Abstract
Objective.
To assess the development of a positive child health index (PCHI) based on 11 adverse outcomes and evaluate PCHI association with quality of life (QoL) scores in a preterm cohort.
Study design.
A total of 889 children enrolled in the Extremely Low Gestational Age Newborn (ELGAN) study in 2002–2004 were followed up at 10 years of age. A parent/caregiver completed questionnaires for child QoL, asthma, visual or hearing impairment, gross motor function impairment, epilepsy, attention deficit/hyperactivity disorder, anxiety, and depression. The child was assessed for cognitive impairment, autism, and obesity. PCHI scores were computed, and linear regression models were used to evaluate the relationship between QoL categories (psychosocial, physical, emotional, social, school, and total) and the PCHI (dichotomized and coded as a multi-level categorical predictor) and to assess sex differences.
Results.
Among ELGAN children, higher PCHI scores were associated with higher reported QoL scores for all QoL categories. Children with no disorders and a PCHI of 100% had PedsQL total scores that were 11 points higher than children with one or more adverse outcomes (PCHI < 100%). Boys had lower QoL scores for categories total, psychosocial, social, and school.
Conclusions.
Positive child health assessed using a quantitative PCHI was associated with QoL across the ELGAN cohort at school age. In the current study, PCHI encompassed 11 outcomes assessed in ELGANs. Future research could include an enhanced panel of child health outcomes to support the use of PCHI as an indicator of positive child health.
Positive child health is the absence of aberrant conditions or disease along with positive physical, cognitive, and social-emotional well-being, and is a foundational element for later health and wellness. Factors that influence positive health outcomes in children born extremely preterm (< 28 weeks of gestation) are understudied. It is known that preterm infants are at increased risk of a variety of adverse developmental and health outcomes [1–6]. Epidemiologic studies involving children born extremely preterm have focused most often on the prevalence and antecedents of adverse outcomes [7]. Such studies can inform efforts to prevent neurodevelopmental impairment, yet they have had limited impact in mitigating impairment.
An alternative approach to improving the quality of life (QoL) for individuals born extremely preterm would be to identify factors associated with favorable outcomes. A first step in the process to optimize the health of preterm infants is a research definition for positive child health which is associated with better QoL. The aim of this study was to develop a quantitative framework, the positive child health index (PCHI), as a metric for assessing positive child health. Data available from the Extremely Low Gestational Age Newborn (ELGAN) study of ten-year-old children born extremely preterm in the United States included information about 11 health and developmental disorders. To assess the construct validity of our approach to positive child health we evaluated the relationship of the number of disorders (the PCHI) to parent-reported QoL [8]. To assess convergent validity, we assessed whether PCHI is higher among girls, because girls born extremely preterm have better neurodevelopmental outcomes than boys. We hypothesized that ELGAN children with a higher PCHI (lower number of disorders) would have higher parent-reported QoL and that girls would have a higher PCHI than boys.
METHODS
STROBE cohort reporting guidelines were utilized for this study [9]. From 2002–2004, women giving birth at <28 weeks of gestation at one of 14 academic medical centers in five states in the United States were asked to enroll in the ELGAN study. Maternal consent was provided either upon hospital admission or before or shortly after delivery. The Institutional Review Board at each participating institution approved procedures employed during this study. Of the mothers approached, approximately 85% gave consent for participation in the original ELGAN study, resulting in a cohort of 1,249 mothers and 1,506 infants.
10-year follow up
As shown in appendix Figure 1, 1,198 children (80% of those enrolled) survived to age 10 years. A subset of 966 eligible children were selected for follow up at 10 years of age because neonatal blood spots had been collected from these children and the primary goal of the ELGAN study was to evaluate associations between neonatal systemic inflammation and cognitive outcome at 10 years of age. Of the 966 children recruited, a total of 889 (92%) participated in some or all the 10-year evaluations.
Participating families completed one visit during which measures reported herein were administered in 3 to 4 hours, including breaks. While the child was tested, the parent or caregiver completed questionnaires. Eleven disorders were investigated: moderate/severe cognitive impairment [6], bilateral blindness [10], hearing impairment [10], gross motor function impairment [6]; epilepsy [5]; attention-deficit/ hyperactivity disorder (ADHD) [11]; autism [4]; anxiety, depression; asthma; and obesity (i.e., body mass index (BMI) above the 95 percentile). These were used to generate a PCHI. Children with no disorders were assigned the highest PCHI of 100%. Any additional disorder reported for a child dropped the PCHI by a percentage based on the number of disorders investigated. Specifically, a 9.1% decrease for each additional disorder was calculated. This calculation based on the inclusion of 11 total disorders (e.g. 100/11 = 9.1). The maximum number of disorders reported was 8 corresponding to a rounded PCHI score of 27%. Extended methods can be found in the appendix.
Pediatric Quality of Life Inventory (PedsQL).
For the quality of life (QoL) measures, a parent or caregiver completed the PedsQL 4.0 generic core scales at 10-year follow-up. The 23-item PedsQL was designed to measure the core dimensions of QoL in areas of physical (8 items), emotional (5 items), social (5 items), and school (5 items) functioning, with each item scored on a five-point Likert scale. Each dimension is summed and linearly transformed to a 0–100 scale with higher scores indicate better QoL. The PedsQL Total summary score incorporates all areas of functioning (23 items), and the PedsQL Psychosocial incorporates the emotional, social and school functioning areas (15 items). The reliability and the validity of the PedsQL generic core scale have been demonstrated in a large population of enrollees in the Children’s Health Insurance Program in the state of California [8].
