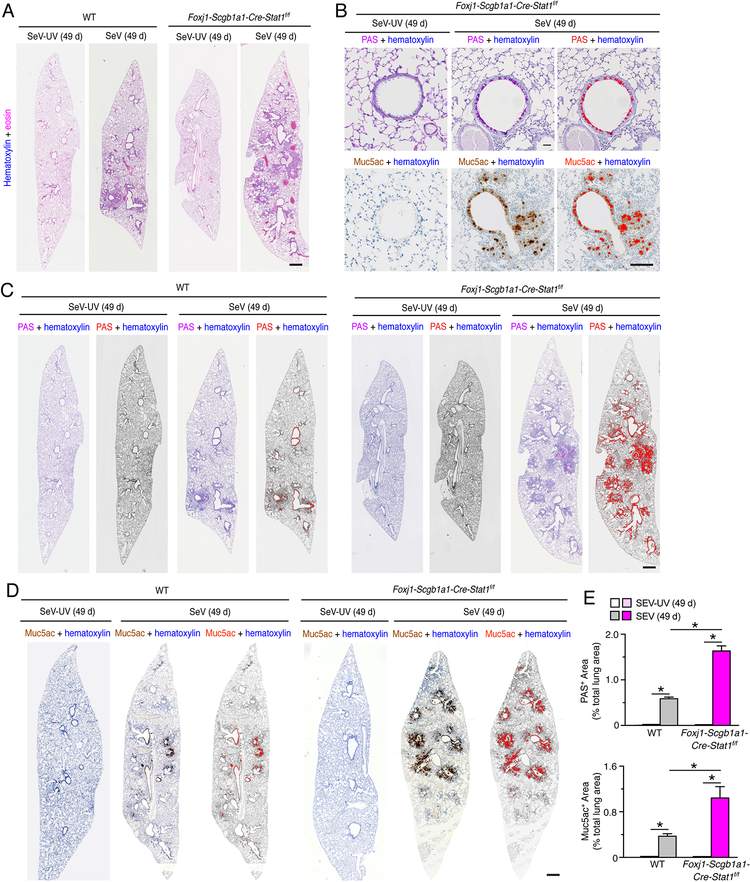
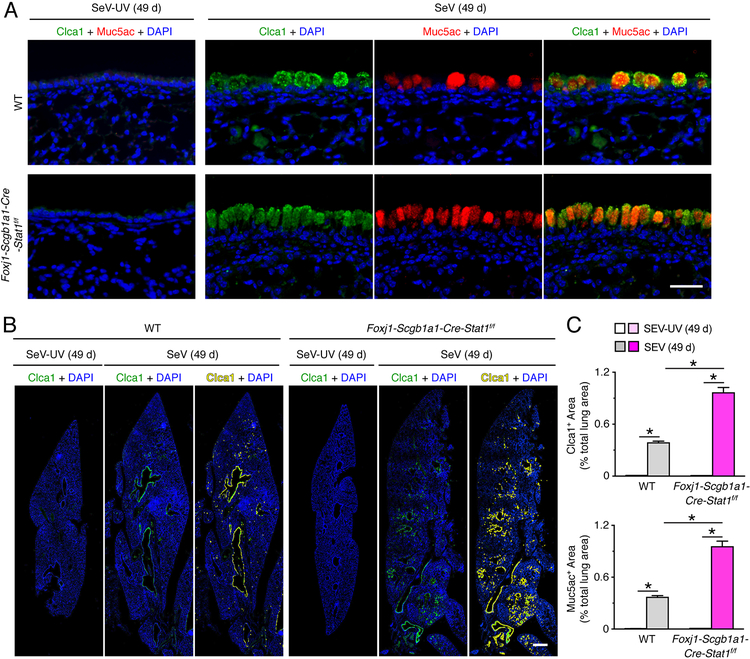
Given the capacity of SeV infection to trigger long-term lung disease (27–30), we also continued study of Foxj1-Scgb1a1-Cre-Stat1f/f and wild-type mice that survived the acute illness due to SeV infection. Here we found that the degree of chronic lung disease was significantly increased in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice marked by airway and adjacent parenchymal immune cell infiltration based on hematoxylin+ staining in hematoxylin and eosin stained lung sections (Fig. 4A) and mucus production marked by PAS+ staining and mucin Muc5ac+ immunostaining (Fig. 4B) in lung sections at 49 d after SeV infection. Quantitative image analysis of whole lung sections demonstrated that PAS+ and Muc5ac+ areas were significantly increased in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice (Fig. 4C, 4D, 4E). In concert with these observations, we found that increases in Muc5ac expression were closely coordinated with increases in Clca1 expression in airway mucous cells based on immunofluorescence microscopy (Fig. 5A). Quantitative image analysis of whole lung sections confirmed that Clca1+ and Muc5ac+ areas were significantly increased in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice (Fig. 5B, 5C). Together, these results indicated that epithelial cell STAT1 defense against acute SeV infection also translated to protection against chronic lung disease that develops after clearance of SeV infection.
FIGURE 4.
STAT1 deficiency in epithelial cells increases susceptibility to chronic lung disease after SeV infection. (A) Hematoxylin and eosin staining of lung sections from WT and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after infection with SeV or SeV-UV. Scale bar, 500 μm. (B) PAS staining and Muc5ac immunostaining for conditions in (A). Scale bar=400 μm. (C) PAS staining and Muc5ac immunostaining for conditions in (A). Scale bar, 500 μm. (D) PAS staining and Muc5ac immunostaining for conditions in (A). Scale bar, 500 μm. (E) Quantitation of PAS staining and Muc5ac immunostaining from conditions in (C, D). For (A-E), data are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (E), * indicates p<0.05.
FIGURE 5.
STAT1 deficiency in epithelial cells increases susceptibility to chronic lung disease after SeV infection. (A)Immunostaining for Clca1 and Muc5ac and counterstaining with DAPI in lung sections from WT and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after infection with SeV or SeV-UV. Scale bar, 100 μm. (B) Corresponding whole lung sections for Clca1 immunostaining for conditions in (A). Scale bar, 500 μm. (C) Quantitation of Clca1 and Muc5ac immunostaining in whole lung sections for conditions in (A). For (A-C), data are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (C), * indicates p<0.05.
