Abstract
Sleep disordered breathing (SDB), which causes sleep deprivation, intermittent hypoxia, and negative intrathoracic pressure swings, can be accompanied by other harmful pathophysiologies relating to cardiovascular diseases (CVD), including sudden death, atrial fibrillation, stroke, and coronary artery disease leading to heart failure. Continuous positive airway pressure (CPAP) therapy for SDB has been reported to provide favorable effects such as lowered systemic blood pressure and improved endothelial function. However, in recent randomized controlled trials, CPAP has failed to demonstrate its beneficial prognostic impact on the primary or secondary setting of CVD. In this review article, we describe the characteristics of SDB complicated with CVD, the prognostic impacts of SDB in CVD, and the beneficial effects of CPAP on CVD.
Keywords: Sleep apnea syndrome, Cerebrovascular disease, Coronary artery disease, Atrial fibrillation, Heart failure
Introduction
Sleep disordered breathing (SDB) is characterized by repetitive episodes of shallow breathing or apnea during sleep1–4), and can result in intermittent hypoxemia. It is typically associated with elevated blood pressure, oxidative stress, inflammation, and hypercoagulation (Fig. 1)2, 3, 5–9). SDB is highly prevalent10–14) and is associated with an elevated risk of serious vascular outcomes2, 3, 15), as well as multiple risk factors, including hypertension5, 16–18), obesity, insulin resistance, and dyslipidemia. Observational studies have indicated that SDB is associated with a high risk of serious cardiovascular disease (CVD), including sudden death, atrial fibrillation (AF), stroke, and coronary artery disease (CAD), leading to heart failure (HF)2–4, 15, 19, 20). Continuous positive airway pressure (CPAP) therapy for SDB has been reported to lower blood pressure14), improve insulin resistance, enhance endothelial function, and increase insulin sensitivity, although improvements in glycemic control and body mass index have not been demonstrated20–23). Thus, patients with SDB are at a significant risk of CVD, and recent randomized controlled trials (RCTs) focusing on treatment using CPAP have failed to demonstrate its beneficial prognostic impact on the primary or secondary setting of CVD18, 23–27). In this review article, we describe the characteristics of SDB complicated with CVD, prognostic impacts of SDB in CVD, and beneficial effects of CPAP on CVD.
Fig. 1.
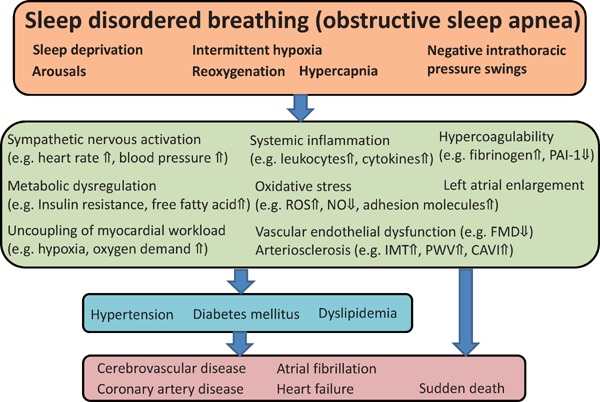
Pathophysiology of the impact of sleep disordered breathing, OSA, on cardiovascular disease
PAI-1, plasminogen activator inhibitor-1; ROS, reactive oxygen species; NO, nitric oxide; FMD, flow-mediated dilatation; IMT, intima-media thickness; PWV, pulse wave velocity; CAVI, cardio-ankle vascular index.
Diagnosis, Definition and Classification of SDB
Overnight polysomnography, which includes assessments of electroencephalogram, electrooculogram, electrocardiogram, and electromyogram, as well as the nasal and oral airflows, respiratory movement (thorax and abdominal respiratory effort), snore, oxygen saturation, body position, and sleep stage, is the gold standard test for SDB (Fig. 2)1, 28). Apnea is the absence of inspiratory airflow for at least 10 s. Hypopnea, a decrease in airflow lasting 10 s or longer, is associated with a drop in arterial oxygen saturation and/or an electroencephalographic arousal.1, 28 The total counts of apnea or hypopnea per hour are defined as the apnea hypopnea index (AHI), and is used to determine SDB: normal, AHI < 5/h; mild, 5 ≤ AHI < 15/h; moderate, 15 ≤ AHI < 30/h; severe, AHI ≥ 30/h. Apnea and hypopnea are classified as obstructive or central, but they both result from an absence or reduction of brainstem neural output to upper airway muscles (e.g., genioglossus) and/or lower thoracic inspiratory pump muscles (diaphragm and intercostal muscles) 1–3). SDB includes obstructive sleep apnea (OSA), central sleep apnea (CSA) with Cheyne–Stokes respiration (CSR), or a combination of both1–3). The pattern of neural output determines the phenotype. OSA is characterized by cessation or marked reduction of airflow in the presence of respiratory effort (Fig. 2). OSA occurs when complete upper airway occlusion occurs (absent airflow, tongue falling backward) in the face of continued activity of inspiratory thoracic pump muscles. In OSA, there is a collapse of the pharynx during sleep with consequent upper airway obstruction, often with snoring. Predisposing factors include obesity, a short neck, and retrognathism. CPAP is well established in clinical guidelines for the treatment of symptomatic OSA (e.g. sleepy) in the non-HF population3), and provides continuous pressure throughout the respiratory cycle, prevents the pharynx from collapsing, and thus suppresses cessation or reduction of airflow and desaturation (Fig. 3). The overnight hypnogram shows an overview of the night's sleep including SDB event, sleep stage, heart rate, and oxygen saturation (Fig. 4). In the present case, CPAP attenuates SDB, and improves sleep quality and heart rate variation (Fig. 4).
Fig. 2.
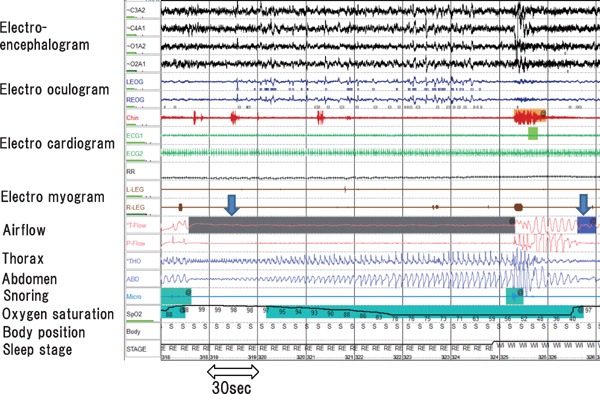
OSA
OSA (downward arrow) is characterized by cessation or marked reduction of the airflow (airflow band) in the presence of respiratory effort (thorax and abdomen band), followed by arousal with breathing resumption.