Statistical analyses.
To assess the relationship between PCHI and QoL, two sets of linear regression models were fit using each QoL outcome (total, psychosocial, physical, emotional, social, school) based on different coding of the PCHI. In the first set of linear regression models, the number of disorders was dichotomized to no disorders vs any disorders (PCHI 100% vs 91% and below). In the second set of linear regression models, the number of disorders was coded as a categorical variable with separate levels for zero to five or more disorders (PCHI 100% to 55% and below). The advantage of this categorical coding of number of disorders rather than a continuous coding is that the impact on QoL is not assumed to be linear in the number of disorders. Furthermore, the PCHI is intrinsically a categorical variable with 11 attainable values. To adjust for potential confounding, we compared the results of models in which no demographic covariates were entered and models that included the binary covariates of public insurance and sex, and the continuous covariates of gestational age, and birth weight z-score. As an indicator of fetal growth restriction, we used the infant’s birth weight z-score, defined as the number of standard deviations (SDs) above or below the median weight of infants of the same gestational age in referent samples not delivered for preeclampsia or fetal indications [12–13].
RESULTS
Study participants (Tables 1, 2 and 3)
Table 1.
ELGAN maternal and newborn demographics (N=889).
| Overall (N=889) |
Male (N=455) |
Female (N=434) |
p-value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age, years | ||||
| < 21 | 115 (13%) | 50 (11%) | 65 (15%) | 0.194 |
| 21 – 35 | 594 (67%) | 313 (69%) | 281 (65%) | |
| > 35 | 180 (20%) | 92 (20%) | 88 (20%) | |
| Education, years | ||||
| <= 12 | 367 (41%) | 184 (40%) | 183 (42%) | 0.313 |
| 13 – 15 | 210 (24%) | 101 (22%) | 109 (25%) | |
| >= 16 | 312 (35%) | 170 (37%) | 142 (33%) | |
| Public insurance | ||||
| Yes | 307 (35%) | 146 (32%) | 161 (38%) | 0.083 |
| No | 568 (65%) | 305 (68%) | 263 (62%) | |
| BMI | ||||
| Underweight | 68 (8%) | 34 (8%) | 34 (8%) | 0.896 |
| Normal | 429 (50%) | 228 (51%) | 201 (49%) | |
| Overweight | 166 (19%) | 83 (19%) | 83 (20%) | |
| Obese | 194 (23%) | 101 (23%) | 93 (23%) | |
| IQ Z-score | ||||
| <= −2 | 33 (4%) | 20 (5%) | 13 (3%) | 0.625 |
| > −2, <= −1 | 62 (7%) | 32 (8%) | 30 (7%) | |
| > −1, <= 1 | 607 (72%) | 300 (71%) | 307 (74%) | |
| > 1 | 139 (17%) | 72 (17%) | 67 (16%) | |
| Single marital status | ||||
| Yes | 353 (40%) | 176 (39%) | 177 (41%) | 0.522 |
| No | 536 (60%) | 279 (61%) | 257 (59%) | |
| Racial identity | ||||
| White | 562 (63%) | 294 (65%) | 268 (62%) | 0.497 |
| Black | 227 (26%) | 115 (25%) | 112 (26%) | |
| Other | 98 (11%) | 45 (10%) | 53 (12%) | |
| Hispanic | ||||
| Yes | 86 (10%) | 37 (8%) | 49 (11%) | 0.104 |
| No | 800 (90%) | 418 (92%) | 382 (89%) | |
| Newborn characteristics | ||||
| Gestational age, weeks | ||||
| 23 | 47 (5%) | 24 (5%) | 23 (5%) | 0.189 |
| 24 | 140 (16%) | 82 (18%) | 58 (13%) | |
| 25 | 179 (20%) | 98 (22%) | 81 (19%) | |
| 26 | 221 (25%) | 105 (23%) | 116 (27%) | |
| 27 | 302 (34%) | 146 (32%) | 156 (36%) | |
| Birth weight, grams | ||||
| <= 750 | 332 (37%) | 154 (34%) | 178 (41%) | 0.027 |
| 751 – 1000 | 382 (43%) | 198 (44%) | 184 (42%) | |
| > 1000 | 175 (20%) | 103 (23%) | 72 (17%) | |
| Birth weight Z-score | ||||
| < −2 | 53 (6%) | 17 (4%) | 36 (8%) | <0.001 |
| >= −2, < −1 | 120 (13%) | 41 (9%) | 79 (18%) | |
| >= −1 | 716 (81%) | 397 (87%) | 319 (74%) | |
| Cesarean delivery | ||||
| Yes | 590 (66%) | 290 (64%) | 300 (69%) | 0.089 |
| No | 299 (34%) | 165 (36%) | 134 (31%) |
Table 2.