STAT1 function defends against acute EV-D68 infection
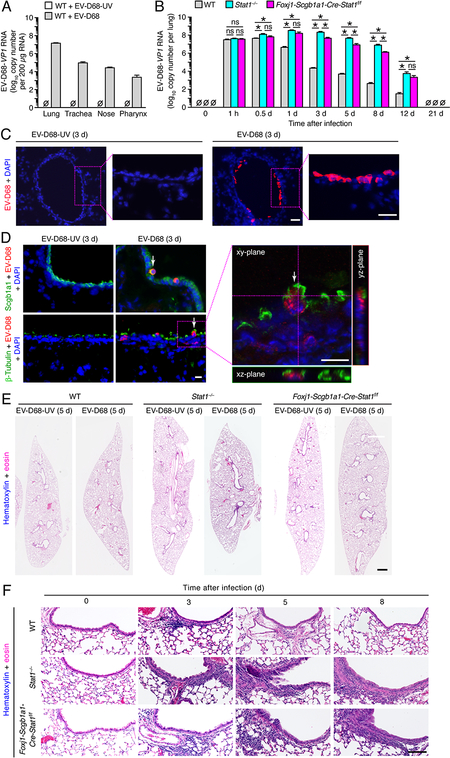
We next aimed to extend our studies of epithelial cell STAT1 to defense against respiratory viruses that are pathogenic in humans, especially in relation to severe airway disease. Given the high levels of EV-D68 replication (1×108-fold increase in viral titer) in primary-culture human airway epithelial cells (data not shown), we selected this virus for further study in mice in vivo. Initial experiments showed no significant body weight loss in Stat1−/−, Foxj1-Scgb1a1-Cre-Stat1f/f, or wild-type mice after EV-D68 infection (data not shown). Nonetheless, we detected significant increases in viral titer monitored by the level of EV-D68-VP1 RNA with the largest increase in lung tissue and smaller increases in tracheal, nasal, and pharyngeal tissue in wild-type mice (Fig. 6A). In addition, we detected a time-dependent increase in EV-D68-VP1 RNA in lung tissue with significant increases in viral RNA in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice, and in turn in Stat1−/− mice compared to Foxj1-Scgb1a1-Cre-Stat1f/f mice at 0.5–12 d after infection (Fig. 6B). Both time- and strain-dependence of EV-D68 titers were similar to the pattern that we found for SeV titers in lung tissue (Fig. 2C). However, the level of replication for EV-D68 was less than SeV, e.g., 1×101-fold versus 1×108-fold increase in viral RNA levels in the lung at 1 h versus 1–3 d after infection in Stat1−/− mice (Fig. 2C and Fig. 6B). Viral titer for EV-D68 was 5.7×107 whereas SeV was 1.2×104 viral RNA copies at 1 h after infection. However, this initial difference is expected since the inoculum for EV-D68 was 1.5×108 whereas SeV was 5.0×105 viral RNA copies, and the 1-h values reflect the inoculum amount since there is no detectable viral RNA replication until 4–14 h after infection (57, 58). In contrast, the viral titer at later time points is lower for EV-D68 than for SeV, but this result is also expected given the lower level of replication for a non-adapted human pathogen compared to a native rodent pathogen in a mouse host.
FIGURE 6.
STAT1-deficiency in epithelial cells increases severity of EV-D68 infection. (A) Tissue levels of EV-D68-VP1 RNA in WT mice at 1 d after infection with EV-D68 (0.5 × 106 ePFU given intranasally) or an equivalent amount of control EV-D68-UV. (B) Lung levels of EV-D68-VP1 RNA for WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at the indicated times after infection with EV-D68. (C) Immunostaining for EV-D68 with DAPI counterstaining in lung sections from Stat1−/− mice at 3 d after infection with EV-D68 or EV-D68-UV. Scale bar, 100 μm. (D) Immunostaining for EV-D68 and Scgb1a1 or β-Tubulin IV and counterstaining with DAPI in lung sections for conditions in (C) imaged with conventional and confocal microscopy. Arrows indicate cells co-staining for EV-D68 and Scgb1a1 or β-Tubulin IV. Scale bars, 50 μm. (E) Hematoxylin and eosin staining in lung sections for conditions in (A). Scale bar, 500 μm. (F) Hematoxylin and eosin staining in airway sections for conditions in (A). Scale bar, 200 μm. For (A-E), data are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (A,C), * indicates p<0.05.
In concert with the increase in viral levels in lung tissue, we also observed immunostaining for EV-D68 in airway epithelium at 3 d after infection in wild-type, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice (Fig. 6C). Moreover, EV-D68 was localized selectively to airway club cells based on co-staining with Scgb1a1 and to airway ciliated cells based on co-staining with β-Tubulin IV using conventional and confocal microscopy (Fig. 6D). Similarly, hematoxylin-eosin staining of lung sections showed immune cell infiltration mainly into the airway epithelium and adjacent subepithelial tissue at 3–8 d after infection in whole lung sections (Fig. 6E) and at higher magnification of airway epithelial sections (Fig. 6E) in all three strains of mice. Similar to viral levels, immune cell infiltration of airways was increased in Stat1−/− and Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice at 3–8 d after EV-D68 infection (Fig. 6F). Together, these results provided evidence of productive infection with EV-D68 in mice that was confined at least primarily to airway epithelial cells and was sensitive to loss of STAT1 function in lung epithelial cells, most likely the same set of host airway epithelial cells.
