Abstract
Objective:
Sex differences in the brain are traditionally treated as binary. We present new evidence that a continuous measure of sex differentiation of the brain can explain sex differences in psychopathology. The degree of sex differentiated brain features (ie, features that are more common in one sex) may predispose individuals toward sex-biased psychopathology and may also be influenced by the genome. We hypothesized that individuals with a female-biased differentiation score would have greater female-biased psychopathology (internalizing symptoms, such as anxiety and depression), whereas individuals with a male-biased differentiation score would have greater male-biased psychopathology (externalizing symptoms, such as disruptive behaviors).
Method:
Using the Philadelphia Neurodevelopmental Cohort database acquired from database of Genotypes and Phenotypes, we calculated the sex differentiation measure, a continuous data-driven calculation of each individual’s degree of sex differentiating features extracted from multimodal brain imaging data (Magnetic resonance imaging (MRI)/Diffusion MRI) from the imaged participants (n=866, 407F/459M).
Results:
In males, higher differentiation scores were correlated with higher levels of externalizing symptoms (r=0.119, p=0.016). The differentiation measure reached genome-wide association study significance (p<5*10−8) in males with single nucleotide polymorphisms Chromsome5:rs111161632:RASGEF1C and Chromosome19:rs75918199:GEMIN7, and in females with Chromosome2:rs78372132:PARD3B and Chromosome15:rs73442006:HCN4.
Conclusion:
The sex differentiation measure provides an initial topography of quantifying male and female brain features. This demonstration that the sex of the human brain can be conceptualized on a continuum has implications for both the presentation of psychopathology and the relation of the brain with genetic variants that may be associated with brain differentiation.
Keywords: Sex, gender, differentiation, mosaic, genotype
Introduction:
The human brain may reflect and be influenced by biological and environmental sex specific differences during development, thus putting the brain on a male-female continuous spectrum.1 Recent work suggests that the human brain may not be strictly male or strictly female; rather, it may be composed of both characteristically male and characteristically female regions.2 Here, we propose that the human brain is not classifiable into strict biological binary male and female groups, and that the composition of sex differentiating traits in the brain is linked to the presentation of psychopathology. We also explore whether the composition of these traits is influenced by genetic differences. Sex differentiating brain traits are brain features that are more common in one sex than in the other. In the present study, we developed a measure to quantify sex-differentiation of the brain using a definition based on a biological sex male-female classification. We then examined the relation between this measure of differentiation and sex-biased psychopathology. Sex differences in psychopathology have been widely reported.3,4 In addition, in order to identify whether genetic variants play a role in the composition of sex differentiating traits, we conducted a genome-wide association (GWA) analysis. Recent studies have shown that genetic effects for complex traits are sex specific, and are also found in genes located not only on the sex-chromosomes but also on the autosomes.5 Therefore, we also examined whether we can detect genetic variants that are associated with brain sex differentiation.
Sex differences in psychopathology often emerge during childhood.6 At key peripubertal stages of development, girls and boys differentiate in their clinical presentation of psychopathology: whereas girls present more often with internalizing disorders (e.g., depression or anxiety), boys present more often with externalizing disorders [e.g., attention deficit with hyperactivity (ADHD), oppositional defiant disorder (ODD), or conduct disorder]. These factor labels (internalizing, externalizing, thought disturbance) emerge as the most frequent factors in investigations of the structure of psychiatric disorders.7–11 These labels were chosen because participants with symptoms of depression or anxiety clustered into a latent variable, the internalizing factor, whereas participants with symptoms of disruptive behavioral disorders including ADHD, ODD, or CD clustered into an externalizing factor. In large cohorts of youth, sex differences have been documented separately in brain development, psychopathology, and cognition;12–15 however, few studies have related how the degree of sex-differentiation in the brain is related to psychopathology.
We posit that the degree of sex-differentiation in the brain is associated with sex differences in psychopathology such that a preponderance of brain features seen more often in females is associated with internalizing psychopathology, and a preponderance of brain features seen more often in males is associated with externalizing psychopathology. That is, individuals with brains comprising more characteristically female traits may be more likely to present with internalizing psychopathology, whereas individuals with brains that comprise more characteristically male traits may be more likely to present with externalizing psychopathology. Although this hypothesis is well supported by the extant literature in which sex is treated as a binary variable,16–18 it has not been tested using sex as a continuous measure, in which a greater degree of features of one sex (i.e. high differentiation) affects sex-biased variance in psychopathology. A brain derived continuous measure may better reflect sex-biased variance in psychopathology than a binary chromosomal assignment of XX or XY.
Genetic influences play an important role during brain development. The majority of brain measures show high heritability.19 Also, sex differences in the heritability of white matter have been found.20 The next step is to identify genetic variants that are associated with the degree of sex differentiating traits in the brain.
In this study we investigated the relations among neuroimaging data, psychopathology scores, and genomic data reported in the Philadelphia Neurodevelopmental Cohort (PNC). First, we generated a composite continuous brain sex differentiation measure of each participant’s sexual differentiation, based on neuroimaging data (structural and diffusion MRI), which reflects the relative degree of female and male features in an individual participant. Second, we explored whether the differentiation measure 1) is related to psychopathology that is known to be sex-biased in childhood, i.e. individuals with a female-scored brain will be more likely exhibit internalizing psychopathology features whereas male-scored brains more likely externalizing psychopathology; and 2) is related to genomic variants, which we investigated with a GWA analysis.
Method:
Participants:
We downloaded the PNC database from the database of Genotypes and Phenotypes (dbGaP) after being approved for controlled access to individual-level data21 (N=8719, mean age = 13.76±3.68, sex distribution = 4498F/4221M).
