Abstract
Background:
Low CD4+ recovery among HIV-positive individuals who achieve virologic suppression is common but has not been studied among individuals initiating treatment at CD4+ counts > 500 cells/mm3.
Setting:
United States, Africa, Asia, Europe & Israel, Australia, Latin America.
Methods:
Among immediate-ART participants in the Strategic Timing of AntiRetroviral Therapy trial, low CD4+ recovery was defined as a CD4+ increase < 50 cells/mm3 from baseline after 8 months despite viral load ≤ 200 copies/mL. Risk factors for low recovery were investigated with logistic regression.
Results:
39.7% of participants had low CD4+ recovery. Male gender (OR 1.53, p = 0.007), lower screening CD4+ (OR 1.09 per 100 fewer cells/mm3, p = 0.004), higher baseline CD8+ (OR 1.05 per 100 more cells/mm3, p < 0.001), and lower HIV RNA (OR 1.93 per log10 decrease, p < 0.001) were associated with low CD4+ recovery. D-dimer had a quadratic association with low CD4+ recovery, with lowest odds occurring at 0.32 μg/mL. At lower HIV RNA levels, odds of low recovery were elevated across levels of screening CD4+ count, but at higher levels, odds of low CD4+ recovery were greater among those with lower versus higher screening CD4+.
Conclusion:
Low CD4+ recovery is frequent among participants starting ART at high CD4+ counts. Risk factors include male gender, lower screening CD4+ cell counts, higher CD8+ cell counts, and lower HIV RNA levels. More follow-up is required to determine the impact of low CD4+ recovery on clinical outcomes.
Keywords: Antiretroviral therapy, CD4, HIV, immune response
Introduction
In the majority of HIV-positive patients treated with antiretroviral therapy (ART), viral suppression is achieved and CD4+ counts recover to > 500 cells/mm3.1–7 The percentage of patients with a low CD4+ count recovery varies between 15% and 30% depending on the definition used and on the time period since the start of ART8–10. Among patients who initiate ART at low CD4+ counts, low CD4+ recovery after initiation of ART is associated with increased risk for AIDS, serious non-AIDS diseases, and death8,11–15. Several risk factors for failure to show a substantial increase in CD4+ counts have been well-characterized among patients who initiate ART at CD4+ levels < 500 cells/mm3. These risk factors include older age, lower HIV RNA level, hepatitis C co-infection, active hepatitis B co-infection, longer duration of HIV infection, and lower nadir CD4+ count8–11,16–19. Genetic factors have also been cited as possible links to low CD4+ recovery14,19–23. To our knowledge, the determinants of low CD4+ recovery following virologic suppression have not been studied specifically among patients who initiate ART at CD4+ counts > 500 cells/mm3.
The goal of this investigation is to estimate the prevalence of low CD4+ count recovery despite virologic suppression after 8 months of ART among participants with HIV who initiate ART at a CD4+ count > 500 cells/mm3, and to determine predictors of low recovery, including pre-ART CD4+ cell count. This will be accomplished using participants randomized to the immediate ART treatment group in the Strategic Timing of Antiretroviral Treatment (START) trial13. Since the number of clinical events in the immediate ART initiation group of the START was very low, we did not have the power to evaluate the clinical implication of low CD4 recovery.
Methods
Design.
The START trial was approved by the institutional review board or ethics committee at each participating site, and written informed consent was obtained from all study participants. The study design and baseline characteristics of participants in START have been reported previously13,24,25. In START, HIV-positive, ART-naïve participants with CD4+ counts > 500 cells/mm3 were randomized to immediate initiation of ART (n = 2,325) or to deferred initiation until the CD4+ count declined to 350 cells/mm3 or AIDS developed (n = 2,359). ART regimens were selected by participants and their providers from a list of approved drug combinations derived from guidelines of the Department of Health and Human Services26. Participants were required to have two CD4+ counts > 500 cells/mm3 at least 2 weeks apart within 60 days before randomization. We refer to the first of these CD4+ counts as the screening CD4+ and the second as the baseline CD4+ count. In addition to CD4+ cell count, CD8+ cell count, and HIV RNA level were measured locally prior to randomization and at 1 month, 4 months, and every 4 months of follow-up thereafter. Similar to Baker et al.11 and Florence et al.16, low CD4+ recovery following ART initiation was defined as CD4+ increase < 50 cells/mm3 8 months after randomization despite HIV RNA ≤ 200 copies/mL at 8 months; high recovery was defined as CD4+ increase ≥ 50 cells/mm3 among those with an HIV RNA ≤ 200 copies/mL. Those who do not achieve large gains in CD4+ count following the initiation of ART have also been referred to as immunologic non-responders.
Statistical Analysis.