Epithelial STAT1 function protects against EV-D68-induced chronic lung disease
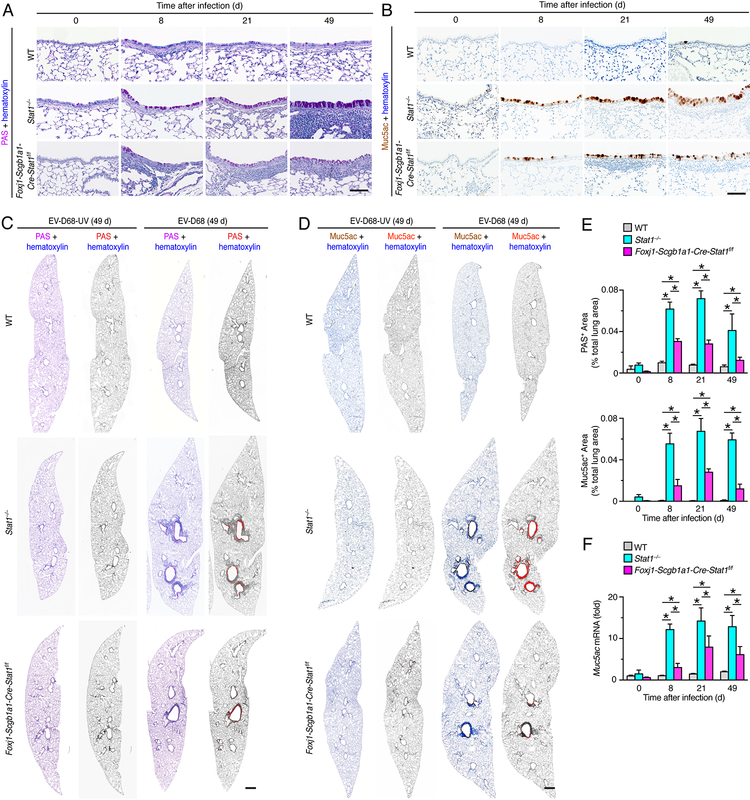
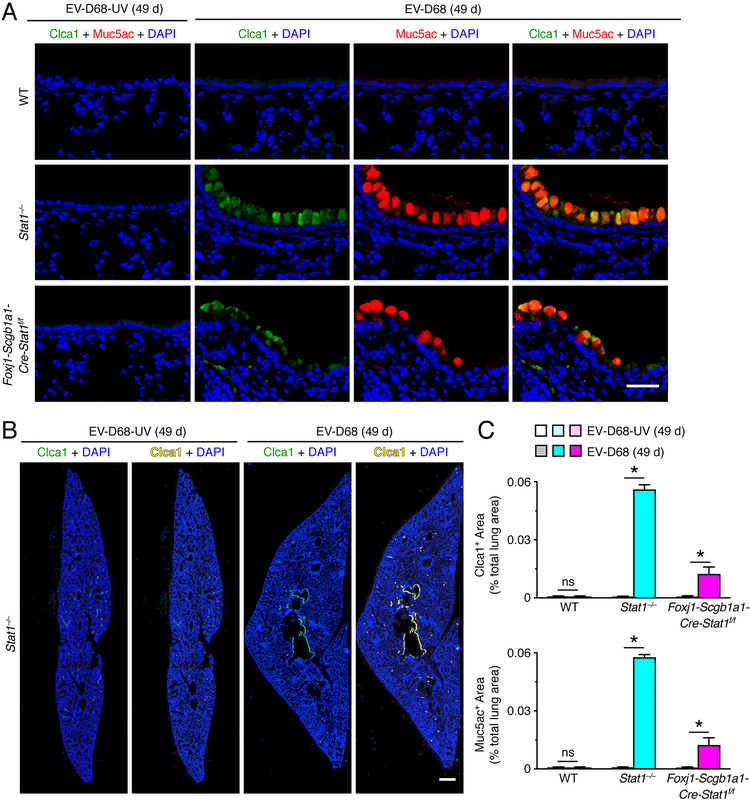
To define whether EV-D68 infection might unexpectedly trigger long-term lung disease, we studied the possibility that excess mucus production might develop as a marker of chronic lung disease after this type of viral infection. Indeed, PAS staining of lung sections showed increases in PAS+ cells (consistent with the morphology of mucous cells) in the airway epithelium generally with adjacent immune cell infiltration in Stat1−/− and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 8, 21, and 49 d after EV-D68 infection (Fig. 7A). Similarly, Muc5ac immunostaining demonstrated specific increases in Muc5ac+ mucous cells in the airway epithelium under these same experimental conditions (Fig. 7B). Corresponding staining of whole lung sections demonstrated that histopathologic changes marked by mucus production areas were confined to airways after EV-D68 infection (Fig. 7B, 7C). Quantitative image analysis of whole lung sections further confirmed that PAS+ and Muc5ac+ areas were significantly increased in Stat1−/− and to a lesser degree in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice at 8–49 d after EV-D68 infection (Fig. 7C, 7D, 7E). In concert with the evidence of chronic lung disease from tissue staining, we also found significant increases in lung levels of Muc5ac mRNA in Stat1−/− and to a lesser degree in Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice at 8–49 d after EV-D68 infection (Fig. 7F). In concert with these observations, we again found that increases in Muc5ac expression were linked to increases in Clca1 expression in airway mucous cells based on immunofluorescence microscopy (Fig. 8A). Quantitative image analysis of whole lung sections confirmed that Clca1+ and Muc5ac+ areas were significantly increased in Stat1−/− and to a lesser degree in Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after EV-D68 infection (Fig. 8B, 8C).
FIGURE 7.
STAT1 deficiency in epithelial cells increases susceptibility to chronic lung disease after EV-D68 infection. (A) PAS-hematoxylin staining of lung sections from WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 8, 21 and 49 d after infection with EV-D68. Scale bar, 100 μm. (B) Muc5ac immunostaining with hematoxylin counterstaining from the conditions in (A). Scale bar, 100 μm. (C) PAS and hematoxylin stained lung sections from WT, Stat1−/−, Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after infection with EV-D68 or EV-D68-UV. Scale bar, 500 μm. (D) Muc5ac and hematoxylin stained lung sections for conditions in (C). Scale bar, 500 μm. (E) Quantitation of PAS+ and Muc5ac+ staining areas for conditions in (C, D). (F) Lung levels of Muc5ac mRNA for conditions in (E). For (A-F), data are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (E,F), * indicates p<0.05.
FIGURE 8.
STAT1 deficiency in epithelial cells increases susceptibility to chronic lung disease after EV-D68 infection. (A) Immunostaining for Clca1 and Muc5ac and counterstaining with DAPI in lung sections for WT, Stat1−/−, Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after infection with EV-D68 or EV-D68-UV. Scale bar, 100 μm. (B) Corresponding whole lung sections for Clca1 immunostaining from Stat1−/− mice for conditions in (A). Scale bar, 500 μm. (C) Quantitation of Clca1 and Muc5ac immunostaining in whole lung sections for conditions in (A). For (A-C), data are representative of 3 separate experiments (n≥8 mice per condition in each experiment). For (C), * indicates p<0.05.
