Abstract
Adult stem cells, also termed as somatic stem cells, are undifferentiated cells, detected among differentiated cells in a tissue or an organ. Adult stem cells can differentiate toward lineage specific cell types of the tissue or organ in which they reside. They also have the ability to differentiate into mature cells of mesenchymal tissues, such as cartilage, fat and bone. Despite the fact that the balance has been comprehensively scrutinized between adipogenesis and osteogenesis and between chondrogenesis and osteogenesis, few reviews discuss the relationship between chondrogenesis and adipogenesis. In this review, the developmental and transcriptional crosstalk of chondrogenic and adipogenic lineages are briefly explored, followed by elucidation of signaling pathways and external factors guiding lineage determination between chondrogenic and adipogenic differentiation. An in-depth understanding of overlap and discrepancy between these two mesenchymal tissues in lineage differentiation would benefit regeneration of high-quality cartilage tissues and adipose tissues for clinical applications.
Keywords: Stem cell; Chondrogenesis; Adipogenesis,; Lineage differentiation
Introduction
Stem cells are gaining importance due to their potential to regenerate damaged tissues [1, 2]. Adult stem cells, which exist in the postnatal organism, have been identified to have multi-lineage or uni-lineage differentiation capacity toward which they are committed to differentiate. Mesenchymal stem cells (MSCs), as part of the multi-lineage differentiation of adult stem cells, have the ability to form articular cartilage, fat and bone [3]. The balances between adipogenesis and osteogenesis and between chondrogenesis and osteogenesis have been comprehensively reviewed [4, 5]; however, few reviews explore the crosstalk between chondrogenesis and adipogenesis.
There is a strong and close relationship between chondrogenesis and adipogenesis. For example, a high concentration of dexamethasone could induce adipogenic differentiation even during chondrogenic induction of human synovium-derived stem cell (SDSC) pellets [6]. Pericytes in pellet cultures in chondrogenic medium also underwent adipogenic differentiation, as evidenced by the fact that some cells within the pellets displayed a signet-ring adipocyte-like morphology [7]. Interestingly, depletion of RUNX2 (Runt-related transcription factor 2), a typical osteogenic marker, resulted in the loss of chondrocyte phenotype and induced adipogenic differentiation in primary chondrocytes in vitro [8]. Furthermore, Qu et al. found that genetic deletion of Vav1, a guanine exchange factor for Rho GTP highly expressed in murine bone marrow-derived MSCs (BMSCs), led to spontaneous adipogenesis but disabled chondrogenic differentiation [9]. They also found that overexpression reversed this phenotype, resulting in increased chondrogenesis but decreased adipogenesis [9].
The transcription factors of chondrogenesis and adipogenesis are interrelated, which can influence stem cell fate. Down-regulation of Sox9 (SRY-Box 9), a classical transcription factor for chondrogenesis, seems to be required for adipocyte differentiation since Sox9 can bind to and suppress the major adipogenic transcription factors CCAAT/enhancer-binding protein beta (C/EBPβ) and C/EBPδ promoter activity directly [10]. On the contrary, Sox9 directly regulates COL2A1 (type II collagen) but it binds to the element overlapping with C/EBP motif in RCS (rat chondrosarcoma) cells [11]; thereby, C/EBPβ and C/EBPδ may participate in interleukin 1β (IL-1β)-induced repression of COL2A1 expression. Furthermore, chondrogenic marker genes COL2A1, ACAN (aggrecan) and SOX9 are reported to be suppressed by C/EBPα, C/EBPβ and C/EBPδ in ATDC5 cells (derived from mouse teratocarcinoma cells and characterized as a chondrogenic cell line) [12, 13]. These findings imply negative regulation between C/EBP family members and Sox9. However, other reports indicate that Sox9 is imperative for adipogenic differentiation by stabilizing C/EBPβ mRNA in rat adult BMSCs [14] and C/EBP family members show potent transactivation of SOX9 in both ATDC5 and Hela cells [15]. Therefore, the interaction of transcription factors between chondrogenesis and adipogenesis is complicated. The in-depth investigation is still in its infancy.
In this review, for the first time, we briefly discuss developmental origins of articular cartilage and adipose tissue, followed by signaling pathways guiding chondrogenic and adipogenic differentiation of stem cells as well as regulators controlling the crosstalk of chondrogenesis and adipogenesis. Further investigations of lineage-specific differentiation may lead to promising applications of MSCs in tissue engineering and regeneration.
Developmental origins of articular cartilage and adipose tissue
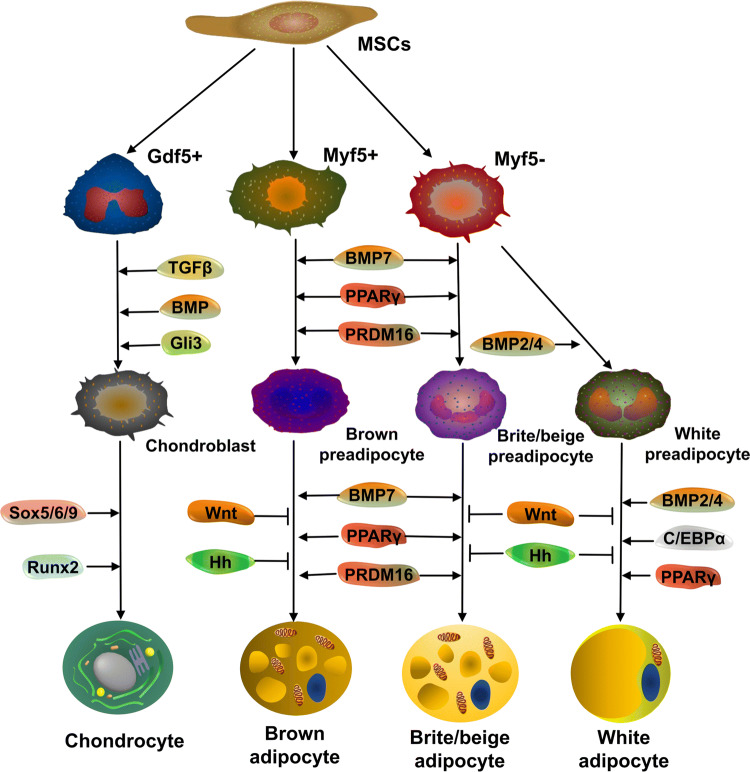
MSCs developing from the mesoderm commit to chondrogenic and adipogenic differentiation (brown, brite/beige and white adipocytes) (Fig. 1) and other lineages. Transcription factors promote the differentiation of chondroblasts and preadipocytes to acquire their specific functions.
Fig. 1.
Developmental origins of articular cartilage and adipose tissue. Adult stem cells develop from the mesoderm and then commit into different lineages, including but not limited to chondrogenic and non-skeletal adipogenic lineage (brown adipocyte, brite/beige adipocyte, white adipocyte). However, in the cephalic region, adipocytes have a neuroectodermal origin. Lineage determination is influenced by a number of transcription factors and growth factors in a spatiotemporal pattern (See text for details)
In the chondrogenic lineage, Sox9 is necessary for induction and maintenance of chondrocytic phenotypes in concert with Sox5 and Sox6 [16]. Transforming growth factor beta (TGFβ), bone morphogenetic protein (BMP), GLI-Kruppel family member 3 (Gli3) and Runx2 also promote chondrogenic differentiation [17]. Cartilage developmental stages can be divided into three phases: mesenchymal condensation, interzone formation and cavitation and stabilization of articular cartilage [18]. During mesenchymal condensation, chondroblasts migrate from the lateral plate of the mesoderm followed by an interruption of continuous cartilage anlagen by interzone formation. The interzone is composed of three layers: two chondrogenic layers and one intermediate layer. The former covers the cartilage while the latter aids intra-articular structure formation [19]. At early stages of joint morphogenesis, GDF5 (growth differentiation factor 5) mRNA is highly expressed in regions flanking future joint sites, within the flattened intermediate interzone [20]. Cells with a GDF5-expressing lineage actively take part in joint tissue formation and constitute a progenitor cell cohort endowed with joint-formation capacity [21].
Brown adipocytes arise from precursors that express myogenic factor 5 (Myf5), a gene that was also expressed in the myogenic lineage with transcriptional co-factor PRDM16 (PRD1-BF-1-RIZ1 homologous domain-containing protein-16) as a dominant regulator [22, 23]. Despite the fact that some white fat cells arise from Myf5 + precursors, most evidence suggests that white and brown adipocytes take different developmental paths [24]. Adipocytes in the cephalic region are ectodermal due to the mesenchyme in this part of the body deriving from the neuroectoderm [25]. Multiple signaling pathways are involved in the fate of adipocyte lineage. For example, Wingless/int (Wnt) and Hedgehog proteins are important for MSC myogenic lineage commitment but prevent MSCs from proceeding toward an adipogenic lineage [26]. In the adipogenic lineage, peroxisome proliferator-activated receptor gamma (PPARγ) is necessary and sufficient for adipogenesis [27]. C/EBPα is required for the differentiation of white but not brown adipocytes [28]. With BMP2 and BMP4 as white adipogenic factors, BMP7 serves as the unique brown fat inducer [29]. Forced expression of PRDM16 in a white preadipocyte cell line or white adipocytes in vivo promotes a robust brown adipocyte phenotype [22].
Signaling pathways guiding chondrogenic and adipogenic differentiation of stem cells
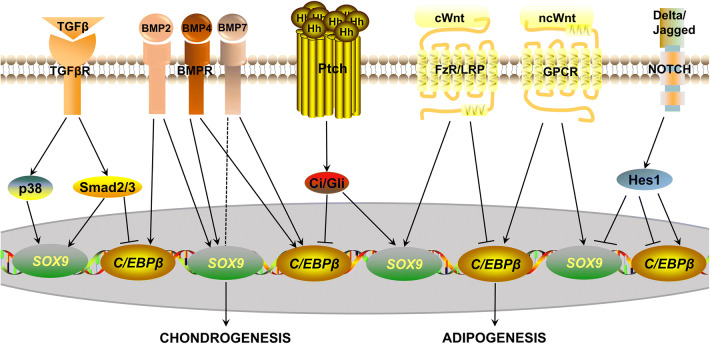
Recent studies have demonstrated that multiple signaling pathways are involved in determining stem cell fate, including TGFβ/BMP signaling, Hedgehog, Wnt and Notch signaling (Fig. 2).
Fig. 2.
Signaling pathways involved in regulating chondrogenic and adipogenic differentiation of MSCs. These signaling pathways maintain a delicate balance between chondrogenesis and adipogenesis through regulating SOX9 or CEBPβ. For example, TGFβ binding with TGFβR results in further activation of p38 and Smad2/3. The p38 signaling and Smad2/3 promotes chondrogenesis while Smad2/3 inhibits adipogenesis. BMP2/4/7 enhance chondrogenic and adipogenic differentiation. Interestingly, BMP7 favors adipogenesis over chondrogenesis (indicated by the dotted line). Hedgehog signaling has pro-chondrogenic and anti-adipogenic properties, which is consistent with canonical Wnt (cWnt) signaling while non-canonical Wnt (ncWnt) pathways promote both differentiations. Notch signaling is a negative regulator of chondrogenesis but plays an inhibitory and obligatory role in adipogenesis
TGFβ
TGFβ1 has the ability to induce chondrogenic differentiation in chick periosteum-derived mesenchymal cells [30] and in human and animal MSCs but in a dose-dependent manner [31–33]. For example, 10 ng/mL of TGFβ1 induced human BMSC differentiation into chondrocytes while lower doses, such as 0.01–0.1 ng/mL and 0.5–1.0 ng/mL, did not work in monolayer culture [34, 35]. TGFβ2 and TGFβ3 were more effective than TGFβ1 in promoting chondrogenic differentiation in human BMSCs [32]. Interestingly, TGFβ1 and TGFβ3 exerted an inhibitory effect on adipogenesis. TGFβ1 induced dominant chondrogenesis while suppressing adipogenic differentiation of CL-1 cells (a cell line that can spontaneously differentiate into chondrocytes and adipocytes) in the presence of fetal calf serum (FCS) [36] or human BMSCs [37]. In vitro treatment of 100 pM TGFβ1 also inhibited 3T3-L1 (mouse cells that resemble preadipocytes) differentiation into mature adipocytes [38] and 1 ng/mL TGFβ1 hampered lipid accumulation in mouse embryonic fibroblasts [39]. TGFβ3 (10 ng/mL) suppressed the induction of adipogenesis-associated genes, such as PPARG (PPARγ) and FABP4 (fatty acid binding protein 4), even in a two-dimensional micromass chondrogenic culture of human SDSCs [6].
Mechanically, TGFβ in chondrogenic differentiation can signal through the canonical Smad2 (small mothers against decapentaplegic homolog 2) or Smad3-mediated pathway or non-canonical p38 mitogen-activated protein kinase (MAPK) pathway [40–43]. However, the MAPK kinase (MEK)/extracellular signal-regulated protein kinase (Erk) signaling pathway in TGFβ-induced chondrogenesis remains controversial and complex [43]. In adipogenesis, however, overexpression of Smad2 or Smad3 inhibited lipid accumulation of 3T3-F442A preadipocytes, with Smad3 exerting a stronger effect [44]. A dominant-negative form of Smad3 is able to suppress the inhibitory function of TGFβ signaling on adipogenesis while adipogenesis proceeds normally in the presence of the dominant-negative form of Smad2, supporting Smad3 as a TGFβ signaling component in inhibiting adipogenic differentiation [44]. Moreover, Smad3, along with Smad4, associated with C/EBPβ and C/EBPδ resulted in decreased PPARγ expression in NIH3T3 cells [45]. The above evidence indicates that TGFβ may exhibit opposite effects on chondrogenic and adipogenic lineage differentiation via Smad3.
BMP
More than 20 different BMP isoforms have now been identified. Among them, BMP2, BMP4 and BMP7 are the well-established determinants of chondrogenic and/or adipogenic differentiation of MSCs.