Psychopathology Data: Variable Clustering and Reduction via Factor Analysis
Demographic, medical, and psychopathology histories were assessed using a structured computerized instrument, GOASSESS,22 which was developed from the Kiddie-Schedule for Affective Disorders and Schizophrenia.23 In addition to standard demographic data, the psychopathology screener assesses symptom- and criterion-related assessments of mood, anxiety, disruptive behavioral, eating, psychotic, and substance use disorders. Both subject and collateral informant data were acquired for children ages 11–17; for children under age 11 only collateral data was acquired, whereas for young adults older than age 18 only subject report was acquired. Psychopathology data were extracted from 252 individual item-level responses to a semi-structured interview from dbGaP).24 We conducted a factor analysis to dimensionalize the psychopathology data using R’s Psych Package25 (See Supplement 1: Factor Methods Details, available online). Factor analysis is useful to organize common processes underlying psychopathology26 and has been previously conducted within this same data sample.27
Five factors emerged that included symptoms in the following broad categories: 1) psychosis; 2) mania; 3) anxiety and depression; 4) disruptive behaviors (ADHD, ODD, and CD); and 5) fear (Figure S1 and S2, available online). Anxiety, depression, and fear were labeled as internalizing factors; disruptive behaviors were labeled externalizing factors; and psychosis and mania were labeled thought disturbance factors.
Structural and Diffusion Image Processing
We processed structural MRI MPRAGE data in BrainSuite (http://brainsuite.org/) using the cortical extraction pipeline. For the brain surface extraction, each brain was individually examined to ensure a satisfactory cortical extraction. Participants with excessive motion, as defined by impaired image clarity or image artifacts, were dropped. For the bias field correction, we applied the iterative option in order to reduce potential image inhomogeneity. SVREG (http://brainsuite.org/processing/svreg/) was used to register data to the Brainsuite BCI-DNI_brain atlas (http://brainsuite.org/svreg_atlas_description/).
Diffusion Tensor Imaging (DTI) data were assessed for quality using DTIPrep (https://www.nitrc.org/projects/dtiprep/). DTIPrep is a program that is designed to addresses data quality problems that affect diffusion MRI and a detailed description of the program is covered in (Oguz et al.).28 During processing of an participant’s brain scan, DTIPrep removes individual diffusion weighted volumes found to be affected by corrupting artifacts. If more than 80% of a participants diffusion weighted volumes were not removed, that subject was considered to have passed quality control (QC). If a participant’s data passed QC, their QC’ed diffusion weighted volumes were then registered to the structural data using BDP (http://brainsuite.org/processing/diffusion/). BDP was used to correct for geometric distortions in diffusion images (registration-based distortion correction) and to co-registers diffusion and anatomical images. BDP registrations were individually inspected to ensure a satisfactory registration. Axial and radial diffusivity were chosen in order to obtain a comprehensive assessment of diffusivity in both gray and white matter regions across the entire brain.29 Finally, we extracted cortical thickness, area, volume, and axial and radial diffusivity values from the 95 regions of interest (ROIs) that were defined from the BCI-DNI atlas for each participant. 66 of these regions are labeled on the surface and cortical thickness was also obtained. SVREG further subdivides some ROIs into gray and white matter based off of T1 tissue intensity values. Details for ROIs are available via http://brainsuite.org/svreg_atlas_description/.
Estimation of Brain Sex Differentiation: Likelihood ratio Approach
We estimated brain sexual differentiation based on adaptations of methods presented previously.2 We used all brain variables and scored the analyses continuously in order to retain the overall characteristic sex differentiation of the brain. This allowed for an automated continuous data-driven calculation of each variable and each participant’s degree of sex differentiating features.
For each brain measurement (axial/radial diffusion, area, volume, thickness) available within the ROIs and using a total of 698 brain features, we estimated sexual differentiation measures for each participant using the likelihood ratio of male and female distributions, as described below. For features influenced by brain volume (area, volume) we normalized with whole brain volume (total brain volume excluding cerebrospinal fluid). This is a significant methodological decision given that, on average, males and females differ in brain volume. By making the decision to control for brain volume, common in research examining sex differences in brain imaging, we attempted to generate a measure of sex differences that is not directly related to volumetric differences. In this likelihood ratio approach, we separately estimated the male and female population distribution of each metric using the Gaussian kernel density estimate with bandwidth selected via Scott’s rule.30 Code replicating this log likelihood computation and all experiments is available at https://github.com/OwenPhillips/differentiation. We then calculated the differentiation measure of each participant for a particular brain feature within the ROI by taking the log likelihood ratio of the two estimated distributions. Thus, the differentiation measure for each brain feature within the ROI is a measure of the odds that the participant’s data came from the distribution of male scores versus the distribution of female scores. At this stage, we implemented two rules for our approach. First, features that did not have an adequate level of difference between males and females were dropped. Variables with nearly identical distributions in the male and female populations provide little basis for differentiation. To smoothly interpolate between including all variables and restricting our differentiation measure to only the most sensitive variables, we introduce a tunable cutoff value and only include those variables for which the male and female distributions differ by more than that cutoff value. We adopt the Hellinger Distance, a standard measure of distributional distance,31 as our dissimilarity measure so that non-differentiating features would be excluded. After eliminating all variables with a Hellinger Distance below 0.12, 502 variables remained. This was done because when the two probability density functions were essentially overlapping, the differentiation measure for these individual features would be essentially zero. The divergence cutoff value is tunable where a lower cutoff would allow more variables to be included and a higher cutoff would further remove more variables. The Hellinger Distance of 0.12 was chosen by inspection of remaining overlap as a compromise between allowing the inclusion of all features and restricting the differentiation measure to the most sensitive features. The benefit of this approach is that it allows for the initial inclusion of all available data while automatically removing non-contributing features31.