Fig. 3.
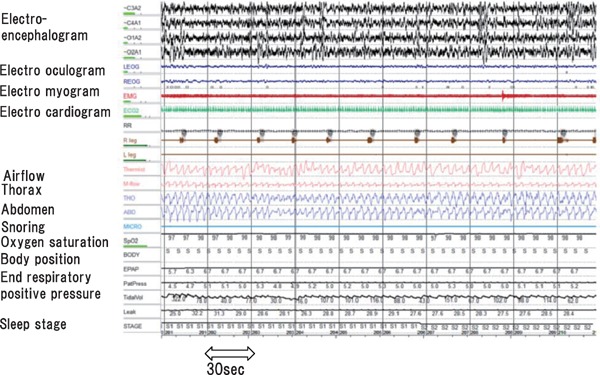
OSA on treatment with positive airway pressure.
CPAP provides continuous pressure throughout the respiratory cycle, preventing the pharynx from collapsing and thus suppresses cessation or reduction of airflow and desaturation.
Fig. 4.
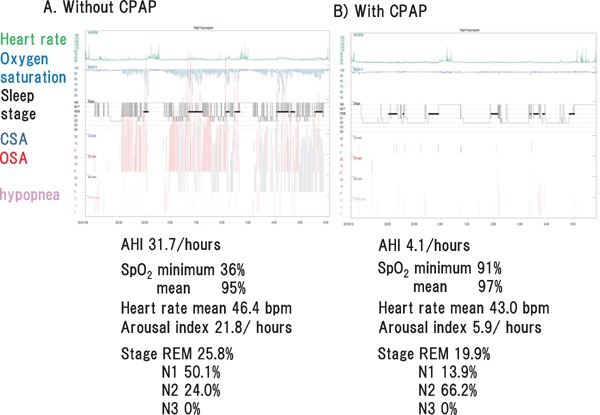
Hypnogram shows an overview of the night's sleep, including SDB, sleep stage, heart rate, and oxygen saturation
In the hypnogram of representative case, CPAP attenuates SDB, and improves both sleep quality and heart rate variation. CSA, central sleep apnea; OSA, obstructive sleep apnea; SpO2, oxygen saturation; AHI, apnea hypopnea index; REM, rapid eye movement.
On the other contrary, CSA is characterized by cessation of both airflow and respiratory effort during sleep (Fig. 5). CSR is recognized as increasing and decreasing gradually repeated respiratory pattern (Fig. 5). CSA occurs when there is a transient reduction of the generation of breathing rhythm by the pontomedullary pacemaker, usually reflecting changes in the partial pressure of CO22, 29). In HF, rostral fluid shift during sleep leads to pharyngeal edema, which may exacerbate obstructive tendencies29, 30). In CSA, the underlying abnormality is in the regulation of breathing in the respiratory centers of the brainstem. In normal physiology, minute ventilation during sleep is primarily regulated by chemoreceptors in the brainstem and carotid bodies, which trigger an increase in respiratory drive in response to a rise in arterial carbon dioxide (PaCO2), thus maintaining PaCO2 within a narrow range4, 30). Patients with HF and CSA tend to have an exaggerated respiratory response to carbon dioxide associated with excess sympathetic nervous activity, such that the modest rise in PaCO2 that may occur during sleep results in appropriate hyperventilation4, 31). This drives the PaCO2 below the “apneic threshold,” at which point the neural drive to respire is too low to stimulate effective inspiration, and apnea or hypopnea ensues.
Fig. 5.
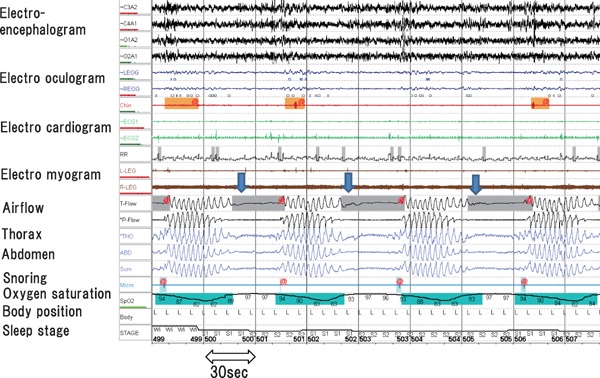
CSA
CSA (downward arrow) is characterized by cessation of both airflow (airflow band) and respiratory effort (thorax and abdomen band) during sleep. Cheyne–Stokes respiration is recognized as increasing and decreasing gradually repeated respiratory pattern.
Although polysomnography provides comprehensive data, it is expensive, laborious, and unavailable in many centers. Moreover, multichannel sleep polygraphs (with oxygen saturation, nasal airflow, and chest and abdominal movement record) is more widely available and can even be set up by the patient at home. Studies comparing the diagnostic accuracy of home polygraphy have shown that it has a sensitivity and specificity of 90%–100% for the diagnosis of significant SDB4, 32). Because of limited access to portable sleep monitors or overnight polysomnography, the majority of subjects with SDB remain undiagnosed. SDB is associated with an altered sympatho-vagal balance determined by the nocturnal cyclic alternating of apneas and hyperventilation-bradycardia during apnea, followed by abrupt tachycardia33). This phenomenon causes cyclic variations in heart rate33). Not only OSA but also CSA-CSR demonstrates heart rate oscillations. Both types of SDB present cyclic lengthening/shortening in the R-R interval during apnea and post-apneic hyperventilation. We have reported that cyclic variation of heart rate score (CVHRS) determined by Holter electrocardiogram is a useful screening index for severe SDB in patients with CVD34). In that study, there was a significant positive correlation between CVHRS and AHI (R = 0.60, P < 0.001)34). In addition, the receiver operating curve analysis revealed that CVHRS (with a cut-off value of 30/h) identified severe SDB with a sensitivity of 82%, a specificity of 77%, and an area under the curve of 0.83 (95% confidence interval [CI]: 0.72–0.93)34). Recently, pacemaker algorithms have been developed to detect and quantify SDB accurately35). It is possible to measure thoracic impedance continually between the right ventricular lead tip and the generator. On inspiration, the increased volume of air in the chest increases the thoracic impedance, with the inverse occurring on expiration, with consequent proportional changes in detected potential differences. It has recently been reported that intrathoracic impedance revealed a sensitivity of 88.9% and a specificity of 84.6% for the diagnosis of moderate to severe SDB35).