BMP2 is a positive regulator in chondrogenic differentiation. In human BMSCs, BMP2 is the most effective in promoting chondrogenic differentiation as compared to BMP4 and BMP6 [46]. BMP2 (500 ng/mL), associated with a thiazolidinedione activator of PPARγ, also stimulated adipogenesis in 3T3-L1 cells and rat BMSCs [47]. BMP2 (50 ng/mL) could even induce adipogenesis on a two-dimensional micromass chondrogenic culture of human SDSCs [6]. In fact, the concentration of BMP2 strongly influences mesenchymal cell differentiation. For example, differentiation of C3H10T1/2 mouse embryonic stem cells (ESCs) into adipocytes occurred at lower concentrations (such as 10 ng/mL) of BMP2, while chondrocyte differentiation was prevalent at higher concentrations (such as 1000 ng/mL) [48]. However, BMP2 (50 ng/mL) was also reported to increase proteoglycan and type II collagen expression but decrease the level of adipocyte-specific aP2 (adipocyte protein-2) expression in human BMSCs [49].
BMP4 is a useful agent for stimulating chondrogenic differentiation both in vitro and in vivo [50–52]. Nakayama et al. demonstrated that BMP4 promotes chondrogenesis of ESC-derived mesodermal cells in a dose-dependent manner. They found that 50 ng/mL of BMP4 enhanced more cartilage formation compared to 20 ng/mL, while 5 ng/mL was not effective [53]. BMP4 also promotes stem cell commitment to the adipocyte lineage. Taha et al. found that 100 ng/mL of BMP4 induced more adipocyte clusters in ESC-derived embryoid body outgrowth compared to 50 and 10 ng/mL, suggesting that BMP4 induces adipogenesis in a dose-dependent manner [54].
BMP7 alone or with TGFβ increased chondrogenesis in bovine synovium explants, human ESCs, SDSCs and BMSCs [55–59]. BMP7 also augments phosphorylation of Smad1/5/8 in white and brown preadipocytes. However, via p38 MAPK, BMP7 initiated a full program of brown adipogenesis including increased expression of UCP1 (uncoupling protein 1), CEBPs and PPARG and blockade of adipogenic inhibitors such as NDN (Necdin), PREF1 (preadipocyte factor 1) and WNT10A (a canonical Wnt signaling molecule) [60]. Interestingly, BMP7 favors adipogenic differentiation over chondrogenic differentiation. For example, BMP7 dose-dependently decreased the level of aggrecan assessed by Alcian blue staining and increased the number of lipid-filled cells in human BMSCs [61]. Moreover, BMP7 (50 ng/mL) initiated adipogenesis instead of chondrogenesis of human BMSCs even in micromass cultures, which usually favors chondrogenic differentiation [61]. In addition, treatment with 100 ng/mL BMP7 alone did not increase chondrogenic gene expression, such as proteoglycan, COL2A1 and SOX9, after a 21-day chondrogenic induction of human BMSCs [62]. Interestingly, even in osteogenic induction, BMP7 elevated the expression of adipogenic genes PPARG, ADIPOQ (adiponectin) and LPL (lipoprotein lipase) of human BMSCs [62].
Hedgehog
Extracellular ligands of the hedgehog protein family are modulators of stem cell chondrogenesis along with potential interactions on adipocyte differentiation pathways [34, 63, 64]. The interaction of Hedgehogs with receptors of the patched (PTCH) family, a conserved transmembrane protein receptor that negatively regulates the Hedgehog signaling pathway, ultimately rescues Ci (in Drosophila)/Gli (in vertebrates) from proteolytic degradation and then promotes their nuclear localization.
Hedgehog signaling is well known to stimulate MSC chondrogenic differentiation. Indian Hedgehog (IHH), expressed and secreted by pre-hypertrophic and early hypertrophic cells, is a key regulator of endochondral ossification [65]. IHH-deficient mice displayed a markedly reduced chondrocyte proliferation and premature chondrocyte hypertrophy [66]. Generally, chondrogenic differentiation of human BMSCs was characterized by an increase of IHH expression [34]. Knockdown of IHH or pharmacological inhibition of Hedgehog signaling with cyclopamine or HhAntag could completely block TGFβ1 or BMP2-induced chondrogenesis in mesenchymal cells [34, 67]. Furthermore, overexpression of IHH was sufficient to drive chondrogenesis, even when TGFβ signaling was inhibited [34]. Likewise, treatment with IHH or the recombinant amino half of SHH (recombinant N-terminal portion of Sonic Hedgehog) induced chondrocyte differentiation in clonal pre-chondrogenic RMD-1 and ATDC5 cells [68]. SHH is also a critical moderator of cell differentiation due to its anti-adipogenic and pro-chondrogenic properties in mouse adipose-derived stem cells (ADSCs) [69].
The anti-adipogenic potential of Hedgehog signaling has been observed in a variety of multipotent cell lineages. Generally, adipogenic differentiation of human ADSCs was characterized by a decrease in Gli1, Gli2, Gli3 and PTCH expression [64]. A dominant negative form of Gli2 was reported to promote adipogenesis of 3T3-L1 cells [70]. Conversely, treatment with purmorphamine, a Hedgehog agonist, decreased adipocyte-specific markers, such as FABP, CFD (complement factor D, Adipsin), CD36, ADIPOQ and LEP (leptin) in human ADSCs [64]. Likewise, SHH resulted in suppression of pro-adipogenic effects of BMP2 in multipotent C3H10T1/2 cells and transgenic activation of Hedgehog signaling in both Drosophila and mammalian models impaired fat formation [70–72]. In summary, current data suggest that Hedgehog signaling promotes stem cell chondrogenic differentiation over adipogenic differentiation, primarily via Gli transcription factor activity.
Wnt
Over the course of several decades, Wnt signaling has been identified as playing an essential role in cell fate determination and differentiation [73, 74]. Collectively, canonical Wnt signaling has demonstrated both pro-chondrogenic and anti-adipogenic activities while the non-canonical pathways promote both of these differentiations.
Many studies showed that Wnt proteins, such as Wnt3a, have been reported to promote chondrogenic differentiation [75]. Moreover, secreted frizzled-related protein 1 (sFRP1) and Dickkopf-related protein 1 (Dkk1) enhanced glycosaminoglycan (GAG) synthesis, SOX9 and COL2A1 expression only in the early chondrogenesis of human BMSC pellet cultures [76]. However, several members of the Wnt signaling family have been identified to inhibit early stage adipogenic differentiation [77]. Wnt1, Wnt6, Wnt10a and Wnt10b have been shown to maintain 3T3-L1 cells in an undifferentiated state via inhibition of PPARγ and C/EBPα [77–81]. Further studies also suggest that canonical ligand Wnt3a inhibits activation of both PPARγ and C/EBPα in order to elicit its anti-adipogenic effects [82]. Additionally, during adipogenic differentiation of human ADSCs, the mRNA levels of SFRP4 and DKK1, two Wnt antagonists, were higher than in the undifferentiated state [83]. Also, a 48-h treatment with sFRP1 and sFRP4 up-regulated adiponectin secretion in human ADSCs [84]. Accordingly, inhibition of Wnt/β-catenin signaling via treatment with Sclerostin or Dkk family proteins positively regulated adipogenesis [77, 78, 85, 86]. A higher level of sFRP2 enhanced adipogenic differentiation and a decrease of sFRP2 suppressed adipogenesis in mouse BMSCs [87]. Interestingly, Kirton et al. found that Wnt/β-catenin signaling enhanced chondrogenesis while attenuating adipogenic differentiation of pericytes in both monolayer and pellet cultures [7].
The non-canonical signaling pathway has also been implicated as a modulator of chondrogenic and adipogenic differentiation of MSCs. To date, the β-catenin independent pathway has been reported to be a determinant of stem cell chondrogenesis. For example, TGFβ up-regulated the level of Wnt5a, which promoted chondrogenesis of chick wing bud mesenchymal cells [88]. Overexpression of Wnt11 promoted chondrogenesis in rat BMSCs [89]. Moreover, hyaluronan-grafted chitosan promoted chondrogenesis by Wnt5a-mediated non-canonical Wnt signaling in rat ADSCs [90]. Furthermore, Wnt5a and Wnt11-mediated non-canonical Wnt signals were actively involved in the enhanced chondrogenesis of decellularized extracellular matrix (ECM) expanded human SDSCs [91]. These results indicated that non-canonical Wnt signaling plays a positive role in promoting the chondrogenic differentiation response. Non-canonical Wnt ligands, Wnt4 and Wnt5a, promoted the adipogenesis of 3T3-L1 cells through the Protein Kinase C (PKC)-CamKII pathway [92]. These data indicated that, different from canonical Wnt signals, the non-canonical Wnt pathway was a positive regulator in adipogenic differentiation.
Notch
Notch signaling has been shown to be active in undifferentiated stem cells and in the early phase of chondrogenic differentiation. Notch signaling was down-regulated when chondrocyte differentiation ensued in pellet cultures of human BMSCs [93]. Correspondingly, overexpression of NICD (Notch intracellular domain), HES1 (Hairy and enhancer of split-1) and HEY1 (Hairy/enhancer-of-split related with YRPW motif protein 1) prevented chondrogenesis of human BMSCs [93]. Further studies found that inhibition of Notch signaling on chondrogenesis of murine limb bud mesenchymal progenitor cells was markedly reduced by knockdown of TWIST1 (Twist-related protein 1) [94]. These results suggest that Notch signaling plays a negative role during chondrogenic differentiation.
Notch receptor mRNA expression, such as NOTCH1, 2, 3 and 4, decreased as adipogenic differentiation of human ADSC clones [95]. However, only Notch1 and 4 increased during adipogenic differentiation of 3T3-L1 cells [96]. Existing evidence indicates that Notch signaling plays an inhibitory and obligatory role in adipogenesis [97, 98]. Exposure to ligand jagged1 in 3T3-L1 cells or jagged1 transgene expression in human BMSCs blocked PPARγ and C/EBPα induction and inhibited adipocyte formation in response to adipogenic induction [98, 99]. N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT, γ-secretase inhibitor) inhibited Notch signaling, induced autophagy and promoted adipogenesis of human BMSCs through the Phosphatase and tensin homolog (PTEN)-phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway [100]. However, exposure of jagged1 to mouse ADSCs stimulated adipogenesis by promoting PPARγ expression [101]. Active form Notch4 promoted adipogenic differentiation of 3T3-L1 cells [96]. Moreover, reduction of HES1 using siRNA or impaired Notch1 expression by antisense constructs was associated with inhibition of adipogenic differentiation in 3T3-L1 cells, which may involve modulation of DLK1 (Delta-like 1 homolog)/Pref1 [97, 98]. Thus, the Notch signaling pathway inhibits or promotes adipogenesis in a complex manner through multiple intracellular signaling pathways.
Regulators controlling the balance of chondrogenic and adipogenic differentiation of stem cells
The crosstalk between chondrogenic and adipogenic differentiation is important and accumulating evidence shows that a multitude of cues direct the lineage commitment. Here, we will discuss the cues controlling the crosstalk between chondrogenic and adipogenic differentiation of MSCs, including biochemical, biophysical and biological factors.
Biochemical factors
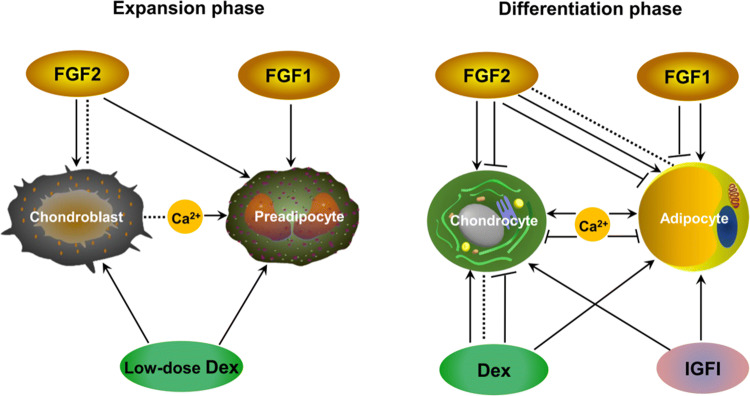
Culture conditions such as culture medium and growth factor supplements are crucial for MSC differentiation toward a specific lineage. To induce chondrogenesis and adipogenesis of MSCs, various combinations have been included such as induction reagents and growth factors (Fig. 3) (Table 1). For example, typical chondrogenic factors, including 40 μg/mL proline, 100 nM dexamethasone, 0.1 mM ascorbic acid-2-phosphate, 1 × ITS Premix and TGFβ3, could promote chondrogenic differentiation as the induction medium [91]. Moreover, stem cells cultured in medium supplemented with 1 mM dexamethasone, 0.5 mM IBMX (isobutyl-1-methyxanthine), 200 mM indomethacin and 10 mM insulin supported adipogenic differentiation [102].
Fig. 3.
Biochemical factors that regulate chondrogenic and adipogenic differentiation of MSCs. During the period of expansion, FGF2 has no effect or pro-chondrogenic properties while playing a pro-adipogenic role. During the differentiation period, the pro- and anti-differentiation roles of FGF2 have been reported in chondrogeneis and adipogenesis. Moreover, no effect (indicated by dotted line) has been reported for the addition of FGF2 in adipogenic differentiation. FGF1 in both the expansion and differentiation periods enhances adipogenesis. However, during the differentiation period, FGF1 treatment has a tendency to inhibit adipogenesis. Low-dose dexamethasone (Dex) treatment during the period of expansion increases both chondrogenic and adipogenic potentials. However, Dex supplementation in the differentiation period can enhance, inhibit or have no effect on chondrogenesis, depending on the tissue sources of MSCs, dosages and growth factors combined. IGFI signaling plays a positive role in both chondrogenesis and adipogenesis. During the period of expansion, calcium plays a positive role in adipogenesis while having no effect for chondrogenesis. During the differentiation period, the pro- and anti-differentiation roles of calcium have been reported in chondrogeneis and adipogenesis
Table 1.