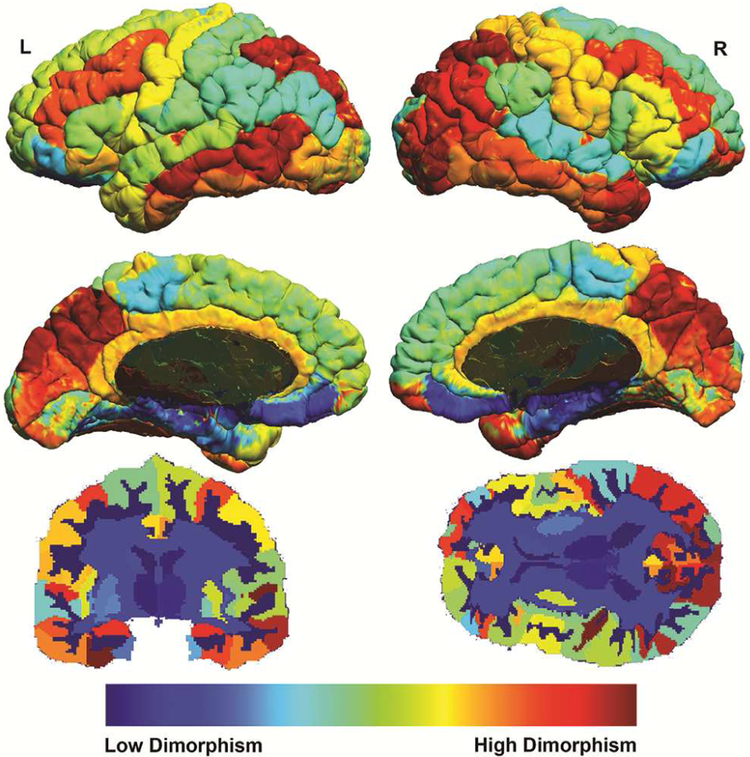
Second, in order to remove bias from outliers in estimated distributions, we winsorized the differentiation measures to within two standard deviations. Two standard deviations was chosen because 95% of values were within this range and, thus, we would minimize the contribution from outliers. Brain measures within each participant were then averaged across all their ROIs to create a single final mean sexual differentiation measure for each participant. This process was also done with the divergence value of each brain measure within each ROI to create a mean effect size for each ROI. The divergence value measures the degree of difference between the two distribution functions. These divergence values were then rank ordered and used in order to generate a whole brain visualization of the differentiation measure on the brain (Figure 1). No statistical tests were done in order to generate this representative image, rather, this represents an ordered ranking of how much the different brain regions vary between the sexes according the “differentiation measure”. The underlying code for the “differentiation score” calculation is available here: https://github.com/OwenPhillips/differentiation.
Figure 1: Mapping the Brain’s Differentiation by Region.
Note: Maps show the order ranked divergence value of mean differentiation within each brain region. Cool colors indicate low differentiation (the spread of the underlying variable for the two populations is low, i.e. male and female brains tend to be similar in these areas). Hot colors indicate high differentiation (the spread of the underlying variable for the two population is high, i.e. male and female brains tend to be different in these areas).
Statistical analysis:
We first computed partial correlations, with age as a covariate, between the factor scores and sex to establish which factors were sex biased. Next, we computed partial correlations within sex, with age as a covariate, between the differentiation measure (a single measure of brain sex differentiation) and the factor scores that were sex biased (i.e. internalizing and externalizing factors).
Genetic Data Processing:
All samples included in this study were genotyped on one of four Illumina arrays: the HumanHap550v1.1, HumanHap550v3.0, Human610_Quadv1_B, and HumanOmniExpress-12v1.0. Genetic data processing steps were applied on the full sample with genotyping data (N=8,741) (see Supplement 2: Genetic Processing, available online) for details. To examine biological consequences of the detected variants we analyzed expression values from GTEx project,32 Encode roadmap methylation data from the Haploreg project33 and single cell expression data from adult and fetal brain using R and previously defined brain cell populations.34
Genome-wide association study (GWA)
Associations of SNPs and the differentiation measure was conducted using linear regression. Calculations were carried out with PLINK (--linear standard-beta --assoc qt-means ) within males only, within females only and as a supplement within all subjects (Figure S3 and S4, available online). Participants of African descent and European descent were analyzed separately and then combined via PLINK’s meta. Age and PCA principal components 1-10 (in order to control for variability in ethnicity) were included as covariates within males and within females separately. The genome-wide significance level was set at 5 × 10−8. Manhattan and quantile-quantile (QQ) plots were generated with the R package qqman (https://CRAN.R-project.org/package=qqman).
In order to examine how much genetic variation explains, we estimated SNP-based observed heritability and correlation with psychiatric disorders using previously published GWAS available on LDhub, which provides an atlas of genetic correlations across complex human traits.35 SNP based observed heritability was calculated with LD score regression.35
Results:
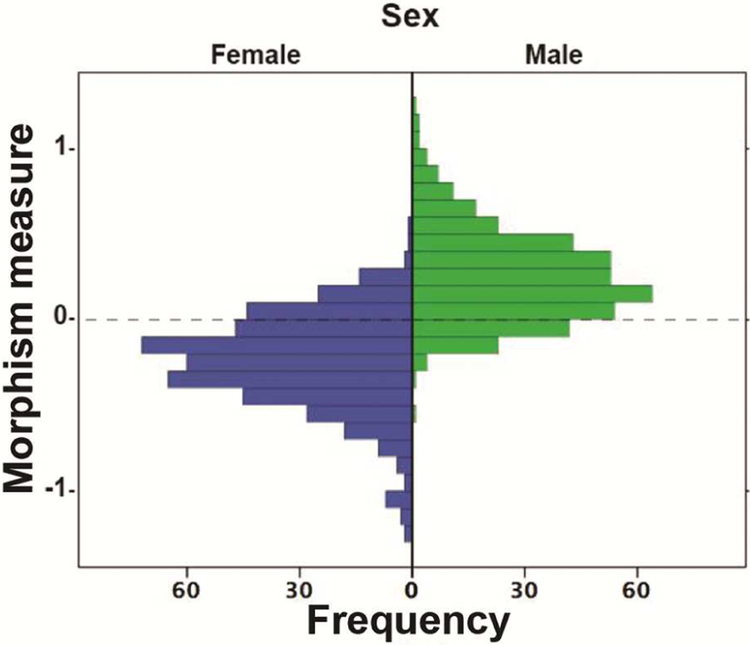
The number of participants with both structural and diffusion data was 883. Of these, 865 (ages 8-21, mean 14.31, SD=3.39), ((407 M, ages 8-21, mean 14.54, SD=3.44), (459 F, ages 8-21, mean 14.06, SD=3.32)) passed quality control. Figure 2 shows a histogram of the brain sex differentiation measure.
