Abstract
Chronic systolic heart failure (HF) with acute decompensation can result in cardiogenic shock (CS) requiring short-term mechanical circulatory support. We sought to identify predictors of survival for acute decompensated HF (ADHF) patients requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Patients >18 years old treated at our institution with VA-ECMO from 2009–2018 for ADHF with CS were studied. Demographic, hemodynamic, and echocardiographic data were collected. The primary outcome was survival to discharge. Fifty-two patients received VA-ECMO for ADHF with CS; twenty-four (46.2%) survived. Seventeen (32.7%) had suffered cardiac arrest, and 37 (71.2%) were mechanically ventilated. Mean lactate was 4.33±3.45 mmol/L and patients were receiving 2.7±1.2 vasopressor/inotropic infusions at ECMO initiation; these did not differ significantly between survivors and non-survivors. Pre-ECMO cardiac index was 1.84±0.56L/min/m2 and 1.94±0.63L/min/m2 in survivors and non-survivors, respectively (p=0.57). In multivariable analysis, only diabetes mellitus (DM; OR 13.25, CI 1.42–123.40, p=0.02) and mineralocorticoid receptor antagonist use (OR 0.12, CI 0.02–0.78, p=0.03) were independent predictors of mortality. Nineteen (79.2%) survivors required durable VAD. Among ADHF patients receiving VA-ECMO, DM is a powerful predictor of outcomes while markers of clinical acuity including hemodynamics, vasopressor/inotrope use, and lactate are not. The vast majority of survivors required durable LVAD.
Introduction:
Cardiogenic shock (CS) portends a poor short-term prognosis.1 Most data detailing CS outcomes have focused on acute myocardial infarction (AMI) patients; relatively little data exists to guide management of chronic heart failure (HF) patients with CS.1–2 Durable left-ventricular assist devices (LVAD) have demonstrated improvements in survival and quality of life for advanced HF patients,3–5 but those presenting with acute decompensated heart failure (ADHF) complicated by CS are often not candidates for this therapy due to the acuity of their presentation. Indeed, INTERMACS profile 1 (i.e. “crash and burn”) patients have a worse overall prognosis compared to others undergoing durable LVAD implantation.6 Instead, attempts are often made to stabilize such patients with short-term circulatory support devices and determine whether they might be candidates for durable LVAD or heart transplant (HT).
Veno-arterial (VA) extra-corporeal membrane oxygenation (ECMO) has been used with increasing frequency to support CS patients and is typically reserved for the most severely affected cohort.2,7 However, there are risks associated with this therapy and despite providing a great deal of circulatory support, mortality remains high.7 Continued growth of ECMO use for CS is anticipated particularly as this therapy receives increasing recognition in organ allocation guidelines.8 Emerging data have begun to guide ECMO use for AMI patients with CS9–13 but relatively little is known about the overall outcomes of ADHF patients with CS and the utility of ECMO for this population.14 As such, we sought to determine predictors of survival for chronic dilated cardiomyopathy patients treated with ECMO for an acute decompensation into CS.
Methods:
This study was approved by Columbia University’s Institutional Review Board. Participants or their surrogate provided written informed consent for inclusion in a CS database. A waiver of consent was granted for those without a surrogate and too critically–ill to provide informed consent prior to death.
Patient Selection and Outcomes
All patients 18 years or older treated at our institution with VA-ECMO who had HF with reduced left ventricular ejection fraction (LVEF) for > 3 months and presented with acute decompensation were included in this retrospective analysis. Patients with an acute coronary syndrome, acute HF (e.g. myocarditis), and HF with preserved ejection fraction were excluded. Data collected included demographic and echocardiographic variables as well as hemodynamics whenever available. The primary outcome was survival to hospital discharge. Secondary outcomes were 1-year survival and durable LVAD implantation or HT during the index admission.
Mechanical Circulatory Support Device Weaning
Our center tests daily whether patients can be weaned from short-term mechanical circulatory support devices like VA-ECMO. Once vasopressors have been weaned to low levels (i.e. norepinephrine <5mcg/min, vasopressin <2U/hr) we perform a daily trial of ECMO flow reduction to determine whether the patient is hemodynamically dependent or not. We utilize pulmonary artery (PA) and radial artery catheters to evaluate the change in hemodynamic status as this is done. If the mean arterial pressure (MAP) falls by more than 15% or below 65mmHg as flows are reduced to 1L/min we consider this a failure to wean from ECMO. Bedside echocardiography is also used during flow reduction to further guide decisions about potential success or failure of device discontinuation. In our institution a patient who fails repeated weaning attempts is transitioned to a durable LVAD or HT whenever possible.
Statistical Analysis
Categorical data are presented as percentages and continuous data as means ± standard deviation or medians with IQR where appropriate. Pearson’s chi–squared test was used to compute the significance of the difference between groups for categorical variables. Normality of continuous variables was tested using the Shapiro-Wilk test. The Student’s t–test and Mann-Whitney U test were used, where appropriate, to compare groups for continuous variables. Logistic regression was used to determine significant predictors of survival to discharge. Variables with a p-value ≤ 0.10 in univariable analysis were included in a multivariable model. For time-to-event analyses, Kaplan-Meier estimates of event-free survival were created for groups of interest and the log-rank test was used to compare survivor functions. A p-value <0.05 was considered statistically significant. Data were analyzed using Stata (StataCorp, College Station, TX).