Studies investigating biochemical factors guiding lineage determination between chondrogenic and adipogenic differentiation
| Cell type | Treatment | Chondrogenic differentiation | Adipogenic differentiation | Possible mechanisms | Refs. |
|---|---|---|---|---|---|
| Dexamethasone | |||||
| 3T3-L1 preadipocyte | 1 μM Dex during differentiation induction | – | Increased | By repressing the transcription of TAZ, a suppressor of PPARγ | [112] |
| Dex (10, 100, 1000 nM) during differentiation induction | – | Increased | By repressing type I RUNX2, which blocked adipocyte differentiation | [116] | |
| ADSC (human) | Dex (10, 100 nM) and TGFβ1 (1, 10 ng/mL) in alginate beads during differentiation induction | Accumulated sulfated GAG on day 9 by combining with TGFβ1 | – | – | [33] |
| ATDC5 | 1 μM Dex during differentiation induction | Suppressed formation of cartilage nodule-like structures | – | By down-regulating Wnt/β-catenin signaling | [106] |
| BMSC (human) | 10 nM Dex during expansion phase | Increased | Increased | – | [103] |
| 100 nM Dex during expansion and differentiation phases (pellets or cells) | Increased | Increased | – | [110] | |
| 100 nM Dex during differentiation induction | Increased | – | By increasing TGFβ3-induced phosphorylation of Smad2 and Smad3 | [115] | |
| BMSC (bovine) | 100 nM Dex and 10 ng/mL TGFβ1 in agarose gels during differentiation induction | Increased | – | By positive regulation through Smad2/3 | [107] |
| BMSC or SDSC (bovine) | 100 nM Dex and 10 ng/mL TGFβ1 or 200 ng/mL BMP2 in micromass culture during differentiation induction | Increased TGFβ1-induced chondrogenesis in BMSCs, while exerting no remarkable effect on either-induced chondrogenesis in SDSCs | – | – | [109] |
| BMSC (equine) | Dex (1, 100 nM) in agarose gel during differentiation induction | Increased matrix accumulation while 100 nM Dex suppressed undesirable hypertrophy | – | – | [105] |
| BMSC (mouse) or C3H10T1/2 | BMSCs isolated from Dex (50 mg/kg daily)-treated mice or C3H10T1/2 treated with 1 μM Dex during differentiation induction | – | Increased | By up-regulating C/EBPα expression through inhibiting its promoter methylation via down-regulating Wnt/β-catenin signaling | [117] |
| D1 cells | Dex (10−9 to 10−7 M) during differentiation induction | – | Increased | – | [113] |
| ROB-C26 | 100 nM Dex during differentiation induction | – | Increased | By inhibiting β-catenin expression | [111] |
| 10 nM Dex and 100 ng/mL BMP2 during differentiation induction | – | Increased | By inhibiting osterix expression | [114] | |
| SDSC (human) | 100 nM Dex combined with 10 ng/mL TGFβ1 or 200 ng/mL BMP2 in alginate during differentiation induction | Suppressed BMP2-induced increase of ACAN and COL2A1 but did not affect TGFβ1-induced expression | – | – | [108] |
| Dex (1 nM to 1 μM) with or without 50 ng/mL BMP2 and 10 ng/mL TGFβ3 in micromass aggregates or 3D pellets during differentiation induction | 1 or 10 nM Dex combined with TGFβ3 or less than 1 nM Dex with BMP2 increased proteoglycan synthesis and type II collagen. 100 nM Dex with TGFβ3 or more than 10 nM Dex with BMP2 disturbed cartilage formation | Oil droplets increased when 1 μM Dex with BMP2 was used and TGFβ3 suppressed adipogenesis induced by Dex in micromass aggregates | – | [6] | |
| TBMPC (human) | 100 nM Dex combined with 10 ng/mL TGFβ3 during differentiation induction | Increased | – | GRα is required for Dex-mediated modulation of chondrogenesis | [104] |
| FGF | |||||
| 3T3-L1 preadipocyte | 1 nM FGF2 during differentiation induction | – | Increased | By phosphorylation of Erk1/2 | [135] |
| ADSC (human) | 10 ng/mL FGF2 or 10 ng/mL BMP6 or 10 ng/mL TGFβ3 during differentiation induction | FGF2 abolished chondrogenic effect of combined BMP6 and TGFβ3 | – | – | [129] |
| FGF2 (1, 10, 100, 1000, 10000 ng/mL) during expansion phase | – | The strongest enhancement in the group of 1000 ng/mL FGF2 | – | [133] | |
| 10 ng/mL FGF2 during differentiation induction | – | No effect | – | [134] | |
| BMSC (human) | 10 ng/mL FGF2 during expansion phase | Increased | – | – | [121, 123] |
| FGF2 (1, 10 ng/mL) during expansion phase | Increased | – | – | [125] | |
| 1 ng/mL FGF2 during expansion phase | No effect | Increased | – | [127] | |
| 10 ng/mL FGF2 or 10 ng/mL TGFβ3 during differentiation induction | FGF2 alone or combined with TGFβ3 decreasing COL2A1 | – | – | [128] | |
| 10 ng/mL FGF2 or 10 ng/mL TGFβ2 during differentiation induction | FGF2 alone or combined with TGFβ2 increasing chondrogenesis | – | – | [130] | |
| 25 ng/mL FGF1 or FGF2 together with 25 μg/mL heparin in 3D collagen gels during differentiation induction | – | Decreased | – | [119] | |
| 10 ng/mL FGF2 during expansion phase | Increased | – | By up-regulating SOX9 | [118] | |
| 10 ng/mL FGF2 during differentiation induction | – | Decreased | By up-regulating HMGA2 | [136] | |
| BMSC (rat) | 3 ng/mL FGF2 during the expansion or differentiation period | – | Increased | – | [132] |
| IPFSC (porcine) | 5 ng/mL FGF2 during expansion phase followed by encapsulation in agarose hydrogels | Increased | – | – | [124] |
| Preadipocyte (human) | 1 ng/mL FGF1 during only expansion or both expansion and differentiation phases | – | Increased | – | [131] |
| 1 ng/mL FGF1 and 90 μg/mL heparin during expansion and differentiation phases | – | Increased | By down-regulating BAMBI in a PI3K-dependent manner | [137] | |
| SDSC (human) | 10 ng/mL FGF2 during expansion or differentiation phase | Increased for the group with treatment in expansion | – | By up-regulating both p-p38 and p-Jnk while p-Erk1/2 was moderately suppressed | [120] |
| FGF2 (0.1, 1, 10, 100 ng/mL) during expansion phase | Increased | – | – | [122] | |
| SFCPC (equine) | 100 ng/mL FGF2 during expansion phase | Accelerated cell expansion without affecting subsequent chondrogenic capacity | – | – | [126] |
| IGFI | |||||
| 3T3-L1 preadipocyte | 7 nM IGFI during expansion and differentiation phases | – | Increased | – | [144] |
| 7 or 10 nM IGFI during expansion and differentiation phases | – | Increased | – | [145] | |
| AC (human) | 50 ng/mL IGFI in alginate gel during differentiation induction | Increased | – | By activating the PI3K/Akt and Erk/MAPK pathways | [143] |
| ATDC5 | IGFI (1, 10, 50 nM) during expansion and differentiation phases | Increased | – | – | [142] |
| BMA (human) | Transduced with rAAV to overexpress IGFI | Increased | Increased | – | [140] |
| BMSC (human) | Transduced with rAAV to overexpress IGFI | Increased | Increased | – | [139] |
| BMSC (rat) | 100 ng/mL IGF1 during differentiation induction | Increased | – | By upregulating p38, Erk1/2 and PI3K signaling in monolayer culture while upregulating PI3K signaling in 3D culture | [147] |
| CL-1 | 100 ng/mL IGFI during differentiation induction with 10% FCS | Increased | Increased | – | [36] |
| LBMC (chick) | 100 ng/mL IGFI during differentiation induction | Increased | – | By activating PI3K | [146] |
| Preadipocyte (human) | 50 ng/mL IGFI during expansion phase | – | Increased | By activating PI3K1A and mTORC1 signaling | [148] |
| Calcium | |||||
| 3T3-L1 preadipocyte | Ca2+ (1.8, 2.5, 5, 10 mM) during expansion and differentiation phases | – | Decreased with treatment of Ca2+ higher than 2.5 mM | Through a G-protein-coupled mechanism mediated by a novel Ca2+ sensor or receptor | [160] |
| ADSC (human) | Ca2+ (1.8 or 8 mM) during differentiation induction | Elevated calcium inhibiting chondrogenesis | – | – | [153] |
| BMSC (human) | 5 mM Ca2+ or PEMF during differentiation induction | Transient calcium exposure increasing chondrogenesis while subsequent exposures to elevated calcium suppressing chondrogenic differentiation | – | Through the TRP channel superfamily by activation of the SOX9 pathway | [154] |
| BMSC (mouse) | Under electrical stimulation (0, 1, 5 or 25 V/cm, with a duration of 8 ms and a frequency of 5.0 Hz) | Increased | – | By driving ATP/Ca2+ oscillations | [156] |
| 9 mM CaCl2 during differentiation induction | – | Increased | By suppressing Erk activity | [161] | |
| – | Increased | Through CaSR followed by a decrease in cAMP | [163] | ||
| CaCl2 (1.8, 5.4, 10.8 mM) during differentiation induction | – | Increased | – | [162] | |
| BMSC (porcine) | Chelating free Ca2+ | Depleting intracellular calcium stores suppressing the beneficial effect of hydrostatic pressure on chondrogenesis | – | Via vimentin adaptation to loading | [155] |
| 4 mM Ca2+ during differentiation induction | – | Increased | By activation of the CaMKII and PI3K/Akt signaling pathway | [164] | |
| Preadipocyte (human) | Ca2+ agonists (30 nM thapsigargin and 2 μM A23187) | – | Increasing Ca2+ inhibiting the early stage while promoting the late stage of differentiation | – | [159] |
| SDSC (porcine) | 5 mM Ca2+ during expansion phase | No effect | Increased | – | [152] |
| UCB-MSC (human) | 1.8 mM CaCl2 during expansion phase | No effect | Increased | By negatively regulating the Wnt5a/β-catenin pathway | [165] |
AC articular chondrocyte, ACAN aggrecan, ADSC adipose-derived stem cell, Akt protein kinase B, ATDC5 derived from mouse teratocarcinoma cells and characterized as a chondrogenic cell line, BAMBI BMP and activin membrane-bound inhibitor, BMA bone marrow aspirates, BMP bone morphogenetic protein, BMSC bone marrow-derived stem cell, C3H10T1/2 a mouse mesenchymal cell line, CaCl2 calcium chloride, CaMKII calmodulin-dependent protein kinase II, cAMP cyclic adenosine 3′,5′-monophosphate, CaSR calcium-sensing receptor, CDM cartilage-derived matrix, C/EBP CCAAT/enhancer-binding protein, CL-1 a bipotent cell line established from tibia of normal adult mouse, CoCl2 cobalt chloride, COL2A1 type II collagen, D1 cells pluripotent mesenchymal cells, which were cloned from BALB/c mouse bone marrow cells, Dex dexmethasone, Erk extracellular signal-regulated protein kinase, FCS fetal calf serum, FGF fibroblast growth factors, GAG glycosaminoglycan, GR glucocorticoid receptor, HMGA2 high mobility group A-2, IGFI insulin-like growth factor I, IPFSC infrapatellar fat pad derived stem cell, Jnk Jun N-terminal kinase, LBMC limb bud mesenchymal cell, MAPK mitogen-activated protein kinase, mTORC mammalian target of rapamycin complex, PEMF pulse electromagnetic fields, PI3K phosphatidylinositol 3-kinase, PPARγ peroxisome proliferator-activated receptor gamma, rAAV recombinant adeno-associated virus, ROB-C26 the clonal rat mesenchymal progenitor cell line, RUNX2 Runt-related transcription factor 2, SDSC synovium-derived stem cell, SFCPC synovial fluid chondroprogenitor cell, Smad small mothers against decapentaplegic homolog, TAZ transcriptional co-activator with PDZ binding motif, TBMPC trabecular bone mesenchymal progenitor cell, TGF transforming growth factor, TRP transient receptor potential, UCB-MSCs umbilical cord blood-derived MSCs, Wnt Wingless/int
Dexamethasone
Dexamethasone typically included in the cocktail of both chondrogenic and adipogenic media is to stimulate chondrogenesis and adipogenesis, indicating that it is a crucial component in differentiation induction. Interestingly, low dose (10 nM) dexamethasone treatment during the expansion period increased chondrogenic and adipogenic potential in human BMSCs [103]. Unexpectedly, adipocyte-like oil droplets were recognized in a three-dimensional micromass aggregate culture of human SDSCs with dexamethasone (100 or 1000 nM) plus BMP2 (50 ng/mL), which indicates that high concentration of dexamethasone could cause adipogenesis in chondrogenic culture of human SDSCs [6].
Many studies found that dexamethasone increased the aggrecan or proteoglycan synthesis rates in human ADSCs, human adult trabecular bone mesenchymal progenitor cells (MPCs) and equine BMSCs [33, 104, 105]. However, dexamethasone (1 μM) inhibited insulin-induced chondrogenesis and decreased chondrogenic potential in ATDC5 cells [106]. In combination with TGFβ1 or TGFβ3, dexamethasone augments the levels of ACAN, COL2A1 and COMP (cartilage oligomeric matrix protein) in human ADSCs and trabecular bone MPCs, and bovine BMSCs [33, 104, 107]. However, Kurth et al. found that the TGFβ1-mediated expression of ACAN and COL2A1 mRNAs was not enhanced in the presence of 100 nM dexamethasone, whereas the BMP2-induced expression of these markers was markedly suppressed in human SDSCs [108]. In fact, tissue sources of MSCs, dosages and growth factors combined have an impact on the role of dexamethasone in chondrogenesis. In aggregates of bovine BMSCs, 100 nM dexamethasone enhanced TGFβ1-induced chondrogenesis, but had little influence on BMP2-induced response [109]. In human ADSCs, dexamethasone (10 or 100 nM) significantly reduced TGFβ1-mediated increases in proteoglycan synthesis rates on days 1 and 5, but notably increased the rates on day 9 [33]. In aggregates of bovine SDSCs, 100 nM dexamethasone exerted no remarkable effect on either TGFβ1- or BMP2-induced chondrogenesis [109]. Dexamethasone concentration also affects chondrogenic differentiation of adult stem cells [6, 105]. For example, less than 10 nM dexamethasone combined with 10 ng/mL TGFβ1 or less than 1 nM dexamethasone with 50 ng/mL BMP2 enhanced the synthesis of proteoglycan and type II collagen in human SDSCs [6]. However, a higher concentration of dexamethasone (100 nM) with TGFβ1 or more than 10 nM dexamethasone with BMP2 disturbed cartilaginous tissue formation [6]. These results indicate that the influence of dexamethasone on chondrogenesis is context dependent.