Associations between SDB and CVD
Endothelial dysfunction can be caused by oxidative stress, systemic inflammation, and sympathetic nervous activation. These factors are affected by SDB (Fig. 1), such as intermittent hypoxia, sleep deprivation, and arousals36). Inflammatory pathways triggered by intermittent hypoxia in OSA might also contribute to the development and progression of atherosclerosis. SDB severity is reportedly associated with endothelial dysfunction determined by flow-mediated dilatation37) or arterial stiffness determined by cardio-ankle vascular index38). With regard to cerebral artery, SDB-related oxidative stress and systemic inflammation promote increased intima-media thickness20, 39). Markers of oxidative stress and inflammation are associated with SDB-related hypoxia and intima-media thickness40). Patients with OSA without other known risk factors for arteriosclerosis have increased intima-media thickness compared with those without OSA41), and intima-media thickness is related to nocturnal hypoxia severity42). In a small RCT, effective CPAP therapy was associated with a significant reduction in intimamedia thickness over a period of < 6 months43). With regard to the coronary arteries, OSA is associated with an increased burden of noncalcified and calcified coronary plaques, determined by coronary computed tomography angiography44, 45). Doubling of the AHI was associated with a 19% increase in coronary artery calcium in men aged < 65 years, and a 17% increase of the same parameter in women of all ages44). It has been reported that SDB severity is significantly associated with coronary atherosclerotic burden severity (Gensini score), and reflected elevated troponin T as silent myocardial ischemia and minute myocardial injury, even in patients with stable CAD46).
In a large observational cohort study, Marin et al. reported that untreated severe OSA was associated with a 3-fold increase in the relative risk of fatal CVD (stroke and myocardial infarction [MI]) and nonfatal CVD (MI, stroke, coronary artery bypass surgery, and percutaneous transluminal coronary angiography), compared with patients without SDB47). CPAP attenuated these CVD risks. Gottlieb et al. reported that OSA was a significant predictor of incident CAD (MI, revascularization procedure, or cardiac death) after adjustment for multiple risk factors in men aged < 70 years, but not in older men or women of any age. Among men aged 40–70 years, those with severe OSA were 68% more likely to develop CAD than those without SDB48).
Pathophysiological and Prognostic Impact of SDB in Patients with CVD
OSA is observed in 30%–83% of patients with hypertension, 38%–65% of those with CAD, 57%–75% of those who had a stroke, 12%–55% of those with HF, and 20%–50% of those with arrhythmias2). Inspiratory efforts in OSA against the occluded upper airway are associated with intrathoracic pressure oscillations that result in increased sympathetic activity49). As shown in Figs. 1 and 2, the hypoxia, hypercapnia, and arousal from sleep that occur at the end of the OSA further increase sympathetic activity. The postapneic period, when a patient recovers upper airway patency, is often characterized by marked increases in blood pressure and heart rate49). During apneic phases, increased cardiac oxygen demand is paralleled by reduced oxygen supply because of excessive desaturation during apnea. Uncoupling of myocardial workload and coronary blood flow have been demonstrated20, 50). Hypoxia-induced vasodilation is reduced in the presence of endothelial dysfunction and arteriosclerosis. In patients with acute MI, SDB was associated with higher work load51), less myocardial salvage, and a smaller reduction in infarct size52). The presence of SDB among Japanese patients with acute coronary syndrome following primary percutaneous coronary intervention has been reported to be associated with a higher incidence of major adverse CVD events during long-term follow-up53). Among 241 patients with acute coronary syndrome, SDB was found in 126 (52.3%)53). The cumulative incidence of major adverse cardiocerebrovascular events (MACCEs) was significantly higher in patients with SDB than in those without (21.4% vs. 7.8%, P = 0.006, hazard ratio [HR]: 2.28, 95% CI: 1.06–4.92, P = 0.035)53). In patients with CAD, OSA is independently associated with subsequent MACCEs in patients undergoing percutaneous coronary intervention (drug-eluting stents were used in 80.1% and bioresorbable vascular scaffolds in 6.3% of these patients)54). Although target lesion revascularization is not associated with the presence of SDB, the crude incidence of MACCEs was higher in the OSA group than in the non-OSA group (3-year estimate, 18.9% vs. 14.0%, P = 0.001). Multivariate Cox regression analysis indicated that OSA was a predictor of MACCEs (adjusted HR: 1.57, 95% CI: 1.10–2.24, P = 0.013)54).
Thus, SDB seems to negatively affecting CVD; however, intermittent hypoxia caused by SDB may lead to ischemic preconditioning in the myocardium in patients with CAD. In a study by Ludka et al., SDB was present in 65.7% (n = 399) of their study population, and non-ST-elevation MI (NSTEMI) was present in 30% (n = 182)55). In that study, the degree of SDB severity was associated with an increasing likelihood of NSTEMI, and with a decreasing likelihood of STEMI (P < 0.001)55). The relative frequency of NSTEMI in the moderate to severe SDB group of that study was 40.6% versus 29.9% for STEMI (P = 0.01)55). NSTEMI prevalence increases with increasing SDB severity. This finding may suggest a cardioprotective role of SDB, which may attenuate the development of STEMI, perhaps through ischemic preconditioning55).
In patients with HF, presence of sleep disturbance including OSA is associated with adverse prognosis3, 56, 57). OSA may accelerate the progression of HF in several ways. The negative intrathoracic pressure generated by the respiratory muscles trying to inspire against closed airways increases venous return to the right heart, increasing preload and causing the septum shift to the left, which may compromise left ventricular ejection fraction (LVEF)4, 49, 58). The ability of the failing left ventricle to cope with enhanced preload is further impaired by increased transmural pressure during episodes of negative intrathoracic pressure, thereby increasing the afterload. Apnea and hypopnea activate the sympathetic nervous system; levels of circulating catecholamines and muscle sympathetic nerve activity are higher in those with HF and SDB than those with HF without SDB4, 59). SDB is associated with latent myocardial damage and alteration of myocardial carnitine metabolism in patients with HF, presented by higher circulation troponin T and carnitine levels60–63). In addition, SDB induces impairment of vagal activity, cardiac electrical instability, and ventricular arrhythmias across a 24-hour period accessed by heart rate variability and heart rate turbulence using Holter electrocardiogram64–66).
The incidence of CSA-CSR has been increasing in the past two decades, and whether it is merely a marker of the severity of underlying diseases or an important risk factor that independently worsens the prognosis of patients with HF, and therefore requires treatment, is still widely debated. Indeed, when multivariate analyses were performed to control for potential confounders involved in determining the outcome in patients with HF, CSA was found to be an independent factor for death or cardiac transplantation in these patients49, 67). Some large-scale studies have demonstrated that SDB is associated with occurrence of ventricular arrhythmias64, 68) and adverse prognosis in subjects with HF69, 70).