Results:
In total, 52 chronic systolic HF patients with a dilated cardiomyopathy received VA-ECMO for CS resulting from ADHF between 2009 and 2018. Of these, 24 (46.2%) survived to discharge and 28 (53.8%) did not. Of survivors, 19 (79.2%) required durable LVAD prior to discharge. An additional 4 patients received a durable LVAD but did not survive to discharge (Figure 1).
Figure 1.
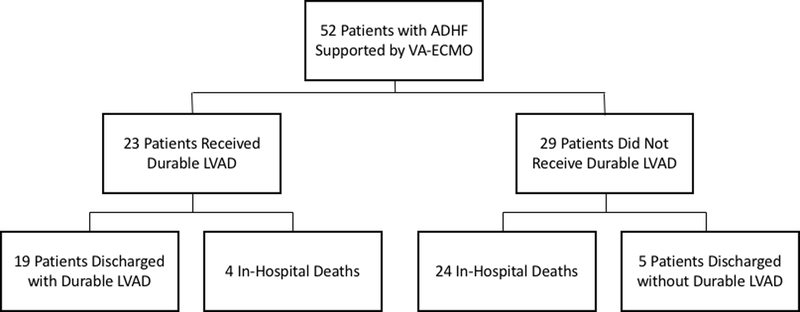
Patient Outcomes. ADHF, acute decompensated heart failure; VA-ECMO, veno-arterial extra-corporeal membrane oxygenation; LVAD, left ventricular assist device.
Prior to ECMO implantation 17 (32.7%) patients had an intra-aortic balloon pump (IABP) and 13 (25.0%) had a percutaneous LVAD. An additional 7 (13.5%) patients had a percutaneous LVAD inserted following ECMO cannulation to decompress the left ventricle while 15 patients (28.8%) remained without a venting device during ECMO support. Seventeen (32.7%) had suffered a cardiac arrest prior to ECMO implantation, and 10 (19.2%) had active CPR during ECMO implantation. Thirty-seven (71.2%) were mechanically ventilated and the mean lactate was 4.33±3.45mmol/L.
Patient demographics and co-morbidities are displayed in Table 1. All patients had a dilated cardiomyopathy; twenty (38.5%) patients had an ischemic etiology for the cardiomyopathy (ICM) and 14 (26.9%) had diabetes mellitus (DM). Survivors and non-survivors differed significantly in DM prevalence and likelihood of pre-ECMO treatment with a mineralocorticoid receptor antagonist (MRA). Those receiving MRA therapy had similar serum creatinine to those who did not (p = NS). In addition, there was a trend towards non-survivors having older age, higher lactate levels, and lower pH. LVEF was 13.7±4.1 percent and left ventricular end-diastolic dimension was 7.1±1.0 cm. Right ventricular function was moderately or severely reduced in 25 (55.5%) patients, 32 (72.7%) had moderate or severe mitral regurgitation, and 18 (40.9%) had moderate or worse tricuspid regurgitation.
Table 1:
Patient Demographics
| Variable | All (52) | Survived (24) | Died (28) | p value |
|---|---|---|---|---|
| Age (years) | 52.0 ± 14.8 | 47.8 ± 14.4 | 55.7 ± 14.3 | 0.052 |
| Male Gender, N (%) | 45 (86.5) | 22 (91.7) | 23 (82.1) | 0.32 |
| Ischemic Dilated Cardiomyopathy, N (%) | 20 (38.5) | 7 (29.2) | 13 (46.4) | 0.20 |
| Hypertension, N (%) | 28 (53.8) | 10 (41.7) | 18 (64.3) | 0.10 |
| Prior Stroke, N (%) | 10 (19.2) | 3 (12.5) | 7 (25.0) | 0.25 |
| Diabetes Mellitus, N (%) | 14 (26.9) | 2 (8.3) | 12 (42.9) | 0.005 |
| Cardiac Arrest, N (%) | 17 (32.7) | 8 (33.3) | 9 (32.1) | 0.93 |
| Ventricular Tachycardia, N (%) | 14 (26.9) | 6 (25.0) | 8 (28.6) | 0.77 |
| Mechanical Ventilation, N (%) | 37 (71.2) | 16 (66.7) | 21 (75.0) | 0.51 |
| ICD, N (%) | 38 (73.1) | 16 (66.7) | 22 (78.6) | 0.34 |
| Beta Blocker, N (%) | 45 (86.5) | 20 (83.3) | 25 (89.3) | 0.53 |
| ACE Inhibitor, N (%) | 35 (67.3) | 15 (62.5) | 20 (71.4) | 0.49 |
| MRA, N (%) | 22 (42.3) | 14 (58.3) | 8 (28.6) | 0.03 |
| Lactate (mmol/L) | 4.33 ± 3.45 | 3.40 ± 2.93 | 5.13 ± 3.52 | 0.06 |
