Abstract
Aim: The impact of international normalized ratio (INR) on prognosis after acute ischemic stroke without anticoagulation therapy is unclear. Herein, the association between baseline INR and stroke outcomes in patients without anticoagulation therapy was investigated.
Methods: A total of 14,782 ischemic stroke patients from the China National Stroke Registry II were included in this analysis. The period of follow-up was 1 year after stroke onset. Multivariate logistic regression models were used to estimate the relationship between INR and stroke outcomes including all-cause death, recurrent stroke, composite end point, and poor functional outcome.
Results: Of 14,782 patients with stroke, all-cause death occurred in 1080 (7.3%), recurrence stroke in 538 (3.9%), combined end point in 1319 (8.9%), and poor functional outcome in 3001 (20.3%). Compared with the medium INR group (0.9–1.1), the odds ratios with confidence intervals of 95% for the high INR group (> 1.1) were 1.58 (1.32–1.98) for all-cause death, 1.40 (1.10–1.79) for stroke recurrence, 1.52 (1.29–1.79) for combined end point, and 1.21 (1.06–1.39) for poor functional outcome. No association between low INR (< 0.9) and any stroke outcomes was found compared with the medium group.
Conclusions: Increased admission INR was associated with adverse stroke outcomes among acute ischemic stroke patients without atrial fibrillation or anticoagulation therapy.
Keywords: Transient ischemic attack, Minor stroke, International normalized ratio, Recurrent stroke, Registries
Introduction
Of all neurological conditions, stroke is one of the most devastating disease1). Almost one half of stroke survivors remain disabled, and a seventh requires the institutional care2). The INR standardizing the prothrombin time has been accepted as a sensitive and reliable marker of coagulation abnormalities3), and is widely used in the management of patients receiving anticoagulation treatment4, 5). Changes in blood coagulation function may accelerate blood clot formation or may influence clinical outcome in acute ischemic stroke patients with atrial fibrillation (AF), especially under the anticoagulation therapy6, 7). Yet, it is critical to understand the potential mechanism, which is related to the self-protection in an attempt to avoid excessive brain injury after acute ischemic stroke8).
Some studies found that the increased admission INR was an independent predictor of poor clinical outcomes in trauma, acute decompensated heart failure, and acute pulmonary embolism patients9–11). Some researchers explored the relation between admission INR and acute infarct volume in ischemic stroke patients with preadmission warfarin use12). However, the instantaneous change of INR after cerebral ischemia was not understood. On the other hand, the prognostic value of INR among ischemic stroke patients without AF or without anticoagulation has not been investigated so far. We assumed that the INR would also have a prognostic value in ischemic stroke patients without AF. Thus, the goal of the current study was to explore the clinical significance and prognostic value of admission INR on adverse stroke outcomes including all-cause death, recurrent stroke, combined end point, and poor functional outcome in patients with acute ischemic cerebrovascular events based on the China national stroke registry phase II.
Methods
Study Design and Participants
The population used for this study was from China National Stroke Registry II (CNSR II). The CNSR II design was described in detail previously13). Briefly, the CNSR II launched in 2012 is a nationwide initiative to establish a reliable national stroke database for evaluating stroke care delivery in clinical practice and identifying areas that need further improvement compared with CNSR I in 200714). Of 25,018 patients participated in CNSR II, 14,782 patients without anticoagulation therapy were eligible for the current study after excluding hemorrhagic stroke (n = 3,246), medical history of AF or diagnosis of AF at discharge (n = 1,822), anticoagulation therapy during hospitalization (n = 100), missing or extreme INR value (an INR > 5.00 is considered as extreme value, n = 2,880), and loss to 1-year follow-up (n = 2,008) (Supplementary Fig. 1). The study was approved by the central Institutional Review Board at Beijing Tiantan Hospital, in compliance with the Declaration of Helsinki. Every participant provided signed informed consent before his/her participation.
Supplementary Fig. 1.
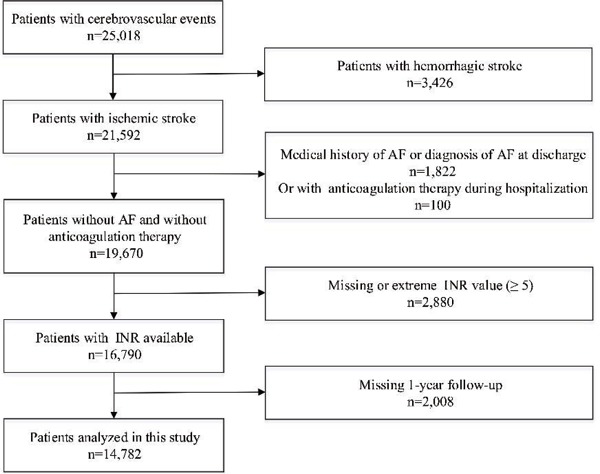
Flow chart showing the patients selection from China National Stroke Registry II AF indicates atrial fibrillation; INR, international normalized ratio.