Characteristics of the 966 children eligible for recruitment classified by whether or not they were assessed at age 10 years. These are column percents.
| Assessed | Row | |||
|---|---|---|---|---|
| Yes | No | n | ||
|
Maternal characteristics | ||||
| Age, years | < 21 | 13 | 13 | 125 |
| 21–35 | 67 | 74 | 654 | |
| > 35 | 20 | 13 | 187 | |
| Education, years | ≤12 | 41 | 55 | 395 |
| > 12, < 16 | 23 | 26 | 221 | |
| ≥ 16 | 36 | 19 | 320 | |
| Single marital status | Yes | 40 | 54 | 398 |
| Public insurance | Yes | 35 | 57 | 352 |
| Racial identity | White | 63 | 50 | 588 |
| Black | 26 | 33 | 256 | |
| Other | 11 | 17 | 111 | |
| Hispanic | Yes | 10 | 12 | 97 |
|
Newborn characteristics | ||||
| Sex | Male | 51 | 57 | 498 |
| Gestational age, weeks | 23–24 | 21 | 19 | 198 |
| 25–26 | 45 | 55 | 446 | |
| 27 | 34 | 26 | 322 | |
| Birth weight, grams | ≤ 750 | 37 | 32 | 353 |
| 751–1000 | 43 | 57 | 431 | |
| > 1000 | 20 | 11 | 182 | |
| Birth weight Z-score | < −2 | 6 | 3 | 54 |
| ≥ −2, < −1 | 13 | 16 | 131 | |
| ≥ −1 | 81 | 81 | 781 | |
|
Postnatal characteristics | ||||
| Echolucent lesion | Yes | 6 | 8 | 62 |
| Ventriculomegaly | Yes | 11 | 2 | 94 |
| Necrotizing enterocolitis, | Yes | 3 | 4 | 34 |
| Bell stage 3b Retinopathy of prematurity, prethreshold | Yes | 13 | 13 | 125 |
| Bronchopulmonary dysplasia, O2 at 36 wks | Yes | 52 | 52 | 500 |
| Maximum column N | 873 | 93 | 966 | |
Table 3.
Missing data by participant. All variables reflect child characteristic at age 10 unless otherwise noted.
| Variable | Missing |
|---|---|
| Maternal Age | 0 (0%) |
| Maternal Education | 0 (0%) |
| Marital Status | 0 (0%) |
| Maternal Public Insurance | 14 (2%) |
| Maternal BMI | 32 (4%) |
| Maternal IQ Z-score | 48 (5%) |
| Maternal Race | 2 (<1%) |
| Maternal Ethnicity | 3 (<1%) |
| Sex | 0 (0%) |
| Birth Weight | 0 (0%) |
| Birth Weight Z-score | 0 (0%) |
| Gestational Age | 0 (0%) |
| Cesarean Delivery | 0 (0%) |
| Cognitive Impairment | 15 (2%) |
| Asthma | 0 (0%) |
| Epileptic Seizures | 1 (<1%) |
| GMF Impairment | 0 (0%) |
| Autism | 32 (4%) |
| BMI 95th percentile | 18 (2%) |
| Deafness | 0 (0%) |
| Bilateral blindness | 0 (0%) |
| ADHD | 15 (2%) |
| Anxiety | 14 (2%) |
| Depression | 15 (2%) |
| Symptom Count (Derived Variable) | 0 (0%) |
| PedsQL Total | 16 (2%) |
| PedsQL Psychosocial | 16 (2%) |
| PedsQL Physical | 16 (2%) |
| PedsQL Emotional | 16 (2%) |
| PedsQL Social | 16 (2%) |
| PedsQL School | 16 (2%) |
Characteristics of the 889 study participants are shown in Table 1. Table 2 compares the maternal and newborn characteristics of the 889 children who were assessed and the 77 children who were not assessed from among the 966 children eligible for study participation. The rates of missing data among the 889 participants are provided in Table 3. Although data were missing for some of the individual disorders (0–4%), each child was assigned a PCHI that reflected their available data.
The majority (63%) of mothers identified as white; 26% identified as black; and 10% identified as Hispanic. A total of 41% had received 12 or fewer years of formal education and 35% were eligible for public medical insurance. Of the enrolled mothers, 19% were obese prior to pregnancy.
Of the ELGANs, 51% percent were boys and 49% were girls. Twenty-one percent were born at 23–24 weeks of gestation. Six percent had fetal growth restriction based on a birth weight z-score below −2 standard deviations.
Health and developmental disorders — (Tables 4 and 5, Figure 2)
Table 4.
ELGAN child health characteristics at age 10 by sex (N=889).
| Disorder | Overall (N=889) n (%) |
Male (N=455) n (%) |
Female (N=434) n (%) |
aORI (95% CI) (M vs F) |
p-value |
|---|---|---|---|---|---|
| Moderate/severe cognitive impairment | 214 (24%) | 127 (28%) | 87 (20%) | 1.7 (1.2, 2.4) | 0.002 |
| Bilateral blindness | 9 (1%) | 5 (1%) | 4 (1%) | 1.2 (0.3, 4.8) | 0.749 |
| Deafness | 41 (5%) | 20 (4%) | 21 (5%) | 0.8 (0.4, 1.5) | 0.464 |
| Gross motor function impairment | 97 (11%) | 55 (12%) | 42 (10%) | 1.2 (0.8, 1.9) | 0.345 |
| Epilepsy | 66 (7%) | 37 (8%) | 29 (7%) | 1.3 (0.8, 2.3) | 0.266 |
| ADHD | 152 (17%) | 98 (22%) | 54 (13%) | 2.0 (1.4, 2.9) | <.001 |
| Autism | 61 (7%) | 41 (9%) | 20 (5%) | 2.1 (1.2, 3.8) | 0.010 |
| Asthma | 335 (38%) | 172 (38%) | 163 (38%) | 1.0 (0.8, 1.3) | 0.982 |
| BMI 95th percentile | 101 (12%) | 46 (10%) | 55 (13%) | 0.7 (0.5, 1.1) | 0.155 |
| Anxiety | 132 (15%) | 68 (15%) | 64 (15%) | 1.1 (0.7, 1.6) | 0.625 |
| Depression | 59 (7%) | 30 (7%) | 29 (7%) | 1.0 (0.6, 1.7) | 0.958 |
Adjusted for public insurance, and child gestational age and birth weight Z-score.