| pH | 7.38 ± 0.13 | 7.41 ± 0.14 | 7.35 ± 0.12 | 0.09 |
| INR | 2.34 ± 1.89 | 2.65 ± 2.28 | 2.07 ± 1.46 | 0.28 |
| Albumin (g/dL) | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.77 |
| Total bilirubin (mg/dL) | 2.5 ± 2.5 | 2.1 ± 1.9 | 2.7 ± 2.9 | 0.39 |
| White Blood Cell (cells x103/uL) | 12.4 ± 6.5 | 13.2 ± 7.5 | 11.7 ± 5.6 | 0.42 |
| Hemoglobin (g/dL) | 11.6 ± 2.1 | 11.5 ± 1.8 | 11.6 ± 2.3 | 0.84 |
| RDW (%) | 16.2 ± 2.5 | 15.6 ± 2.2 | 16.7 ± 2.6 | 0.12 |
| Platelet (cells x103/uL) | 175.8 ± 65.0 | 188.5 ± 79.1 | 165.0 ± 48.7 | 0.20 |
| Creatinine (mg/dL) | 2.13 ± 1.48 | 2.18 ± 2.05 | 2.09 ± 0.71 | 0.83 |
| Sodium (mmol/L) | 133.3 ± 5.9 | 133.8 ± 3.7 | 132.9 ± 7.2 | 0.62 |
ICD, implantable cardioverter-defibrillator; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist; INR, international normalized ratio; RDW, red cell distribution width.
Hemodynamics
Forty-two (80.8%) patients had an invasive hemodynamic assessment with pulmonary artery catheterization before ECMO initiation (Table 2). Only 9 (17.3%) patients underwent ECMO initiation more than 24 hours after this invasive evaluation. Amongst patients with invasive hemodynamic monitoring, there was evidence of severe hemodynamic compromise. The MAP was 67.3±14.2mmHg, mean cardiac index (CI) 1.90±0.60L/min/m2, and mean cardiac power index (CPI) 0.29±0.11W despite 30 (57.7%) patients already having either IABP or percutaneous LVAD. Forty-five (90.0%) were on at least one vasopressor and 27 (54.0%) were on multiple vasopressors; forty-one (82.0%) were on an inotrope. Patients were receiving an average of 2.7±1.2 inotropic or vasopressor infusions at ECMO initiation.
Table 2:
Hemodynamics and Echocardiographic Characteristics at ECMO Initiation
| Variable | All (52) | Survived (24) | Died (28) | p value |
|---|---|---|---|---|
| Heart Rate (beats/min) | 101.7 ± 25.7 | 102.7 ± 24.5 | 101.0 ± 27.1 | 0.82 |
| Systolic Blood Pressure (mmHg) | 87.6 ± 16.6 | 90.0 ± 14.7 | 85.6 ± 18.0 | 0.36 |
| Diastolic Blood Pressure (mmHg) | 57.4 ± 14.9 | 60.1 ± 13.6 | 55.1 ± 15.8 | 0.23 |
| Right Atrial Pressure (mmHg) | 16.6 ± 8.2 | 16.3 ± 7.7 | 17.0 ± 8.9 | 0.80 |
| Systolic PA Pressure (mmHg) | 55.1 ± 14.0 | 55.0 ± 15.2 | 55.1 ± 13.3 | 0.98 |
| Diastolic PA Pressure (mmHg) | 30.1 ± 8.4 | 31.1 ± 8.8 | 29.3 ± 8.0 | 0.50 |
| PCW Pressure (mmHg) | 28.7 ± 7.0 | 28.2 ± 6.4 | 29.1 ± 7.5 | 0.73 |
| Cardiac Output (L/min) | 3.89 ± 1.33 | 3.73 ± 1.14 | 4.01 ± 1.48 | 0.52 |
| Cardiac Index (L/min/m2) | 1.90 ± 0.60 | 1.84 ± 0.56 | 1.94 ± 0.63 | 0.57 |
| Cardiac Power Output (W) | 0.61 ± 0.24 | 0.58 ± 0.20 | 0.63 ± 0.27 | 0.57 |
| Cardiac Power Index (W/m2) | 0.29 ± 0.11 | 0.29 ± 0.10 | 0.30 ± 0.12 | 0.80 |
| Number of vasopressors | 1.7 ± 1.0 | 1.6 ± 1.1 | 1.7 ± 0.9 | 0.66 |
| Number of inotropes | 1.0 ± 0.6 | 1.0 ± 0.6 | 1.0 ± 0.7 | 0.80 |
| PA Pulsatility Index | 2.43 ± 2.94 | 2.47 ± 3.67 | 2.40 ± 2.17 | 0.94 |
| PVR (Wood units) | 3.04 ± 3.30 | 2.88 ± 2.82 | 3.14 ± 3.66 | 0.85 |
| LVEDd (cm) | 7.1 ± 1.0 | 7.2 ± 1.2 | 7.0 ± 0.8 | 0.63 |
| LVEF (%) | 13.7 ± 4.1 | 13.1 ± 3.3 | 14.2 ± 4.6 | 0.36 |