Data Collection
Trained research coordinators in each hospital collected the baseline data including patient demographics, vascular risk factors, stroke severity, lab test, medication use, complication, and diagnosis at discharge. The vascular risk factors included the history of stroke, hypertension, dyslipidemia, diabetes mellitus, coronary heart disease, current or previous smoking, and moderate or heavy alcohol consumption (≥ 2 standardized alcohol drinks per day). The hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, any use of continuous antihypertensive drug for more than 2 weeks before the onset of cerebrovascular diseases, or self-reported history of hypertension. The dyslipidemia was defined as serum triglyceride ≥ 150 mg/dL, low-density lipoprotein cholesterol ≥ 130 mg/dL, high-density lipoprotein cholesterol ≤ 40 mg/dL, any use of lipid-lowering drugs, or any self-reported history of dyslipidemia. The diabetes mellitus was defined as fasting glucose level ≥ 7.0 mmol/L (126 mg/dL), the nonfasting glucose concentration ≥ 11.1 mmol/L (200 mg/dL) with classic symptoms of hyperglycemia or hyperglycemic crisis, any use of glucose-lowering drugs, or any self-reported history of diabetes mellitus. The height and weight were measured with participants wearing a scrub suit and no shoes, and then the body mass index (BMI) was calculated by dividing the weight in kilograms at the square of height in meters.
Measurements of INR
The venous blood was drawn from patients who fasted within the first 24 h of admission for routine laboratory measurements including INR. The blood samples were obtained, preserved, and processed in a manner recommended by the clinical site laboratory's policies and procedures. The 3.2%citrate tubes are used and filled properly to maintain a blood/citrate ratio of 9/1. INR was measured on a STA-R EVOLUTION automated coagulation analyzer (Diagnostica Stago, Asnières, France) with an international sensitivity index of approximately 1.0 and using HemosIL RecombiPlasTin 2G as reagent (Instrumentation Laboratory, Bedford, MA, USA). The reference range of INR was roughly 0.9–1.1 in all clinical laboratories.
Study Outcomes
The participants were followed up by telephone interview by trained research personnel who were blinded to patient baseline clinical status. The patients were asked standardized follow-up questions 12 months after the onset of stroke. The study outcomes included the all-cause mortality, stroke recurrence, and poor functional outcome. Death was confirmed either by the death certificates from local citizen registries or from the treating hospitals. If such records were unavailable, the death was confirmed if it was reported on 2 consecutive follow-up contacts with ≥ 2 different proxies. The recurrent stroke included the ischemic stroke, intracranial hemorrhage, and subarachnoid hemorrhage. The recurrent end points of the stroke comprised fatal and nonfatal stroke. We also evaluated the composite of death or recurrent stroke in the prognostic analysis. The poor functional outcome was defined as the modified Rankin Scale ranging from 3 to 6.
Statistical Analysis
The subjects were divided into the following three groups based on the reference range of INR - low INR (< 0.9), medium INR group (0.9–1.1), and high INR (> 1.1). The baseline characteristics were compared between the three INR groups. The continuous variables were expressed as mean (SD) or median (interquartile range, IQR) values, and compared using the analysis of variance or Kruskal–Wallis test. The categorical variables were expressed as frequency (percent), and compared using the chi-square test.
The multivariable logistic regression models were used to assess the association of INR with 12-month clinical outcomes in stroke patients. The covariates in the multivariable analysis included the age, sex, history of stroke, hypertension, dyslipidemia, diabetes mellitus, coronary heart disease, current or previous smoking, moderate or heavy alcohol consumption, BMI, admission NIHSS and mRS scores, pneumonia, antihypertensive, antidiabetic and lipid-lowing therapy, as well as the time from onset to the blood sampling. The odds ratios (ORs) were calculated with confidence intervals (CIs) of 95% for low and high INR groups compared to the medium group.