Effects of CPAP Therapy for OSA on CVD Outcome in Patients with CVD or AF
Although CPAP is the gold standard therapy for OSA, the effects of CPAP on the morbidity and mortality of CVD remain unproven. In a study that included patients with moderate to severe OSA without daytime sleepiness, the prescription of CPAP compared with usual care did not result in a statistically significant reduction in the incidence of hypertension or cardiovascular events (HR: 0.83, 95% CI: 0.63–1.1, P = 0.20)71). However, in an adherence analysis, patients who used CPAP for at least 4 h/night showed a lower risk of CVD (HR: 0.72, 95% CI: 0.52–0.98, P = 0.04)71).
With regard to patients with CAD, the Continuous Positive Airway Pressure Treatment of OSA to Prevent Cardiovascular Disease (SAVE) trial randomized 2,602 patients with a prior history of CVD and without moderate sleepiness who received either CPAP or usual care72). After a mean follow-up of 3.7 years, there was no benefit regarding any cause-specific CVD outcome (CPAP vs. usual care, HR: 1.10, 95% CI: 0.91–1.32, P = 0.34), despite improved sleepiness scores and quality-of-life measures. After propensity-score matching, the group of patients who used CPAP for at least 4 h/night showed a lower risk of CVD (HR: 0.52, 95% CI: 0.30–0.90, P = 0.02). Possible explanations for the lacking benefit for CVD were assumed to be because of the exclusion of study subjects with sleepy or severe SDB, a relatively short mean follow-up period, poor CPAP compliance (mean usage, 3.3 h/night), diagnosis determined by portable sleep monitor, and study subjects with more than 84% of the patients being nonsmokers, being of Asian descent (63.7%), and those with a prescription of effective medication including aspirin (75%) or antihypertensive agents (77%). In addition, a similar favorable trend with adequate nightly CPAP time (> 4 h/night) was noticed in the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnea (RICCADSA) study, which involved patients with newly revascularized CAD and moderate to severe OSA71, 73). In this study, although the use of a CPAP compared with usual care did not result in a significant reduction in the incidence of CVD, including the incidence of MI, repeat revascularization, stroke, or cardiovascular mortality (95% CI: 0.63–1.1, P = 0.20), CPAP with better adherence (> 4 h/night) may reduce the CVD (HR: 0.29; 95% CI: 0.10–0.86, P = 0.026)71, 73).
With regard to patients with AF, CPAP is associated with a significantly decreased recurrence rate of AF, even after electrical cardioversion or catheter ablation74–77). In addition, patients are less likely to progress to more permanent forms of AF and have significantly reduced occurrence of paroxysmal AF compared with untreated patients74–77). A recent metaanalysis has revealed that OSA treated with CPAP after AF intervention has a reduced AF risk78). With regard to patients with stroke, there is some evidence that CPAP improves long-term survival in patients with ischemic stroke having moderate to severe OSA79, 80). Parra et al.81) followed up patients with stroke for 24 months, and found that, compared with nonusers, CPAP users had notable interval delay until the occurrence of the first CVD event after initial stroke (14.9 vs. 7.9 months, P = 0.044). Although CPAP users had lower cardiovascular mortality (0% vs. 4.3%, P = 0.161) and lower MACE (including cardiac ischemic events, stroke recurrence, and cardiovascular death; 12.3% vs. 11.6%, P = 0.560) compared with nonusers, the differences did not reach statistical significance.
Effects of SDB Therapy on CVD Outcome in Patients with HF
Regarding HF, positive end-expiratory pressure prevents alveoli collapsing secondary to pulmonary edema and maintains alveoli at a greater diameter, and reducing breathing workload. It also increases alveolar recruitment, improves gas exchange, and reduces right to left intrapulmonary shunting of blood2, 4, 29). The positive intrathoracic pressure reduces venous return (preload) and LV transmural pressure (afterload), and may therefore benefit cardiac function in some patients4). CPAP caused abolition of negative intrathoracic pressure swings and reductions in nocturnal blood pressure, which resulted in a dramatic reduction in LV afterload that was accompanied by a decrease in heart rate. We have previously reported that CPAP improves right ventricular systolic function, pulmonary function, and exercise capacity, resulting in reduction of all-cause mortality in patients with HF having preserved EF82). Taken together, it seems that CPAP for OSA in patients with HF ameliorates LVEF, and possibly improves cardiovascular prognosis83). On the contrary, treatment of CSA or CSA-CSR in HF improves not only LV systolic84–87) and diastolic function88) but also pulmonary82), renal89, 90), and vascular functions88, 91); thus, it also potentially improves prognosis in patients with HF having reduced or preserved EF4, 29, 82, 84–89, 92–94, 95) However, a recent RCT failed to demonstrate that treatment of CSA improves the prognosis of patients with HF having CSA96).
Interpretation of Recent Randomized Clinical Trials and Future Perspectives
We propose the possible explanations for the lacking benefit for CVD as follows: 1) CPAP adherence, 2) the method of SDB treatment, 3) a relatively short follow-up period, 4) the selection of study subjects, and 5) diagnosis or risk stratification of SDB. First, recent meta-analysis24–26) suggests that CPAP compliance is key in reducing the risk of adverse cardiovascular outcomes in patients with OSA, with decreased incidence of MACE having been observed in patients using CPAP ≥ 4 h compared with those not on CPAP. Thus, patients with OSA should be extensively counseled on the importance of compliance with CPAP use24–26). Second, other treatments, including oral appliances97) or upper airway stimulation devices98), may lead to the improvement of CVD outcome. Third, the short follow-up duration of most trials may have given insufficient time for CPAP to have affected CVD outcomes99), and the meta-regression identified no association between follow-up time and the HR of CPAP versus control in the contributing trials24–26). Fourth, recent RCTs do not include severe SDB or sleepy subjects, who are generally recommended CPAP therapy. Fifth, with regard to the risk stratification or therapeutic targets, parameters of hypoxia (time with oxygen saturation), rather than classic AHI, seems to be an appropriate therapeutic target100–103). In addition, the use of continuous blood pressure measurement by using pulse transit time (PTT) has recently been reported104, 105). Arterial PTT was measured between the R-spike of the electrocardiogram and the plethysmographic curve of finger pulse-oximetry104, 105). Both PTT and pulse wave velocity were shown to have a correlation with blood pressure, and have been reported to be suitable for indirect blood pressure measurements104, 105). Comparing blood pressure measured using the PTT-based method and that measured using a cuff resulted in a significant correlation104, 105). In a representative case (Fig. 6), CPAP improved not only SDB but also both PTT index and mean or maximum blood pressure. Thus, arterial PTT is useful for detecting night-time blood pressure, which may be a useful risk stratification or therapeutic target in patients with SDB.