Figure 2: Histogram of Likelihood ratio “Differentiation measure”.
Note: On the left of the histogram the distribution of the differentiation measure (degree of sex differentiating features) is shown for females. Females with a very negative differentiation measure have a high level of female skewed brain features; females with scores closer to zero have a greater mix of male and female-biased features and females with very positive scores have a high level of male-biased features. On the right is the distribution of the differentiation measure for males. Males with a very positive differentiation measure have a high level of male skewed brain features; males with scores closer to zero have a greater mix of male and female-biased features and males with very positive scores have a high level of male-biased features.
Factor Analysis:
Additional details for the Factor Analysis are provided in the Supplementary Material, available online (See Table S1, Figure S1-2, available online). In brief, the factor analysis yielded the following results: Thought disorder factors: factor 1: mean=4.90 sd=5.90, factor 2: mean=3.18 sd=4.71, Internalizing Factors (depression, anxiety): factor 3: mean=5.42 sd=5.66, factor 5: mean=5.49 sd=5.1, Externalizing Factor (disruptive behavior): factor 4: mean=5.68 sd=5.00.
Correlations:
Relation between Factor Scores and Sex
Partial correlations between sex and the externalizing factor for disruptive behavioral symptoms (r=0.125, p=0.001) and between sex and the internalizing factors for anxiety/depression symptoms (r=−0.154, p=0.001) and for fear symptoms (r=−0.217, p=0.001) were all significant. Partial correlations between sex and the thought disorder factors of psychosis-related symptoms (r=0.018, p=0.60) and mania-related symptoms (r=−0.007, p=0.827) were not significant.
Relation between the differentiation measure and externalizing symptoms
Partial correlations within males were significant between the differentiation measure and the externalizing factor for disruptive behavioral symptoms (r=0.119, p=0.016). Partial correlations within females were not significant (r=0.009, p=0.854).
Relation between the differentiation measure and internalizing symptoms
Partial correlations within males between the differentiation measure and the internalizing factors for anxiety/depression symptoms (r=−0.026, p=0.597) and fear symptoms (r=0.049, p=0.321) were not significant nor were they within females for anxiety/depression symptoms (r=0.043, p=0.354) or fear symptoms (r=−0.030, p=0.516).
GWA
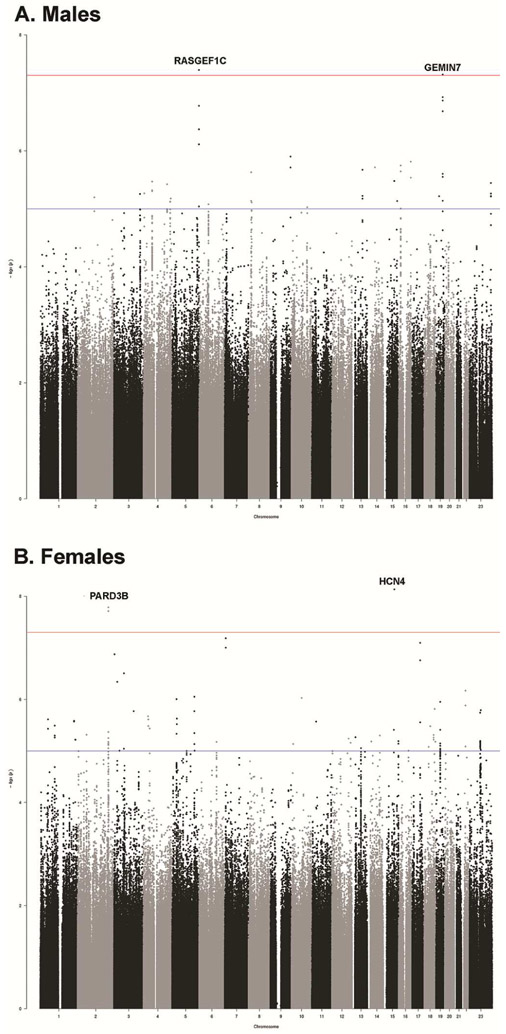
Of the 407 M and 459 F who had MRI data that passed QC, 336 male and 396 female subjects also had GWA data from autosomal and the X chromosome (Figure 3), consisting of 3,543,016 imputed SNPs that passed our stringent QC.
Figure 3: Genome Wide Association for the Differentiation Measure.
Note: A) Manhattan plot of the meta-analyses for males and the differentiation measure. B) Manhattan plot of the meta-analyses for females and the differentiation measure.
Horizontal line indicates threshold for genome-wide significance (P<5 × 10−8).