Table 5.
Number of disorders for ELGANs at 10 years of age by sex. (N=889)
| Number of disorders | Overall (N=889) n (%) |
Male (N=455) n (%) |
Female (N=434) n (%) |
aORI (95% CI) (M vs F) |
p-value |
|---|---|---|---|---|---|
| 1+ vs. 0 | 603 (68%) | 318 (70%) | 285 (66%) | 1.3 (0.9, 1.7) | 0.132 |
| 2+ vs. 0–1 | 353 (40%) | 200 (44%) | 153 (35%) | 1.4 (1.1, 1.9) | 0.013 |
| 3+ vs. 0–2 | 191 (21%) | 113 (25%) | 78 (18%) | 1.6 (1.1, 2.2) | 0.009 |
| 4+ vs. 0–3 | 77 (9%) | 47 (10%) | 30 (7%) | 1.5 (0.9, 2.5) | 0.103 |
| 5+ vs. 0–4 | 29 (3%) | 17 (4%) | 12 (3%) | 1.2 (0.6, 2.7) | 0.578 |
| 6+ vs. 0–5 | 10 (1%) | 4 (1%) | 6 (1%) | 0.6 (0.2, 2.2) | 0.440 |
| 7+ vs. 0–6 | 3 (<1%) | 3 (1%) | 0.945 | ||
| 8+ vs. 0–7 | 1 (<1%) | 1 (<1%) | 0.493 |
Adjusted for public insurance, and child gestational age and birth weight Z-score.
Figure 2.
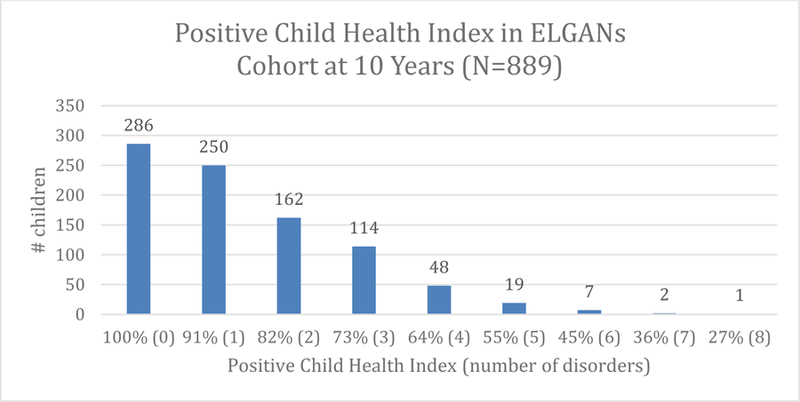
Positive Child Health Index (PCHI) in ELGANs cohort at 10 years (n = 889).
Prevalence for the 11 health and developmental disorders that we considered in this study ranged from 1% for bilateral blindness to 38% for asthma. Boys were more likely to have moderate/severe cognitive impairment, ADHD, and autism; the other 8 outcomes were not associated with sex (Table 4). Boys were more likely to have two or more disorders and three or more disorders than girls (Table 5). Statistical significance for sex differences in Tables 4 & 5 were derived from logistic regression models which adjusted for public insurance, gestational age and birth weight z-score. When participants were classified according to the PCHI, 32% of children had a PCHI of 100% (0 of the 11 disorders) (Figure 2).
Parent-reported QoL as a function of disorder assessment – (Table 6 and 7)
Table 6.
Parent reported QoL from PedsQL assessment linear regression with disorders (n = 859). Analysis is adjusted for sex, public insurance, gestational age, and birth weight. Separate models are fit for binary and categorical classifications of disorders.