To explore the association between INR distribution and clinical outcomes, the INR grading categories were created by 0.05 increments i.e., ≤ 0.85, 0.85–0.90, 0.90–0.95, 0.95–1.00, 1.00–1.05, 1.05–1.10, 1.10–1.15, 1.15–1.20, and > 1.20. The adjusted OR and 95% CIs were assessed for each category using an INR value of 0.95–1.00 as the reference group. Additionally, we performed a sensitivity analysis in a subgroup of patients without intravenous (IV) recombinant tissue plasminogen activator (rtPA) treatment, because the current guidelines accept IV rtPA treatment for patients treated within 4.5 h of onset with an INR ≤ 1.715).
All analyses were conducted with SAS Version 9.2 software (SAS Institute). Two-tailed P values < 0.05 were considered to be statistically significant.
Results
Baseline Characteristics
A total of 14,782 patients without anticoagulation therapy were eligible for our study (Supplementary Fig. 1). Supplementary Table 1 shows the baseline characteristics of ischemic patients included and excluded due to missing INR or 1-year follow-up. The baseline characteristics of patients included in this study and those from CNSR II were basically similar, except the patients included in this subgroup analysis for which higher prevalence of vascular risk factors including history of stroke, dyslipidemia, diabetes mellitus, coronary heart disease, and moderate or heavy drinking was noticed. More patients included in this study had a mRS score of 3–5 at discharge (Supplementary Table 1). The demographic and clinical characteristics of eligible patients are shown in Table 1. As compared with those with the low INR, the patients with high INR were more likely to be older, male, to have higher prevalence of history of stroke and coronary heart disease, pneumonia, shorter time from symptom onset to the blood sampling, higher discharge mRS score, and higher NIHSS on admission, whereas the prevalence of hypertension, diabetes mellitus, and medications (antihypertensive, antidiabetic, and lipid-lowering), and BMI were lower in patients with high INR than those with low INR.
Supplementary Table 1. Baseline characteristics of patients included vs. excluded in the study.
| Characteristics | Patients included (n = 14,782) | Patients excluded (n = 4,868) | P-value |
|---|---|---|---|
| Age, years, mean (SD) | 64.2 (11.9) | 63.8 (11.9) | 0.05 |
| Male sex | 9511 (64.3) | 3096 (63.6) | 0.35 |
| Vascular risk factors, n (%) | |||
| Stroke | 5283 (35.7) | 1599 (32.8) | < 0.001 |
| Hypertension | 11218 (75.9) | 3657 (75.1) | 0.28 |
| Dyslipidemia | 1844 (12.5) | 547 (11.2) | 0.02 |
| Diabetes mellitus | 3081 (20.8) | 947 (19.5) | 0.04 |
| Coronary heart disease | 1772 (12.0) | 522 (10.7) | 0.02 |
| Current or previous smoking | 6655 (45.0) | 2184 (44.9) | 0.85 |
| Moderate or heavy drinkers | 4548 (30.8) | 1396 (28.7) | 0.01 |
| mRS score of 3–5 at discharge, n (%) | 3076 (20.8) | 928 (19.1) | 0.01 |
| Body mass index, kg/m2, median (IQR) | 24.1 (22.1–25.8) | 24.1 (22.0–25.7) | 0.38 |
| Time to blood collection, hour, median (IQR) | 43 (24–72) | 44 (24–80) | 0.94 |
| Admission NIHSS score | 3 (1–6) | 3 (1–6) | 0.05 |
| Medications | |||
| Antihypertensive | 7242 (49.0) | 2423 (49.8) | 0.34 |
| Antidiabetic | 3017 (20.4) | 937 (19.2) | 0.08 |
| Lipid-lowering | 7848 (53.1) | 2629 (54.0) | 0.27 |
| Pneumonia | 868 (5.9) | 275 (5.6) | 0.56 |
Abbreviations: mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Table 1. Baseline characteristics of the participants to the study classified based on the admission INR value.