Association between the differentiation measure and genome wide SNPs within males
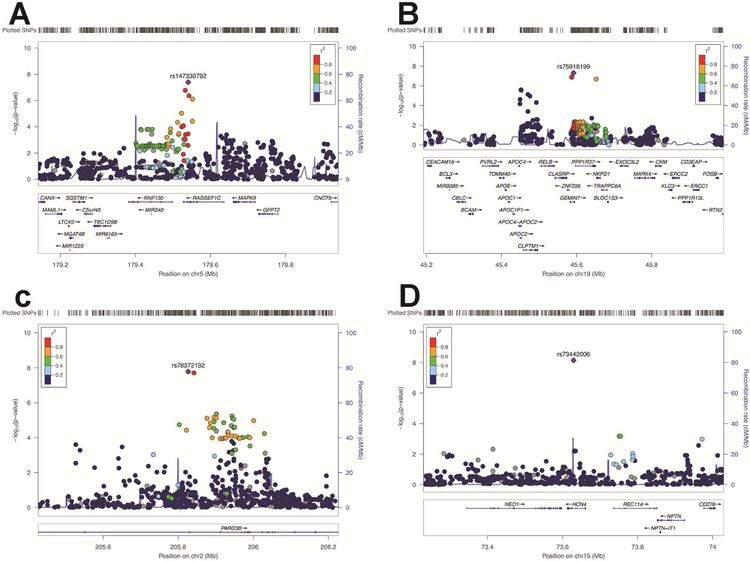
Two SNPs reached genome-wide significance (Figure 3 and Figure 4, and see Figure S5, available online): 1) SNP rs111161632 (p=4.032E-8), from chromosome 5 located on RasGEF Domain Family Member 1C (RASGEF1C). RASGEF1C, is expressed in the Brain (Cerebellum (x9.8) and Cerebellar Hemisphere (x9.3) (GTExPortal https://gtexportal.org). RASGEF1C has been shown in both male and female hypothalamic neural-progenitor/stem cells36 to be glucocorticoid regulated (see Figure S6 and S7, available online).
Figure 4: LocusZoom of Regional Hits for the Differentiation Measure.
Note: Regional plots of the top hit in the association results based on the meta-analysis. Blue lines indicate the recombination rate for the ASN population in the 1000 Genome Project. The Y axis is −log10(P-values) of the single nucleotide polymorphisms (SNPs) and the X axis is chromosomal position (hg19). The linkage disequilibrium (r2) between the top and the remaining SNPs is indicated by color. (a) RASGEF1C gene cluster, (b) GEMIN7, (c) PARD3B, (d) HCN4; SNPs, single nucleotide polymorphism.
2) SNP rs75918199 (p=4.82E-8) from chromosome 19 located on the Gem Nuclear Organelle Associated Protein 7 (GEMIN7). GEMIN7 is a component of the core survival motor neuron protein (SMN) complex, which is required for pre-mRNA splicing in the nucleus and involved in neuron-specific functions, like neurite outgrowth and axonal transport.37 GEMIN7 is expressed in all tissues including the pituitary and neurons.38,39 (see Figure S8 and S9, available online). Furthermore, this SNP is located on H3K4me1 binding site in neuronal tissues based on Encode 15 state model (http://archive.broadinstitute.org/mammals/haploreg/detail_v4.1.php?query=&id=rs75918199).33 The relation between SNPs in RASGEF1C and GEMIN7 and the externalizing factor score was not significant.
Association between the differentiation measure and genome wide SNPs within females
Two SNPs reached GWA significance (Figure 3, Figure 4, and Figure S10, available online): 1) SNP rs78372132 (p=1.64E-8) (chromosome 2) located on intron of Par-3 Family Cell Polarity Regulator Beta (PARD3B). The expression by UniProt/SwissProt (http://www.uniprot.org/)shows intermediate levels in the brain and the Allen brain Atlas shows widespread expression throughout the gray matter of the brain (see Figure S11 and S12, available online).40,41 Furthermore, this SNP is located on H3K4me1 binding site in neuronal tissues based on Encode 15 state model ((http://archive.broadinstitute.org/mammals/haploreg/detail_v4.1.php?query=&id=rs78372132).
2) SNP rs73442006 (p=7.34E-9) from chromosome 15 located on the Hyperpolarization Activated Cyclic Nucleotide Gated Potassium Channel 4 (HCN4). HCN4 encodes a member of the hyperpolarization-activated cyclic nucleotide-gated potassium channels and HCN4 subunits may also play a physiological role in the developing hippocampus and may help control the rhythmic activation of pacemaker neurons during brain development.42,43 The protein differential expression in normal tissues indicates expression in the frontal cortex and there is evidence for expression in subcortical regions of the brain (see Figure S13 and S14 available online)..44 Furthermore, this SNP is located on H3K4me1 binding site in neuronal tissues based on Encode 15 state model (http://archive.broadinstitute.org/mammals/haploreg/detail_v4.1.php?query=&id=rs73442006).
Observed Heritability
While common variants did not explain heritability in brain sex differentiation in the total sample, there was a significant proportion of heritability in males, specifically h2snp=0.015 [se=0.011]. We then examined whether genome-wide variants in brain sex differentiation were shared with psychiatric disorders using previously published GWAS available on LDhub. In LDhub, a significant genetic correlation in males was obtained between our differentiation score and schizophrenia (p=0.047, r=−0.28 [se=0.14]). The lack of heritability estimate in the females or our total PNC sample is likely reflected by lack of power due to relatively small N.
Discussion:
We developed a continuous measure to quantify the level of heterogeneity due to sex differences in the brain and then applied this measure to explore how brain sex differences were related to differences in psychopathology and the genome in youth. Two main findings emerged from this investigation: 1) within males, the brain sex differentiation measure was associated with an externalizing “disruptive behavior” factor; and 2) within both males and females, variants in different genes were associated with the differentiation measure.