| Binary | Total | Psychosocial | Physical | Emotional | Social | School | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val |
| PCHI 100% | 78.1 | 1.8 | <.001 | 74.7 | 1.8 | <.001 | 84.6 | 2.3 | <.001 | 78.7 | 2.3 | <.001 | 77.1 | 2.4 | <.001 | 68.3 | 2.3 | <.001 |
| PCHI 91% and below | 67.0 | 1.5 | <.001 | 63.5 | 1.5 | <.001 | 73.5 | 1.9 | <.001 | 71.8 | 1.9 | <.001 | 63.6 | 1.9 | <.001 | 55.2 | 1.9 | <.001 |
| Sex = Female | 2.6 | 1.0 | 0.012 | 3.6 | 1.1 | <.001 | 0.7 | 1.3 | 0.618 | 0.6 | 1.3 | 0.662 | 3.8 | 1.4 | 0.006 | 6.4 | 1.3 | <.001 |
| No Public Insurance | 6.0 | 1.1 | <.001 | 6.1 | 1.1 | <.001 | 5.9 | 1.4 | <.001 | 3.0 | 1.4 | 0.036 | 5.6 | 1.4 | <.001 | 9.7 | 1.4 | <.001 |
| +1 Week Gestation | 1.5 | 0.4 | <.001 | 1.5 | 0.4 | <.001 | 1.5 | 0.5 | 0.004 | 0.7 | 0.5 | 0.180 | 2.5 | 0.5 | <.001 | 1.3 | 0.5 | 0.012 |
| Birth Weight Z-Score | 0.2 | 0.5 | 0.676 | 0.3 | 0.5 | 0.539 | −0.0 | 0.6 | 0.997 | −0.7 | 0.6 | 0.253 | 0.3 | 0.6 | 0.640 | 1.3 | 0.6 | 0.030 |
|
Tests for Group Differences | ||||||||||||||||||
| PCHI 100% vs 91% and below | 11.2 | 1.1 | <.001 | 11.2 | 1.2 | <.001 | 11.1 | 1.4 | <.001 | 6.8 | 1.4 | <.001 | 13.5 | 1.5 | <.001 | 13.2 | 1.4 | <.001 |
| Categorical | Total | Psychosocial | Physical | Emotional | Social | School | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val |
| PCHI 100% | 83.0 | 1.6 | <.001 | 79.3 | 1.7 | <.001 | 89.8 | 2.2 | <.001 | 83.0 | 2.3 | <.001 | 82.3 | 2.3 | <.001 | 72.6 | 2.2 | <.001 |
| PCHI 91% | 78.1 | 1.6 | <.001 | 74.4 | 1.7 | <.001 | 84.9 | 2.2 | <.001 | 81.7 | 2.3 | <.001 | 75.8 | 2.3 | <.001 | 65.7 | 2.2 | <.001 |
| PCHI 82% | 70.2 | 1.6 | <.001 | 65.8 | 1.7 | <.001 | 78.3 | 2.1 | <.001 | 74.1 | 2.2 | <.001 | 67.2 | 2.2 | <.001 | 56.2 | 2.1 | <.001 |
| PCHI 73% | 66.5 | 1.7 | <.001 | 62.1 | 1.8 | <.001 | 74.7 | 2.3 | <.001 | 72.6 | 2.4 | <.001 | 60.3 | 2.4 | <.001 | 53.3 | 2.3 | <.001 |
| PCHI 64% | 62.4 | 2.3 | <.001 | 61.7 | 2.4 | <.001 | 63.9 | 3.0 | <.001 | 67.2 | 3.2 | <.001 | 62.3 | 3.2 | <.001 | 55.6 | 3.1 | <.001 |
| PCHI 55% and below | 44.6 | 2.7 | <.001 | 43.5 | 2.9 | <.001 | 46.7 | 3.6 | <.001 | 51.4 | 3.7 | <.001 | 40.6 | 3.7 | <.001 | 38.5 | 3.7 | <.001 |
| Sex = Female | 1.8 | 0.9 | 0.051 | 2.9 | 1.0 | 0.004 | −0.2 | 1.2 | 0.878 | −0.1 | 1.3 | 0.953 | 2.9 | 1.3 | 0.023 | 5.8 | 1.3 | <.001 |
| No Public Insurance | 3.4 | 1.0 | <.001 | 3.6 | 1.1 | <.001 | 3.1 | 1.3 | 0.022 | 0.6 | 1.4 | 0.655 | 2.8 | 1.4 | 0.043 | 7.4 | 1.4 | <.001 |
| +1 Week Gestation | 0.7 | 0.4 | 0.059 | 0.7 | 0.4 | 0.067 | 0.7 | 0.5 | 0.188 | −0.0 | 0.5 | 0.991 | 1.6 | 0.5 | 0.002 | 0.6 | 0.5 | 0.245 |
| Birth Weight Z-Score | 0.4 | 0.4 | 0.396 | 0.5 | 0.5 | 0.312 | 0.2 | 0.6 | 0.758 | −0.5 | 0.6 | 0.389 | 0.4 | 0.6 | 0.488 | 1.5 | 0.6 | 0.011 |
|
Tests for Group Differences | ||||||||||||||||||
| PCHI 100% vs 91% | 4.9 | 1.2 | <.001 | 4.9 | 1.2 | <.001 | 4.9 | 1.6 | 0.002 | 1.3 | 1.6 | 0.430 | 6.5 | 1.6 | <.001 | 6.9 | 1.6 | <.001 |
| PCHI 91% vs 82% | 7.9 | 1.4 | <.001 | 8.6 | 1.5 | <.001 | 6.7 | 1.8 | <.001 | 7.6 | 1.9 | <.001 | 8.6 | 1.9 | <.001 | 9.6 | 1.9 | <.001 |
| PCHI 82% vs 73% | 3.7 | 1.7 | 0.027 | 3.8 | 1.8 | 0.036 | 3.6 | 2.2 | 0.111 | 1.5 | 2.3 | 0.506 | 6.9 | 2.3 | 0.003 | 2.9 | 2.3 | 0.208 |
| PCHI 73% vs 64% | 4.0 | 2.4 | 0.095 | 0.4 | 2.6 | 0.884 | 10.8 | 3.2 | <.001 | 5.4 | 3.3 | 0.105 | −1.9 | 3.3 | 0.562 | −2.3 | 3.3 | 0.477 |
| PCHI 64% vs 55% and below | 17.8 | 3.2 | <.001 | 18.2 | 3.4 | <.001 | 17.1 | 4.3 | <.001 | 15.8 | 4.5 | <.001 | 21.6 | 4.5 | <.001 | 17.1 | 4.4 | <.001 |
Table 7.