| Characteristics | Total | INR < 0.9 | INR = 0.9.1.1 | INR > 1.1 | P-value |
|---|---|---|---|---|---|
| (n = 14782) | (n = 2137) | (n = 10563) | (n = 2082) | ||
| Age, years, mean (SD) | 64.2 (11.9) | 61.8 (11.2) | 64.3 (11.9) | 66.2 (12.0) | < 0.001 |
| Male | 9511 (64.3) | 1243 (58.2) | 6863 (64.7) | 1432 (68.8) | < 0.001 |
| INR, mean (SD) | 1.01 (0.18) | 0.85 (0.05) | 0.99 (0.06) | 1.26 (0.33) | < 0.001 |
| ALT, U/L, median (IQR) | 19 (13–27) | 20 (14–29) | 18 (13–26) | 18 (13–26) | < 0.001 |
| AST, U/L, median (IQR) | 21 (17–26) | 21 (17–27) | 20 (17–26) | 21 (17–28) | < 0.001 |
| Vascular risk factors, n (%) | |||||
| Stroke | 5283 (35.7) | 696 (32.6) | 3812 (36.1) | 775 (37.2) | 0.003 |
| Hypertension | 11218 (75.9) | 1661 (77.7) | 8033 (76.0) | 1524 (73.2) | 0.002 |
| Dyslipidemia | 1844 (12.5) | 276 (12.9) | 1319 (12.5) | 249 (12.0) | 0.64 |
| Diabetes mellitus | 3081 (20.8) | 506 (23.7) | 2221 (21.0) | 354 (17.0) | < 0.001 |
| Coronary heart disease | 1772 (12.0) | 242 (11.3) | 1252 (11.9) | 278 (13.4) | 0.09 |
| Current or previous smoking | 6655 (45.0) | 923 (43.2) | 4770 (45.2) | 962 (46.2) | 0.72 |
| Moderate or heavy drinkers | 4548 (30.8) | 632 (29.6) | 3273 (31.0) | 642 (30.8) | 0.46 |
| Discharge mRS score = 3–5, n (%) | 3076 (20.8) | 351 (16.4) | 2213 (21.0) | 512 (24.6) | < 0.001 |
| BMI, kg/m2, median (IQR) | 24.1 (22.1–25.8) | 24.1 (22.3–26.0) | 24.1 (22.2–25.8) | 23.8 (21.7–25.6) | < 0.001 |
| Mean time to blood collection, hour, median (IQR) | 43 (24–72) | 45 (25–84) | 43 (24–72) | 40 (24–71) | < 0.001 |
| Admission NIHSS score, median (IQR) | 3 (1–6) | 3 (1–5) | 3 (1–6) | 4 (17) | < 0.001 |
| Medications, n (%) | |||||
| Antiplatelet | 12249 (82.9) | 1871 (87.6) | 8642 (81.8) | 1736 (83.4) | < 0.001 |
| Antihypertensive | 7242 (49.0) | 1081 (50.6) | 5202 (49.2) | 959 (46.1) | 0.008 |
| Antidiabetic | 3017 (20.4) | 516 (24.1) | 2192 (20.8) | 309 (14.8) | < 0.001 |
| Lipid-lowering | 7848 (53.1) | 1286 (60.2) | 5626 (53.3) | 936 (45.0) | < 0.001 |
| Pneumonia, n (%) | 868 (5.9) | 82 (3.8) | 585 (5.5) | 201 (9.7) | < 0.001 |
Abbreviations IN-international normalized ratio, ALT-alanine transaminase, AST-aspartate transaminase, mRS-modified Rankin Scale, NIHSS-National Institutes of Health Stroke Scale.
One-Year Rates of Adverse Stroke Outcomes
Table 2 summarizes the 1-year incidences of stroke outcomes including all-cause death, stroke recurrence, composite outcome of death and stroke recurrence, and poor functional outcome. Of 14,782 patients with stroke, all-cause death occurred in 1080 (7.3%), recurrence stroke in 538 (3.9%), combined end point in 1319 (8.9%), and poor functional outcome in 3001 (20.3%). The incidence of all outcomes increased in INR groups (P < 0.001 for all). The incidence of both ischemic stroke and intracranial hemorrhage also increased in INR groups (P < 0.001 for ischemic stroke, P = 0.01 for intracranial hemorrhage). Supplementary Fig. 2 displays 1-year events rate in INR grading categories. The risk of all-cause death, recurrent stroke, combined end point, and poor functional outcome was obviously increased for the INR grading category of 1.05–1.10 and higher.