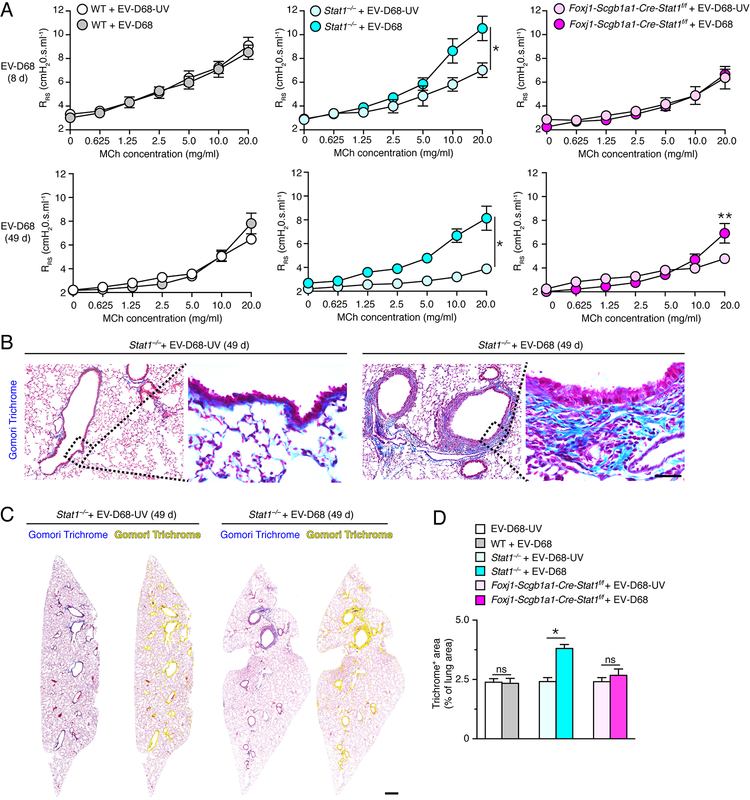
In addition to immune cell infiltration and excess mucus production, we also found significant increases in airway reactivity to inhaled methacholine in Stat1−/− mice at 8 and 49 d after infection with EV-D68 compared to EV-D68-UV (Fig. 9A). Similar to the pattern observed for regulation of Muc5ac expression, the development of airway hyper-reactivity was not detectable in wild-type mice and was also found at only the highest concentration of methacholine in Foxj1-Scgb1a1-Cre-Stat1f/f mice (Fig. 9A). We detected no significant increase in baseline respiratory system resistance (RRS) at 8 or 49 d after viral infection in wild-type, Stat1−/−, or Foxj1-Scgb1a1-Cre-Stat1f/f mice (data not shown), consistent with our studies of SeV and IAV infections (28, 29, 31). To further address the comparison between the post-EV-D68 mouse model with chronic lung disease in humans, we also assessed the development of fibrosis as a feature of airway disease. Here we detected a significant increase in Gomori trichrome staining for the blue-tone of collagen expression in Stat1−/− mice at 49 d after EV-D68 infection (Fig. 9B). Corresponding staining of whole lung sections demonstrated that trichrome+ staining was confined to airways (Fig. 9C), and quantitative image analysis of these sections confirmed that trichrome+ areas were significantly increased in Stat1−/− but not Foxj1-Scgb1a1-Cre-Stat1f/f or wild-type mice at 49 d after EV-D68 infection (Fig. 9D). Together, the findings show that key features of airway disease, i.e., immune cell infiltration, mucus production, hyper-reactivity, and fibrosis can be triggered in response to EV-D68 infection if protection from STAT1 function in barrier epithelial cells is lost. The localized nature of the acute infection, chronic disease, and STAT1 deficiency in the EV-D68 model further suggests that the critical function of STAT1 to protect against these outcomes is linked in particular to airway epithelial cells.
FIGURE 9.
STAT1 deficiency in epithelial cells increases susceptibility to chronic lung disease marked by airway hyperreactivity and fibrosis after EV-D68 infection. (A) Levels of airway reactivity using response of respiratory system resistance (RRS) to inhaled methacholine (MCh) in WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 8 d and 49 d after infection with EV-D68 or EV-D68-UV. (B) Gomori trichrome staining of lung sections from Stat−/− mice at 49 d after infection with EV-D68 or EV-D68-UV. Scale bar, 200 μm. (C) Gomori trichome staining of whole lung sections for conditions in (B) with yellow colorization indicating computer-assigned collagen-containing light-blue+ staining areas. Scale bar, 500 μm. (D) Quantitation of trichome light-blue+ staining areas in lung sections for WT, Stat1−/−, and Foxj1-Scgb1a1-Cre-Stat1f/f mice at 49 d after infection with EV-D68 or EV-D68-UV. For (A,D), values are representative of 3 separate experiments (n=5–8 mice per condition in each experiment). For (A), * indicates p<0.05 by ANOVA for entire MCh concentration-response and ** indicates p<0.05 by post-hoc Bonferroni for single MCh concentration versus EV-D68-UV condition. For (D), * indicates p<0.05.
Discussion
The present study develops key evidence that STAT1 function in barrier epithelial cells, and in particular airway epithelial cells, provides a fundamental mechanism for host defense against respiratory viral infection and in turn a critical safeguard against chronic lung disease that develops after clearance of infectious virus. These concepts derive from a series of specific findings: (1) loss of STAT1 function in barrier epithelial cells is achieved with newly developed Foxj1-Scgb1a1-Cre-Stat1f/f transgenic mice and results in increased viral titers of both mouse and human viral pathogens (SeV and EV-D68, respectively); (2) increases in viral titers caused by epithelial STAT1 deficiency is associated with more severe acute infectious illness marked by exaggerated weight loss after SeV and immune cell infiltration after SeV and EV-D68 infections; (3) loss of STAT1 control over acute infection also leads to more severe manifestations of the chronic lung disease that can develop after clearance of SeV and EV-D68 infection and is marked by immune cell infiltration and mucus production for at least as long as 7 wk after infection; (4) STAT1-deficiency limited to epithelial cells coupled with the limitation of acute infection to airway epithelial cells (ciliated and club cells) and chronic disease to airways in the case of EV-D68 infection further suggests that STAT1 protection is specific at least in part to airway epithelial cells within the lung epithelial barrier. Here we discuss the novelty and significance of these findings for the acute infection, chronic lung disease, and implications for comparable conditions in humans.
In regard to the acute infection, we expected that global loss of STAT1 function and compromise in interferon signal transduction would lead to an increase in viral replication and titer as we described previously for SeV (7). Similarly, there was a transient delay in viral clearance in Stat−/− mice after SARS-CoV infection that persisted in bone marrow chimeras with STAT1 deficiency in stromal cells (59). Thus, as expected, we observed that both SeV and EV-D68 titers were significantly increased in conventional Stat1−/− mice compared to wild-type mice. For both types of viruses, we also found an increase in viral titer in Foxj1-Scgb1a1-Cre-Stat1f/f transgenic mice. Of note, the level of compromise was less than for Foxj1-Scgb1a1-Cre-Stat1f/f transgenic mice than Stat1−/− mice, but this result can be explained by the fact that the viruses infect both Foxj1+ ciliated cells and Scgb1a1+ club cells and the efficiency Cre recombination for the Scgb1a1-driver is only 63% in this cell population, consistent with previous reports by others (56). Moreover, in the case of SeV infection, the virus also infects distal Sftpc+ AT2 cells, and the efficiency is only 39%, thereby providing for further viral replication. In contrast, EV-D68 infection is limited to airway epithelial cells based on immunostaining for viral protein that selectively co-localizes with club cell and ciliated cell proteins in the airway epithelium. Thus, a protective effect under these conditions indicates that airway epithelial cells (rather than lung epithelial barrier cells in general) must provide a key protective site. This finding establishes long-sought evidence for this concept.