Studies also found that dexamethasone could increase adipogenic potential in human BMSCs, ROB-C26 (the clonal rat mesenchymal progenitor cell line) and 3T3-L1 cells [110–112]. A high concentration of dexamethasone (100 nM) favors adipogenic differentiation over osteogenic differentiation of human BMSCs or mouse pluripotent mesenchymal cells [113]. Combined with BMP2, dexamethasone treatment (10 nM) increased the early phase of differentiation of adipocytes in ROB-C26 [114].
A glucocorticoid receptor (GR) is required for dexamethasone-mediated modulation of chondrogenesis. Dexamethasone could promote chondrogenic differentiation of MSCs through enhancing TGFβ3-induced phosphorylation of Smads [115]. However, the mechanisms of dexamethasone in chondrogenic studies are not clear. There is more information about how dexamethasone induces adipogenesis. Type I Runx2 kept 3T3-L1 cells in a growth-arrested state and was significantly down-regulated during adipocyte differentiation [116]. Knockdown of RUNX2 stimulated adipogenesis of 3T3-L1 cells; dexamethasone repressed type I Runx2 through direct binding of GR in the RUNX2 P2 promoter at the transcriptional level [116]. These results indicate that Runx2 may be a downstream target of dexamethasone in the adipogenic differentiation of 3T3-L1 cells. Moreover, C/EBPα was significantly up-regulated in dexamethasone-induced osteoporotic BMSCs by a mechanism that involved inhibited DNA hypermethylation of its promoter [117].
FGF
In mammals, fibroblast growth factors (FGFs) are heparin-binding growth factors which contain 23 members. In general, FGF2, FGF9 and FGF18 have been primarily studied in chondrogenic differentiation while FGF1 and FGF2 are most investigated in adipogenic differentiation [118, 119].
Many studies showed that FGF2 has a positive role in chondrogenic differentiation in human SDSCs and BMSCs, and equine synovial fluid chondroprogenitor cells (SFCPCs) [120–122]. FGF2 has been shown to retain chondrogenic potential when supplemented during expansion [121, 123–125]. Our study discovered that 10 ng/mL FGF2 treatment during expansion, but not differentiation, increased GAG deposition, pellet size and chondrogenic gene expression during chondrogenic induction in human SDSCs [120]. However, another study found that treatment with 100 ng/ml FGF2 during the expansion period significantly accelerated cell expansion without affecting subsequent chondrogenic capacity in equine SFCPCs [126]. Similarly, supplementation of 1 ng/mL FGF2 during the expansion period increased adipogenic rather than chondrogenic differentiation in human BMSCs [127]. Moreover, supplementation with 10 ng/mL FGF2 alone or combined with 10 ng/mL TGFβ3 during the differentiation period decreased COL2A1 in human BMSCs [128]. Hildner et al. also found that the addition of 10 ng/mL FGF2 during the differentiation period abolished the chondrogenic effect of combined 10 ng/mL BMP6 and 10 ng/mL TGFβ3 in human ADSCs [129]. However, 10 ng/mL FGF2 and 10 ng/mL TGFβ2 have a synergistic effect in chondrogenic differentiation of human BMSCs [130].
FGFs, such as FGF1 and FGF2, have been demonstrated to have strong adipogenic effects in the presence of adipocyte differentiation stimuli [131–133]. FGF2 seems to enhance adipogenic potential of human ADSCs when present (1000 ng/mL) during expansion [133]. However, addition of FGF2 in the differentiation phase only was not effective for adipogenesis of human ADSCs (at 10 ng/mL) [134] and even inhibited adipogenic differentiation of human BMSCs (at 25 ng/mL) in collagen gels in the presence of heparin (25 μg/mL) [119]. FGF1 has been shown to promote adipogenic differentiation for preadipocytes. Hutley et al. demonstrated that FGF1 treatment (1 ng/ml) only during the expansion phase or a continuous treatment with FGF1 in the expansion and differentiation periods enhanced primary human preadipocyte adipogenic differentiation [131]. However, FGF1 treatment (25 ng/mL) in the presence of heparin (25 μg/mL) only during differentiation induction had a tendency to inhibit adipogenic differentiation of human BMSCs [119].
Exposure of FGF2 to MSCs during expansion up-regulated SOX9 [118]. In addition, Pizzute et al. found FGF2 pretreatment significantly up-regulated both p-p38 and p-Jnk (Jun N-terminal kinase) signals in human SDSCs; total Erk1/2 was markedly reduced, while p-Erk1/2 was moderately suppressed [120]. Similarly, short-term treatment of FGF2 to 3T3-L1 cells promoted adipogenesis by phosphorylation of Erk1/2 and increased the expression of PPARG and CEBPA [135]. Another study showed that the inhibitory effect of FGF2 on adipogenesis of human BMSCs is dependent on high mobility group A-2 (HMGA2) [136]. Moreover, FGF1 down-regulates BAMBI (BMP and activin membrane-bound inhibitor homolog) in a PI3K-dependent manner to induce adipogenic differentiation [137].
IGFI
Insulin-like growth factor I (IGFI) binding with IGF receptor (IGFR), which belongs to the family of receptor tyrosine kinases [138], has been shown to stimulate chondrogenic and adipogenic differentiation [36, 139, 140]. Treatment using 100 ng/mL IGFI alone for 3 weeks did not induce dominant chondrogenesis in CL-1 cells; however, in the presence of 10% FCS, IGFI increased both Alcian blue staining intensity and fractional Oil Red O positive area [36]. Furthermore, Frisch et al. utilized recombinant adeno-associated virus (rAAV) vectors to overexpress IGFI and found that application of IGFI vector significantly increased pellet diameters, proteoglycan and type II collagen in the aggregates and the intensities of Oil Red O staining in human BMSCs and bone marrow aspirates [139, 140]. These data indicate that IGFR signaling is involved in chondrogenesis and adipogenesis.
MAPK pathways are a downstream signal of the IGFR pathway [141]. A marked reduction of IGFI-mediated Erk1/2 activation has been demonstrated to occur during chondrogenesis [142]. Moreover, ATDC5 cells continually exposed to PD98059, a selective inhibitor of MAPK kinases, plus IGFI showed a greater degree of chondrogenic differentiation, as demonstrated by both Alcian blue staining and COL10A1 (type X collagen) expression, than cells exposed to IGFI alone [142]. Moreover, an alginate-chondrocyte system with IGFI treatment inhibited Erk1/2 and resulted in an increase in proteoglycan synthesis [143]. Similarly, there was a dramatic decrease in IGFI-stimulated MAPK activity during early differentiation of 3T3-L1 cells, which was permissive for IGFI-mediated adipogenic differentiation [144]. PD98059 enhanced adipogenic markers, such as PPARG, aP2 and LPL, suggesting that inhibition of MAPK in subconfluent, proliferating 3T3-L1 cells accelerates adipogenic differentiation [145]. These data indicate that down-regulation of the Erk1/2 pathway is indispensable for IGFI-stimulated chondrogenesis and adipogenesis.
IGFI has also been shown to stimulate chondrogenic and adipogenic differentiation through the PI3K pathway. In the absence of serum, 100 ng/mL IGFI activated PI3K and its downstream signaling molecule, Akt (protein kinase B), in chick limb bud mesenchymal cells. Moreover, inhibition with LY294002, a selective PI3K inhibitor, blocked the ability of IGFI to stimulate the accumulation of proteoglycan in chick mesenchymal cells, human ankle articular chondrocytes and rat BMSCs, implying that IGFI induces chondrogenesis of mesenchymal cells via the PI3K/Akt pathway [143, 146, 147]. Similarly, LY294002 abolished the LPL expression, which functions as a terminal marker of adipogenesis in human orbital preadipocytes [148]. Moreover, activation of insulin receptor substrate 1 (IRS1) and IRS2 and associated PI3K pathways led to activation of PPARγ and C/EBPα and thereby resulted in the induction of adipogenic differentiation [149]. Thus, IGFI signaling is involved in both chondrogenic and adipogenic differentiation.
Calcium ions
Calcium ions (Ca2+) play a pivotal role in regulating cell differentiation potential [150, 151]. Calcium homeostasis during chondrogenesis is complicated. Ca2+ concentrations influenced the response of MSCs to chondrogenic induction. A low concentration (1.8 mM) showed no effect for chondrogenic differentiation while a higher one (5 or 8 mM) showed no difference or negative influence [152, 153]. Moreover, transient Ca2+ exposure (5 mM) enhanced chondrogenesis while subsequent exposures to elevated Ca2+ (5 mM) suppressed chondrogenic differentiation [154]. Calcium channels, which are regulated by physical stimuli, such as hydrostatic pressure, electrical stimulation and pulse electromagnetic fields, seemed to play a crucial role in chondrogenic differentiation of MSCs [154–156]. Voltage-operated calcium channels, transient receptor potential channels and purinergic receptors have been reported to be regulated by physical stimuli [150, 151, 157, 158]. Modulating these channels or receptors can influence the concentration of intracellular Ca2+, which may have an impact on chondrogenesis. For example, transient receptor potential channel antagonists could effectively block chondrogenesis of the first exposition to pulse electromagnetic fields [154].
As for adipogenesis, increasing Ca2+ inhibited the early stages while promoting the late stage of differentiation, thus exerting a biphasic regulatory role [159]. What is more, continuous high concentrations of Ca2+ inhibited adipogenic differentiation of 3T3-L1 preadipocytes [160]. Calcium ions have been reported to play a positive role in adipogenic differentiation of porcine SDSCs and BMSCs, mouse BMSCs and human umbilical cord blood-derived MSCs through different pathways [152, 161–165].
Biophysical factors
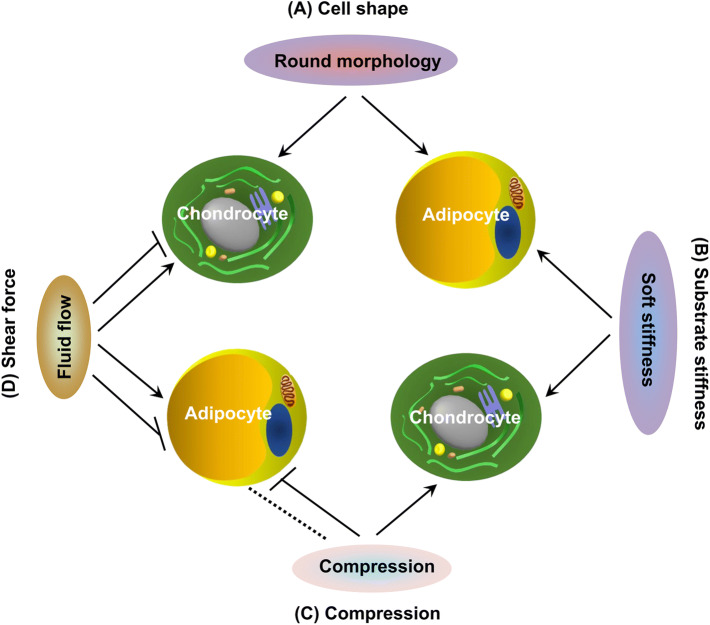
Given physical interaction with elements in the microenvironment, the shape of a stem cell is one of the biophysical factors implicated in cell fate decision. Cell shape can be influenced through micropatterned substrates [166], chitosan/polycaprolactone blended materials [167], gelatin/hyaluronic acid cryogels [168], ECM composition and mechanical properties [169] and chemicals [169, 170], indicating that spherical morphology encourages chondrogenic and adipogenic differentiation. There is increasing evidence showing that stem cell fate is also influenced by macro-mechanical stimulation, such as compression and shear forces, and by micro-mechanical stress, such as substrate stiffness [171] (Fig. 4) (Table 2).
Fig. 4.
Biophysical factors that regulate chondrogenic and adipogenic differentiation of MSCs. a Cell shape: round morphology encourages chondrogenic and adipogenic differentiation. b Substrate stiffness: soft stiffness guides MSC differentiation down corresponding tissue lineages of similar stiffness, such as both chondrogenesis and adipogenesis. c Compression: compression promoted chondrogenesis; however, compression immediately after adipogenic induction does not have an effect (indicated by dotted line) but before stimulation significantly inhibits adipogenesis. d Shear force: oscillatory fluid flow up-regulates chondrogenic and adipogenic differentiation while a higher magnitude resulted in decreased chondrogenic and adipogenic differentiation
Table 2.