Fig. 6.
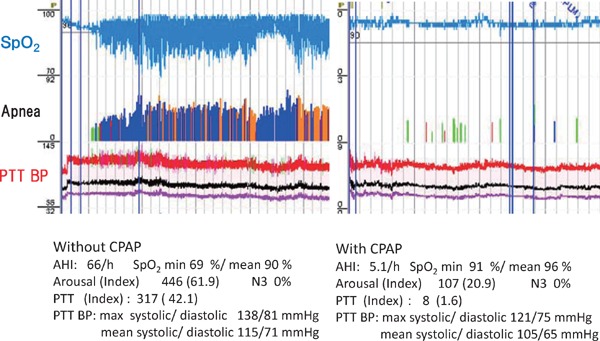
Effect of CPAP on SDB and arterial blood pressure measured by the PTT method
Improvement of SDB by CPAP leads to decreased PTT-based blood pressure and PTT index. AHI, apnea hypopnea index; SpO2, oxygen saturation; REM, rapid eye movement.
Further studies are needed to determine whether managing SDB improves the prognosis of patients with SDB. Several studies in patients with CAD106) or HF107, 108) are ongoing and their results are expected.
Conclusion
SDB causes sleep deprivation, intermittent hypoxia, and negative intrathoracic pressure swings, accompanied by other harmful pathophysiology that leads to CVD. Further studies are needed to determine whether appropriately managing SDB improves the prognosis of patients with SDB and cardiovascular diseases.
Conflict of Interest
Akiomi Yoshihisa belongs to the Department of Advanced Cardiac Therapeutics, supported by Fukuda-denshi Co, Ltd.
References
- 1). Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG: Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med, 2017; 13: 479-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK: Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol, 2017; 69: 841-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T, American Heart Association Council for High Blood Pressure Research Professional Education Committee CoCC, American Heart Association Stroke C, American Heart Association Council on Cardiovascular N and American College of Cardiology F : Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation, 2008; 118: 1080-1111 [DOI] [PubMed] [Google Scholar]
- 4). Pearse SG, Cowie MR: Sleep-disordered breathing in heart failure. Eur J Heart Fail, 2016; 18: 353-361 [DOI] [PubMed] [Google Scholar]
- 5). Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S: Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA, 2012; 307: 2169-2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Lavie L: Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev, 2015; 20: 27-45 [DOI] [PubMed] [Google Scholar]
- 7). Phillips CL, McEwen BJ, Morel-Kopp MC, Yee BJ, Sullivan DR, Ward CM, Tofler GH, Grunstein RR: Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax, 2012; 67: 639-644 [DOI] [PubMed] [Google Scholar]
- 8). Maruyama K, Morishita E, Sekiya A, Omote M, Kadono T, Asakura H, Hashimoto M, Kobayashi M, Nakatsumi Y, Takada S, Ohtake S: Plasma levels of platelet-derived microparticles in patients with obstructive sleep apnea syndrome. J Atheroscler Thromb, 2012; 19: 98-104 [DOI] [PubMed] [Google Scholar]
- 9). Oga T, Chin K, Tabuchi A, Kawato M, Morimoto T, Takahashi K, Handa T, Takahashi K, Taniguchi R, Kondo H, Mishima M, Kita T, Horiuchi H: Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb, 2009; 16: 862-869 [DOI] [PubMed] [Google Scholar]
- 10). Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med, 1993; 328: 1230-1235 [DOI] [PubMed] [Google Scholar]
- 11). Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM: Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol, 2013; 177: 1006-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW: The Sleep Heart Health Study: design, rationale, and methods. Sleep, 1997; 20: 1077-1085 [PubMed] [Google Scholar]
- 13). Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A: Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med, 2001; 163: 608-613 [DOI] [PubMed] [Google Scholar]
- 14). Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J: Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med, 2015; 3: 310-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T: Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol, 2008; 52: 686-717 [DOI] [PubMed] [Google Scholar]
- 16). Pataka A, Riha RL: Continuous positive airway pressure and cardiovascular events in patients with obstructive sleep apnea. Curr Cardiol Rep, 2013; 15: 385. [DOI] [PubMed] [Google Scholar]
- 17). Pack AI, Gislason T: Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis, 2009; 51: 434-451 [DOI] [PubMed] [Google Scholar]
- 18). Sundstrom J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B, Blood Pressure Lowering Treatment Trialists C : Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med, 2015; 162: 184-191 [DOI] [PubMed] [Google Scholar]
- 19). Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, Humayun C, Anugula D, Abbas G, Salami J, Elizondo JV, Veledar E, Nasir K: Association of Subjective and Objective Sleep Duration as well as Sleep Quality with Non-Invasive Markers of Sub-Clinical Cardiovascular Disease (CVD): A Systematic Review. J Atheroscler Thromb, 2017; 24: 208-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Arzt M, Hetzenecker A, Steiner S, Buchner S: Sleep-Disordered Breathing and Coronary Artery Disease. Can J Cardiol, 2015; 31: 909-917 [DOI] [PubMed] [Google Scholar]
- 21). Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M: Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology, 2015; 20: 889-895 [DOI] [PubMed] [Google Scholar]
- 22). Feng Y, Zhang Z, Dong ZZ: Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med, 2015; 25: 15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Quan SF, Budhiraja R, Clarke DP, Goodwin JL, Gottlieb DJ, Nichols DA, Simon RD, Smith TW, Walsh JK, Kushida CA: Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med, 2013; 9: 989-993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B: Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA, 2017; 318: 156-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E: A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J, 2018; 39: 2291-2297 [DOI] [PubMed] [Google Scholar]
- 26). Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, Elgendy A, Mahmoud AN, Mentias A, Barakat A, Lal C: Meta-Analysis of Cardiovascular Outcomes With Continuous Positive Airway Pressure Therapy in Patients With Obstructive Sleep Apnea. Am J Cardiol, 2017; 120: 693-699 [DOI] [PubMed] [Google Scholar]
- 27). Blood Pressure Lowering Treatment Trialists C: Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet, 2014; 384: 591-598 [DOI] [PubMed] [Google Scholar]
- 28). Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M : Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med, 2012; 8: 597-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Yoshihisa A, Takeishi Y: Heart failure and sleep disordered breathing. Fukushima J Med Sci, 2017; 63: 32-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, Mak S, Bradley TD: Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation, 2010; 121: 1598-1605 [DOI] [PubMed] [Google Scholar]