In regard to chronic lung disease, we found that the increased severity of acute infection translated to more likely and severe development of chronic lung disease that persisted after clearance of infectious virus for both SeV and EV-D68. In the case of SeV infection, the signs of chronic lung disease such as mucus production are not evident until 21 d after infection and become maximal at 49 d after infection (27–30). In contrast, for EV-D68 infection, mucus production develops during the acute infection illness at 8 d after infection and persists for at least 49 d after infection. However, this apparent difference might reflect the acute destruction of epithelium after more severe infection with SeV and hence the inability to manifest epithelial cell differentiation towards mucous cells until there is epithelial repair by 8–12 d after infection (60). Indeed, in the case of EV-D68 infection, there were no significant signs of chronic disease unless the severity of infection was enhanced through the loss of STAT1 function using Stat1−/− or Foxj1-Scgb1a1-Cre-Stat1f/f mice. Nonetheless, despite the relatively mild nature of the acute infection with EV-D68, it is striking that signs of chronic airway disease marked by excess immune cell infiltration and mucous cell metaplasia persisted for at least 7 wk after initial infection. The limitation of EV-D68 replication to airway club and ciliated cells further provides for a specific role of airway epithelial cells in host defense and protection from lung disease. Moreover, the focal nature of the disease likely reflects localized sites of viral replication in the absence of IFN-dependent control and in turn localized reprogramming of host cells that is needed for long-term virus-induced disease.
Our findings also significantly advance previous studies of SeV, EV-D68, and related respiratory viruses in mouse models. For example, our own studies of SeV provided initial evidence that STAT1 function was required to control viral replication and acute illness based on STAT1 expression in the stromal cell compartment, but did not study epithelial cell-specific approaches or the development of chronic disease (7). Similarly, our studies of SARS-coronavirus detected increases in viral titer in Stat1−/− mice but did not define any phenotype beyond 9 d after infection (59). Similarly, a previous study detected airway neutrophil infiltration and hyperreactivity at 2 d after EV-D68 infection in female, wild-type Balbc/J mice but did not study host defense or chronic disease (61). This transient effect was slightly suppressed with anti-IL-17A blocking antibody, consistent with neutrophil-linked hyperreactivity found after short-term airway injury even in response to non-infectious agents (62), but the immune basis for subsequent longer-term disease after EV-D68 infection still needs to be defined. In contrast to the case for EV-D68, there is considerable evidence for SeV and IAV infections that the immune mechanism for post-viral disease is based on a prolonged type 2 immune response (and not an IL-17 response) that proceeds from epithelial stem cell expansion to innate immune cell activation and feed-forward to airway progenitor epithelial cell (APEC) differentiation towards mucous cells (28–31, 63). Further studies will be needed to determine whether the same mechanism drives chronic disease after EV-D68 infection. Nonetheless, the present finding for induction of Clca1 gene expression, which is highly dependent on IL-13 stimulation (29, 31, 64, 65), suggests that the type 2 immune response might also drive chronic mucus production and airway hyper-reactivity after EV-D68 similar to SeV and IAV infections (28–31). The same mechanism is also proposed to mediate short-term airway disease after human rhinovirus (RV) infections in mice (66).
In contrast, the present model for EV-D68 infection exhibits significant viral replication, e.g., at least a 1×101-fold increase from initial viral dose and 2–3×103-104-fold higher viral titers for 3–8 d in Stat1−/− and Foxj1-Scgb1a1-Cre-Stat1f/f mice compared to wild-type mice. The model thereby represents an advance over mouse models for infections with closely related RVs (RV-A, RV-B, and RV-C strains) that replicate poorly in mice with peak viral titer that is generally 1×103-fold lower than the initial inoculum and is characterized by viral clearance at 4 d after infection (67–70). These results highlight the frontline role of anti-viral host defense at the airway epithelial cell barrier in controlling infection, but this system is complemented by the actions of immune cells and in particular the adaptive immune response that allows for eventual clearance of the virus via cytotoxic T cell and antibody-producing B cell and T cell function. Presumably, at least some aspects of this adaptive immune response do not depend on IFN signal transduction and are therefore sufficient to clear infection even when initial defenses are compromised. Nonetheless, the persistently increased viral loads found with epithelial cell compromise appear to be enough to cause chronic lung disease based on the present mouse model.
The clearance of infectious virus also raises the issue of persistent viral-RNA remnants that are likely non-infectious but might still have a role in post-viral disease. In that context, we reported (and confirm here) that viral-RNA remnants are found long after clearance of infectious virus in the case of SeV (28), and we found the same persistence more recently for influenza A virus (31). In addition, our report for IAV includes data for active viral RNA replication with detection of both positive and negative-strand viral RNA and for localization of these viral-RNA remnants to sites of chronic lung disease. In each case, viral-RNA remnants are not detectable after infection with UV-inactivated virus, thereby further excluding any artifact of the PCR-based detection assay Moreover, many other labs have reported similar persistence for other RNA viruses, including RSV (73), measles virus (74), Ebola virus (75), and chikungunya virus (76). The role of active viral-RNA remnants in chronic disease is a subject of intense investigation in our lab and others. Notably, we do not detect persistence of EV-D68 RNA despite the development of chronic lung disease, suggesting that viral-RNA remnants are a marker versus a cause of long-term disease.
The present findings have significant implications for both acute illness and chronic disease that develops after viral infection and respiratory viral infection in particular in humans. In particular, EV-D68 can cause severe acute illness in humans, particularly asthmatics. The present model might therefore provide evidence that IFN-signaling deficiency is a factor in susceptibility to this type of infection. The basis for any possible IFN deficiency in asthma is less certain, but differences based on the type of infection might be highly relevant given the varying capacity of different viruses to subvert IFN production and signaling, including EV-D68 and SeV (25, 26, 71). In that context, the results provide the intriguing possibility that EV-D68 similar to SeV and IAV might be particularly prone to cause the severity of acute infection that leads to chronic disease. This experimental relationship fits with studies of viral infection in children where the type of virus does not correlate with the development of subsequent asthma (72). Additional work must particularly address, the likely scheme for viral reprogramming of APECs towards mucous cells and the possible upstream and downstream influence of immune cells. The present findings should serve as a new experimental model for these directions based on viral infections that provide significant replication and severity of illness that are key to chronic disease in the mouse model and likely in comparable airway disease due to asthma and chronic obstructive pulmonary disease.