Our results partially support our hypothesis that the degree of sex differentiated brain features is related to psychopathology, but sex biologically defined may be a stronger determinant of psychopathology. Numerous studies have found significant differences in brain structure between the sexes,16,45–47 however, recently Joel et al. analyzed four large imaging datasets and found that most brains are composed of unique mosaics of characteristics with some characteristics more common in females, others more common in males.2 Although the methodological approach taken is not without criticism,48 other research supports the notion that sex as a simple categorical variable can be problematic in studies of the function and structure of the human brain.49–51 Elucidating the biological sex of the brain is increasingly relevant to the field of psychiatry, in which specific recommendations have been proposed to incorporate sex as a variable in psychiatric research.52
In this study we developed a single quantitative differentiation measure that seeks to capture the degree of sex-bias of an individual brain. We correlated this measure with factor scores of psychopathology (internalizing factors: depression, anxiety, fear, and an externalizing factor: disruptive behaviors) that are known to be impacted by sex. We found that within males a differentiation measure indicative of a higher degree of male skewed features was significantly correlated with externalizing “disruptive behavior” symptoms. A recent study focused on autism, that utilized a multivariate probabilistic classification to compute the biological sex from cortical thickness suggested male neuroanatomical characteristics carry a higher risk increases for autism.53 Another study using the same dataset as the present work used a probability classification approach and also found a link between classification for male or female brain sex and neurocognitive function across a number of behavioral domains.15 Although the methodological approaches taken and the behavior under investigation by our own and these recent studies vary, they are similar in that they all suggest that there is a link between the degree of sex differentiating features in the brain and behavior. Our study adds novel findings that relate brain measures specifically to psychopathology in males but not in females, in whom the differentiation measure was not associated with increased internalizing symptoms. Thus, although a female brain is linked to internalizing symptoms, a more “female-type” brain does not appear to be associated with internalizing psychopathology. However, it is possible that there are sex-specific pathways leading to a similar psychopathology phenotype in males and females. Such pathways have been described previously in the context of psychiatric disorders.54,55 These pathways may in turn interact with the sexual differentiation of the brain. Future work will be needed to investigate this possibility.
We also found that the differentiation measure was associated with different genetic variants in males and females. To our knowledge, this the first study to link the degree of sex differentiating traits in the brain to the genome. Specifically, in males, two SNPs were genome-wide significant. One of them was (rs111161632) located in RASGEF1C and the other was rs75918199 located in GEMIN7. In females two SNPs were genome-wide significant. One of them was rs78372132, located in PARD3B, the other was rs73442006 located in HCN4. Overall, the association between the differentiation measure and the genes identified suggest that these genes may play a role in the natural variation of brain sex differentiation; however, these genes have not been previously linked to sex differences and should be replicated in a larger independent sample. Interestingly, all four significant SNPs were located on genes that are active in the brain as evidenced both by expression and methylation data. For example, GEMIN7 is expressed in the pituitary and HCN4 is expressed in the hippocampus, both are brain regions that contain important biochemical pathways that are critical for the expression and regulation of stress and have been shown to have sex specific differences in stress response.56,57 Together, this suggests that variants in the identified genes may influence the degree of sex differentiating traits in the brain.
Overall, a single in vivo measure of the brain’s sex heterogeneity (our differentiation measure) may capture more of the complexity of how different systems interact through the influence of sex compared to the classical binary measure and we hope that this initial manuscript can be a step towards understanding that complexity. However, given the complexity of the brain and the difficulty in obtaining and incorporating brain data from children and adolescents, a single measure is not completely explanatory and is limited by several methodological and conceptual considerations, which should be addressed in future work.
This research demonstrates that the relative degree of characteristically male or female features can be represented by a continuous brain differentiation measure, however, this single score is limited in determining precisely what drives the differentiation measure to be high or low. For example, an individual with an even mix of highly male and highly female features would have a similarly low differentiation measure as an individual with many features that were not characteristically male or female. Further, with the single score, we cannot determine whether nonfocal brain regions and/or features are driving the effects we have observed. Through the open source software we have made freely available at (https://github.com/OwenPhillips/differentiation), and the publicly accessible PNC data, these questions can be probed in future work.
The brain’s plasticity is likely variable across the lifespan, which suggests that the differentiation measure is also variable across lifespan. This is especially true in the developing brain where developmental patterns can vary significantly and beyond the strict effects of age we did not have pubertal stage information or testosterone data available, which also affects development.58 Previous research has also indicated that sex steroid hormones have an impact on sex specific differentiation of the brain.1 In future work, it will be important to control for the stage of pubertal development and consider contributions from other sociodemographic factors such as socioeconomic status, education level, and occupation for older youth.
Beyond the developmental impact on the differentiation measure, there are interactions between age/sex and the development of psychopathology59 that were likely not captured in this analysis. In our analyses, we accounted for age by its use as a confounding variable; however, future research that seeks to identify how the brain’s sex changes with age would be of significant interest. Similarly, in relation to the genome and the differentiation measure, we have provided evidence that the underlying genome is associated with the presentation of degree of sex differentiated brain features, however, it is likely that societal and environmental influences also have an impact. Future work across a wider spectrum of development and through the lifespan is needed. It is also important to emphasize that the brain sex differentiation measure is a measure of biological sex classification, not a gender sex classification.
The differentiation measure was characterize by incorporating multiple metrics from both structural and diffusion imaging. It is likely that incorporating more information, such as data from higher resolution scanners, more precise ROIs, other imaging modalities (functional/spectroscopy), would increase the accuracy of the measure. Furthermore, within the participants’ brain scans there may be subtle variations in the amount of movement that could influence the assessment of the quantitative measures (e.g., participants with more externalizing symptoms may experience more motion in the scanner, which in turn could influence the cortical thickness measurement).60 This movement in turn could influence the differentiation measure. Future analyses of the PNC cohort may benefit from more granular assessments and matching of participant characteristics as related to motion within the scanner.