- 31). Javaheri S: A mechanism of central sleep apnea in patients with heart failure. N Engl J Med, 1999; 341: 949-954 [DOI] [PubMed] [Google Scholar]
- 32). Pinna GD, Robbi E, Pizza F, Taurino AE, Pronzato C, La Rovere MT, Maestri R: Can cardiorespiratory polygraphy replace portable polysomnography in the assessment of sleep-disordered breathing in heart failure patients? Sleep Breath, 2014; 18: 475-482 [DOI] [PubMed] [Google Scholar]
- 33). Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A: Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet, 1984; 1: 126-131 [DOI] [PubMed] [Google Scholar]
- 34). Shimizu T, Yoshihisa A, Iwaya S, Abe S, Sato T, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Cyclic variation in heart rate score by holter electrocardiogram as screening for sleep-disordered breathing in subjects with heart failure. Respir Care, 2015; 60: 72-80 [DOI] [PubMed] [Google Scholar]
- 35). Defaye P, de la Cruz I, Marti-Almor J, Villuendas R, Bru P, Senechal J, Tamisier R, Pepin JL: A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: the DREAM European study. Heart Rhythm, 2014; 11: 842-848 [DOI] [PubMed] [Google Scholar]
- 36). Budweiser S, Enderlein S, Jorres RA, Hitzl AP, Wieland WF, Pfeifer M, Arzt M: Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med, 2009; 6: 3147-3157 [DOI] [PubMed] [Google Scholar]
- 37). Yoshihisa A, Owada T, Hoshino Y, Miyata M, Misaka T, Sato T, Suzuki S, Sakamoto N, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Ishibashi T, Takeishi Y: Flow-mediated dilatation identifies impaired endothelial function in patients with sleep apnea syndrome. Fukushima J Med Sci, 2010; 56: 115-120 [DOI] [PubMed] [Google Scholar]
- 38). Iguchi A, Yamakage H, Tochiya M, Muranaka K, Sasaki Y, Kono S, Shimatsu A, Satoh-Asahara N: Effects of weight reduction therapy on obstructive sleep apnea syndrome and arterial stiffness in patients with obesity and metabolic syndrome. J Atheroscler Thromb, 2013; 20: 807-820 [DOI] [PubMed] [Google Scholar]
- 39). Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH: Vascular inflammation in obesity and sleep apnea. Circulation, 2010; 121: 1014-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M: Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med, 2005; 172: 625-630 [DOI] [PubMed] [Google Scholar]
- 41). Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G: Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med, 2005; 172: 613-618 [DOI] [PubMed] [Google Scholar]
- 42). Ayas NT, Patel SR, Malhotra A, Schulzer M, Malhotra M, Jung D, Fleetham J, White DP: Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep, 2004; 27: 249-253 [DOI] [PubMed] [Google Scholar]
- 43). Hui DS, Shang Q, Ko FW, Ng SS, Szeto CC, Ngai J, Tung AH, To KW, Chan TO, Yu CM: A prospective cohort study of the long-term effects of CPAP on carotid artery intima-media thickness in obstructive sleep apnea syndrome. Respir Res, 2012; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Weinreich G, Wessendorf TE, Erdmann T, Moebus S, Dragano N, Lehmann N, Stang A, Roggenbuck U, Bauer M, Jockel KH, Erbel R, Teschler H, Mohlenkamp S, Heinz Nixdorf Recall study g : Association of obstructive sleep apnoea with subclinical coronary atherosclerosis. Atherosclerosis, 2013; 231: 191-197 [DOI] [PubMed] [Google Scholar]
- 45). Kent BD, Garvey JF, Ryan S, Nolan G, Dodd JD, McNicholas WT: Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J, 2013; 42: 1263-1270 [DOI] [PubMed] [Google Scholar]
- 46). Inami T, Seino Y, Otsuka T, Yamamoto M, Kimata N, Murakami D, Takano M, Ohba T, Ibuki C, Mizuno K: Links between sleep disordered breathing, coronary atherosclerotic burden, and cardiac biomarkers in patients with stable coronary artery disease. J Cardiol, 2012; 60: 180-186 [DOI] [PubMed] [Google Scholar]
- 47). Marin JM, Carrizo SJ, Vicente E, Agusti AG: Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet, 2005; 365: 1046-1053 [DOI] [PubMed] [Google Scholar]
- 48). Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E: Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation, 2010; 122: 352-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Parati G, Lombardi C, Castagna F, Mattaliano P, Filardi PP, Agostoni P, Italian Society of Cardiology Working Group on Heart Failure m : Heart failure and sleep disorders. Nature reviews Cardiology, 2016; 13: 389-403 [DOI] [PubMed] [Google Scholar]
- 50). Hamilton GS, Meredith IT, Walker AM, Solin P: Obstructive sleep apnea leads to transient uncoupling of coronary blood flow and myocardial work in humans. Sleep, 2009; 32: 263-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Hetzenecker A, Buchner S, Greimel T, Satzl A, Luchner A, Debl K, Husser O, Hamer OW, Fellner C, Riegger GAJ, Pfeifer M, Arzt M: Cardiac workload in patients with sleep-disordered breathing early after acute myocardial infarction. Chest, 2013; 143: 1294-1301 [DOI] [PubMed] [Google Scholar]
- 52). Buchner S, Satzl A, Debl K, Hetzenecker A, Luchner A, Husser O, Hamer OW, Poschenrieder F, Fellner C, Zeman F, Riegger GA, Pfeifer M, Arzt M: Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J, 2014; 35: 192-199 [DOI] [PubMed] [Google Scholar]
- 53). Mazaki T, Kasai T, Yokoi H, Kuramitsu S, Yamaji K, Morinaga T, Masuda H, Shirai S, Ando K: Impact of Sleep-Disordered Breathing on Long-Term Outcomes in Patients With Acute Coronary Syndrome Who Have Undergone Primary Percutaneous Coronary Intervention. Journal of the American Heart Association, 2016; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, Loo G, Koo CY, Gao XF, Chandra S, Yang XX, Furlan SF, Ge Z, Mundhekar A, Zhang WW, Uchoa CH, Kharwar RB, Chan PF, Chen SL, Chan MY, Richards AM, Tan HC, Ong TH, Roldan G, Tai BC, Drager LF, Zhang JJ: Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation, 2016; 133: 2008-2017 [DOI] [PubMed] [Google Scholar]
- 55). Ludka O, Stepanova R, Sert-Kuniyoshi F, Spinar J, Somers VK, Kara T: Differential likelihood of NSTEMI vs STEMI in patients with sleep apnea. Int J Cardiol, 2017; 248: 64-68 [DOI] [PubMed] [Google Scholar]
- 56). Kanno Y, Yoshihisa A, Watanabe S, Takiguchi M, Yokokawa T, Sato A, Miura S, Shimizu T, Nakamura Y, Abe S, Sato T, Suzuki S, Oikawa M, Saitoh S, Takeishi Y: Prognostic Significance of Insomnia in Heart Failure. Circ J, 2016; 80: 1571-1577 [DOI] [PubMed] [Google Scholar]