Together, the present study establishes a new experimental model that provides proof-of-concept for the role of barrier epithelial cells in defense against respiratory viral infection based on the capacity for STAT1-dependent IFN signal transduction. The model provides for protection against acute infectious illness as well as the fundamental features of chronic respiratory disease, i.e., airway inflammation, mucus production, hyperreactivity, and fibrosis. Thus, the model should allow further development of precise therapeutic strategies for acute and chronic lung disease in humans, ranging from gene-based to small-molecule IFN-signal enhancers to target upstream events (10, 77, 78) to cytokine- and kinase-inhibitor strategies to target downstream events such as mucous cell metaplasia (65, 79, 80). These approaches will require definition of expression and function of epithelial cell-derived cytokines in the post-EV-D68 model as was done for SeV infection and was translated to humans with COPD (29). In fact, comparison of mechanism for post-viral lung disease after EV-D68 versus SeV and IAV and related RVs will likely prove useful for stratification of patients with viral exacerbations of chronic lung disease.
Supplementary Material
Acknowledgements
We thank Bradford C. Bemiss and Derek E. Byers for providing human clinical samples and Xiaohua Jin and Rose M. Tidwell for expert technical assistance.
This work was supported by grants from the National Institutes of Health (National Institute of Allergy and Infectious Diseases U19-AI070412, R01-AI111605, and R01-AI130591 and National Heart, Lung, and Blood Institute R01-HL121791 and R01-HL120153).
Abbreviations used in this article:
- Clca1
chloride channel accessory 1
- COPD
chronic obstructive pulmonary disease
- EV
enterovirus
- IAV
influenza A virus
- PAS
periodic acid-Schiff
- qPCR
quantitative PCR
- RV
rhinovirus
- MUC5AC
mucin 5AC
- SeV
Sendai virus
Footnotes
Conflict of Interest Disclosures
The authors have no conflicts of interest.
References
- 1.Stark GR, and Darnell JE Jr. 2012. The JAK-STAT pathway at twenty. Immunity 36: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider WM, Chevillotte MD, and Rice CM. 2014. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol 32: 513–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durbin JE, Hackenmiller R, Simon MC, and Levy DE. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84: 443–450. [DOI] [PubMed] [Google Scholar]
- 4.Park C, Li S, Cha E, and Schindler C. 2000. Immune response in Stat2 knockout mice. Immunity 13: 795–804. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda K, Kato K, Aoki K, Matsuda T, Miyamoto A, Shibamori M, Yamashita M, Numata A, Takase K, Kobayashi S, Shibata S, Asano Y, Gondo H, Sekigchi K, Nakayama K, Nakayama T, Okamura T, Koamura S, Niho Y, and Nakayama K. 2000. Tyk2 plays a restricted role in IFNa signaling, although it is required for IL-12-mediated T cell function. Immunity 13: 561–571. [DOI] [PubMed] [Google Scholar]
- 6.Durbin JE, Fernandez-Sesma A, Lee C-K, Dharma Rao T, Frey AB, Moran TM, Vukmanovic S, Garcia-Sastre A, and Levy DE. 2000. Type I IFN modulates innate and specific antiviral immunity. J. Immunol 164: 4220–4228. [DOI] [PubMed] [Google Scholar]
- 7.Shornick LP, Wells AG, Zhang Y, Patel AC, Huang G, Takami K, Sosa M, Shukla NA, Agapov E, and Holtzman MJ. 2008. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J. Immunol 180: 3319–3328. [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Gopal R, Manni ML, McHugh KJ, Mandalapu S, Robinson KM, and Alcorn JF. 2017. STAT1 is required for suppression of Type 17 immunity during influenza and bacterial superinfection. Immunohorizons 1: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look DC, Roswit WT, Frick AG, Gris-Alevy Y, Dickhaus DM, Walter MJ, and Holtzman MJ. 1998. Direct suppression of Stat1 function during adenoviral infection. Immunity 9: 871–880. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Mao D, Roswit WT, Jin X, Patel AC, Patel DA, Agapov E, Wang Z, Tidwell RM, Atkinson JJ, Huang G, McCarthy R, Yu J, Yun NE, Paessler SL, Lawson TG, Omattage NS, Brett TJ, and Holtzman MJ. 2015. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat. Immunol 16: 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Ghonaium AA, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, and Casanova J-L. 2003. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat. Genet 33: 388–391. [DOI] [PubMed] [Google Scholar]
- 12.Sancho-Shimizu V, Perez de Diego R, Jouanguy E, Zhang S-Y, and Casanova J-L. 2011. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol 1: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casanova J-L, Holland SM, and Notaragelo LD. 2012. Inborn errors of human JAKS and STATs. Immunity 36: 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, and Davies DE. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med 201: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel DA, Huang G, Byers DE, You Y, Kim HJ, Agapov E, Moore ML, Peebles RS, Castro M, Sumino K, Shifren A, Brody SL, and Holtzman MJ. 2014. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J. Allergy Clin. Immunol 134: 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, and Brody SL. 2004. The role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol 286: L650–657. [DOI] [PubMed] [Google Scholar]
- 17.Singh G, and Katyal SL. 1997. Clara cells and Clara cell 10 kD protein (CC10). Am. J. Respir. Cell Mol. Biol 17: 141–143. [DOI] [PubMed] [Google Scholar]
- 18.Imamura T, Fuji N, Suzuki A, Tamaki R, Saito M, Aniceto R, Galang H, Sombrero L, S. L, and Oshitani. H 2011. Enterovirus 68 among children with severe respiratory infection, the Phillipines. Emerg. Infect. Dis 17: 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Q-B, Wo Y, Wang H-Y, Wei M-T, Zhang L, Yang H, Liu E-M, Li T-Y, Zhao Z-T, Liu W, and Cao W-C. 2014. Detection of enterovirus 68 as one of the commonest types of enterovirus in patients with acute respiratory tract infection in China. J. Med. Microbiol 63: 408–414. [DOI] [PubMed] [Google Scholar]
- 20.Poelman R, Scholvinck EH, Borger R, Niesters HGM, and van Leer-Buter C. 2015. The emergence of enterovirus D68 in a Dutch University Medical Center and the necessity for routinely screening for respiratory viruses. J. Clin. Virol 62: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster JE, Miller JO, Selvarangan R, Weddle G, Thompson MT, Hassan F, Rogers SL, Oberste MS, Nix WA, and Jackson MA. 2015. Severe enterovirus 68 respiratory illness in children requiring intensive care management. J. Clin. Virol 70: 77–82. [DOI] [PubMed] [Google Scholar]
- 22.Oermann CM, Schuster JE, Conners GP, Newland JG, Selvarangan R, and Jackson MA. 2015. Enterovirus D68: a focused review and clinical highlights from the 2014 U.S. outbreak. Ann. Am. Thorac. Soc 12: 775–781. [DOI] [PubMed] [Google Scholar]