| Right Ventricular size, N (%) Normal Mild Moderate Severe |
13 (28.9) 10 (22.2) 16 (35.6) 6 (13.3) |
6 (30.0) 4 (20.0) 7 (35.0) 3 (15.0) |
7 (28.0) 6 (24.0) 9 (36.0) 3 (12.0) |
0.98 |
| Right Ventricular function, N (% Normal Mild Moderate Severe |
8 (17.8) 12 (26.7) 19 (42.2) 6 (13.3) |
4 (20.0) 4 (20.0) 10 (50.0) 2 (10.0) |
4 (16.0) 8 (32.0) 9 (36.0) 4 (16.0) |
0.82 |
| Tricuspid Regurgitation Normal Mild Moderate Severe |
7 (15.9) 19 (43.2) 18 (40.9) 0 (0) |
4 (20.0) 7 (35.0) 9 (45.0) 0 (0) |
3 (12.5) 12 (50.0) 9 (37.5) 0 (0) |
0.58 |
| Mitral Regurgitation Normal Mild Moderate Severe |
2 (4.6) 10 (22.7) 18 (40.9) 14 (31.8) |
1 (5.0) 3 (15.0) 9 (45.0) 7 (35.0) |
1 (4.2) 7 (29.2) 9 (37.5) 7 (29.2) |
0.74 |
min, minute; PA, pulmonary artery; PCW, pulmonary capillary wedge; L, liters; m, meter; W watts; PVR, pulmonary vascular resistance; LVEDd, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction.
ECMO support
Thirty (57.7%) patients underwent ECMO initiation either on the day of ICU admission or the following day. The median duration of ECMO support was 4 (IQR: 1.5–8) days and the range was 1 to 29 days (Table 3). Length of support on ECMO was similar between survivors and non-survivors (5 days vs 3 days, p=0.45). Thirty-seven (71.2%) patients had a concomitant IABP or percutaneous LVAD during ECMO support and 15 (28.8%) patients remained without either during ECMO support. The median ICU length of stay was 27.5 (IQR: 11.5–39.5) days and the median hospital length of stay was 37.5 (IQR: 20.5–61.5) days. The median hospital length of stay was 51.5 (IQR: 37.5–77) days for survivors and 26 (IQR: 6–49.5) for non-survivors (p = 0.002).
Table 3:
Patient Outcomes
| Variable | |
|---|---|
| Survival to discharge | 24 (46.2%) |
| 30-day survival | 32 (61.5%) |
| Durable LVAD during index admission | 23 (44.2%) |
| Heart Transplant during index admission | 1 (1.9%) |
| ECMO support duration | 4 days (IQR: 1.5 – 8) |
| ICU length of stay | 27.5 days (IQR: 11.5 – 39.5) |
| Hospital length of stay | 37.5 days (IQR: 20.5 – 61.5) |
LVAD, left ventricular assist device; ECMO, extra-corporeal membrane oxygenation; ICU, intensive care unit.
Survival
Thirteen (25.0%) patients died within 1 week of ECMO initiation. The 30-day and 1-year survival estimates were 61.5% and 39.4% for the entire cohort, respectively. Survival to discharge was similar among patients with and without a venting device during ECMO support (p = NS). In univariable analysis, predictors of survival to discharge included DM and MRA use. In addition to these factors and in accordance with our criteria for model inclusion, age, serum lactate, and pH were included in a multivariable model (Table 4). After adjustment, DM and MRA use remained significant predictors of survival to discharge. Among diabetics, 30-day and 1-year survival estimates were 35.7% and 9.5% whereas among non-diabetics, they were 71.1% and 49.6%, respectively (p = 0.0004, Figure 2). A history of cerebrovascular accident (CVA) was more common among diabetic patients though this was not statistically significant (28.6% vs 15.8%, p = 0.30).