If change is measured from baseline, the relationship of change in CD4+ count with baseline CD4+ count is influenced by measurement error and within-participant variability (i.e., regression to the mean)27,28. To reduce the effect of regression to the mean for studying the association of CD4+ count change (value at 8 months minus baseline value) with pre-ART CD4+ count, we used two methods. The first approach used the screening CD4+ count (the 1st of the two pre-ART counts), which was not used to calculate CD4+ count change, as the predictor of change in CD4+ count and of low CD4+ recovery. Based on work by Ederer,29 we assumed that if the correlation between baseline CD4+ count and 8 month CD4+ count was similar to the correlation between screening CD4+ count and 8 month CD4+ count, the effect of regression to the mean could be largely eliminated with this approach. The bias in estimating an association between an initial value and a change from that initial value arises in part due to mathematical coupling: the initial value is both the predictor and is part of the derived response, and this can create bias in the estimated association. This can be partially avoided by using as a predictor a second “initial” value provided that it was obtained in close temporal proximity to the initial value from which change is measured. The second approach used a method proposed by Blomqvist27,30 to estimate the association between “true” or “usual” pre-ART CD4+ cell count levels (with correction for measurement error and short-term intra-person variability) and change in CD4+ count. The second approach has the advantage of not requiring two measurements of pre-ART CD4+ counts for assessing change. However, it does require an estimate of the reliability coefficient (ratio of between person to total variability). In this investigation, the reliability coefficient was estimated by the correlation of the screening and baseline CD4+ counts.
Linear mixed effects models31 were used to analyze CD4+ differences between groups over follow-up. Risk factors for low CD4+ count recovery were studied with logistic regression; odds ratios (ORs) and 95% confidence intervals (CIs) are cited. Because odds ratios are not good approximations of the relative risk when the outcome is common, relative risks from log-binomial and Poisson regression models are also cited. Univariable (i.e., unadjusted) and multivariable (adjusted) analyses were carried out. As potential baseline (pre-ART) risk factors for low recovery, we considered age, gender, race (Asian, black, Hispanic, white/other), geographic location, treatment regimen, body mass index (BMI, kg/m2), co-infection with hepatitis C, co-infection with hepatitis B, screening CD4+ count, baseline CD8+ count, HIV RNA level (copies/mL), baseline interleukin-6 (IL-6, pg/mL), baseline D-dimer (μg/mL), and duration of time since HIV diagnosis. Geographic region was categorized as United States, Africa, Asia, Europe & Israel, Australia, or Latin America. Treatment regimen was categorized as non-nucleoside reverse transcriptase inhibitor (NNRTI) + 2 nucleoside reverse transcriptase inhibitors (NRTIs), protease inhibitor (PI) + 2 NRTIs, integrase inhibitor + 2 NRTIs, or other. The great majority of participants were prescribed tenofovir and emtricitabine as the 2 NRTIs. Thus, further categorization of this class of drugs was not carried out. (See supplemental table 2 in reference 13 for further details of the treatment regimens used in START.) The adjusted analysis included all these variables. For variables with associations that changed between the univariable and multivariable models, we explored interactions among variables as possible explanations of the changes. For continuous predictors, we checked linearity against the logit function using empirical logit plots: for each continuous predictor, observations were grouped based on deciles of the predictor, and observed logits of probability of low CD4+ recovery were plotted against the mean values of the predictor within each decile. All analyses were done with SAS statistical software, versions 9.4 (SAS Institute, Cary, NC, USA).
Results
Study Cohort
Of the 2,325 participants randomized to the immediate ART group of START, 2,063 started ART within 30 days of randomization; of these, 1,983 had a CD4+ count and HIV RNA level at the month 8 visit, and of these, 1,884 (95%) had an HIV RNA ≤ 200 copies/ml at month 8. These 1,884 patients comprise the cohort for this study.
Overall, for these 1,884 participants, the mean (standard deviation) screening, baseline and 8-month CD4+ cell counts were 709 (191), 694 (193) and 806 (261) cell/mm3, respectively. The median (25th, 75th percentile) number of days between screening and baseline CD4+ counts was 21 (16, 33) days. Correlations between screening and 8-month CD4+ count and between baseline CD4+ count and 8 month CD4+ count were 0.51, and 0.50 respectively.
Prevalence of Low CD4+ Count Recovery and Change in CD4+ Count Over Follow-up for those with Low versus High Recovery
Among the 1,884 participants in the immediate ART group who had an HIV RNA ≤ 200 copies/ml 8 months after initiating ART, 748 (39.7%; 95% CI: 37.5% to 42.0%) had low CD4+ recovery. The average change in CD4+ count from baseline to 8 months was −93 cells/mm3 (95% CI: −102 to −84) in low responders vs. +247 cells/mm3 (95% CI: 236 to 258) in high responders. Following an initial increase in CD4+ cell count for low responders and a decrease in CD4+ count for high responders between month 8 and month 12, which we attribute to regression to the mean, over 12 – 60 months of follow-up (median follow-up = 3.0 years, 25th and 75th percentiles are 2.3 and 4.1 years), the mean CD4+ count was 228 cells/mm3 higher in high responders than low responders (95% CI: 207 to 249) controlling for baseline CD4+, and CD4+ counts increased more rapidly among high responders with an average increase of 3.3 cells/mm3/month (95% CI: 2.9 to 3.6) vs 2.2 cells/mm3/month in low responders (95% CI: 1.8 to 2.7) (Figure 1). Thirty-six months after initiating ART, only 17% of low responders had CD4+ counts over 1000 cells/mm3, vs. 37% of high responders (p < 0.0001 for difference).