- 57). Yoshihisa A, Suzuki S, Kanno Y, Takiguchi M, Sato A, Miura S, Masuda A, Yokokawa T, Shimizu T, Nakamura Y, Yamauchi H, Owada T, Abe S, Sato T, Oikawa M, Saitoh S, Takeishi Y: Prognostic significance of periodic leg movements during sleep in heart failure patients. Int J Cardiol, 2016; 212: 11-13 [DOI] [PubMed] [Google Scholar]
- 58). Arzt M, Woehrle H, Oldenburg O, Graml A, Suling A, Erdmann E, Teschler H, Wegscheider K, Schla HFI: Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart failure, 2016; 4: 116-125 [DOI] [PubMed] [Google Scholar]
- 59). Spaak J, Egri ZJ, Kubo T, Yu E, Ando S, Kaneko Y, Usui K, Bradley TD, Floras JS: Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension, 2005; 46: 1327-1332 [DOI] [PubMed] [Google Scholar]
- 60). Miyata M, Yoshihisa A, Yamauchi H, Owada T, Sato T, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Impact of sleep-disordered breathing on myocardial damage and metabolism in patients with chronic heart failure. Heart Vessels, 2015; 30: 318-324 [DOI] [PubMed] [Google Scholar]
- 61). Nakamura Y, Yoshihisa A, Takiguchi M, Shimizu T, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: High-sensitivity cardiac troponin T predicts non-cardiac mortality in heart failure. Circ J, 2014; 78: 890-895 [DOI] [PubMed] [Google Scholar]
- 62). Aimo A, Januzzi JL, Jr., Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Brunner-La Rocca HP, Bayes-Genis A,, Lupon J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Tang WHW, Grodin J, Passino C, Emdin M: Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation, 2018; 137: 286-297 [DOI] [PubMed] [Google Scholar]
- 63). Aimo A, Januzzi JL, Jr., Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Rocca HB, Bayes-Genis A, Lupon J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Wilson Tang WH, Grodin JL, Passino C, Emdin M: High-sensitivity troponin T, NT-proBNP and glomerular filtration rate: A multimarker strategy for risk stratification in chronic heart failure. Int J Cardiol, 2019; 277: 166-172 [DOI] [PubMed] [Google Scholar]
- 64). Yamada S, Suzuki H, Kamioka M, Suzuki S, Kamiyama Y, Yoshihisa A, Saitoh S, Takeishi Y: Sleep-disordered breathing increases risk for fatal ventricular arrhythmias in patients with chronic heart failure. Circ J, 2013; 77: 1466-1473 [DOI] [PubMed] [Google Scholar]
- 65). Yoshihisa A, Suzuki S, Takiguchi M, Shimizu T, Abe S, Sato T, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Impact of sleep-disordered breathing on heart rate turbulence in heart failure patients. PLoS One, 2014; 9: e101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Yamada S, Yoshihisa A, Sato Y, Sato T, Kamioka M, Kaneshiro T, Oikawa M, Kobayashi A, Suzuki H, Ishida T, Takeishi Y: Utility of heart rate turbulence and T-wave alternans to assess risk for readmission and cardiac death in hospitalized heart failure patients. J Cardiovasc Electrophysiol, 2018; 29: 1257-1264 [DOI] [PubMed] [Google Scholar]
- 67). Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD: Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation, 2000; 102: 61-66 [DOI] [PubMed] [Google Scholar]
- 68). Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O: Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J, 2011; 32: 61-74 [DOI] [PubMed] [Google Scholar]
- 69). Javaheri S, Shukla R, Zeigler H, Wexler L: Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol, 2007; 49: 2028-2034 [DOI] [PubMed] [Google Scholar]
- 70). Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, Ruttanaumpawan P, Tomlinson G, Bradley TD: Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol, 2007; 49: 1625-1631 [DOI] [PubMed] [Google Scholar]
- 71). Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, Chiner E, Masa JF, Gonzalez M, Marin JM, Garcia-Rio F, Diaz de Atauri J, Teran J, Mayos M, de la Pena M, Monasterio C, del Campo F, Montserrat JM, Spanish S, Breathing N : Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA, 2012; 307: 2161-2168 [DOI] [PubMed] [Google Scholar]
- 72). McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Investigators S and Coordinators : CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med, 2016; 375: 919-931 [DOI] [PubMed] [Google Scholar]
- 73). Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E: Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med, 2016; 194: 613-620 [DOI] [PubMed] [Google Scholar]
- 74). Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R, Steigner M, John RM, Jerosch-Herold M, Malhotra A, Kwong RY: Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. Journal of the American Heart Association, 2013; 2: e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E: Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol, 2013; 62: 300-305 [DOI] [PubMed] [Google Scholar]
- 76). Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y, Ito Y, Kuroki K, Machino T, Yamasaki H, Igarashi M, Sekiguchi Y, Sato A, Aonuma K: Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm, 2013; 10: 331-337 [DOI] [PubMed] [Google Scholar]
- 77). Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, Holmes DN, Peterson ED, Piccini JP, Gersh BJ, Investigators O-A : Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J, 2015; 169: 647-654 e642 [DOI] [PubMed] [Google Scholar]
- 78). Qureshi WT, Nasir UB, Alqalyoobi S, O'Neal WT, Mawri S, Sabbagh S, Soliman EZ, Al-Mallah MH: Meta-Analysis of Continuous Positive Airway Pressure as a Therapy of Atrial Fibrillation in Obstructive Sleep Apnea. Am J Cardiol, 2015; 116: 1767-1773 [DOI] [PubMed] [Google Scholar]
- 79). Lyons OD, Ryan CM: Sleep Apnea and Stroke. Can J Cardiol, 2015; 31: 918-927 [DOI] [PubMed] [Google Scholar]
- 80). Kim Y, Koo YS, Lee HY, Lee SY: Can Continuous Positive Airway Pressure Reduce the Risk of Stroke in Obstructive Sleep Apnea Patients? A Systematic Review and Meta-Analysis. PLoS One, 2016; 11: e0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Parra O, Sanchez-Armengol A, Bonnin M, Arboix A, Campos-Rodriguez F, Perez-Ronchel J, Duran-Cantolla J, de la Torre G, Gonzalez Marcos JR, de la Pena M, Carmen Jimenez M, Masa F, Casado I, Luz Alonso M, Macarron JL: Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J, 2011; 37: 1128-1136 [DOI] [PubMed] [Google Scholar]