- 23.Midgley CM, Watson JT, Nix WA, Curns AT, Rogers SL, Brown BA, Conover C, Dominguez SR, Feikin DR, Gray S, Hassan F, Hoferka S, Jackson MA, Johnson D, Leshem E, Miller L, Nichols JB, Nyquist A-C, Obringer E, Patel A, Patel M, Rha B, Schneider E, Schuster JE, Selvarangan R, Seward JF, Turabelidze G, Oberste MS, Pallansch MA, and Gerber SI. 2015. Severe respiratory illness associated with a nationwide outbreak of entervirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir. Med 3: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W, Guo H, Chang J, Yu Y, Liu G, Zhang N, Willard SH, Zheng S, and Yu X-F. 2016. ICAM-5/Telencephalin is a function entry receptor for enterovirus D68. Cell Host & Microbe 20: 631–841. [DOI] [PubMed] [Google Scholar]
- 25.Rui Y, Su J, Wang H, Chang J, Wang S, Zheng W, Cai Y, Wei W, Gordy JT, Markham R, Kong W, Zhang W, and Yu X-F. 2017. Disruption of MDA5 mediated innate immune responses by the 3C proteins of Coxsackievirus A16, Coxsackievirus A6, and Enterovirus D68. J. Virol 91: e00546–00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Z, Liu L, Lei X, Zhou Z, He B, and Wang J. 2016. 3C protease of enterovirus D68 inhibits cellular defense mediated by interferon regulatory factor 7. J. Virol 90: 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter MJ, Morton JD, Kajiwara N, Agapov E, and Holtzman MJ. 2002. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest 110: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, and Holtzman MJ. 2008. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med 14: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy YG, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, and Holtzman MJ. 2013. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest 123: 3967–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu K, Byers DE, Jin X, Agapov E, Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober DL, Brett TJ, and Holtzman MJ. 2015. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med 212: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keeler SP, Agapov EV, Hinojosa ME, Letvin AN, Wu K, and Holtzman MJ. 2018. Influenza A virus infection causes chronic lung disease linked to sites of active viral RNA remnants. J. Immunol 201: 2354–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtzman MJ, Byers DE, Alexander-Brett J, and Wang X. 2014. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol 14: 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodruff PG, van den Berge M, Brightling C, Burchard EG, Christenon SA, Han MK, Holtzman MJ, Kraft M, Lynch DA, Martinez FD, Reddel HK, Sin DD, Washko GR, Wenzel SE, Punturieri A, Freemer M, and Wise RA. 2017. American Thoracic Society/National Heart, Lung, and Blood Institute asthma-chronic obstructive pulmonary disease overlap workshop report. Am. J. Respir. Crit. Care Med. 196: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brito-Mutunayagam R, Appleton SL, Wilson DH, Ruffin RE, and Adams RJ. 2010. Global initiative for chronic obstructive lung disease stage 0 is associated with excess FEV1 decline in a representative population sample. Chest 138: 605–613. [DOI] [PubMed] [Google Scholar]
- 35.Aikawa T, Shimura S, Sasaki H, Ebina M, and Takishima T. 1992. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101: 916–921. [DOI] [PubMed] [Google Scholar]
- 36.Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, and Chung KF. 2002. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 40: 367–373. [DOI] [PubMed] [Google Scholar]
- 37.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, and Bai TR. 2003. Characterization of airway plugging in fatal asthma. Am J Med 115: 6–11. [DOI] [PubMed] [Google Scholar]
- 38.James AL, Elliot JG, Abramson MJ, and Walters EH. 2005. Time to death, airway wall inflammation and remodelling in fatal asthma. Eur. Respir. J 26: 429–434. [DOI] [PubMed] [Google Scholar]
- 39.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, Raymond WW, Lachowicz-Scroggins ME, Di Maio S, Hoffman EA, Castro M, Fain SB, Jarjour NN, Israel E, Levy BD, Erzurum SC, Wenzel SE, Meyers DA, Bleecker ER, Phillips BR, Mauger DT, Gordon ED, Woodruff PG, Peters MC, and Fahy JV. 2018. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Invest 128: 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, and Pare PD. 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Eng. J. Med 350: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 41.Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Sharafkhaneh A, Luketich JD, Coxson HO, Elliott WM, and Sciurba FC. 2007. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 176: 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R. r., Woodruff PG, O’Neal WK, and Boucher RC. 2017. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 377: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graubert TA, Hug BA, Wesselschmidt R, Hsieh CL, Ryan TM, Townes TM, and Ley TJ. 1998. Stochastic, stage-specific mechanisms account for the variegation of a human globin transgene. Nucl. Acids Res. 26: 2849–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, and Holtzman MJ. 2007. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. (Rapid Communication). Am. J. Respir. Cell Mol. Biol 36: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, El-Bardeesy N, Bronson RT, Sugarbaker D, Maser RS, Shapiro SD, and Wong K-K. 2006. K-ras activation generates an inflammatory response in lung tumors. Oncogene 25: 2105–2112. [DOI] [PubMed] [Google Scholar]
- 46.Pan J, You Y, Huang T, and Brody SL. 2007. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J. Cell Sci. 120: 1868–1876. [DOI] [PubMed] [Google Scholar]
- 47.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, and Eliceiri KW. 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herold J, and Andino R. 2000. Poliovirus requires a precise 5’ end for efficient strand RNA synthesis. J. Virol 74: 6394–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griggs TF, Bochkov YA, Nakagome K, Palmenberg AC, and Gern JE. 2015. Production, purification, and capsid stability of rhinovirus C types. J. Virol. Methods 217: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Sheng J, Baggen J, Meng G, Xiao C, Thibaut HJ, van Kuppeveld FJM, and Rossmann MG. 2015. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Comm 6: 8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans WJ, Hurst BL, Peterson CJ, Van Wettere AJ, Day CW, Smee DF, and Tarbet EB. 2018. Development of a respiratory disease model for enterovirus D68 in 4-week-old mice for evaluation of antiviral therapies. Antiviral Res 162: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrey JD, Wang H, Hurst BL, Zukor K, Siddharthan V, Van Wettere AJ, Sinex DG, and Tarbet EB. 2018. Causation of acute flaccid paralysis by myelitis and myositis in enterovirus-D68 infected mice deficient in interferon ab/g receptor deficient mice. Viruses 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurst BL, Evans WJ, Smee DF, and Van Wettere AJ. 2019. Evaluation of antiviral therapies in respiratory and neurological disease models of Enterovirus D68 infection in mice. Virol. 526: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Nunen MCJ, and van der Veen J. 1967. Experimental infection with Sendai virus in mice. Arch Gesamte Virusforsch 22: 388–397. [DOI] [PubMed] [Google Scholar]