Studies investigating biophysical factors guiding lineage determination between chondrogenic and adipogenic differentiation
| Cell type | Treatment | Chondrogenic differentiation | Adipogenic differentiation | Possible mechanisms | Refs. |
|---|---|---|---|---|---|
| Micro-mechanical stress | |||||
| Articular chondrocyte (bovine) | Seeded on 3D fiber deposition PEOT/PBT scaffold | Cartilage mechanically matching scaffolds (E’ = 10 MPa) increased chondrogenesis. | – | – | [179] |
| ADSC (human) | CDM within multi-layer electrospun PCL versus PCL construct | Increased | – | – | [174] |
| Cultured on adipose matrix-coated PA gel or tissue culture plastic | – | Gels mimicking the stiffness of adipose tissue (2 kPa) increased adipogenesis most | – | [176] | |
| BMSC (human) | PA gels with varied stiffness (1, 3, 15 kPa) | Soft substrates (1 kPa) increasing chondrogenesis | Soft substrates (1 kPa) increasing adipogenesis | By weaker cell adhesion | [175] |
| Cultured on a 1.37 or 4.47 kPa hydrogel matrix | – | Soft substrate increasing adipogenesis | – | [177] | |
| Cross-linked hydrogel based on thiol modified HA and gelatin with varied stiffness (0.15, 1.5 or 4 kPa) | – | Hydrogel substrates with 4 kPa increasing adipogenesis | – | [183] | |
| MSC (neonatal rat) | Cultured on PA, PDMS substrates or PA-PEG-RGD gels with varied stiffness (~ 130, ~ 3170 kPa) | – | Soft substrate increasing adipogenesis | – | [178] |
| SDSC (human) | dECM deposited by human adult SDSCs during expansion phase | Increased differentiation potential | – | – | [91] |
| dECM deposited by human fetal SDSCs versus human adult SDSCs | dECM deposited by human fetal SDSCs increasing chondrogenesis | dECM deposited by human fetal SDSCs increasing adipogenesis | – | [102] | |
| Macro-mechanical stress | |||||
| BMSC (human) | Dynamic compression (an intermittent regimen, a strain amplitude of 15% and frequency of 1 Hz) | Increased | – | – | [187] |
| Dynamic compression (1 Hz with 10 static offset strain) | Increased | – | – | [188] | |
| Dynamic compression (1 Hz with 10% sinusoidal strain, superimposed on a 10% static offset strain) | Increased | – | By up-regulating TGFβ pathway | [189] | |
| Under free swelling or dynamic compression conditions (5% of strain, 1 Hz of frequency) | Increased chondrogenesis in chitosan-coated PLCL | – | Through TGFβ/Smad and integrin signaling | [190] | |
| Dynamic compressive loading (10% peak compressive sinusoidal strain at 1 Hz frequency, superimposed on a 5% compressive tare strain) | Increased chondrogenesis in encapsulated HA hydrogels | – | – | [191] | |
| BMSC and AC (rat) | Fluid shear stimulation (from 0.009 to 1.089 dyne/cm2) | An increase in magnitude of stimulation initiating dedifferentiation for chondrocytes | An increase in magnitude of stimulation decreasing adipogenic differentiation | By increasing YAP expression to regulate differentiation | [194] |
| C3H10T1/2 | Oscillatory fluid flow (1 Hz, peak shear stresses of 1.0 Pa, 1 h) | Increased | Increased | By inhibiting tension within the actin cytoskeleton | [193] |
| SGBS | A compressive force of 226 Pa for 12 h | – | Compressive force before induction significantly inhibiting adipogenesis | By suppressing PPARG2 and CEBPA in a COX-2-dependent manner | [192] |
AC articular chondrocyte, ADSC adipose-derived stem cell, BMSC bone marrow-derived stem cell, C3H10T1/2 a mouse mesenchymal cell line, CDM cartilage-derived matrix, CEBP CCAAT/enhancer-binding protein, COX-2 cyclooxygenase 2, dECM decellularized extracellular matrix, HA hyaluronic acid, MAPK mitogen-activated protein kinase, PA polyacrylamide, PCL poly(ε-caprolactone), PDMS polydimethylsiloxane, PEG poly(ethylene glycol), PEOT/PBT poly(ethylene oxide-terephtalate)/poly(butylene terephtalate), PLCL poly L-lactide-co-ε-caprolactone, PPARG: PPARγ peroxisome proliferator-activated receptor gamma, RGD Arg-Gly-Asp, SDSC synovium-derived stem cell, SGBS Simpson–Golabi–Behmel syndrome cells, a preadipocyte cell line derived from human adipose tissue, Smad small mothers against decapentaplegic homolog, TGF transforming growth factor, Wnt Wingless/int, YAP Yes-associated protein
Micro-mechanical stress
As an external signal, substrate stiffness of ECM, usually represented by elastic modulus or Young’s modulus, is a determinant of stem cell lineage differentiation [172]. Many studies show that, compared to stiffer ones, softer substrates, such as hydrogel, porous/fibrous scaffold and decellularized ECM (dECM), were more likely to support MSC chondrogenesis [171]. Moreover, less stiff acellular ECM scaffolds, such as cartilage-derived dECMs and cell-derived dECMs, also enhanced chondrogenic differentiation by increasing chondrogenic gene expression compared to stiffer scaffolds [91, 102, 173, 174]. Similarly, softer matrix also promotes stem cell differentiation into adipogenic lineage. For instance, Park et al. showed that, compared to stiffer substrates (3, 15 kPa), human BMSCs seeded onto a soft substrate (1 kPa) had a higher expression of the adipogenic marker LPL as well as chondrogenic marker COL2A1 expression [175]. Moreover, MSCs on softer substrates, such as adipose matrix-coated polyacrylamide gel and hydrogel substrates, exhibited more adipogenic markers and fat droplets compared to a stiffer matrix [176–178].
Direct evidence showed that dECM deposited by fetal SDSCs with lower elasticity promoted chondrogenic and adipogenic differentiation [102]. Generally, substrate stiffness may likely guide MSC differentiation down corresponding tissue lineages of similar stiffness. For example, substrates approximating the elastic moduli of cartilage (0.4–0.8 MPa) may be more likely to enhance stem cells toward chondrogenesis [179–181], while scaffolds closely mimicking that of adipose tissue (2–8 kPa) might promote adipogenesis [176, 182–184].
The exact mechanisms of substrate stiffness underlying chondrogenesis and adipogenesis are unknown, but recent studies indicate that actin and ROCK (Rho-associated protein kinase)/RhoA might be involved. Cytochalasin D disrupted the actin cytoskeleton and promoted chondrogenic and adipogenic differentiation [170, 185]; treatment with Y27632, a selective inhibitor of ROCK, increased GAG production and decreased the number of actin fibers [186]. Inhibiting both ROCK and RhoA also promoted MSCs toward adipogenic differentiation [170]. These studies indicate that MSCs with a less organized and less stiff actin cytoskeleton organization are more prone to differentiate into chondrocytes and adipocytes.
Macro-mechanical stress
Many studies showed that direct compression promoted chondrogenesis [187–189]. For instance, human BMSCs were seeded in either chitosan-coated poly L-lactide-co-ε-caprolactone scaffolds [190] or hyaluronic acid hydrogel [191] with dynamic compression (5 or 10% strain, 1 Hz) enhanced cartilage formation and suppressed chondrocyte hypertrophy. However, the effects of mechanical stimuli on adipogenic differentiation are not well known. One study subjected SGBS (a human preadipocyte cell line) to a compressive force of 226 Pa for 12 h [192]. They found that compressive force immediately after adipogenic induction did not affect adipogenic differentiation; however, compressive force before adipogenic induction significantly inhibited PPARγ and C/EBPα through the up-regulation of cyclooxygenase-2.
Oscillatory fluid flow (1 Hz, peak shear stresses of 1.0 Pa, 1 h) has also been shown to induce the up-regulation of SOX9 and PPARG in C3H10T1/2 progenitor cells and its promotion of chondrogenic and adipogenic differentiation depended on inhibiting tension within the actin cytoskeleton, indicating it has the potential to regulate stem cell fate [193]. However, with the increasing magnitude of fluid shear stimulation (from 0.009 to 1.089 dyne/cm2), higher YAP (Yes associated protein) decreased adipogenic differentiation and initiated dedifferentiation for chondrocytes [194]. Further research is needed to clarify the role of shear force in chondrogenic and adipogenic differentiation.
Biological factors
Biological factors, such as hypoxia and aging, provide a better way to understand MSC differentiation toward chondrogenesis versus adipogenesis due to pathophysiologic conditions (Fig. 5) (Table 3).
Fig. 5.
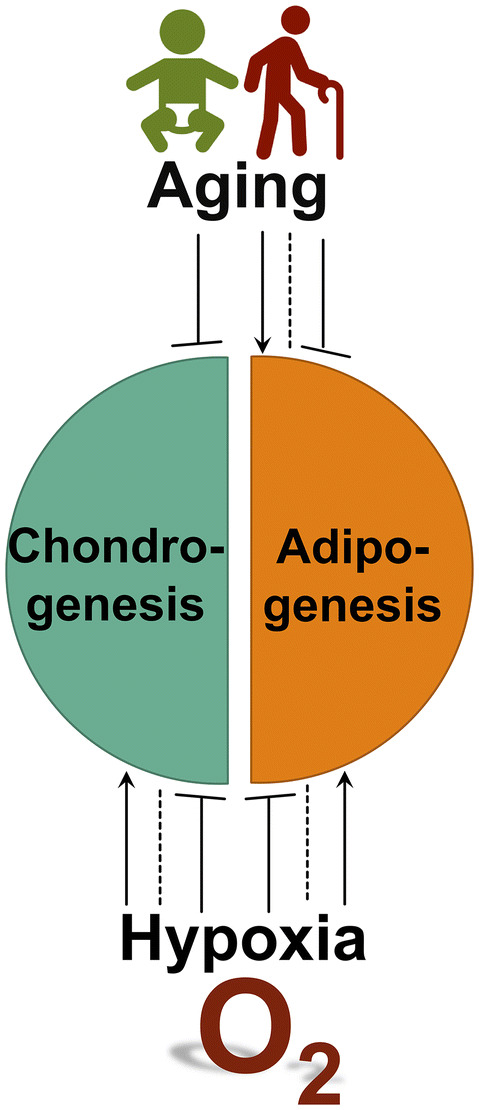
Biological factors that regulate chondrogenic and adipogenic differentiation of MSCs. Both hypoxia and aging have complex effects on chondrogensis and adipogenesis. In general, hypoxia promotes chondrogenic differentiation while suppressing adipogenic differentiation, although differential roles in either differentiation have been reported. Interestingly, aging impairs chondrogenic potential of MSCs while it has complex roles in adipogenic differentiation
Table 3.
Studies investigating biological factors guiding lineage determination between chondrogenic and adipogenic differentiation
| Cell type | Treatment | Chondrogenic differentiation | Adipogenic differentiation | Possible mechanisms | Refs. |
|---|---|---|---|---|---|
| Hypoxia | |||||
| 3T3-L1 preadipocyte | 2% O2 during differentiation induction | – | Decreased | By regulating DEC1/Stra13 to repress PPARG2 promoter | [201] |
| Chemical hypoxia (CoCl2) during differentiation induction | – | Decreased | By inhibiting PPARG in a HDAC-independent manner | [202] | |
| 1 or 4% O2 during differentiation induction | – | Mild, but not severe, promoting differentiation | Through excess of acetyl-CoA independently of HIF activation | [205] | |
| 1% O2 during differentiation induction | – | Decreased | By increasing miR-27a and miR-27b to inhibit PPARγ and C/EBPα | [213] | |
| ADSC (human) | 5% O2 during differentiation induction | No effect | – | – | [199] |
| 2% O2 during expansion phase | – | Increased | – | [209] | |
| ADSC (murine) | 2% O2 during expansion phase | – | Increased | By increasing Sca-1/CD44 | [208] |
| BMSC (equine) | 5% O2 during expansion phase | Increased | No effect | By up-regulating HIF1α | [210] |
| BMSC (human) | 5% O2 during differentiation induction | Increased | – | – | [197] |
| 3% O2 during expansion and differentiation phases | Increased | – | By up-regulating HIF2α | [198] | |
| 1% O2 during differentiation induction | Decreased | Decreased | – | [200] | |
| 0.2% O2 during differentiation induction | – | Increased | – | [206] | |
| 5% O2 during differentiation induction | Increased | – | By up-regulating HIF1α | [211] | |
| 1% O2 during differentiation induction | Increased | – | By activating the PI3K/Akt/FoxO pathway | [212] | |
| BMSC (rat) | 2% O2 during differentiation induction | Increased | – | By up-regulating HIF1α | [196] |
| C2C12 and G8 (murine) | 1% O2 during differentiation induction | – | Increased | – | [204] |
| IPFP cells (human) | 5% O2 during differentiation induction | Increased | – | By up-regulating HIF2α | [195] |
| MSC (human) | 3% O2 during expansion phase | – | Increased | – | [207] |
| Preadipocyte (human) | 1% O2 or chemical hypoxia (DFO) during differentiation induction | – | Decreased | – | [204] |
| Aging | |||||
| ADSC (bovine) | Passages 2, 5 | Passage 2 > passage 5 | Passage 2 > passage 5 | – | [222] |
| BMSC (porcine) | Passage 5–15 | Early late passage | – | – | [214] |
| BMSC (bovine) | Fetal, juvenile and adult bovine donors | Fetal > juvenile > adult donor | – | – | [215] |
| BMSC (human) | Passages 3, 5, 6, 7, 9, 11 | Early and mid > late passage | Early and mid < late passage | By increasing CDKN2A level | [217] |
| 80 patients (14–79 year-old) | – | Young < old donor | – | [218] | |
| passage 1–10 | – | Differentiation potential dropped from the 6th passage on | – | [220] | |
| Passages 3, 5, 8, 14 | – | Early > late passage | – | [221] | |
| Young (aged 18–29 years) and old (aged 68–81 years) | – | No effect | – | [223] | |
| BMSC (murine) | Adult (6–8 month-old) and old (20–26 month-old) | – | Adult < old donor | By altering TGFβ and BMP2/4 signaling pathways | [219] |
| TDSC (rat) | Early (P5), mid (P10) and late passages (P20, P30) of TDSCs | Early and mid > late passages | Early and mid > late passages | – | [216] |
ADSC adipose-derived stem cell, Akt protein kinase B, BMSC bone marrow-derived stem cell, CEBP CCAAT/enhancer-binding protein, CDKN2A cycline-dependent kinase inhibitor 2A, CoCl2 cobalt chloride, DEC1/Stra13 differentiated embryo-chondrocyte expressed gene 1/stimulated with retinoic acid 13, DFO desferrioxamine, FoxO forkhead box protein O, HDAC histone deacetylase, HIF hypoxia inducible factor, IPFP infrapatellar fat pad, PI3K phosphatidylinositol 3-kinase, PPARG PPARγ, peroxisome proliferator-activated receptor gamma, TDSC tendon-derived stem cells
Hypoxia
In general, hypoxia promotes chondrogenic differentiation while suppressing adipogenic differentiation, although with some inconsistent results. Hypoxia during the differentiation period could enhance chondrogenic marker genes, transcription factors and ECM deposition in rat BMSCs and human infrapatellar fat pad stem cells and BMSCs [195–197]. Hypoxia (3% O2) during the expansion and differentiation periods could enhance expression of COL2A1, ACAN and SOX9 in human BMSCs [198]. However, results are inconsistent. For example, hypoxia during chondrogenic induction of human ADSCs did not significantly alter the levels of COL2A1 and ACAN [199]. Cicione et al. even found that expression of SOX9 and ACAN in human BMSCs decreased during chondrogenic induction under severe hypoxia (1% O2) [200].