Figure 1.
Mean CD4+ counts (cells/mm3 ± standard error of the man) during follow-up for participants with high recovery (CD4+ increase ≥ 50 cells/mm3 8 months after initiating ART) vs. low recovery (CD4+ increase < 50 cells/mm3 8 months after initiating ART). All participants had HIV RNA ≤ 200 copies/ml at month 8.
Association of Pre-ART Screening and Baseline CD4+ Count with Change in CD4+ Count at 8 Months
Table 1 gives estimates of slopes for the regression of change in CD4+ cell count from baseline to 8 months on screening and baseline CD4+ cell count levels. The slope is positive and not significant for screening CD4+ cell count indicating for each 100 cell higher screening CD4+ count, the change in CD4+ count at 8 months was +2.1 cells/mm3 greater. In contrast, as a result of regression to the mean, for baseline CD4+ cell count, the slope is negatively biased at −32.5 cells/mm3 (95% CI: −37.8, −27.2; p < 0.001). The slope estimated with Blomqvist’s method, which adjusts the baseline CD4+ count for measurement error, is similar to the slope for the screening CD4+ cell count: +1.7 cells/mm3 (95% CI: −11.6, +16.9; p = 0.82) for a 100 cell higher baseline CD4+ count.
Table 1.
Change in CD4+ Cell Count from Baseline to 8 Months Per 100 cells/mm3 higher in Screening and Baseline CD4+ Count Levels
| Predictor | Estimate | S.E. | 95% CI | p-value |
|---|---|---|---|---|
| Screening CD4+ (cells/mm3) | 2.1 | 2.8 | (−3.4, 7.7) | 0.45 |
| Baseline CD4+ (cells/mm3) | −32.5 | 2.7 | (−37.8, −27.2) | <.001 |
| Baseline CD4+(Blomqvist Estimator) | 1.7 | 7.3 | (−11.6, 16.9) | 0.82 |
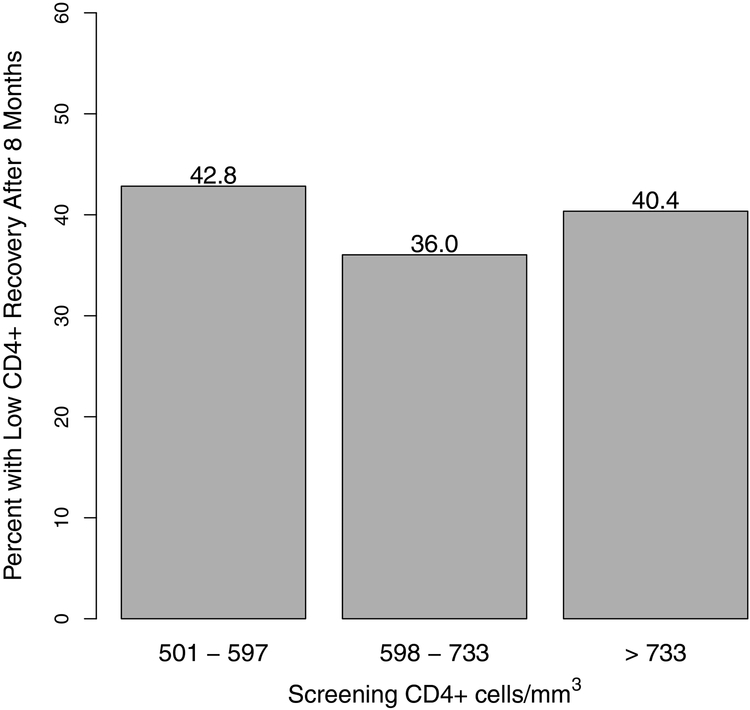
Similar to the results shown in Table 1, the univariate association between the percent with a low CD4+ response at 8 months and screening CD4+ cell count shows no clear relationship (p = 0.85 for trend) (Figure 2).
Figure 2.
Percent of participants with low CD4+ recovery after 8 months by tertiles of screening CD4+ (1st of 2 pre-ART CD4+ values).
Univariable and Multivariable Predictors of Low CD4+ Count Recovery
Supplementary Table 1 gives baseline characteristics for those with low and high recovery. Table 2 shows the results for univariable and multivariable logistic regression models for baseline predictors of low CD4+ recovery. In multivariable analyses, males had significantly greater odds of low CD4+ recovery than females (odds ratio [OR] 1.53, p = 0.007), but the association was not significant in univariable analysis (OR 0.93, p = 0.47). The striking difference in the effect of gender between univariable and multivariable models is explained by the confounding effect of HIV RNA – itself a strong predictor of CD4+ recovery - between females and males: the median (25th, 75th percentile) of log10 viral load was 3.81 (3.05, 4.36) for females and 4.26 (3.69, 4.70) for males.