Table 2. Events rate at 1 year in participants classified based on the admission INR value.
| Outcomes | Total | INR < 0.9 | INR = 0.9–1.1 | INR > 1.1 | P-value |
|---|---|---|---|---|---|
| (n = 14782) | (n = 2137) | (n = 10563) | (n = 2082) | ||
| All-cause death | 1080/14767 (7.3) | 99/2136 (4.6) | 713/10551 (6.8) | 268/2080 (12.9) | < 0.001 |
| Recurrent Stroke | 538/13788 (3.9) | 50/2030 (2.5) | 380/9907 (3.8) | 108/1851 (5.8) | < 0.001 |
| Ischemic Stroke | 510/13788 (3.7) | 47/2030 (2.3) | 365/9907 (3.7) | 98/1851 (5.3) | < 0.001 |
| Intracranial hemorrhage | 28/13788 (0.2) | 3/2030 (0.2) | 15/9907 (0.2) | 1 /1851 (0.5) | 0.01 |
| Combined end point | 1319/14782 (8.9) | 121/2137 (5.7) | 889/10563 (8.4) | 309/2082 (14.8) | < 0.001 |
| Poor functional outcome | 3001/14767 (20.3) | 315/2136 (14.7) | 2114/10551 (20.0) | 572/2080 (27.5) | < 0.001 |
Abbreviations INR - international normalized ratio.
Supplementary Fig. 2.
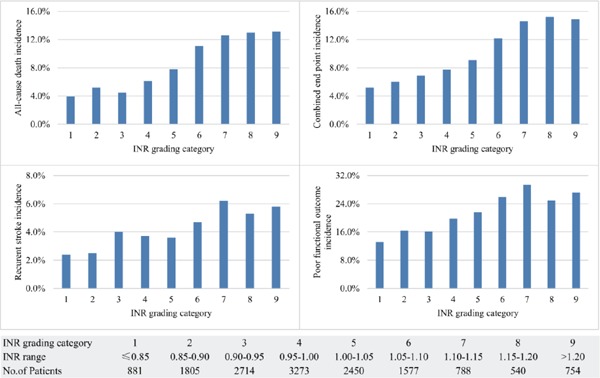
Rates of one-year events among patients with ischemic stroke or TIA according to INR grading category
Association between INR and Clinical Outcomes
Table 3 shows the associations between the INR groups and all stroke outcomes. The adjusted ORs with 95% CIs for high vs medium INR were 1.58 (1.32–1.98) for all-cause death, 1.40 (1.10–1.79) for stroke recurrence, 1.52 (1.29–1.79) for combined end point, and 1.21 (1.06–1.39) for poor functional outcome. The adjusted ORs with 95% CIs for low vs medium INR were 0.93 (0.73–1.19) for all-cause mortality, 0.80 (0.58–1.09) for stroke recurrence, 0.87 (0.70–1.07) for combined end point, and 0.89 (0.76–1.04) for poor functional outcome.
Table 3. ORs and 95% CIs for risk of events according to admission INR value in patients with acute cerebrovascular events.
| INR < 0.9 | INR = 0.9–1.1 | INR > 1.1 | |
|---|---|---|---|
| All-cause death | |||
| Unadjusted | 0.67 (0.54–0.83) | 1.00 | 2.04 (1.76–2.37) |
| Adjusted | 0.93 (0.73–1.19) | 1.00 | 1.58 (1.32–1.89) |
| Recurrent stroke | |||
| Unadjusted | 0.63 (0.47–0.85) | 1.00 | 1.55 (1.25–1.94) |
| Adjusted | 0.80 (0.58–1.09) | 1.00 | 1.40 (1.10–1.79) |
| Combined end point | |||
| Unadjusted | 0.69 (0.61–0.79) | 1.00 | 1.51 (1.36–1.69) |
| Adjusted | 0.87 (0.70–1.07) | 1.00 | 1.52 (1.29–1.79) |
| Poor functional outcome | |||
| Crude | 0.69 (0.61–0.79) | 1.00 | 1.51 (1.36–1.69) |
| Adjusted | 0.89 (0.76–1.04) | 1.00 | 1.21 (1.06–1.39) |
Adjusted for age, sex, history of stroke, hypertension, dyslipidemia, diabetes mellitus, coronary heart disease, current or previous smoking, moderate or heavy alcohol consumption, BMI, liver function, admission NIHSS, mRS scores at discharge, pneumonia, antiplatelet, antihypertensive, antidiabetic and lipid-lowing therapy, and mean time from onset to the blood sampling.
Fig. 1 shows that the adjusted ORs for all-cause death for INR grading category of 1.05–1.10, 1.10–1.15, 1.15–1.20, and > 1.2 were 1.44 (95% CIs, 1.12–1.85), 1.71 (95% CIs, 1.27–2.31), 2.08 (95% CIs, 1.48–2.92), and 1.60 (95% CIs, 1.18–2.18), respectively. The adjusted ORs for combined end point were 1.27 (95% CIs, 1.01–1.59), 1.59 (95% CIs, 1.21–2.07), 1.90 (95% CIs, 1.40–2.57), and 1.49 (95% CIs, 1.13–1.96), respectively.