Many studies showed that adipogenic induction of stem cells under hypoxia resulted in attenuated adipogenic differentiation [201–203]. However, other studies showed that hypoxic conditions in the myogenic cell lines, C2C12 and G8, increased adipocyte differentiation [204]; in addition, mild hypoxia (4% O2) in 3T3-L1 cells or extreme hypoxia (0.2% O2) in human BMSCs promoted adipogenesis [205, 206]. These observations indicate that the effect of oxygen on adipogenic differentiation is extensive and cell-type specific. Interestingly, stem cells that had previously been cultured in hypoxia could subsequently be stimulated to exhibit normal or even significantly higher differentiation capacity, indicating that hypoxic preconditioning may represent a strategy to enhance MSC adipogenic differentiation [207–209].
In terms of mechanism, hypoxia inducible factors (HIFs) may play critical roles in stem cell chondrogenic and adipogenic differentiation under hypoxia. The expression level of HIF1α was significantly increased on day 21 during chondrogenic differentiation of equine hypoxia-expanded BMSCs [210]. Besides, HIF1α was found to be able to bind to a Sox9 promoter and up-regulate this key transcription factor [211]. Except for HIFs, hypoxia was demonstrated to enhance chondrogenic differentiation by inhibiting apoptosis via activating the PI3K/Akt/FoxO pathway [212]. During adipogenic differentiation, HIF1 was increased to regulate DEC1 (Differentiated embryo-chondrocyte expressed gene 1)/Stra13 (Stimulated with retinoic acid 13), thereby repressing PPARγ2 promoter activation [201]. This finding indicates that HIF1 mediates hypoxia-induced inhibition of adipogenic differentiation. Moreover, hypoxia increased miR-27a and miR-27b, which strongly inhibited PPARγ and C/EBPα in preadipocytes [213]. Interestingly, extreme hypoxia (0.2% O2) induced adipogenic differentiation of human BMSCs through HIF1α and C/EBPs [206].
Aging
Many studies showed that in vitro aging (passage number in culture) and in vivo aging (donor age) influenced the differentiation potential of adult stem cells. Several reports showed that aging impaired stem cell chondrogenic potential in porcine, bovine and human BMSCs, and rat tendon-derived stem cells [214–217]. Different from chondrogenic differentiation, which declines with age, adipogenic differentiation seems to accelerate with aging. Aging up-regulated adipocyte specific genes such as PPARG and aP2 [218, 219]. In line with these molecular changes, the number and size of adipocytes increased in late passage MSCs compared to early passage ones [217]. However, no difference or a general decrease has been reported in adipogenic differentiation capacity of aged stem cells [216, 220–223].
Stable gene expression levels of cyclin-dependent kinase inhibitor 2A (CDKN2A) and CDKN2C, the senescence-associated marker genes, in early passages contributed to effective chondrogenic differentiation [217]. Human BMSCs preserved chondrogenic potential with low CDKN2C and stable CDKN2A expression level. Increased CDKN2A expression led to impaired chondrogenic potential [217]. Adipogenic differentiation was less affected by CDKN2A and CDKN2C expression, but higher expression of CDKN2A resulted in a more effective adipogenic differentiation [217].
As aging progresses, reactive oxygen species (ROS) and oxidative stress have been reported to increase and to play vital roles in stem cell differentiation. Suppression of Heme oxygenase-1 (HO-1), an agent known to neutralize oxidative stress [224], strongly increased PPARγ expression and adipogenesis in human BMSCs [225]. Correspondingly, overexpressing HO-1 suppressed adipogenic differentiation in porcine ADSCs [226]. Adenovirus-mediated expression of HO-1 in human BMSCs slightly decreased adipogenic differentiation of MSCs, but did not affect chondrogenic differentiation [227]. The above-mentioned reports indicate that increasing intracellular oxidative stress may be one of the major drivers of adipogenesis during the aging process.
Conclusions
Over decades of studies, the relationship between chondrogenesis and adipogenesis is still not clear. For example, TGFβ and Hedgehog signaling pathways can promote chondrogenesis and inhibit adipogenesis; interestingly, BMP signaling can promote both chondrogenesis and adipogenesis simultaneously through the Smad1/5/8 pathway and the p38 pathway. These findings indicate that chondrogenic and adipogenic differentiations are competing and reciprocal. To make matters more complicated, some external chemical factors have differing roles in stem cell differentiation through different pathways. For example, dexamethasone can promote adipocyte differentiation through C/EBPα but inhibits adipogenesis via Runx2. Studies have demonstrated that biochemical, biophysical and biological cues can exert their effects on the crosstalk between chondrogenesis and adipogenesis via a variety of signaling pathways. These signals approach at a controlled cascade of transcription events, including Sox9 for chondrogenesis and C/EBPs and PPARγ for adipogenesis. Unlike the relationship between chondrogenesis and osteogenesis or adipogenesis and osteogenesis, studies on the decision between chondrogenesis and adipogenesis are few. Understanding the mechanisms underlying the balance between chondrogenic and adipogenic differentiation is more meaningful via in vivo studies and in vitro studies at a clonal level. These new data will be of great significance to identify the pathogenic causes of cartilage and fat-related diseases and will lead to better clinical applications of adult stem cells in cartilage and fat tissue engineering.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R01AR067747-01A1) to M.P., and Natural Science Foundation of China (81601889) to S.C.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Wagers AJ, Weissman IL. Plast Adult Stem Cells. Cell. 2004;116(5):639–648. doi: 10.1016/S0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 6.Chijimatsu R, Kobayashi M, Ebina K, Iwahashi T, Okuno Y, Hirao M, Fukuhara A, Nakamura N, Yoshikawa H. Impact of dexamethasone concentration on cartilage tissue formation from human synovial derived stem cells in vitro. Cytotechnology. 2018;70(2):819–829. doi: 10.1007/s10616-018-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101(6):581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 8.Enomoto H, Furuichi T, Zanma A, Yamana K, Yoshida C, Sumitani S, Yamamoto H, Enomoto-Iwamoto M, Iwamoto M, Komori T. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci. 2004;117(Pt 3):417–425. doi: 10.1242/jcs.00866. [DOI] [PubMed] [Google Scholar]
- 9.Qu P, Wang L, Min Y, McKennett L, Keller JR, Lin PC. Vav1 regulates mesenchymal stem cell differentiation decision between adipocyte and chondrocyte via sirt1. Stem Cells. 2016;34(7):1934–1946. doi: 10.1002/stem.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9(3):287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki K, Li J, Yu H, Fukui N, Sandell LJ. CCAAT/enhancer-binding proteins beta and delta mediate the repression of gene transcription of cartilage-derived retinoic acid-sensitive protein induced by interleukin-1 beta. J Biol Chem. 2002;277(35):31526–31533. doi: 10.1074/jbc.M202815200. [DOI] [PubMed] [Google Scholar]
- 12.Okuma T, Hirata M, Yano F, Mori D, Kawaguchi H, Chung UI, Tanaka S, Saito T. Regulation of mouse chondrocyte differentiation by CCAAT/enhancer-binding proteins. Biomed Res. 2015;36(1):21–29. doi: 10.2220/biomedres.36.21. [DOI] [PubMed] [Google Scholar]
- 13.Ushijima T, Okazaki K, Tsushima H, Iwamoto Y. CCAAT/enhancer-binding protein beta regulates the repression of type II collagen expression during the differentiation from proliferative to hypertrophic chondrocytes. J Biol Chem. 2014;289(5):2852–2863. doi: 10.1074/jbc.M113.492843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockl S, Bauer RJ, Bosserhoff AK, Gottl C, Grifka J, Grassel S. Sox9 modulates cell survival and adipogenic differentiation of multipotent adult rat mesenchymal stem cells. J Cell Sci. 2013;126(Pt 13):2890–2902. doi: 10.1242/jcs.124305. [DOI] [PubMed] [Google Scholar]
- 15.Ushita M, Saito T, Ikeda T, Yano F, Higashikawa A, Ogata N, Chung U, Nakamura K, Kawaguchi H. Transcriptional induction of SOX9 by NF-kappaB family member RelA in chondrogenic cells. Osteoarthr Cartil. 2009;17(8):1065–1075. doi: 10.1016/j.joca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;5:6. doi: 10.1007/s00441-017-2613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharjee M, Coburn J, Centola M, Murab S, Barbero A, Kaplan DL, Martin I, Ghosh S. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–122. doi: 10.1016/j.addr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37(2):127–133. [PubMed] [Google Scholar]
- 20.Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122(12):3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- 21.Decker RS. Articular cartilage and joint development from embryogenesis to adulthood. Semin Cell Dev Biol. 2017;62:50–56. doi: 10.1016/j.semcdb.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16(3):348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134(12):2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 28.Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, Darlington GJ. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci USA. 2001;98(22):12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20(5–6):523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, Caplan AI, Ono K. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132(4):1603–1608. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- 31.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 32.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 33.Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9(6):1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 34.Handorf AM, Chamberlain CS, Li WJ. Endogenously produced Indian Hedgehog regulates TGFbeta-driven chondrogenesis of human bone marrow stromal/stem cells. Stem Cells Dev. 2015;24(8):995–1007. doi: 10.1089/scd.2014.0266. [DOI] [PubMed] [Google Scholar]
- 35.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80(12):1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura H. Establishment of a bipotent cell line CL-1 which differentiates into chondrocytes and adipocytes from adult mouse. Osteoarthr Cartil. 2004;12(1):25–37. doi: 10.1016/j.joca.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19(3):463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 38.Ignotz RA, Massague J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci USA. 1985;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsurutani Y, Fujimoto M, Takemoto M, Irisuna H, Koshizaka M, Onishi S, Ishikawa T, Mezawa M, He P, Honjo S, Maezawa Y, Saito Y, Yokote K. The roles of transforming growth factor-beta and Smad3 signaling in adipocyte differentiation and obesity. Biochem Biophys Res Commun. 2011;407(1):68–73. doi: 10.1016/j.bbrc.2011.02.106. [DOI] [PubMed] [Google Scholar]
- 40.Coricor G, Serra R. TGF-beta regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuli R, Seghatoleslami MR, Tuli S, Howard MS, Danielson KG, Tuan RS. p38 MAP kinase regulation of AP-2 binding in TGF-beta1-stimulated chondrogenesis of human trabecular bone-derived cells. Ann N Y Acad Sci. 2002;961:172–177. doi: 10.1111/j.1749-6632.2002.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim BS, Kang KS, Kang SK. Soluble factors from ASCs effectively direct control of chondrogenic fate. Cell Prolif. 2010;43(3):249–261. doi: 10.1111/j.1365-2184.2010.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43(4):333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol. 2000;149(3):667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278(11):9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 46.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 47.Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475(3):201–204. doi: 10.1016/S0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 48.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9(1):57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Res Biol Divers. 2003;71(9–10):567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 50.Kuroda R, Usas A, Kubo S, Corsi K, Peng HR, Rose T, Cummins J, Fu FH, Huard J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 51.Steinert A, Weber M, Dimmler A, Julius C, Schutze N, Noth U, Cramer H, Eulert J, Zimmermann U, Hendrich C. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthopaed Res. 2003;21(6):1090–1097. doi: 10.1016/S0736-0266(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 52.Semba I, Nonaka K, Takahashi I, Takahashi K, Dashner R, Shum L, Nuckolls GH, Slavkin HC. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by Sox9 and Msx2. Dev Dynam. 2000;217(4):401–414. doi: 10.1002/(SICI)1097-0177(200004)217:4<401::AID-DVDY7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.Nakayama N, Duryea D, Manoukian R, Chow G, Han CYE. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci. 2003;116(10):2015–2028. doi: 10.1242/jcs.00417. [DOI] [PubMed] [Google Scholar]
- 54.Taha MF, Valojerdi MR, Mowla SJ. Effect of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse embryonic stem cells. Anat Histol Embryol. 2006;35(4):271–278. doi: 10.1111/j.1439-0264.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 55.Shintani N, Hunziker EB. Chondrogenic differentiation of bovine synovium—bone morphogenetic proteins 2 and 7 and transforming growth factor beta 1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007;56(6):1869–1879. doi: 10.1002/art.22701. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto C, Matsumoto T, Sakimura K, Shindo H. Osteogenic protein-1 with transforming growth factor-beta 1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J Orthop Sci. 2007;12(6):555–561. doi: 10.1007/s00776-007-1176-4. [DOI] [PubMed] [Google Scholar]
- 57.Brown PT, Squire MW, Li WJ. Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res. 2014;358(1):149–164. doi: 10.1007/s00441-014-1926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cicione C, Muinos-Lopez E, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Alternative protocols to induce chondrogenic differentiation: transforming growth factor-beta superfamily. Cell Tissue Bank. 2015;16(2):195–207. doi: 10.1007/s10561-014-9472-7. [DOI] [PubMed] [Google Scholar]
- 59.Lee PT, Li WJ. Chondrogenesis of embryonic stem cell-derived mesenchymal stem cells induced by TGF1 and BMP7 through increased TGF receptor expression and endogenous TGF1 production. J Cell Biochem. 2017;118(1):172–181. doi: 10.1002/jcb.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1044. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neumann K, Endres M, Ringe J, Flath B, Manz R, Haupl T, Sittinger M, Kaps C. BMP7 promotes adipogenic but not osteo-/chondrogenic differentiation of adult human bone marrow-derived stem cells in high-density micro-mass culture. J Cell Biochem. 2007;102(3):626–637. doi: 10.1002/jcb.21319. [DOI] [PubMed] [Google Scholar]
- 62.Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, Diwan AD. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem. 2010;109(2):406–416. doi: 10.1002/jcb.22412. [DOI] [PubMed] [Google Scholar]
- 63.Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114(Pt 11):2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 64.Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells. 2008;26(4):1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- 65.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 66.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mundy C, Bello A, Sgariglia F, Koyama E, Pacifici M. HhAntag, a hedgehog signaling antagonist, suppresses chondrogenesis and modulates canonical and non-canonical BMP signaling. J Cell Physiol. 2016;231(5):1033–1044. doi: 10.1002/jcp.25192. [DOI] [PubMed] [Google Scholar]