It is important to note that overall a conceptual choice was made where the brain sex differentiation score was initially developed from a biological sex male-female classification; however, a different type of classification approach that does not adhere to an initial binary construct of biological sex may be more useful. Furthermore, we made another conceptual decision to calculate a single whole brain differentiating measure using a multi-regional approach; however, an approach focused on specific regions of interest may be more useful in identifying associations between psychopathology and genetics. Further, we made a methodological decision to remove 'non-differentiating' information from the calculation of the differentiating measure; however, what is or what is not differentiating may vary across development. In addition, the methodological decisions and the likelihood that the differentiation measure is influenced throughout development make it possible that both significant and non-significant associations with the differentiation measure vary across lifespan. Overall, future studies that explore regional variation in differentiation across lifespan would be helpful.
We should also emphasize that the relation between an individual’s differentiation measure and psychopathology is not preclusive. For example, if an individual has a high differentiation score reflecting a high degree of characteristically male brain features, it does not necessarily mean they will exhibit disruptive behavior. This is reflected in the statistical analysis in which the correlation between the measure of sex differentiating brain features and externalizing symptoms in males is significant but weak (r=0.119, p=0.016). Therefore, this association is likely to be more useful for understanding population-level sex differences in the presentation of psychopathology, but limited for understanding any particular individual’s psychopathology. Furthermore, due to the exploratory nature of our analysis, we did not adjust our analyses for multiple comparisons to generate novel hypotheses in this nascent field.
Finally, though this study contains a large number of participants for an MRI study, it is comparatively small for a GWA; this is further complicated by the fact that it is an ethnically diverse population. We made an effort to minimize this complication by including only by the two largest ethnic group (Europen and African decent), while still maintaining the value of including a diverse group of participants. However, future studies including more participants would be beneficial in confirming the effects of the genome on the differentiation measure. Ultimately, this study has significant limitations and before strong conclusions can be made revolving how the brain’s sex “differentiation score”, relates to psychopathology and the genome, this research should be replicated in a larger dataset with participants across a wider age range. The now in collection UK BioBank (http://www.ukbiobank.ac.uk/), or the The Lifespan Human Connectome Project Development (https://www.humanconnectome.org/study/hcp-lifespan-development) would be particularly appealing to deal with a number of the limitations mentioned and to further investigate how the brain’s sex is related to psychopathology, age effects, and the underlying genome.
In this paper we describe an initial attempt to move beyond the observation that the brain is a mosaic of male and female traits to quantifying the level of heterogeneity and its relation to sex-differentiated psychopathology. To do so, we developed an automated continuous data-driven calculation of each participant’s degree of sex differentiating brain features, which we called the “differentiation measure.” Although the differentiation measure we developed has clear limitations, it provides an initial topography of male and female brain features, their associations with psychopathologies that are sex biased, and the underlying genetic influence on the presentation of the brain sex topography. Taken together, our research supports the formulation that the sex of the human brain can be conceptualized along a continuum rather than as binary. An individuals’ placement on this continuum can have important implications for the presentation of psychopathology. Furthermore, genetic variants can impact an individual’s placement on this continuum.
Supplementary Material
Acknowledgments
This work was supported by the Office of Research on Women’s Health and the National Institute of Mental Health (R56MH107243). Dr. Ollila has been supported by the Academy of Finland grant #309643.
Footnotes
Disclosure: Dr. Singh has received grant or research support from the National Institute of Mental Health, the National Institute on Aging, the Child Health Research Institute at Stanford University, Neuronetics, Johnson and Johnson, and the Brain and Behavior Research Foundation. She has served on the advisory board for Sunovion. She has served on the editorial boards of Bipolar Disorders and the Journal of Abnormal Child Psychology. Drs. Phillips, Ollila, Hallmayer, Gotlib, Taylor, Mackey, Mr. Onopa, Ms. Hsu, and Mr. Hillary report no biomedical financial interests or potential conflicts of interest.