Table 2.
Univariable and Multivariable Odds Ratios for Low CD4 Recovery.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Risk Factor | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, OR per 10 years higher | 1.06 (0.97 – 1.16) | 0.23 | 1.10 (0.98 – 1.24) | 0.11 |
| Female | 1.00 (ref.) | 1.00 (ref.) | ||
| Male | 0.93 (0.75 – 1.14) | 0.47 | 1.53 (1.12 – 2.10) | 0.007 |
| Race | ||||
| White/Other | 1.00 (ref.) | 1.00 (ref.) | ||
| Asian | 1.28 (0.89 – 1.83) | 0.18 | 1.12 (0.33 – 3.78) | 0.86 |
| Black | 1.51 (1.22 – 1.87) | <.001 | 1.08 (0.73 – 1.59) | 0.70 |
| Hispanic | 1.17 (0.88 – 1.55) | 0.27 | 1.28 (0.90 – 1.81) | 0.17 |
| Geographic Location | ||||
| United States | 1.00 (ref.) | 1.00 (ref.) | ||
| Africa | 1.70 (1.19 – 2.44) | 0.004 | 1.62 (1.01 – 2.58) | 0.043 |
| Asia | 1.26 (0.79 – 2.01) | 0.33 | 1.27 (0.34 – 4.81) | 0.72 |
| Europe & Israel | 1.02 (0.72 – 1.44) | 0.92 | 1.03 (0.68 – 1.58) | 0.88 |
| Australia | 1.09 (0.57 – 2.07) | 0.80 | 1.09 (0.52 – 2.27) | 0.82 |
| Latin America | 1.17 (0.82 – 1.67) | 0.38 | 1.15 (0.75 – 1.76) | 0.52 |
| Treatment Regimen | ||||
| NNRTI + 2 NRTIs | 1.00 (ref.) | 1.00 (ref.) | ||
| Protease Inhibitor + 2 NRTIs | 0.67 (0.52 – 0.86) | 0.002 | 0.78 (0.59 – 1.04) | 0.09 |
| Integrase Inhibitor + 2 NRTIs | 0.72 (0.44 – 1.17) | 0.18 | 0.90 (0.52 – 1.56) | 0.70 |
| Other | 2.80 (0.25 – 30.99) | 0.40 | 1.52 (0.09 – 25.75) | 0.77 |
| Body Mass Index, OR per kg/m2 higher | 0.99 (0.98 – 1.01) | 0.55 | 0.98 (0.96 – 1.00) | 0.08 |
| Hepatitis C Co-infection | 1.06 (0.64 – 1.75) | 0.84 | 0.99 (0.55 – 1.76) | 0.96 |
| Hepatitis B Co-infection | 1.60 (0.95 – 2.72) | 0.08 | 1.45 (0.79 – 2.67) | 0.24 |
| Screening CD4+, OR per 100 fewer cells/mm3 | 1.00 (0.95 – 1.04) | 0.85 | 1.09 (1.03 – 1.15) | 0.004 |
| Baseline CD8+, OR per 100 more cells/mm3 | 1.03 (1.02 – 1.05) | <.001 | 1.05 (1.03 – 1.07) | <.001 |
| Baseline HIV RNA copies/mL, OR per log10 lower | 1.68 (1.50 – 1.87) | <.001 | 1.93 (1.68 – 2.22) | <.001 |
| Baseline IL-6 pg/mL, OR per log2 higher | 1.01 (0.91 – 1.12) | 0.89 | 1.07 (0.95 – 1.20) | 0.28 |
| †Baseline log2 D-dimer μg/mL | 1.25 (1.03 – 1.52) | 0.021 | 1.27 (1.03 – 1.57) | 0.028 |
| †Baseline (log2 D-dimer μg/mL)2 | 1.09 (1.02 – 1.17) | 0.013 | 1.11 (1.03 – 1.19) | 0.006 |
| Time since HIV diagnosis, OR per 1 year higher | 1.04 (1.01 – 1.07) | 0.011 | 1.03 (0.99 – 1.06) | 0.15 |
Low Recovery: Initial CD4 Recovery ≤ 50 cells/mm3 8 months after initiating ART
High Recovery: Initial CD4 Recovery > 50 cells/mm3 8 months after initiating ART
OR: odds ratio; 95% CI: 95% confidence interval.