Unadjusted Parent reported QoL from PedsQL assessment linear regression with disorder (n = 873). Separate models are fit for binary and categorical classifications of disorders.
| Total | Psychosocial | Physical | Emotional | Social | School | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | Parm Est |
Std Err |
P-val | |
| Binary Model | ||||||||||||||||||
| PCHI 100% | 88.6 | 0.9 | <.001 | 85.7 | 0.9 | <.001 | 94.0 | 1.1 | <.001 | 83.4 | 1.1 | <.001 | 90.7 | 1.2 | <.001 | 82.9 | 1.2 | <.001 |
| PCHI 91% and below | 75.7 | 0.6 | <.001 | 72.7 | 0.7 | <.001 | 81.2 | 0.8 | <.001 | 75.9 | 0.8 | <.001 | 75.2 | 0.8 | <.001 | 67.1 | 0.8 | <.001 |
|
Tests for Group Differences | ||||||||||||||||||
| PCHI 100% vs 91% and below | 12.9 | 1.1 | <.001 | 13.0 | 1.1 | <.001 | 12.8 | 1.4 | <.001 | 7.5 | 1.4 | <.001 | 15.6 | 1.4 | <.001 | 15.8 | 1.4 | <.001 |
|
| ||||||||||||||||||
|
Categorical Model | ||||||||||||||||||
| PCHI 100% | 88.6 | 0.8 | <.001 | 85.7 | 0.8 | <.001 | 94.0 | 1.0 | <.001 | 83.4 | 1.1 | <.001 | 90.7 | 1.1 | <.001 | 82.9 | 1.1 | <.001 |
| PCHI 91% | 83.5 | 0.8 | <.001 | 80.5 | 0.9 | <.001 | 88.9 | 1.1 | <.001 | 82.3 | 1.2 | <.001 | 84.1 | 1.2 | <.001 | 75.3 | 1.2 | <.001 |
| PCHI 82% | 74.5 | 1.1 | <.001 | 70.8 | 1.1 | <.001 | 81.4 | 1.4 | <.001 | 74.4 | 1.5 | <.001 | 73.9 | 1.5 | <.001 | 64.1 | 1.5 | <.001 |
| PCHI 73% | 70.4 | 1.3 | <.001 | 66.6 | 1.4 | <.001 | 77.6 | 1.7 | <.001 | 72.9 | 1.8 | <.001 | 66.8 | 1.8 | <.001 | 60.2 | 1.8 | <.001 |
| PCHI 64% | 66.0 | 2.0 | <.001 | 66.0 | 2.1 | <.001 | 66.0 | 2.6 | <.001 | 68.0 | 2.7 | <.001 | 68.2 | 2.8 | <.001 | 61.9 | 2.8 | <.001 |
| PCHI 55% and below | 48.1 | 2.5 | <.001 | 47.6 | 2.7 | <.001 | 49.1 | 3.3 | <.001 | 51.6 | 3.5 | <.001 | 46.3 | 3.5 | <.001 | 44.8 | 3.5 | <.001 |
|
Tests for Group Differences | ||||||||||||||||||
| PCHI 100% vs 91% | 5.1 | 1.2 | <.001 | 5.1 | 1.2 | <.001 | 5.0 | 1.5 | 0.001 | 1.1 | 1.6 | 0.478 | 6.7 | 1.6 | <.001 | 7.6 | 1.6 | <.001 |
| PCHI 91% vs 82% | 9.0 | 1.4 | <.001 | 9.8 | 1.5 | <.001 | 7.5 | 1.8 | <.001 | 8.0 | 1.9 | <.001 | 10.2 | 1.9 | <.001 | 11.1 | 1.9 | <.001 |
| PCHI 82% vs 73% | 4.0 | 1.7 | 0.016 | 4.2 | 1.8 | 0.021 | 3.8 | 2.2 | 0.085 | 1.5 | 2.3 | 0.523 | 7.1 | 2.3 | 0.002 | 3.9 | 2.3 | 0.093 |
| PCHI 73% vs 64% | 4.4 | 2.4 | 0.062 | 0.6 | 2.5 | 0.814 | 11.6 | 3.1 | <.001 | 4.9 | 3.3 | 0.132 | −1.4 | 3.3 | 0.659 | −1.7 | 3.3 | 0.615 |
| PCHI 64% vs 55% and below | 17.9 | 3.2 | <.001 | 18.5 | 3.4 | <.001 | 16.9 | 4.2 | <.001 | 16.4 | 4.4 | <.001 | 22.0 | 4.4 | <.001 | 17.1 | 4.5 | <.001 |
The PCHI was associated with each PedsQL measure when dichotomized and when evaluated as a multi-level categorical predictor. Linear models were fit without an overall intercept term so that the PCHI parameter estimates correspond to the average PedsQL score for individuals in the associated PCHI category when matched on other characteristics. Covariates were included in each regression analysis to address potential confounding. Within the models, 859 participants are reported in the adjusted analysis (Table 6) and 873 in the unadjusted analysis (Table 7) due to missing demographic data for 14 subjects (public insurance). There is complete agreement between the statistical significance of all tests for group differences between the adjusted and unadjusted analyses for both the dichotomized and coded as a multi-level categorical representation for PCHI.
In the linear regression model using dichotomized PCHI, a PCHI of 100% is associated with an 11.2-point increase in PedsQL total score when compared with subjects with PCHI of 91% and below (p<0.001) and increases across the other domains of QoL between 6.8 and 13.5 points (p<0.001). Girls were estimated to have scores 2.6 points higher on the PedsQL total assessment (p=0.012), and statistically significantly higher scores were also observed in the psychosocial, social, and school domains.