Table 4:
Predictors of In-Hospital Mortality
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.04 | 1.00 – 1.08 | 0.06 | 1.01 | 0.96 – 1.06 | 0.74 |
| Gender | 2.39 | 0.42 – 13.64 | 0.33 | |||
| Ischemic Dilated Cardiomyopathy | 2.10 | 0.67 – 6.65 | 0.21 | |||
| DM | 8.25 | 1.62 – 42.09 | 0.01 | 13.25 | 1.42 – 123.40 | 0.02 |
| Lactate | 1.20 | 0.98 – 1.47 | 0.08 | 1.10 | 0.85 – 1.43 | 0.45 |
| Total Bilirubin | 1.11 | 0.87 – 1.42 | 0.39 | |||
| Albumin | 0.87 | 0.33 – 2.28 | 0.77 | |||
| Creatinine | 0.96 | 0.66 – 1.39 | 0.83 | |||
| INR | 0.84 | 0.62 – 1.15 | 0.28 | |||
| pH | 0.01 | 0.00 – 2.09 | 0.09 | 0.90 | 0.00 – 808.43 | 0.98 |
| RDW | 1.21 | 0.95 – 1.55 | 0.13 | |||
| Cardiac Arrest | 0.95 | 0.30 – 3.02 | 0.93 | |||
| Cardiac Index at implant | 1.38 | 0.47 – 4.06 | 0.56 | |||
| Pulmonary Artery Pulsatility Index | 0.99 | 0.80 – 1.22 | 0.93 | |||
| ICD | 1.83 | 0.53 – 6.33 | 0.34 | |||
| Beta Blocker Use | 1.67 | 0.34 – 8.32 | 0.53 | |||
| ACE Inhibitor Use | 1.50 | 0.47 – 4.80 | 0.50 | |||
| MRA use | 0.29 | 0.09 – 0.91 | 0.03 | 0.12 | 0.02 – 0.78 | 0.03 |
OR odds ratio; CI confidence interval; DM, diabetes mellitus; INR, international normalized ratio; RDW, red blood cell distribution width; ICD, intra-cardiac cardioverter-defibrillator; ACE, angiotensin-converting enzyme; MRA, mineralocorticoid receptor antagonist.
Figure 2.
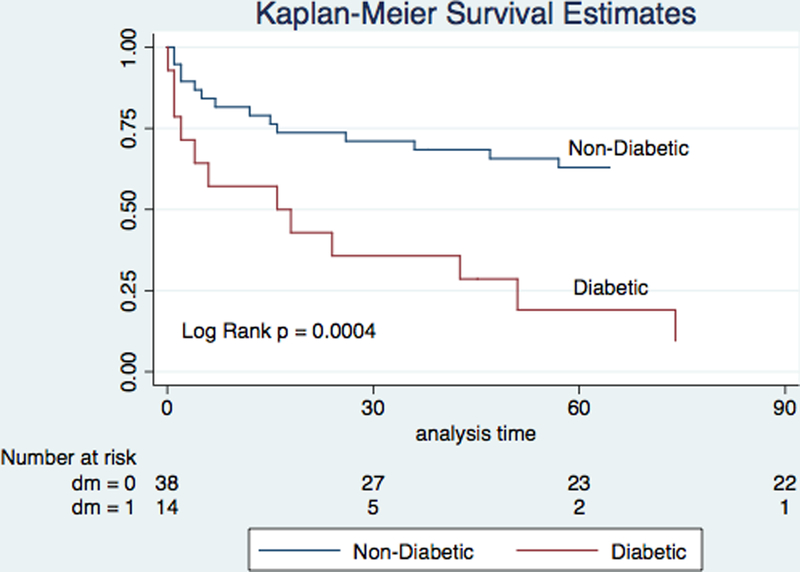
Kaplan–Meier Survival Estimates After ECMO Implantation Among Diabetic and Non-Diabetic Patients. ECMO, extra-corporeal membrane oxygenation.
Among those undergoing active CPR at the time of ECMO initiation, 3 (30.0%) patients survived to discharge compared to 21 (50.0%) of those not undergoing CPR (p = 0.25). The duration of support with ECMO was shorter in those who received this therapy during active CPR (2.0 days vs 5.0 days, p = 0.03) but all those surviving to discharge in this subset of patients required durable LVAD implantation.
Median follow-up after discharge was 381 (IQR: 141–908) days. Following discharge, patients weaned from ECMO without LVAD did not require durable LVAD implantation or HT. Among those requiring durable LVAD, 5 (21.7%) died in follow-up. None of the patients with durable LVAD at discharge underwent explant for significant recovery on device support. The causes of in-hospital death were multi-organ failure in 19 patients (67.9%), neurologic injury in 4 (14.3%), asystole in 2 (7.1%), sepsis in 2 (7.1%), and uncontrolled bleeding in 1 (3.6%). The causes of death did not differ significantly between those patients with and without DM.
Bridging Strategy
Following initial stabilization with ECMO, 24 patients (46.2%) were transitioned to either short-term surgical biventricular assist device (CentriMag, Abbott Vascular, formerly Thoratec, Pleasanton, CA) or incorporation of a minimally invasive short-term ventricular assist device (VAD; CentriMag) into the ECMO circuit.15 Of these, 14 (58.3%) were bridged to durable LVAD and 10 (41.7%) expired. A total of twenty-three patients received a durable LVAD during the index admission (Figure 1). Of those who bridged to durable LVAD directly from ECMO, 4 of 9 (44.4%) died while all 14 patients (100%) who bridged to durable LVAD from short-term surgical VAD survived to discharge. Among those ultimately receiving durable LVAD, the MAP at the time of ECMO initiation was 70.5±11.4 mmHg on 2.5±1.1 vasopressor or inotrope infusions, the mean lactate was 3.30±2.24 mmol/L, the mean serum creatinine was 1.80±0.73 mg/dL, 14 (60.9%) were mechanically ventilated, 13 (59.1%) had IABP or percutaneous LVAD, and 8 (34.8%) had suffered a recent cardiac arrest or were actively undergoing CPR at the time of ECMO cannulation. 1–year survival of this cohort was 69.9% after durable LVAD insertion (Figure 3), compared to a 1-year survival of 83.4%_among the remainder of durable LVAD recipients not treated with ECMO during the study period (p = 0.16). Among patients bridged to durable LVAD, median duration of ECMO support in survivors was 5.0 days and in non-survivors was 2.0 (p = 0.19).