Considering both the linear and quadratic regression coefficients in the adjusted model, the OR associated with a doubling of D-dimer from 0.323 (median) to 0.646 is 1.003. In the model with adjustment for other covariates and only a linear term for D-dimer, this OR was 1.01
In univariable analyses, black participants had significantly higher odds of low CD4+ recovery than white/other participants (OR 1.51, p < 0.001), and Africans had higher odds of low recovery than participants in the United States (OR 1.70, p = 0.004). In the multivariable model, black race was no longer significant; the association remained significant when African participants were compared with participants in the United States (OR 1.62, p = 0.043). The univariable OR for black vs. white/other was attenuated in the multivariable model because the model included both race and geographic region. In a multivariable model excluding geographic region but including all other variables (results not shown), the OR for black vs. white/other was 1.34 (95% CI: 1.00 – 1.78, p = 0.047).
In univariable analysis, participants treated with a PI + 2 NRTIs had lower odds of low CD4+ recovery compared to participants treated with NNRTI + 2 NRTIs (OR 0.67, p = 0.002), but the effect was not significant in the multivariable model. The reduced association in the multivariable analysis is largely due to the adjustment for geographic region. Participants in the three geographic areas with the lowest odds of low recovery (United States, Europe & Israel, and Australia) were significantly more likely to be treated with a PI + 2 NRTIs ( = 222.5, p < 0.001 for test of homogeneity). Aside from race and treatment regimen, odds ratios for other covariates considered were not materially modified with the exclusion of geographic region from the multivariable model.
Higher baseline CD8+ was associated with increased odds of low CD4+ recovery in both univariable analysis (OR 1.03 per 100 more cells/mm3, p < 0.001) and multivariable analysis (OR 1.05 per 100 more cells/mm3, p < 0.001). Lower baseline HIV RNA level was associated with increased odds of low recovery in both univariable analysis (OR 1.68, p < 0.001) and in multivariable analysis (OR 1.93, p < 0.001). Time since HIV diagnosis was weakly associated with odds of low CD4+ recovery in univariable analysis (OR 1.04 per 1-year longer, p = 0.011) but not in multivariable analysis (OR 1.03, p = 0.15).
As previously described, screening CD4+ cell count was not associated with CD4+ change at 8 months. Similarly, in univariable analysis screening CD4+ was not associated with odds of low recovery (p = 0.85). However, in the multivariable model, lower screening CD4+ was associated with increased odds of low recovery (OR 1.09 per 100 fewer cells/mm3, p = 0.004). This was explored further and a significant interaction between screening CD4+ cell count and baseline HIV RNA level was found (p = 0.005 for interaction term from logistic model when both screening CD4+ and baseline log10 HIV RNA were continuous predictors for low recovery). Figure 3 illustrates the interaction. Among those with lower baseline HIV RNA levels, the association between screening CD4+ count and low CD4+ recovery at 8 months was weaker (red bars). Similarly, we checked for an interaction between baseline CD8+ cell count and baseline HIV RNA levels (Supplemental Figure 1), but the effect of the interaction was not significant (p = 0.62).
Figure 3.
Percent of participants with low CD4+ recovery after 8 months by tertiles of screening CD4+ (1st of 2 pre-ART CD4+ values) and baseline HIV RNA copies/mL. Samples sizes are indicated for each subgroup.
In univariable and multivariable analysis, we included a quadratic term for D-dimer, because empirical logit plots suggested a possible quadratic association between D-dimer and odds of low CD4+ recovery. Both analyses indicated a quadratic effect of D-dimer. From the univariable analysis, the estimated odds of low CD4+ recovery were lowest for D-dimer of 0.27 μg/mL (median D-dimer was 0.32 μg/mL); the odds of low CD4+ recovery for the 25th percentile (0.22 μg/mL) and 75th percentile (0.50 μg/mL) of D-dimer versus 0.27 μg/mL were 6.7% and 0.7% higher, respectively. From the multivariable analysis, the estimated odds of low CD4+ recovery were lowest for D-dimer of 0.32 μg/mL; the odds of low CD4+ recovery for the 25th percentile and 75th percentile of D-dimer versus 0.32 μg/mL were 11.5%% and 0.2% higher, respectively, assuming fixed values of all other variables.
The empirical logit plots also suggested a possible quadratic effect of screening CD4+ (this is also suggested by Figure 2); a quadratic effect was found in univariable analysis (data not shown), but the association was no longer present after considering the interaction between screening CD4+ and viral load.
Estimated relative risks for comparison with odds ratios are given in Supplementary Table 2. Relative risks are lower than the odds ratios, but risk factors for low recovery identified from these analyses are similar.
Conclusion
To our knowledge, this is the first study investigating the prevalence and risk factors for CD4+ recovery specifically among individuals who initiate ART at CD4+ counts > 500 cells/mm3. We showed that low CD4+ recovery after starting ART at CD4+ counts > 500 cells/mm3 is frequent despite virologic suppression, and the lower CD4+ cell counts following ART initiation for those with low recovery compared to those with high recovery at 8 months persisted through 5 years of follow-up. Similar to other studies, we found that male gender32,33, lower screening CD4+ cell count16,33,34 and lower baseline HIV RNA11,16,35 increased the odds of low CD4+ recovery. We also found that race and/or geographic region may be an important predictor of poor CD4 recovery and could inform studies to identify potential genetic or environmental etiologies. The association with screening CD4+ cell count was not large – for a 100 cell lower screening count the odds of low response was increased by 9%. In addition, we found that the association with pre-ART screening CD4+ count depends on HIV RNA level. Associations of low CD4+ response with screening CD4+ counts were weaker among those with low HIV RNA levels. This interaction and lack of recognition of the impact of measurement error and within-person variability on studies of the association between change and initial value (see below) may explain differences among studies of low responders, some which identified low baseline or nadir CD4+ count with low CD4+ recovery and some which did not.