In the multi-level categorical predictor, a PCHI of 100% was associated with a 4.9-point increase in PedsQL total score when compared with subjects with PCHI of 91% (p<0.001), and there was a 7.9-point increase from PCHI 91% to PCHI 82% (p<0.001). Across all domains of PedsQL, a PCHI of 64% vs 55% and below was associated with an increase of 15.8–21.6 points of QoL (p<0.001). The tests for group differences show the magnitude and statistical significance of the differences in each adjacent category of PCHI for each domain of PedsQL. These tests demonstrate a consistent positive association between PCHI and QoL that has varying magnitudes across PCHI groups and domains of QoL. PedsQL domains for psychosocial, social, and school (p<0.05) were significantly higher for girls, and PedsQL total (p=0.051) was borderline significantly higher for girls.
DISCUSSION
The aim of this study was to develop a quantitative index for defining positive child health using data from the ELGAN cohort, a large and diverse cohort of ten-year-old children born extremely preterm in the United States. We considered parent-reported QoL and data on the absence or presence of asthma, obesity, and nine neurodevelopmental and neurobehavioral outcomes, including cognitive impairment, bilateral blindness, hearing impairment, gross motor function impairment, epilepsy, ADHD, autism, anxiety, and depression. From these 11 disorders, we established a quantitative tool, the PCHI, and assessed if high PCHI scores captured children with high QoL scores. The utility of the PCHI is supported by our finding that it was positively associated with parent-reported QoL.
PCHI scores were higher among ELGAN girls compared with boys, as would be expected based on the higher prevalence of some developmental impairments among boys. Preterm boys have higher risks for autism, ADHD, and cognitive impairment compared with girls, and similar sex differences have been reported by others [6, 14–18]. The reasons for higher vulnerability of boys are not well understood. Irrespective of the mechanisms that underlie the higher risk among boys, the implication for clinicians is that preterm boys are a particularly vulnerable group that warrants enhanced monitoring of health and development. In addition to lower PCHI scores, boys had lower QoL scores for categories total, psychosocial, social, and school. Success in both domains probably depends in part on cognitive abilities, and in the ELGAN cohort cognitive impairment is more frequent among boys than girls. In addition, the increased prevalence of ADHD and autism among ELGAN boys likely impact boys’ performances in school and social aspects of daily life. Other QoL studies have shown girls tend to score lower in physical functioning health related QoL compared with boys [19], although some studies do not observe any sex differences in health-related QoL [20].
The National Research Council defines child health as the extent to which children can develop and realize their potential, satisfy their needs, and develop the capacities that allow them to interact successfully with their surrounding environments [21]. Similarly, the World Health Organization (WHO) defines child heath as a state of complete physical, mental, and social well-being [22]. Although the absence of any of the disorders assessed for our study should not be regarded as the sole indicator of “positive child health,” the PCHI identified many of the children with high parent-reported QoL, and because positive child health is an outcome of interest in National Institutes of Health’s recently launched Environmental Influences on Child Health Outcomes (ECHO) program, the PCHI could be used to identify children with positive health and help identify factors that promote positive child health outcomes in future studies.
An encouraging finding was that 32% of our extremely high-risk ELGAN cohort were free from all 11 of the disorders that we examined (PCHI 100%), and were more likely to have higher QoL scores. It is promising that a large proportion of ELGAN infants were thriving despite their extremely preterm status. The focus and goal of future analyses will be to identify early life antecedents associated with their high positive health results at 10 years of age using the established PCHI.
An important limitation of our study is that the outcomes were obtained from a cohort evaluated primarily for neurodevelopmental outcomes, rather than a broader profile of disorders, such as cardiometabolic and respiratory illnesses. In addition, we did not collect precise information about the severity of most of the disorders we studied, except for cognitive impairment. Thus, a diagnosis of asthma probably included a wide range of severity whereas for most of the neurodevelopmental outcomes, the diagnosis included only the most severely affected individuals. We expect that the severity of an impairment could be related to the QoL and the likelihood of positive child health. Thus, we posit that a more informative approach than the one described here would include more detailed evaluation of the severity of asthma, obesity, psychiatric, and neurodevelopmental disorders. Last, of the original 966, the 77 study participants lost-to-follow-up were more likely to have indicators of social disadvantage, such as eligibility for public assistance. The bias from lost-to-follow-up children would therefore be expected to result in an underestimation of adverse outcomes in the cohort. However, given the low frequency of lost-to-follow-up children (8%), the magnitude of this bias was likely to be small. Despite these limitations, the significant relationship of the PCHI with QoL shows promise for its use in measuring positive child health outcomes. Current ELGAN follow-up studies are collecting child-reported QoL measures at ages 15 and above because the relationship between parent and child QoL reports has been shown to be complex [23–24]. Strengths of our study include a large sample that was relatively diverse with respect to sociodemographic attributes, and that neurodevelopmental assessments were performed by individuals who were blinded to the child’s parent-reported QoL.
This study revealed that at 10 years of age, ELGAN children with overall fewer disorders and higher PCHI had higher parent-reported QoL, and 32% of the cohort had none of the disorders examined. In addition, girls were more likely to have higher PCHI and higher QoL than boys for PedsQL categories total, psychosocial, social and school. The approach we used to assess positive child health lays a foundation to identify characteristics associated with optimal outcomes among children born extremely preterm at high risk for adverse outcomes. A PCHI, such as the one created here, can be utilized to help further understand influencing factors on positive child health. Additional efforts to utilize this index across populations warrants consideration.
Supplementary Material
Figure 1.