- 82). Yoshihisa A, Suzuki S, Yamauchi H, Sato T, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Beneficial Effects of Positive Airway Pressure Therapy for Sleep-Disordered Breathing in Heart Failure Patients With Preserved Left Ventricular Ejection Fraction. Clin Cardiol, 2015; 38: 413-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83). Kasai T: Sleep apnea and heart failure. J Cardiol, 2012; 60: 78-85 [DOI] [PubMed] [Google Scholar]
- 84). Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS, Investigators C : Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med, 2005; 353: 2025-2033 [DOI] [PubMed] [Google Scholar]
- 85). Suzuki S, Yoshihisa A, Miyata M, Sato T, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Adaptive servo-ventilation therapy improves long-term prognosis in heart failure patients with anemia and sleep-disordered breathing. Int Heart J, 2014; 55: 342-349 [DOI] [PubMed] [Google Scholar]
- 86). Yoshihisa A, Shimizu T, Owada T, Nakamura Y, Iwaya S, Yamauchi H, Miyata M, Hoshino Y, Sato T, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J, 2011; 52: 218-223 [DOI] [PubMed] [Google Scholar]
- 87). Miyata M, Yoshihisa A, Suzuki S, Yamada S, Kamioka M, Kamiyama Y, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Adaptive servo ventilation improves Cheyne-Stokes respiration, cardiac function, and prognosis in chronic heart failure patients with cardiac resynchronization therapy. J Cardiol, 2012; 60: 222-227 [DOI] [PubMed] [Google Scholar]
- 88). Yoshihisa A, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail, 2013; 15: 543-550 [DOI] [PubMed] [Google Scholar]
- 89). Owada T, Yoshihisa A, Yamauchi H, Iwaya S, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep-disordered breathing. J Card Fail, 2013; 19: 225-232 [DOI] [PubMed] [Google Scholar]
- 90). Yoshihisa A, Suzuki S, Owada T, Iwaya S, Yamauchi H, Miyata M, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Short-term use of adaptive servo ventilation improves renal function in heart failure patients with sleep-disordered breathing. Heart Vessels, 2013; 28: 728-734 [DOI] [PubMed] [Google Scholar]
- 91). Suzuki S, Yoshihisa A, Sato Y, Watanabe S, Yokokawa T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Takeishi Y: Association between sleep-disordered breathing and arterial stiffness in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail, 2018; 5: 284-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD, Investigators C : Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation, 2007; 115: 3173-3180 [DOI] [PubMed] [Google Scholar]
- 93). Sharma BK, Bakker JP, McSharry DG, Desai AS, Javaheri S, Malhotra A: Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis. Chest, 2012; 142: 1211-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94). Iwaya S, Yoshihisa A, Nodera M, Owada T, Yamada S, Sato T, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y: Suppressive effects of adaptive servo-ventilation on ventricular premature complexes with attenuation of sympathetic nervous activity in heart failure patients with sleep-disordered breathing. Heart Vessels, 2014; 29: 470-477 [DOI] [PubMed] [Google Scholar]
- 95). Yoshida M, Ando SI, Kodama K, Ebihara K, Tanaka K, Hayashi A, Taguchi E, Kadokami T, Nakao K, Sakamoto T: Adaptive servo-ventilation therapy reduces hospitalization rate in patients with severe heart failure. Int J Cardiol, 2017; 238: 173-176 [DOI] [PubMed] [Google Scholar]
- 96). Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H: Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med, 2015; 373: 1095-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97). Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD: Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J Clin Sleep Med, 2015; 11: 773-827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98). Strollo PJ, Jr., Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, Group ST : Upper-airway stimulation for obstructive sleep apnea. N Engl J Med, 2014; 370: 139-149 [DOI] [PubMed] [Google Scholar]
- 99). Parra O, Sanchez-Armengol A, Capote F, Bonnin M, Arboix A, Campos-Rodriguez F, Perez-Ronchel J, Duran-Cantolla J, Martinez-Null C, de la Pena M, Jimenez MC, Masa F, Casadon I, Alonso ML, Macarron JL: Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res, 2015; 24: 47-53 [DOI] [PubMed] [Google Scholar]
- 100). Sawatari H, Chishaki A, Nishizaka M, Tokunou T, Adachi S, Yoshimura C, Ohkusa T, Ando S: Cumulative Hypoxemia During Sleep Predicts Vascular Endothelial Dysfunction in Patients With Sleep-Disordered Breathing. Am J Hypertens, 2016; 29: 458-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101). Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, Ancoli-Israel S, Ensrud K, Purcell S, White DP, Redline S, Wellman A: The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J, 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102). Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, Horstkotte D, Wegscheider K: Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J, 2016; 37: 1695-1703 [DOI] [PubMed] [Google Scholar]
- 103). Yamaguchi T, Takata Y, Usui Y, Asanuma R, Nishihata Y, Kato K, Shiina K, Yamashina A: Nocturnal Intermittent Hypoxia Is Associated With Left Ventricular Hypertrophy in Middle-Aged Men With Hypertension and Obstructive Sleep Apnea. Am J Hypertens, 2016; 29: 372-378 [DOI] [PubMed] [Google Scholar]
- 104). Gehring J, Gesche H, Drewniok G, Kuchler G, Patzak A: Nocturnal blood pressure fluctuations measured by using pulse transit time in patients with severe obstructive sleep apnea syndrome. Sleep Breath, 2018; 22: 337-343 [DOI] [PubMed] [Google Scholar]
- 105). Gesche H, Grosskurth D, Kuchler G, Patzak A: Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol, 2012; 112: 309-315 [DOI] [PubMed] [Google Scholar]
- 106). Esquinas C, Sanchez-de-la Torre M, Aldoma A, Flores M, Martinez M, Barcelo A, Barbe F, Spanish Sleep N : Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol, 2013; 36: 495-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107). Lyons OD, Floras JS, Logan AG, Beanlands R, Cantolla JD, Fitzpatrick M, Fleetham J, John Kimoff R, Leung RS, Lorenzi Filho G, Mayer P, Mielniczuk L, Morrison DL, Ryan CM, Series F, Tomlinson GA, Woo A, Arzt M, Parthasarathy S, Redolfi S, Kasai T, Parati G, Delgado DH, Bradley TD, Investigators A-H : Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail, 2017; 19: 579-587 [DOI] [PubMed] [Google Scholar]
- 108). Haruki N, Floras JS: Sleep-Disordered Breathing in Heart Failure- A Therapeutic Dilemma. Circ J, 2017; 81: 903-912 [DOI] [PubMed] [Google Scholar]