While perhaps counter-intuitive, the finding that lower baseline HIV RNA increased the odds of low CD4+ recovery has been reported previously33. This effect may be due to prior innate suppression of HIV replication, such that lack of CD4 decline may have already been achieved, blunting any additional increase. This is in line with the findings that HLA-Bw4 is associated with lower HIV RNA during natural history and blunted CD4 response on cART19. One other possible explanation is that patients with lower HIV RNA levels at entry in START had much longer duration of untreated HIV infection when compared to those with higher HIV RNA levels. A widely held hypothesis is that HIV associated damage from immune activation within lymphatic tissues leads to fibrosis that impairs T-cell homeostasis and immune recovery. Longer duration of untreated HIV infection may worsen the process of LN fibrosis and, in part, contribute to reduced CD4 recovery during ART treatment36,37.
In contrast to most studies reporting age as risk factor for low CD4+ recovery11,16,33–35,38,39, possibly due to thymic senescence, we did not find a strong association between older age and low CD4+ recovery. This may be because the great majority of participants in START were < 45 years of age.
Previous studies have not investigated inflammatory and coagulation markers such as IL-6 and D-dimer. We did not observe an association of IL-6 with low CD4+ count recovery; however, we found a curvilinear association of D-dimer with low recovery. We have previously reported that both markers are associated with HIV viral load at baselines40. With adjustment for HIV viral load and other factors this association persisted.
In linear regression analysis, we demonstrated that screening and baseline CD4+ counts had different relationships with CD4+ change from baseline to 8 months. The inverse relationship with baseline CD4+ cell count can be explained by regression to the mean. When this association was corrected for measurement error and temporal variability27,30, the slope estimate was nearly identical with the slope for screening CD4+ count. This finding may prove useful in other investigations that do not have the luxury of multiple readings for assessing the relationship of change with initial value.
In addition to having two pre-ART CD4+ cell counts to consider as predictors of low CD4+ response, other strengths of our study are the large sample size and the geographic and demographic diversity of the cohort. A limitation of our study is that we do not have sufficient follow-up data to investigate the clinical implications of low recovery due to the small number of events in the immediate ART initiation group of the START trial13. Longer follow-up is required. One might presume that low CD4+ recovery among those who initiate ART at counts > 500 cells does not carry the same risk of morbidity and mortality as those who have low recovery after initiating ART at lower counts. Indeed, CD4+ counts below 500 are not uncommon in HIV-negative individuals41. Furthermore, we do not know the pre-HIV-infection CD4+ values, and hence the extent that “return” to health as a reason for lack of relatively poorer improvement in CD4+ cell count is not possible to determine. This issue has been less of an issue in prior studies assessing impact from ART in patients with lower nadir CD4+ counts, where it is more likely that there may have been an impact from the HIV infection on the CD4+ count. Based on this, it is noteworthy that screening CD4+ count did not predict low CD4+ count recovery for those with lowest HIV RNA levels. This suggests that there may be a subgroup for whom HIV does not impact CD4+ count and who remained at their pre-HIV set-point when ART was initiated. Conversely, the reduced odds of low recovery for higher screening CD4+ cell count in those with higher HIV RNA levels is not readily explainable this way. It is possible these participants may have levels below their pre-HIV value, and their reduced odds of low CD4+ recovery would reflect variation in irrecoverable harm within the population from HIV. Overall, our results are supportive of this interpretation since a greater percentage of those with lower screening CD4+ cell counts (and hence more injury from HIV) had low CD4+ recovery. An alternative explanation is that the results Figure 3 merely reflect variation in the populations selected. It is also possible that factors not yet identified are associated with low recovery and risk of disease. These include host genetic factors9, and these in turn could influence risk of clinical outcomes. Future host genetic analyses may elucidate this issue.
In summary, low CD4+ recovery among HIV-positive patients is frequent even among those who initiate ART at CD4+ levels > 500 cells/mm3. The large number of participants with low recovery with early ART initiation suggests that there is much we do not know about the determinants of low recovery. The risk of morbidity and mortality among those who initiate ART at higher CD4+ counts and have low CD4+ recovery remains to be determined.
Supplementary Material
Supplementary Figure 1. Percent of participants with low CD4+ recovery after 8 months by tertiles of baseline CD8+ and baseline RNA copies/mL. Samples sizes are indicated for each subgroup.