- 68.Enomoto-Iwamoto M, Nakamura T, Aikawa T, Higuchi Y, Yuasa T, Yamaguchi A, Nohno T, Noji S, Matsuya T, Kurisu K, Koyama E, Pacifici M, Iwamoto M. Hedgehog proteins stimulate chondrogenic cell differentiation and cartilage formation. J Bone Miner Res. 2000;15(9):1659–1668. doi: 10.1359/jbmr.2000.15.9.1659. [DOI] [PubMed] [Google Scholar]
- 69.James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, Longaker MT. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16(8):2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3(1):25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140(1):148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 72.Zehentner BK, Leser U, Burtscher H. BMP-2 and sonic hedgehog have contrary effects on adipocyte-like differentiation of C3H10T1/2 cells. DNA Cell Biol. 2000;19(5):275–281. doi: 10.1089/10445490050021186. [DOI] [PubMed] [Google Scholar]
- 73.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 74.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 75.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277(34):30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 76.Im GI, Quan Z. The effects of Wnt inhibitors on the chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2010;16(7):2405–2413. doi: 10.1089/ten.tea.2009.0359. [DOI] [PubMed] [Google Scholar]
- 77.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol. 2011;46(2):R65–R72. doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 78.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279(43):45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 80.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376(Pt 3):607–613. doi: 10.1042/bj20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50(2):477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawai M, Mushiake S, Bessho K, Murakami M, Namba N, Kokubu C, Michigami T, Ozono K. Wnt/Lrp/beta-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARgamma and C/EBPalpha. Biochem Biophys Res Commun. 2007;363(2):276–282. doi: 10.1016/j.bbrc.2007.08.088. [DOI] [PubMed] [Google Scholar]
- 83.Park JR, Jung JW, Lee YS, Kang KS. The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells. Cell Prolif. 2008;41(6):859–874. doi: 10.1111/j.1365-2184.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ehrlund A, Mejhert N, Lorente-Cebrian S, Astrom G, Dahlman I, Laurencikiene J, Ryden M. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98(3):E503–E508. doi: 10.1210/jc.2012-3416. [DOI] [PubMed] [Google Scholar]
- 85.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277(34):30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 86.Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, Mohanty ST, Croucher P, Kramer I, Kneissel M, Rosen CJ, Reagan MR. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233(2):1156–1167. doi: 10.1002/jcp.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cianferotti L, Demay MB. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem. 2007;101(1):80–88. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]
- 88.Jin EJ, Park JH, Lee SY, Chun JS, Bang OS, Kang SS. Wnt-5a is involved in TGF-beta3-stimulated chondrogenic differentiation of chick wing bud mesenchymal cells. Int J Biochem Cell Biol. 2006;38(2):183–195. doi: 10.1016/j.biocel.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Liu S, Zhang E, Yang M, Lu L. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-beta. Mol Cell Biochem. 2014;390(1–2):123–131. doi: 10.1007/s11010-014-1963-0. [DOI] [PubMed] [Google Scholar]
- 90.Hsu SH, Huang GS. Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials. 2013;34(20):4725–4738. doi: 10.1016/j.biomaterials.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. doi: 10.1016/j.actbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishizuka M, Koyanagi A, Osada S, Imagawa M. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 2008;582(21–22):3201–3205. doi: 10.1016/j.febslet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Grogan SP, Olee T, Hiraoka K, Lotz MK. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. 2008;58(9):2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian Y, Xu Y, Fu Q, Chang M, Wang Y, Shang X, Wan C, Marymont JV, Dong Y. Notch inhibits chondrogenic differentiation of mesenchymal progenitor cells by targeting Twist1. Mol Cell Endocrinol. 2015;403:30–38. doi: 10.1016/j.mce.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osathanon T, Subbalekha K, Sastravaha P, Pavasant P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol Int. 2012;36(12):1161–1170. doi: 10.1042/CBI20120288. [DOI] [PubMed] [Google Scholar]
- 96.Lai PY, Tsai CB, Tseng MJ. Active form Notch4 promotes the proliferation and differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2013;430(3):1132–1139. doi: 10.1016/j.bbrc.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 97.Garces C, Ruiz-Hidalgo MJ, Font de Mora J, Park C, Miele L, Goldstein J, Bonvini E, Porras A, Laborda J. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J Biol Chem. 1997;272(47):29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 98.Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol. 2004;24(8):3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhauser M, Brenner S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37(7):867–875. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ, Li JJ, Chen F, Wu HH, Han LX, Lu SH, Zheng YZ, Han ZC. Inhibition of notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem. 2015;36(5):1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 101.Ba K, Yang X, Wu L, Wei X, Fu N, Fu Y, Cai X, Yao Y, Ge Y, Lin Y. Jagged-1-mediated activation of notch signalling induces adipogenesis of adipose-derived stem cells. Cell Prolif. 2012;45(6):538–544. doi: 10.1111/j.1365-2184.2012.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35(2):642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 103.Xiao Y, Peperzak V, van Rijn L, Borst J, de Bruijn JD. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med. 2010;4(5):374–386. doi: 10.1002/term.250. [DOI] [PubMed] [Google Scholar]
- 104.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24(6):1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 105.Tangtrongsup S, Kisiday JD. Effects of dexamethasone concentration and timing of exposure on chondrogenesis of equine bone marrow-derived mesenchymal stem cells. Cartilage. 2016;7(1):92–103. doi: 10.1177/1947603515595263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naito M, Ohashi A, Takahashi T. Dexamethasone inhibits chondrocyte differentiation by suppression of Wnt/beta-catenin signaling in the chondrogenic cell line ATDC5. Histochem Cell Biol. 2015;144(3):261–272. doi: 10.1007/s00418-015-1334-2. [DOI] [PubMed] [Google Scholar]
- 107.Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25(3):655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- 108.Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, Martinovic S, Hunziker EB. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthr Cartil. 2007;15(10):1178–1189. doi: 10.1016/j.joca.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 109.Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells influence of microenvironment, tissue origin and growth factor. Eur Cells Mater. 2011;22:302–319. doi: 10.22203/eCM.v022a23. [DOI] [PubMed] [Google Scholar]
- 110.Oshina H, Sotome S, Yoshii T, Torigoe I, Sugata Y, Maehara H, Marukawa E, Omura K, Shinomiya K. Effects of continuous dexamethasone treatment on differentiation capabilities of bone marrow-derived mesenchymal cells. Bone. 2007;41(4):575–583. doi: 10.1016/j.bone.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 111.Naito M, Omoteyama K, Mikami Y, Takahashi T, Takagi M. Inhibition of Wnt/beta-catenin signaling by dexamethasone promotes adipocyte differentiation in mesenchymal progenitor cells, ROB-C26. Histochem Cell Biol. 2012;138(6):833–845. doi: 10.1007/s00418-012-1007-3. [DOI] [PubMed] [Google Scholar]
- 112.He Q, Huang HY, Zhang YY, Li X, Qian SW, Tang QQ. TAZ is downregulated by dexamethasone during the differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2012;419(3):573–577. doi: 10.1016/j.bbrc.2012.02.074. [DOI] [PubMed] [Google Scholar]
- 113.Wang GJ, Cui Q, Balian G. The nicolas andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 114.Mikami Y, Lee M, Irie S, Honda MJ. Dexamethasone modulates osteogenesis and adipogenesis with regulation of osterix expression in rat calvaria-derived cells. J Cell Physiol. 2011;226(3):739–748. doi: 10.1002/jcp.22392. [DOI] [PubMed] [Google Scholar]
- 115.Hara ES, Ono M, Pham HT, Sonoyama W, Kubota S, Takigawa M, Matsumoto T, Young MF, Olsen BR, Kuboki T. Fluocinolone acetonide is a potent synergistic factor of TGF-beta3-associated chondrogenesis of bone marrow-derived mesenchymal stem cells for articular surface regeneration. J Bone Miner Res. 2015;30(9):1585–1596. doi: 10.1002/jbmr.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang YY, Li X, Qian SW, Guo L, Huang HY, He Q, Liu Y, Ma CG, Tang QQ. Down-regulation of type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol Endocrinol. 2012;26(5):798–808. doi: 10.1210/me.2011-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Zhang N, Huang X, Xu J, Fernandes JC, Dai K, Zhang X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Disease. 2013;4:e832. doi: 10.1038/cddis.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Correa D, Somoza RA, Lin P, Greenberg S, Rom E, Duesler L, Welter JF, Yayon A, Caplan AI. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthr Res Soc. 2015;23(3):443–453. doi: 10.1016/j.joca.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le Blanc S, Simann M, Jakob F, Schutze N, Schilling T. Fibroblast growth factors 1 and 2 inhibit adipogenesis of human bone marrow stromal cells in 3D collagen gels. Exp Cell Res. 2015;338(2):136–148. doi: 10.1016/j.yexcr.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Pizzute T, Li JT, Zhang Y, Davis ME, Pei M. Fibroblast growth factor ligand dependent proliferation and chondrogenic differentiation of synovium-derived stem cells and concomitant adaptation of wnt/mitogen-activated protein kinase signals. Tissue Eng Pt A. 2016;22(15–16):1036–1046. doi: 10.1089/ten.tea.2016.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16(3):1009–1019. doi: 10.1089/ten.tea.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JH, Lee MC, Seong SC, Park KH, Lee S. Enhanced Proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;17(7–8):991–1002. doi: 10.1089/ten.tea.2010.0277. [DOI] [PubMed] [Google Scholar]
- 123.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 124.Buckley CT, Kelly DJ. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J Mech Behav Biomed. 2012;11:102–111. doi: 10.1016/j.jmbbm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 125.Cheng T, Yang C, Weber N, Kim HT, Kuo AC. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem Biophys Res Commun. 2012;426(4):544–550. doi: 10.1016/j.bbrc.2012.08.124. [DOI] [PubMed] [Google Scholar]
- 126.Bianchessi M, Chen Y, Durgam S, Pondenis H, Stewart M. Effect of fibroblast growth factor 2 on equine synovial fluid chondroprogenitor expansion and chondrogenesis. Stem Cells Int. 2016;2016:9364974. doi: 10.1155/2016/9364974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coipeau P, Rosset P, Langonne A, Gaillard J, Delorme B, Rico A, Domenech J, Charbord P, Sensebe L. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11(5):584–594. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 128.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223(1):84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 129.Hildner F, Peterbauer A, Wolbank S, Nurnberger S, Marlovits S, Redl H, van Griensven M, Gabriel C. FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. J Biomed Mater Res, Part A. 2010;94(3):978–987. doi: 10.1002/jbm.a.32761. [DOI] [PubMed] [Google Scholar]
- 130.Bosetti M, Boccafoschi F, Leigheb M, Bianchi AE, Cannas M. Chondrogenic induction of human mesenchymal stem cells using combined growth factors for cartilage tissue engineering. J Tissue Eng Regen Med. 2012;6(3):205–213. doi: 10.1002/term.416. [DOI] [PubMed] [Google Scholar]
- 131.Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, Herington A, Cameron D, Whitehead J, Prins J. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–3106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 132.Neubauer M, Fischbach C, Bauer-Kreisel P, Lieb E, Hacker M, Tessmar J, Schulz MB, Goepferich A, Blunk T. Basic fibroblast growth factor enhances PPARgamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 2004;577(1–2):277–283. doi: 10.1016/j.febslet.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 133.Inoue S, Hori Y, Hirano Y, Inamoto T, Tabata Y. Effect of culture substrate and fibroblast growth factor addition on the proliferation and differentiation of human adipo-stromal cells. J Biomater Sci Polym Ed. 2005;16(1):57–77. doi: 10.1163/1568562052843366. [DOI] [PubMed] [Google Scholar]
- 134.Kakudo N, Shimotsuma A, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359(2):239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 135.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002;277(48):46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 136.Kalomoiris S, Cicchetto AC, Lakatos K, Nolta JA, Fierro FA. Fibroblast growth factor 2 regulates high mobility group A2 expression in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2016;117(9):2128–2137. doi: 10.1002/jcb.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo X, Hutley LJ, Webster JA, Kim YH, Liu DF, Newell FS, Widberg CH, Bachmann A, Turner N, Schmitz-Peiffer C, Prins JB, Yang GS, Whitehead JP. Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes. 2012;61(1):124–136. doi: 10.2337/db11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61(2):203–212. doi: 10.1016/0092-8674(90)90801-K. [DOI] [PubMed] [Google Scholar]
- 139.Frisch J, Venkatesan JK, Rey-Rico A, Schmitt G, Madry H, Cucchiarini M. Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2014;5(4):103. doi: 10.1186/scrt491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frisch J, Rey-Rico A, Venkatesan JK, Schmitt G, Madry H, Cucchiarini M. Chondrogenic differentiation processes in human bone marrow aspirates upon rAAV-mediated gene transfer and overexpression of the insulin-like growth factor I. Tissue Eng Part A. 2015;21(17–18):2460–2471. doi: 10.1089/ten.tea.2014.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Giorgetti S, Ballotti R, Kowalski-Chauvel A, Tartare S, Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993;268(10):7358–7364. [PubMed] [Google Scholar]
- 142.Phornphutkul C, Wu KY, Yang X, Chen Q, Gruppuso PA. Insulin-like growth factor-I signaling is modified during chondrocyte differentiation. J Endocrinol. 2004;183(3):477–486. doi: 10.1677/joe.1.05873. [DOI] [PubMed] [Google Scholar]
- 143.Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389(Pt 3):723–729. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boney CM, Smith RM, Gruppuso PA. Modulation of insulin-like growth factor I mitogenic signaling in 3T3-L1 preadipocyte differentiation. Endocrinology. 1998;139(4):1638–1644. doi: 10.1210/endo.139.4.5920. [DOI] [PubMed] [Google Scholar]
- 145.Boney CM, Gruppuso PA, Faris RA, Frackelton AR., Jr The critical role of Shc in insulin-like growth factor-I-mediated mitogenesis and differentiation in 3T3-L1 preadipocytes. Mol Endocrinol. 2000;14(6):805–813. doi: 10.1210/mend.14.6.0487. [DOI] [PubMed] [Google Scholar]