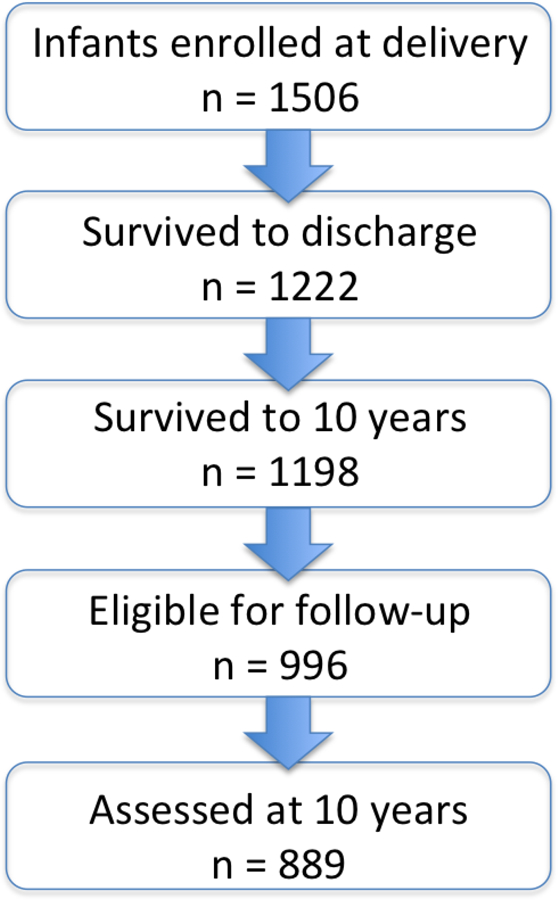
Flow chart of ELGANs enrollment from delivery (2002–2004) to follow up at 10 years of age.
Acknowledgments
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2), the National Institute of Child Health and Human Development (5P30HD018655-34), and the Office of the NIH Director (1UG3OD023348-01)
References
- 1.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med 2014; 19:90–6. [DOI] [PubMed] [Google Scholar]
- 2.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr 2015; 169:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KC. Cognitive functioning at the age of 10 years among children born extremely preterm: a latent profile approach. Pediatr Res 2017; 82:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph RM, O’Shea TM, Allred EN, Heeren T, Hirtz D, Paneth N, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res 2017; 10:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglass LM, Heeren TC, Stafstrom CE, DeBassio W, Allred EN, Leviton A, et al. Cumulative Incidence of Seizures and Epilepsy in Ten-Year-Old Children Born Before 28 Weeks’ Gestation. Pediatr Neurol 2017. [DOI] [PMC free article] [PubMed]
- 6.Kuban KC, Joseph RM, O’shea TM, Allred EN, Heeren T, Douglass L, et al. Girls and boys born before 28 weeks gestation: Risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr 2016; 173:69–75. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child 2017; 102:97–102. [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3:329–41. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies
- 10.Bright HR, Babata KB, Allred EN, Erdei C, Kuban KCK, Joseph RM, et al. Neurocognitive Outcomes at 10 Years of Age in Extremely Preterm Newborns with Late-Onset Bacteremia. J Pediatr 2017; 187:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott MN, Hunter SJ, Joseph RM, O’Shea TM, Hooper SR, Allred EN, et al. Neurocognitive Correlates of Attention-Deficit Hyperactivity Disorder Symptoms in Children Born at Extremely Low Gestational Age. J Dev Behav Pediatr 2017; 38:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev 1987; 15:45–52. [DOI] [PubMed] [Google Scholar]
- 13.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr Res 1999; 46:566. [DOI] [PubMed] [Google Scholar]
- 14.Leviton A, Hooper SR, Hunter SJ, Scott MN, Allred EN, Joseph RM, et al. Antecedents of Screening Positive for Attention Deficit Hyperactivity Disorder in 10-Year Old Children Born Extremely Preterm. Pediatr Neurol 2017. [DOI] [PMC free article] [PubMed]
- 15.Stephens BE, Bann CM, Watson VE, Sheinkopf SJ, Peralta-Carcelen M, Bodnar A, et al. Screening for autism spectrum disorders in extremely preterm infants. J Dev Behav Pediatr 2012; 33: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism, 2015; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsell L, Malouf R, Morris J, Kurinczuk JJ, & Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA pediatr 2015; 169: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loke H, Harley V, & Lee J Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol 2015; 65: 139–150. [DOI] [PubMed] [Google Scholar]
- 19.Tsiros MD, Olds T, Buckley JD, Grimshaw P, Brennan L, Walkley J, et al. Health-related quality of life in obese children and adolescents. Int J Obes 2009; 33: 387. [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Limbers CA, & Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Out 2007; 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council, Children’s health, the nation’s wealth: assessing and improving child health 2004: National Academies Press. [PubMed] [Google Scholar]
- 22.Chatterji S, Ustün BL, Sadana R, Salomon JA, Mathers CD, & Murray CJ. The conceptual basis for measuring and reporting on health. Global Programme on Evidence for Health Policy Discussion Paper 2002; 45. [Google Scholar]
- 23.Berman AH, Liu B, Ullman S, Jadback I, & Engstrom K. Children’s Quality of Life Based on the KIDSCREEN-27: Child Self-Report, Parent Ratings and Child-Parent Agreement in a Swedish Random Population Sample. PloS one 2016; 11; e0150545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sons Lundberg S., Daughters, and Parental Behaviour. Oxf Rev Econ Policy 2005; 21: 340–356. 10.1093/oxrep/gri020 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