Acknowledgements
We thank all the patients who participated in the START study. We are also grateful to all the START investigators13 and to the two anonymous reviewers whose comments substantially improved this research
Conflicts of Interest and Source of Funding
Funding provided by National Institutes of Health Grants UM1-AI068641 and UM1-AI120197. Supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Clinical Center, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundes ministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and University of Minnesota. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
References
- 1.Moore RD, Keruly JC. CD4+ Cell Count 6 Years after Commencement of Highly Active Antiretroviral Therapy in Persons with Sustained Virologic Suppression. Clin Infect Dis. 2007;44(3):441–446. doi: 10.1086/510746 [DOI] [PubMed] [Google Scholar]
- 2.Lok JJ, Bosch RJ, Benson CA, et al. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS. 2010;24(12):1867–1876. doi: 10.1097/QAD.0b013e32833adbcf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouteloup V, Sabin C, Mocroft A, et al. Reference curves for CD4 T-cell count response to combination antiretroviral therapy in HIV-1-infected treatment-naïve patients. HIV Med. 2017;18(1):33–44. doi: 10.1111/hiv.12389 [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Phillips A, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370(9585):407–413. doi: 10.1016/S0140-6736(07)60948-9 [DOI] [PubMed] [Google Scholar]
- 5.Costagliola D, Lacombe J-M, Ghosn J, et al. CD4+ cell count recovery in naïve patients initiating cART, who achieved and maintained plasma HIV-RNA suppression. J Int AIDS Soc Vol 17, No 4 Suppl 3 2014. http://www.jiasociety.org/index.php/jias/article/view/19481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-Cell Recovery with Earlier HIV-1 Antiretroviral Therapy. N Engl J Med. 2013;368(3):218–230. doi: 10.1056/NEJMoa1110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1–infected individuals. JAMA Intern Med. 2015;175(1):88–99. 10.1001/jamainternmed.2014.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzola L, Tincati C, Bellistré GM, d’Arminio Monforte A, Marchetti G. The Absence of CD4+ T Cell Count Recovery Despite Receipt of Virologically Suppressive Highly Active Antiretroviral Therapy: Clinical Risk, Immunological Gaps, and Therapeutic Options. Clin Infect Dis. 2009;48(3):328–337. doi: 10.1086/695852 [DOI] [PubMed] [Google Scholar]
- 9.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117(21):5582 LP–5590. http://www.bloodjournal.org/content/117/21/5582.abstract. [DOI] [PubMed] [Google Scholar]
- 10.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete Immune Recovery in HIV Infection: Mechanisms, Relevance for Clinical Care, and Possible Solutions. Clin Dev Immunol. 2012;2012:670957. doi: 10.1155/2012/670957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker JV, Peng G, Rapkin J, et al. Poor Initial CD4+ Recovery With Antiretroviral Therapy Prolongs Immune Depletion and Increases Risk for AIDS and Non-AIDS Diseases. J Acquir Immune Defic Syndr. 2008;48(5):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takuva S, Maskew M, Brennan AT, Long L, Sanne I, Fox MP. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Soc Vol 17 2014. http://www.jiasociety.org/index.php/jias/article/view/18651/3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helleberg M, Kronborg G, Larsen CS, et al. Poor CD4 response despite viral suppression is associated with increased non-AIDS-related mortality among HIV patients and their parents. AIDS. 2013;27(6). http://journals.lww.com/aidsonline/Fulltext/2013/03270/Poor_CD4_response_despite_viral_suppression_is.19.aspx. [DOI] [PubMed] [Google Scholar]
- 15.Zoufaly A, Cozzi-Lepri A, Reekie J, et al. Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One. 2014;9(1). doi: 10.1371/journal.pone.0087160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–262. [DOI] [PubMed] [Google Scholar]
- 17.Grabar S, Moing V, Goujard C, Al E. CLinical outcome of patients with hiv-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133(6):401–410. 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 18.Khanna N, Opravil M, Furrer H, et al. CD4 + T Cell Count Recovery in HIV Type 1–Infected Patients Is Independent of Class of Antiretroviral Therapy. Clin Infect Dis. 2008;47(8):1093–1101. doi: 10.1086/592113 [DOI] [PubMed] [Google Scholar]
- 19.Rauch A, Nolan D, Furrer H, et al. HLA-Bw4 Homozygosity Is Associated with an Impaired CD4 T Cell Recovery after Initiation of Antiretroviral Therapy. Clin Infect Dis. 2008;46(12):1921–1925. 10.1086/588479. [DOI] [PubMed] [Google Scholar]