Drs. Taylor and Mackey served as the statistical experts for this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci 2011;14(6):677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav 2009;97(2):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: Unifying issues and research strategies. J Child Psychol Psychiatry Allied Discip 2003;44(8):1092–1115. [DOI] [PubMed] [Google Scholar]
- 5.Randall JC, Winkler TW, Kutalik Z, et al. Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits. PLoS Genet 2013;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merikangas KR, He J-P, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspi A, Houts RM, Belsky DW, et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2014;2(2):119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotov R, Ruggero CJ, Krueger RF, Watson D, Yuan Q, Zimmerman M. New dimensions in the quantitative classification of mental illness. Arch Gen Psychiatry. 2011;68(10):1003–1011. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach TM, Edelbrock CS. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev 1981;46(1):1–82. [PubMed] [Google Scholar]
- 10.Kotov R, Chang SW, Fochtmann LJ, et al. Schizophrenia in the internalizing-externalizing framework: A third dimension? Schizophr Bull. 2011;37(6):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbush KT, Watson D. The structure of common and uncommon mental disorders. Psychol Med 2013;43(1):97–108. [DOI] [PubMed] [Google Scholar]
- 12.Vijayakumar N, Allen NB, Youssef G, et al. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Human Brain Mapping. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterthwaite TD, Wolf DH, Roalf DR, et al. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb Cortex. 2015;25(9):2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaczkurkin AN, Moore TM, Ruparel K, et al. Elevated Amygdala Perfusion Mediates Developmental Sex Differences in Trait Anxiety. Biol Psychiatry. 2016;80(10):775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunç B, Solmaz B, Parker D, et al. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos Trans R Soc B Biol Sci 2016;371(1688):20150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 2014;39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jäncke L, Mérillat S, Liem F, Hänggi J. Brain size, sex, and the aging brain. Hum Brain Mapp 2015;36(1):150–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci 2009;29(45):14265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen AG, Mous SE, White T, Posthuma D, Polderman TJC. What Twin Studies Tell Us About the Heritability of Brain Development, Morphology, and Function: A Review. Neuropsychol Rev 2015;25(1):27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochunov P, Jahanshad N, Marcus D, et al. Heritability of fractional anisotropy in human white matter: A comparison of Human Connectome Project and ENIGMA-DTI data. Neuroimage. 2015;111:300–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014;86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 24.Satterthwaite TD, Connolly JJ, Ruparel K, et al. The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revelle W psych: Procedures for Personality and Psychological Research. R Packag. 2016:1–358. [Google Scholar]
- 26.Beesdo-Baum K, Hoefler M, Gloster AT, et al. The structure of common mental disorders: A replication study in a community sample of adolescents and young adults. Int J Methods Psychiatr Res 2009;18(4):204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calkins ME, Merikangas KR, Moore TM, et al. The Philadelphia Neurodevelopmental Cohort: Constructing a deep phenotyping collaborative. J Child Psychol Psychiatry Allied Discip 2015;56(12):1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oguz I, Farzinfar M, Matsui JT, et al. DTIPrep: quality control of diffusion-weighted images. Front Neuroinform 2014;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities Magn Reson Med 2009;61(5):1255–1260. [DOI] [PubMed] [Google Scholar]
- 30.Scott DW. Multivariate Density Estimation: Theory, Practice, and Visualization. Vol 156; 1992. [Google Scholar]
- 31.Xiang L, Yau KKW, Van Hui Y, Lee AH. Minimum Hellinger distance estimation for k-component poisson mixture with random effects. Biometrics. 2008;64(2):508–518. [DOI] [PubMed] [Google Scholar]
- 32.Lonsdale J, Thomas J, Salvatore M, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013;45(6):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward LD, Kellis M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res 2016;44(D1):D877–D881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darmanis S, Sloan SA, Zhang Y, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci 2015;112(23):201507125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47(11):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frahm KA, Peffer ME, Zhang JY, et al. Research Resource: The Dexamethasone Transcriptome in Hypothalamic Embryonic Neural Stem Cells. Mol Endocrinol 2015;30(February):me20151258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briese M, Esmaeili B, Sattelle DB. Is spinal muscular atrophy the result of defects in motor neuron processes? BioEssays. 2005;27(9):946–957. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa C, Usui K, Ito F, Itoh M, Hayashizaki Y, Suzuki H. Role of survival motor neuron complex components in small nuclear ribonucleoprotein assembly. J Biol Chem 2009;284(21):14609–14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giavazzi A, Setola V, Simonati A, Battaglia G. Neuronal-Specific Roles of the Survival Motor Neuron Protein. J Neuropathol Exp Neurol 2006;65(3):267–277. [DOI] [PubMed] [Google Scholar]
- 40.Miller JA, Ding S-L, Sunkin SM, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo H, Seol M-J, Lee K. Differential expression of hyperpolarization-activated cyclic nucleotide-gated channel subunits during hippocampal development in the mouse. Mol Brain. 2015;8(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick D a, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 1997;20:185–215. [DOI] [PubMed] [Google Scholar]
- 44.Seifert R, Scholten a, Gauss R, Mincheva a, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc Natl Acad Sci U S A. 1999;96(16):9391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luders E, Toga AW, Thompson PM. Why size matters: Differences in brain volume account for apparent sex differences in callosal anatomy. The sexual dimorphism of the corpus callosum. Neuroimage. 2014;84:820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips OR, Clark KA, Luders E, et al. Superficial white matter: effects of age, sex, and hemisphere. Brain Connect. 2013;3(2):146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ 2012;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Giudice M, Lippa RA, Puts DA, Bailey DH, Bailey JM, Schmitt DP. Joel etal’s method systematically fails to detect large, consistent sex differences. Proc Natl Acad Sci 2016;(March):201525534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joel D, Fausto-Sterling A. Beyond sex differences: New approaches for thinking about variation in brain structure and function. Philos Trans R Soc Lond B Biol Sci 2016;371:20150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan-Young R, Rumiati RI. Hardwired for sexism? Approaches to sex/gender in neuroscience. Neuroethics. 2012;5(3):305–315. [Google Scholar]
- 51.Springer KW, Mager Stellman J, Jordan-Young RM. Beyond a catalogue of differences: A theoretical frame and good practice guidelines for researching sex/gender in human health. Soc Sci Med 2012;74(11):1817–1824. [DOI] [PubMed] [Google Scholar]
- 52.Zagni E, Simoni L, Colombo D, Zagni E, Simoni L, Colombo D. Sex and Gender Differences in Central Nervous System-Related Disorders. Neurosci J. 2016;2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ecker C, Andrews DS, Gudbrandsen CM, et al. Association Between the Probability of Autism Spectrum Disorder and Normative Sex-Related Phenotypic Diversity in Brain Structure. JAMA Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R, Ma X, Wang G, Yang J, Wang C. Why sex differences in schizophrenia? J Transl Neurosci 2016;1(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- 55.Li M, Lu S, Wang G, Zhong N. The Effects of Gender Differences in Patients with Depression on Their Emotional Working Memory and Emotional Experience. Behav Neurol 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bangasser DA, Curtis A, Reyes BAS, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen TV, McCracken J, Ducharme S, et al. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013;23(6):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ducharme S, Hudziak JJ, Botteron KN, et al. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70(3):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.