Figure 3.
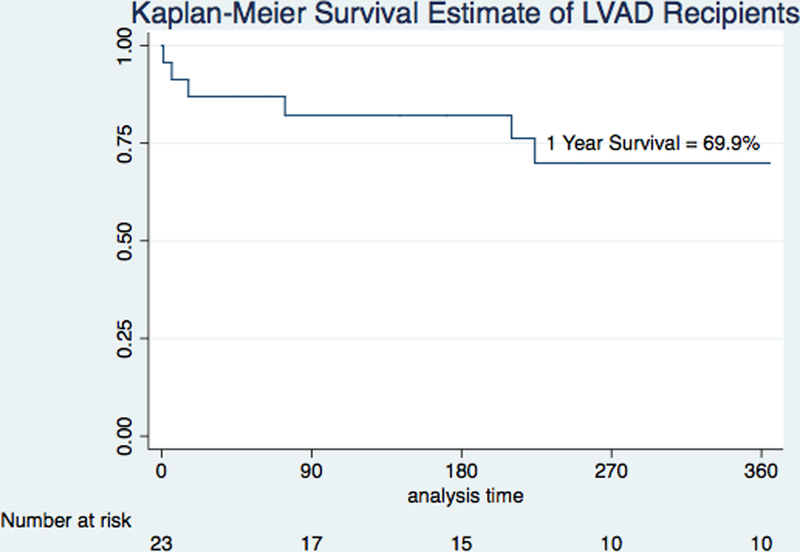
1–Year Kaplan–Meier Survival Estimates Among Patients Receiving Durable Left Ventricular Assist Device.
While none of these patients underwent HT during the index admission, 9 (39.1%) of those who underwent durable LVAD implantation ultimately underwent HT after discharge. The median time to transplant from ECMO initiation was 214 days (IQR: 161 – 499 days). In addition, 4 (17.4%) are currently awaiting HT while on durable LVAD support and 3 (13.0%) are not candidates for HT but are living with destination therapy LVAD support.
Five (9.6%) patients requiring ECMO support ultimately survived to discharge without durable LVAD or HT. These patients had a MAP of 66.0±12.6mmHg and all but 1 were on at least 4 inotropes and vasopressors. In addition, 2 had IABP prior to ECMO initiation and 1 had a percutaneous LVAD. The mean serum lactate was 5.84±5.39mmol/L. The average time of ECMO support was 5.0±1.2 days. None of these 5 patients were discharged on an inotrope or to hospice and none received durable LVAD or HT following discharge. The median follow-up of this cohort was 1221 days. One patient was lost to follow up after discharge, one relocated to another state after 147 days, and the remaining patients are alive and well 1221, 1491, and 2039 days following discharge. Of these patients who recovered from ECMO without need for durable LVAD, 3 presented with rapid, uncontrolled atrial tachyarrhythmias and quickly deteriorated into severe refractory CS, and one developed refractory ventricular arrhythmias. The remaining patient suffered a cardiac arrest in the hospital and had ECMO support initiated immediately following this. For those with uncontrolled atrial tachyarrhythmias, control of the arrhythmia facilitated successful wean from device in all cases.
Discussion:
Our data demonstrate the following:
As a result of a high illness severity including elevated lactate, high prevalence of mechanical ventilation, and an average use of nearly 3 vasoactive medications, chronic dilated cardiomyopathy patients presenting with acute decompensation complicated by CS requiring ECMO have a high in-hospital mortality.
Markers of acute illness severity like the degree of hemodynamic compromise, vasopressor/inotrope requirement, serum lactate, and pH did not predict survival but instead DM emerged as a strong predictor of in-hospital mortality.
The majority of patients who survived required implantation of a durable LVAD but were likely too acutely ill at presentation to undergo LVAD implantation, highlighting the potential use of ECMO to stabilize INTERMACS 1 patients prior to implantation.
While several studies have examined predictors of survival in patients with AMI and CS,9–13,16 little is known about the outcomes of chronic dilated cardiomyopathy patients who present with CS and require ECMO for hemodynamic stabilization. Data have begun to emerge for this patient population but are still largely lacking.14,17 It is important to understand predictors of outcomes for each phenotype of CS in order to refine patient selection for mechanical circulatory support devices. Prior data has demonstrated that etiology of CS is one of the most important predictors of outcomes when using VA-ECMO18–19 and importantly, predictors of prognosis may differ by CS etiology. For this reason, we sought to limit our dataset to a specific CS phenotype: acute decompensation of chronic systolic HF with evidence of CS.