- 20.Haas DW, Geraghty DE, Andersen J, et al. Immunogenetics of CD4 Lymphocyte Count Recovery during Antiretroviral Therapy: An AIDS Clinical Trials Group Study. J Infect Dis. 2006;194(8):1098–1107. 10.1086/507313. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez S, Rosenow AA, James IR, et al. Recovery of CD4+ T Cells in HIV Patients With a Stable Virologic Response to Antiretroviral Therapy Is Associated With Polymorphisms of Interleukin-6 and Central Major Histocompatibility Complex Genes. JAIDS J Acquir Immune Defic Syndr. 2006;41(1). http://journals.lww.com/jaids/Fulltext/2006/01010/Recovery_of_CD4__T_Cells_in_HIV_Patients_With_a.1.aspx. [DOI] [PubMed] [Google Scholar]
- 22.Rajasuriar R, Booth D, Solomon A, et al. Biological Determinants of Immune Reconstitution in HIV‐Infected Patients Receiving Antiretroviral Therapy: The Role of Interleukin 7 and Interleukin 7 Receptor α and Microbial Translocation. J Infect Dis. 2010;202(8):1254–1264. doi: 10.1086/656369 [DOI] [PubMed] [Google Scholar]
- 23.Hartling HJ, Thørner LW, Erikstrup C, et al. Polymorphism in interleukin-7 receptor α gene is associated with faster CD4+ T-cell recovery after initiation of combination antiretroviral therapy. Aids. 2014;28(12):1739–1748. doi: 10.1097/QAD.0000000000000354 [DOI] [PubMed] [Google Scholar]
- 24.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10:S5–S36. doi: 10.1177/1740774512440342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S, Babiker AG, Emery S, et al. Demographic and HIV-specific characteristics of participants enrolled in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl. 1):30–36. doi: 10.1111/hiv.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. [Google Scholar]
- 27.Svärdsudd K, Blomqvist N. A New Method for Investigating the Relation between Change and Initial Value in Longitudinal Blood Pressure Data: I. Description and Application of the Method. Scand J Soc Med. 1978;6(2):85–95. doi: 10.1177/140349487800600207 [DOI] [PubMed] [Google Scholar]
- 28.Hayes RJ. Methods for assessing whether change depends on initial value. Stat Med. 1988;7(9):915–927. doi: 10.1002/sim.4780070903 [DOI] [PubMed] [Google Scholar]
- 29.Ederer F Serum cholesterol changes: Effects of diet and regression toward the mean. J Chronic Dis. 1972;25(5):277–289. doi: 10.1016/0021-9681(72)90164-6 [DOI] [PubMed] [Google Scholar]
- 30.Blomqvist N On the Relation Between Change and Initial Value. J Am Stat Assoc. 1977;72(360):746–749. doi: 10.2307/2286454 [DOI] [Google Scholar]
- 31.Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38(4):963–974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 32.Gandhi RT, Spritzler J, Chan E, et al. Effect of Baseline- and Treatment-Related Factors on Immunologic Recovery After Initiation of Antiretroviral Therapy in HIV-1-Positive Subjects: Results From ACTG 384. J Acquir Immune Defic Syndr. 2006;42(4):426–434. http://journals.lww.com/jaids/Fulltext/2006/08010/Effect_of_Baseline__and_Treatment_Related_Factors.6.aspx. [DOI] [PubMed] [Google Scholar]
- 33.Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17(13). http://journals.lww.com/aidsonline/Fulltext/2003/09050/Continued_CD4_cell_count_increases_in_HIV_infected.9.aspx. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 2002;16(3). http://journals.lww.com/aidsonline/Fulltext/2002/02150/The_extent_of_HIV_1_related_immunodeficiency_and.7.aspx. [DOI] [PubMed] [Google Scholar]
- 35.Asmelash A, Zheng Y, Kaloustian KW, et al. Predictors of suboptimal CD4 response among women achieving virologic suppression in a randomized antiretroviral treatment trial, Africa. BMC Infect Dis. 2014;14(1):1–7. doi: 10.1186/1471-2334-14-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estes J, Baker JV, Brenchley JM, et al. Collagen Deposition Limits Immune Reconstitution in the Gut. J Infect Dis. 2008;198(4):456–464. doi: 10.1086/590112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schacker T The role of secondary lymphatic tissue in immune deficiency of HIV infection. Aids. 2008;22(SUPPL. 3):13–18. doi: 10.1097/01.aids.0000327511.76126.b5 [DOI] [PubMed] [Google Scholar]
- 38.Engsig FN, Zangerle R, Katsarou O, et al. Long-term Mortality in HIV-Positive Individuals Virally Suppressed for >3 Years With Incomplete CD4 Recovery. Clin Infect Dis. 2014;58(9):1312–1321. doi: 10.1093/cid/ciu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engsig FN, Gerstoft J, Kronborg G, et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: A cohort study. BMC Infect Dis. 2010;10(1):318. doi: 10.1186/1471-2334-10-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JV, Sharma S, Grund B, et al. Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect Dis. 2017;4(1):1–9. doi: 10.1093/ofid/ofx262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maini MK, Gilson RJ, Chavda N, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med. 1996;72(1):27–31. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1195587/. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Percent of participants with low CD4+ recovery after 8 months by tertiles of baseline CD8+ and baseline RNA copies/mL. Samples sizes are indicated for each subgroup.