- 55.Marino DJ 2011. Age-specific absolute and relative organ weight distributions for B6C3F1 mice. J. Toxicol. Environ. Health 75: 76–99. [DOI] [PubMed] [Google Scholar]
- 56.Sinha M, and Lowell CA. 2017. Efficiency and specificity of gene deletion in lung epithelial doxycycline-inducible Cre mice. Am. J. Respir. Cell Mol. Biol 57: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato A, Kiyotani K, Sakai Y, Yoshida T, and Nagai Y. 1997. The paramxyovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J 16: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J, He Y-Q, Yi L-N, Zan H, Kung H-F, and He M-L. 2011. Viral kinetics of Enterovirus 71 in human rhabdomysarcoma cells. World J. Gastroenterol 17: 4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page C, Goicochea L, Matthews K, Zhang Y, Klover P, Holtzman MJ, Hennighausen L, and Frieman M. 2012. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respriatory syndrome coronavirus infection. J. Virol 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Look DC, Walter MJ, Williamson MR, Pang L, You Y, Sreshta JN, Johnson JE, Zander DS, and Brody SL. 2001. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am. J. Pathol 159: 2055–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajput C, Han M, Bentley JK, Lei J, Ishikawa T, Wu Q, Hinde JL, Callear AP, Stillwell TL, Jackson WT, Martin ET, and Hershenson MB. 2018. Enterovirus D68 infection induces IL-17-dependent neutrophilic airway inflammation and hyperresponsiveness. JCI Insight 3: e121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Byrne PM, Walters EH, Gold BD, Aizawa H, Fabbri LM, Nadel JA, and Holtzman MJ. 1984. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am. Rev. Respir. Dis 130: 214–219. [DOI] [PubMed] [Google Scholar]
- 63.Byers DE, Wu K, Dang-Vu G, Jin X, Agapov E, Zhang X, Battaile JT, Schechtman KB, Yusen R, Pierce RA, and Holtzman MJ. 2018. Triggering receptor expressed on myeloid cells-2 (TREM-2) expression tracks with M2-like macrophage activity and disease severity in COPD. Chest 153: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel AC, Morton JD, Kim EY, Alevy Y, Swanson S, Tucker J, Huang G, Agapov E, Phillips TE, Fuentes ME, Iglesias A, Aud D, Allard JD, Dabbagh K, Peltz G, and Holtzman MJ. 2006. Genetic segregation of airway disease traits despite redundancy of chloride channel calcium-activated (CLCA) family members. Physiol. Genomics 25: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alevy Y, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, Miller CA, Heier RF, Byers DE, Brett TJ, and Holtzman MJ. 2012. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest 122: 4555–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han M, Rajput C, Hong JY, Lei J, Hinde JL, Wu Q, Bentley JK, and Hershenson MB. 2017. The innate cytokines IL-25, IL-33, and TSLP coooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected mice. J. Immunol 199: 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffrey PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blain ED, Clarke NJ, and Johnston SL. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med 14: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, and Hershenson MB. 2008. Human rhinovirus 1B exposure induces phophatidylinositol 3-kinase-dependent airway inflammation in mice. Am. J. Respir. Crit. Care Med. 177: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q, Miller DJ, Bowman ER, Nagarkar DR, Schneider D, Zhao Y, Linn MJ, Goldsmith AM, Bentley JK, Sajjan US, and Hershenson MB. 2011. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 7: e1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han M, Hong JY, Jaipalli S, Rajput C, Lei J, Hinde JL, Chen Q, Bentley JK, and Hershenson MB. 2016. IFN-g blocks development of an asthma phenotype in rhinovirus-infected baby mice by inhibiting type 2 innate lymphoid cells. Am. J. Respir. Cell Mol. Biol 56: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato A, Kiyotani K, Kubota T, Yoshida T, Tashiro M, and Nagai Y. 2007. Importance of anti-interferon capacity of Sendai virus C protein for pathogenicity in mice. J Virol 81: 3264–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonnelykke K, Vissing NH, Sevelsted A, Johnston SL, and Bisgaard H. 2015. Association between respiratory infections in early life and later asthma is independent of virus type. J. Allergy Clin. Immunol 136: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estripeaut D, Torres JP, Somers CS, Tagliabue C, Shokhar S, Bhoj VG, Grube SM, Wozniakowski A, Gomez AM, Ramilo O, Jafri HS, and Mejias A. 2008. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J. Inf. Dis 198: 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin WW, Kouyos RD, Adams RJ, Grenfell BT, and Griffin DE. 2012. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. American Review of Respiratory Disease 109: 14989–14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A, Gifford RJ, Hopkins S, Hughes J, Johannessen JFI, Karageorgopoulos D, Lackenby A, Lester R, Liu R, MacConnachie A, Mahungu T, Martin D, Marshall N, Mepham S, Orton R, Palmarini M, Patel M, Perry C, Peters S, Porter D, Ritchie D, Ritchie N, Seaton R, Sreenu V, Templeton K, Warren S, Wilkie G, Zambon M, Gopal R, and Thomson E. 2016. Late Ebola virus relapse causing meningoencephalitis; a case report. Lancet 388: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawman DW, Fox J, Ashbrook A, May N, Schroeder K, Torres R, Crowe JJ, Dermody T, Diamond M, and Morrison T. 2016. Pathogenic Chikungunya virus evades B cell responses to establish persistence. Cell Rep 16: 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel DA, Patel AC, Nolan WC, Zhang Y, and Holtzman MJ. 2012. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PloS ONE 7: e36594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel DA, Patel AC, Nolan WC, Huang G, Romero AG, Charlton N, Agapov E, Zhang Y, and Holtzman MJ. 2014. High-throughput screening normalized to biological response: application to antiviral drug discovery. J. Biomol. Screen 19: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawrence MG, Steinke JW, and Borish L. 2018. Cytokine-targeting biologics for allergic diseases. Ann Allergy Asthma Immunol 120: 376–381. [DOI] [PubMed] [Google Scholar]
- 80.Pavord ID 2018. Biologics and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol 141: 1983–1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.