Our patient population is notable for its severity of illness. A significant proportion had suffered a cardiac arrest prior to ECMO initiation and the majority was mechanically ventilated. Furthermore, there was evidence of severe hemodynamic compromise; the mean CPI was similar to that of the SHOCK registry.20 In addition, there was evidence of end-organ dysfunction with markedly elevated serum creatinine and lactate. Not surprisingly, short-term mortality approached 50%, typical of numerous CS reports where case-fatality rates have ranged between 40 and 60%.1–2 For this reason, there has been an increased utilization of mechanical circulatory support devices for CS patients in an effort to improve upon this poor prognosis.2 However, as circulatory support devices including ECMO become more commonly used in the setting of CS, it is important to select patients carefully to optimize outcomes and avoid using support devices for those with exceedingly low likelihood of survival; otherwise these devices may serve only to prolong the dying process. While our data do not support strict exclusion criteria for ECMO support in ADHF patients, it is notable that only 20% of survivors were discharged without a durable LVAD. Patients with strict contra-indications to durable LVAD implantation or HT might therefore be viewed as marginal ECMO candidates.
Interestingly, unlike other studies detailing predictors of survival for those with AMI and shock,10,20 hemodynamics were not predictive of outcomes. In fact, there was no significant difference in any hemodynamic measure between survivors and non-survivors. Instead, the presence of DM emerged as a potent predictor of outcomes for this population; less than 20% of diabetics survived to discharge and their 1–year survival was less than 10%, highlighting this comorbidity as a marker of overall poor prognosis. Interestingly, this risk factor had a greater ability to predict overall outcomes than markers of acute illness severity like serum lactate and pH. Importantly, DM is not an exclusion criterion for durable LVAD insertion at our institution and did not preclude durable LVAD implantation for any patients in our cohort. However, patients with DM might be at a higher risk of developing multi-organ failure, the leading cause of death in our study, in response to severe hemodynamic compromise. In addition, though not statistically significant, diabetic patients in our cohort more frequently had other manifestations of vascular co-morbidities like CVAs. While DM should not be viewed as a contra-indication to ECMO initiation, this patient population might be better served with earlier initiation of circulatory support before multi-organ failure develops, precluding their candidacy for durable LVAD implantation and thereby limiting survival. Furthermore, if DM patients with chronic heart failure supported by ECMO do not demonstrate early signs of improvement, they and/or their families should be counseled that their likelihood of survival to discharge is significantly diminished.
MRA use prior to shock onset was also an independent predictor of survival to discharge among our cohort. MRA therapy has been shown to improve symptoms and mortality in patients with systolic heart failure.21,22 However, it is unclear if prior MRA therapy itself conferred a survival benefit to patients or whether it may have been associated with other factors influencing prognosis. Importantly, those with and without MRA use prior to ECMO had similar renal function. While the majority of patients were treated chronically with beta-blockers and ACE inhibitors, less than half of patients were on MRA therapy. Those treated chronically with MRA therapy might have represented patients under more comprehensive care prior to their decompensation and thus in a better condition to survive such an event.
Our center, like others, has used ECMO in attempts to salvage INTERMACS profile 1 HF patients to determine whether they may be stabilized to undergo durable LVAD implantation, HT, or recovery from short-term support without either of these therapies. Under new organ allocation guidelines, ECMO may be used increasingly as a bridge to HT.8 In light of this, it is important to note that one quarter of patients had early mortality within 1 week of ECMO initiation. In addition, a subset of patients weaned successfully from ECMO and had a good long-term outcome without LVAD or HT. Further study of ECMO support for acute decompensated heart failure will be required to find optimal ways to utilize this therapy to bridge patients directly to HT.
Our center has adopted a practice of initial stabilization with percutaneous support devices for patients with refractory CS instead of immediate implantation of a durable LVAD or short-term invasive surgical biventricular assist device. The majority of patients had ECMO initiated shortly after admission and within 24 hours of measurement of invasive hemodynamics. In addition, the majority of patients in our cohort were also supported by a concomitant percutaneous LV venting device whether present prior to ECMO or inserted following ECMO initiation. Though the outcomes were similar for those with and without a venting device, we expect that this is a result of the fact that the majority of patients received such a device and because of our institutional practice of early implantation of a venting device if signs of LV pressure overload are apparent. Almost half of patients subsequently underwent transition to a short-term surgical VAD prior to bridging to durable LVAD but those who had a short-term VAD following ECMO had better outcomes than those who bridged directly from ECMO to durable LVAD. Identifying the optimal management of the INTERMACS 1 chronic HF patient with a dilated cardiomyopathy remains an important focus within the field of mechanical circulatory support and deserves further study to improve ways of salvaging even the most critically-ill patients.
For patients with advanced systolic HF, durable device therapy has emerged as an important option as destination therapy, bridge to transplant, or bridge to recovery and overall outcomes have been excellent though acuity of illness at implantation significantly impacts outcomes.6,23 For patients undergoing durable LVAD implantation, the timing of device insertion is crucial to maximizing outcomes. An acute hemodynamic deterioration may lead to end-organ failures that would preclude durable LVAD insertion or significantly increase risk for this surgery in a short period of time. Indeed, those patients in our cohort who ultimately underwent durable LVAD implantation were likely too acutely ill at initial presentation (when they received ECMO) to undergo LVAD or HT. However, patient selection is paramount to optimizing outcomes and to avoiding the “bridge to nowhere” situation where a patient cannot be weaned from short-term circulatory support but is not a candidate for durable device therapy or HT.24 Future studies identifying additional prognostic markers for this patient population are urgently needed to help guide the optimal use of this therapy.