- 146.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. 2003;278(38):36563–36571. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 147.McMahon LA, Prendergast PJ, Campbell VA. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;368(4):990–995. doi: 10.1016/j.bbrc.2008.01.160. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L, Grennan-Jones F, Draman MS, Lane C, Morris D, Dayan CM, Tee AR, Ludgate M. Possible targets for nonimmunosuppressive therapy of Graves’ orbitopathy. J Clin Endocrinol Metab. 2014;99(7):E1183–E1190. doi: 10.1210/jc.2013-4182. [DOI] [PubMed] [Google Scholar]
- 149.Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, Terauchi Y, Kamon J, Kaburagi Y, Matsui J, Akanuma Y, Nagai R, Kimura S, Tobe K, Kadowaki T. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21(7):2521–2532. doi: 10.1128/MCB.21.7.2521-2532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Viti F, Landini M, Mezzelani A, Petecchia L, Milanesi L, Scaglione S. Osteogenic differentiation of MSC through calcium signaling activation: transcriptomics and functional analysis. PLoS One. 2016;11(2):e0148173. doi: 10.1371/journal.pone.0148173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M. Characterization of Ca(2 +) signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32(4):165–174. doi: 10.1016/S0143416002001240. [DOI] [PubMed] [Google Scholar]
- 152.Dry H, Jorgenson K, Ando W, Hart DA, Frank CB, Sen A. Effect of calcium on the proliferation kinetics of synovium-derived mesenchymal stromal cells. Cytotherapy. 2013;15(7):805–819. doi: 10.1016/j.jcyt.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 153.Mellor LF, Mohiti-Asli M, Williams J, Kannan A, Dent MR, Guilak F, Loboa EG. Extracellular calcium modulates chondrogenic and osteogenic differentiation of human adipose-derived stem cells: a novel approach for osteochondral tissue engineering using a single stem cell source. Tissue Eng Part A. 2015;21(17–18):2323–2333. doi: 10.1089/ten.tea.2014.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parate D, Franco-Obregon A, Frohlich J, Beyer C, Abbas AA, Kamarul T, Hui JHP, Yang Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep. 2017;7(1):9421. doi: 10.1038/s41598-017-09892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Steward AJ, Kelly DJ, Wagner DR. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. Eur Cells Mater. 2014;28:358–371. doi: 10.22203/eCM.v028a25. [DOI] [PubMed] [Google Scholar]
- 156.Kwon HJ, Lee GS, Chun H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci Rep. 2016;6:39302. doi: 10.1038/srep39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131(1):142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17(8):958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi H, Halvorsen YD, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genom. 2000;3(2):75–82. doi: 10.1152/physiolgenomics.2000.3.2.75. [DOI] [PubMed] [Google Scholar]
- 160.Jensen B, Farach-Carson MC, Kenaley E, Akanbi KA. High extracellular calcium attenuates adipogenesis in 3T3-L1 preadipocytes. Exp Cell Res. 2004;301(2):280–292. doi: 10.1016/j.yexcr.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 161.Hashimoto R, Katoh Y, Miyamoto Y, Itoh S, Daida H, Nakazato Y, Okada T. Increased extracellular and intracellular Ca(2)(+) lead to adipocyte accumulation in bone marrow stromal cells by different mechanisms. Biochem Biophys Res Commun. 2015;457(4):647–652. doi: 10.1016/j.bbrc.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 162.Hashimoto R, Katoh Y, Nakamura K, Itoh S, Iesaki T, Daida H, Nakazato Y, Okada T. Enhanced accumulation of adipocytes in bone marrow stromal cells in the presence of increased extracellular and intracellular [Ca(2)(+)] Biochem Biophys Res Commun. 2012;423(4):672–678. doi: 10.1016/j.bbrc.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 163.Hashimoto R, Katoh Y, Miyamoto Y, Nakamura K, Itoh S, Daida H, Nakazato Y, Okada T. High extracellular Ca(2 +) enhances the adipocyte accumulation of bone marrow stromal cells through a decrease in cAMP. Cell Calcium. 2017;67:74–80. doi: 10.1016/j.ceca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 164.Zhang F, Ye J, Meng Y, Ai W, Su H, Zheng J, Liu F, Zhu X, Wang L, Gao P, Shu G, Jiang Q, Wang S. Calcium supplementation enhanced adipogenesis and improved glucose homeostasis through activation of Camkii and PI3K/Akt signaling pathway in porcine bone marrow mesenchymal stem cells (pBMSCs) and mice fed high fat diet (HFD) Cellular Physiol Biochem. 2018;51(1):154–172. doi: 10.1159/000495171. [DOI] [PubMed] [Google Scholar]
- 165.Bae YK, Kwon JH, Kim M, Kim GH, Choi SJ, Oh W, Yang YS, Jin HJ, Jeon HB. Intracellular Calcium determines the adipogenic differentiation potential of human umbilical cord blood-derived mesenchymal stem cells via the Wnt5a/beta-Catenin signaling pathway. Stem Cells Int. 2018;2018:6545071. doi: 10.1155/2018/6545071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28(3):564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shao HJ, Ho CC, Lee YT, Chen CS, Wang JH, Young TH. Chondrogenesis of human bone marrow mesenchymal cells by transforming growth factors beta1 through cell shape changes on controlled biomaterials. J Biomed Mater Res, Part A. 2012;100(12):3344–3352. doi: 10.1002/jbm.a.34291. [DOI] [PubMed] [Google Scholar]
- 168.Chang KH, Liao HT, Chen JP. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: in vitro and in vivo studies. Acta Biomater. 2013;9(11):9012–9026. doi: 10.1016/j.actbio.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 169.Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18(6):436–444. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Y, Chen S, Pei M. Biomechanical signals guiding stem cell cartilage engineering: from molecular adaption to tissue functionality. Eur Cells Mater. 2016;31:59–78. doi: 10.22203/eCM.v031a05. [DOI] [PubMed] [Google Scholar]
- 172.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 173.Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, Durselen L, Ignatius A, Breiter R, Rotter N. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med. 2015;9(12):E239–E251. doi: 10.1002/term.1650. [DOI] [PubMed] [Google Scholar]
- 174.Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J Biomed Mater Res, Part A. 2014;102(11):3998–4008. doi: 10.1002/jbm.a.35068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32(16):3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34(34):8581–8588. doi: 10.1016/j.biomaterials.2013.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hwang JH, Byun MR, Kim AR, Kim KM, Cho HJ, Lee YH, Kim J, Jeong MG, Hwang ES, Hong JH. Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS One. 2015;10(8):e0135519. doi: 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ye K, Cao L, Li S, Yu L, Ding J. Interplay of matrix stiffness and cell-cell contact in regulating differentiation of stem cells. ACS Appl Mater Interfaces. 2016;8(34):21903–21913. doi: 10.1021/acsami.5b09746. [DOI] [PubMed] [Google Scholar]
- 179.Hendriks JA, Moroni L, Riesle J, de Wijn JR, van Blitterswijk CA. The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration. Biomaterials. 2013;34(17):4259–4265. doi: 10.1016/j.biomaterials.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 180.Younesi M, Goldberg VM, Akkus O. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration. Acta Biomater. 2016;30:212–221. doi: 10.1016/j.actbio.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Muller WE, Neufurth M, Wang S, Tolba E, Schroder HC, Wang X. Morphogenetically active scaffold for osteochondral repair (polyphosphate/alginate/N, O-carboxymethyl chitosan) Eur Cells Mater. 2016;31:174–190. doi: 10.22203/eCM.v031a12. [DOI] [PubMed] [Google Scholar]
- 182.Kim JS, Choi JS, Cho YW. Cell-free hydrogel system based on a tissue-specific extracellular matrix for in situ adipose tissue regeneration. ACS Appl Mater Interfaces. 2017;9(10):8581–8588. doi: 10.1021/acsami.6b16783. [DOI] [PubMed] [Google Scholar]
- 183.Zhao W, Li X, Liu X, Zhang N, Wen X. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater Sci Eng C. 2014;40:316–323. doi: 10.1016/j.msec.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 184.Shoham N, Girshovitz P, Katzengold R, Shaked NT, Benayahu D, Gefen A. Adipocyte stiffness increases with accumulation of lipid droplets. Biophys J. 2014;106(6):1421–1431. doi: 10.1016/j.bpj.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lim YB, Kang SS, Park TK, Lee YS, Chun JS, Sonn JK. Disruption of actin cytoskeleton induces chondrogenesis of mesenchymal cells by activating protein kinase C-alpha signaling. Biochem Biophys Res Commun. 2000;273(2):609–613. doi: 10.1006/bbrc.2000.2987. [DOI] [PubMed] [Google Scholar]
- 186.Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281(19):13134–13140. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- 187.Campbell JJ, Lee DA, Bader DL. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43(4):455–470. [PubMed] [Google Scholar]
- 188.Kupcsik L, Stoddart MJ, Li Z, Benneker LM, Alini M. Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16(6):1845–1855. doi: 10.1089/ten.tea.2009.0531. [DOI] [PubMed] [Google Scholar]
- 189.Li Z, Kupcsik L, Yao SJ, Alini M, Stoddart MJ. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med. 2010;14(6A):1338–1346. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang T, Wen F, Wu Y, Goh GS, Ge Z, Tan LP, Hui JH, Yang Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials. 2015;38:72–85. doi: 10.1016/j.biomaterials.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 191.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18(7–8):715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hossain MG, Iwata T, Mizusawa N, Shima SW, Okutsu T, Ishimoto K, Yoshimoto K. Compressive force inhibits adipogenesis through COX-2-mediated down-regulation of PPARgamma2 and C/EBPalpha. J Biosci Bioeng. 2010;109(3):297–303. doi: 10.1016/j.jbiosc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 193.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122(Pt 4):546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhong W, Tian K, Zheng X, Li L, Zhang W, Wang S, Qin J. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by yes-associated protein. Stem Cells Dev. 2013;22(14):2083–2093. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- 195.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9(3):R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216(3):708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 197.Khan WS, Adesida AB, Tew SR, Lowe ET, Hardingham TE. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J Orthop Res. 2010;28(6):834–840. doi: 10.1002/jor.21043. [DOI] [PubMed] [Google Scholar]
- 198.Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3(2):9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, Cherel Y, Weiss P, Guicheux J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298(2):C355–C364. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 200.Cicione C, Muinos-Lopez E, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013;2013:232896. doi: 10.1155/2013/232896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2(3):331–341. doi: 10.1016/S1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 202.Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun. 2005;333(4):1178–1184. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 203.Gentil C, Le Jan S, Philippe J, Leibowitch J, Sonigo P, Germain S, Pietri-Rouxel F. Is oxygen a key factor in the lipodystrophy phenotype? Lipids Health Disease. 2006;5:27. doi: 10.1186/1476-511X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Itoigawa Y, Kishimoto KN, Okuno H, Sano H, Kaneko K, Itoi E. Hypoxia induces adipogenic differentitation of myoblastic cell lines. Biochem Biophys Res Commun. 2010;399(4):721–726. doi: 10.1016/j.bbrc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 205.Weiszenstein M, Musutova M, Plihalova A, Westlake K, Elkalaf M, Koc M, Prochazka A, Pala J, Gulati S, Trnka J, Polak J. Adipogenesis, lipogenesis and lipolysis is stimulated by mild but not severe hypoxia in 3T3-L1 cells. Biochem Biophys Res Commun. 2016;478(2):727–732. doi: 10.1016/j.bbrc.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 206.Jiang C, Sun J, Dai Y, Cao P, Zhang L, Peng S, Zhou Y, Li G, Tang J, Xiang J. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res Ther. 2015;6:21. doi: 10.1186/s13287-015-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6(6):745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 208.Valorani MG, Germani A, Otto WR, Harper L, Biddle A, Khoo CP, Lin WR, Hawa MI, Tropel P, Patrizi MP, Pozzilli P, Alison MR. Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res. 2010;341(1):111–120. doi: 10.1007/s00441-010-0982-8. [DOI] [PubMed] [Google Scholar]
- 209.Valorani MG, Montelatici E, Germani A, Biddle A, D’Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45(3):225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ranera B, Remacha AR, Alvarez-Arguedas S, Castiella T, Vazquez FJ, Romero A, Zaragoza P, Martin-Burriel I, Rodellar C. Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet J. 2013;195(2):248–251. doi: 10.1016/j.tvjl.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 211.Duval E, Bauge C, Andriamanalijaona R, Benateau H, Leclercq S, Dutoit S, Poulain L, Galera P, Boumediene K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012;33(26):6042–6051. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 212.Lee HH, Chang CC, Shieh MJ, Wang JP, Chen YT, Young TH, Hung SC. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci Rep. 2013;3:2683. doi: 10.1038/srep02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. The FEBS J. 2009;276(8):2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205(2):194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 215.Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res. 2011;469(10):2744–2753. doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tan Q, Lui PP, Rui YF. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering. Stem Cells Dev. 2012;21(5):790–800. doi: 10.1089/scd.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bertolo A, Mehr M, Janner-Jametti T, Graumann U, Aebli N, Baur M, Ferguson SJ, Stoyanov JV. An in vitro expansion score for tissue-engineering applications with human bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2016;10(2):149–161. doi: 10.1002/term.1734. [DOI] [PubMed] [Google Scholar]
- 218.Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J Orthop Res. 2008;26(7):910–917. doi: 10.1002/jor.20623. [DOI] [PubMed] [Google Scholar]
- 219.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3(6):379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 222.Zhao Y, Waldman SD, Flynn LE. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells, Tissues, Organs. 2012;195(5):414–427. doi: 10.1159/000329254. [DOI] [PubMed] [Google Scholar]
- 223.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 224.Morse D, Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27(1):8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 225.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Rese Therapy. 2013;4(2):28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Park EJ, Koo OJ, Lee BC. Overexpressed human heme Oxygenase-1 decreases adipogenesis in pigs and porcine adipose-derived stem cells. Biochem Biophys Res Commun. 2015;467(4):935–940. doi: 10.1016/j.bbrc.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 227.Hamedi-Asl P, Halabian R, Bahmani P, Mohammadipour M, Mohammadzadeh M, Roushandeh AM, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones. 2012;17(2):181–190. doi: 10.1007/s12192-011-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]