Limitations
Our study has several limitations. It is single–center and subject to inherent limitations of practice pattern and bias. Sample size was a significant limitation and it is possible that important predictors of outcomes were missed due to this. While patients with other phenotypes of chronic HF were supported with ECMO at our institution (e.g. restrictive cardiomyopathy or allograft failure patients) we limited our analysis to only those with a dilated cardiomyopathy and acute on chronic systolic HF because we believe that different phenotypes of CS patients may respond differently to ECMO therapy. While this restriction limited our sample size, it allowed us to identify predictors unique to this patient population. There is potential selection bias for patients supported with ECMO and data here is not necessarily generalizable to all CS patients. Furthermore, there is selection bias for patients’ candidacy for durable LVAD and a patient’s suitability for durable LVAD therapy is likely an important factor in determining their prognosis. Lastly, it is possible that some patients might have been weanable from ECMO without the need for durable LVAD. While our weaning protocol for ECMO is institution-specific, we observed that no patients with durable LVAD at discharge demonstrated any signs of ventricular recovery in follow up.
Conclusions:
Among patients with acute on chronic systolic HF complicated by severe refractory CS requiring ECMO, early mortality is similar to that of patients with AMI-related CS but predictors of outcomes differ substantially. DM is a powerful predictor of in-hospital mortality, possibly related to the difficulty achieving end-organ salvage despite ECMO among diabetics. The majority of survivors required durable LVAD therapy for hospital discharge but were likely too acutely ill on presentation to undergo this surgery. This highlights the importance of considering candidacy for durable LVAD and/or HT at the time of ECMO evaluation and initiation of acute circulatory support devices to stabilize patients prior to the onset of multi-organ failure.
Acknowledgements:
Dr. Garan is supported by National Institutes of Health Grant No. KL2TR001874, has previously received honoraria from Abiomed (Danvers, MA, USA) and is now an unpaid consultant for Abiomed. Dr. Naka has received consulting fees from St. Jude Medical (St. Paul, MN, USA).
Disclosures:
Dr. Garan is supported by National Institutes of Health Grant No. KL2TR001874, has previously received honoraria from Abiomed (Danvers, MA) and is now an unpaid consultant for Abiomed.
Dr. Naka has received consulting fees from St. Jude Medical/Abbott Vascular (St. Paul, MN).
References:
- 1.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-Long Trends (2001–2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 5.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. [DOI] [PubMed] [Google Scholar]
- 6.Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–7. [DOI] [PubMed] [Google Scholar]
- 7.Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–11. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW, Kormos RL, Young JB, Kirklin JK & Hunt SA Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant 2016;35:547–9. [DOI] [PubMed] [Google Scholar]
- 9.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42:370–8. [DOI] [PubMed] [Google Scholar]
- 10.Garan AR, Eckhardt C, Takeda K, et al. Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2017; on-line ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truby L, Naka Y, Kalesan B, et al. Important role of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Eur J Cardiothorac Surg 2015;48:322–8. [DOI] [PubMed] [Google Scholar]
- 12.Lee WC, Fang CY, Chen HC, et al. Associations with 30-day survival following extracorporeal membrane oxygenation in patients with acute ST segment elevation myocardial infarction and profound cardiogenic shock. Heart Lung. 2016;45:532–537. [DOI] [PubMed] [Google Scholar]
- 13.Chung SY, Tong MS, Sheu JJ, et al. Short-term and long-term prognostic outcomes of patients with ST-segment elevation myocardial infarction complicated by profound cardiogenic shock undergoing early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention. Int J Cardiol. 2016;223:412–417. [DOI] [PubMed] [Google Scholar]
- 14.Dangers L, Bréchot N, Schmidt M, et al. Extracorporeal Membrane Oxygenation for Acute Decompensated Heart Failure. Crit Care Med 2017;45:1359–1366. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 2017;52:1055–1061. [DOI] [PubMed] [Google Scholar]
- 16.Pöss J, Köster J, Fuernau G, et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:1913–1920. [DOI] [PubMed] [Google Scholar]
- 17.Bermudez CA, Rocha RV, Toyoda Y, et al. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg 2011;92:2125–31. [DOI] [PubMed] [Google Scholar]
- 18.Truby L, Mundy L, Kalesan B, et al. Contemporary Outcomes of Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock at a Large Tertiary Care Center. ASAIO J 2015;61:403–9. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246–56. [DOI] [PubMed] [Google Scholar]
- 20.Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340–8. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 23.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 24.Abrams DC, Prager K, Blinderman CD, Burkart KM, Brodie D. Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest. 2014;145:876–882. [DOI] [PubMed] [Google Scholar]