Fig. 1.
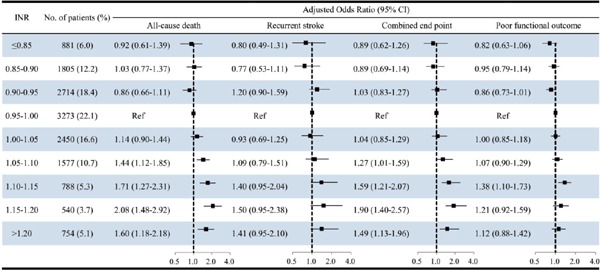
ORs (95% CIs) for all-cause death, stroke recurrence, combined end point of recurrent stroke or death, and poor functional outcome at 1 year according to the level of INR based on a 0.05 increment, with INR of 0.95–1.00 serving as the reference group among patients with ischemic cerebrovascular events.
Sensitivity Analysis
The data listed in Supplementary Table 2 shows that the high INR (> 1.1) was independent of all the stroke outcomes in the subgroup of patients without IV rtPA treatment, which was consistent with the primary analysis. The multivariable analysis showed that the ORs with 95% CIs for the high vs medium INR were 1.61(1.34–1.95) for all-cause death, 1.46 (1.14–1.87) for stroke recurrence, 1.55 (1.31–1.83) for combined end point, and 1.25 (1.08–1.44) for poor functional outcome. No association between low INR (< 0.9) and any stroke outcomes was found compared with the medium group.
Supplementary Table 2. Adjusted odds ratios and 95% confidence intervals for risk of events according to admission INR value in patients without rtPA treatment.
| INR groups |
|||
|---|---|---|---|
| INR < 0.9 (n = 1869) | INR: 0.9–1.1 (n = 9292) | INR > 1.1 (n = 1852) | |
| All-cause death | 0.97 (0.75–1.25) | 1.00 | 1.61(1.34–1.95) |
| Recurrent stroke | 0.85 (0.62–1.16) | 1.00 | 1.46 (1.14–1.87) |
| Combined end point | 0.88 (0.71–1.11) | 1.00 | 1.55 (1.31–1.83) |
| Functional outcome | 0.93 (0.79–1.10) | 1.00 | 1.25 (1.08–1.44) |
Adjusted for age, sex, history of stroke, hypertension, dyslipidemia, diabetes mellitus, coronary heart disease, current or previous smoking, moderate or heavy alcohol consumption, body mass index, liver function, admission NIHSS, mRS scores at discharge, pneumonia, antiplatelet, antihypertensive, antidiabetic, lipid-lowing therapy and mean time from onset to blood collection.
Discussion
In this post hoc analysis of the national cohort study of stroke, it was observed that the high INR is associated with increased risk of 1-year all-cause mortality, stroke recurrence, the combined end point, and poor functional outcome compared with medium INR. However, the low INR did not have a significant impact on the all stroke outcomes. In INR grading categories, only relationships between the all-cause death and combined end point with the elevated grading increment of INR remained significant, although the rates of all stroke outcomes increased with the increase in INR increment. To the best of our knowledge, this is the first national study that defines the prognostic value of baseline INR in patients with ischemic cerebrovascular events.
It was noticed that compared with median INR, the high INR was significantly associated with the high risk of 1-year stroke outcomes including all-cause mortality, stroke recurrence, combined end point, and poor functional outcome. In previously published studies, the association between elevated baseline INR and poor outcomes in patients with acute decompensated heart failure and acute pulmonary embolism was investigated10, 11). A plausible explanation for this phenomenon might be that after an ischemic event, the activation of the coagulation system may result in the consumption of coagulation factors, which is manifested as an elevated INR. Therefore, the thrombosis could be inhibited. In this conditions, either the blood clot enlargement is prevented or thrombolysis is stimulated to increase the blood flow perfusion to the distal vessel in patients after stroke onset16). In this analysis, the patients with higher INR had a more serious stroke measured by the baseline NIHSS score (Supplementary Table 3). Our results suggest that the admission INR would be an important index for prognosis in stroke patients and might be a therapeutic target in those without AF.
Supplementary Table 3. Associations between INR and baseline patient characteristics using the ordinal logistic regression model.
| Characteristics | Odds ratios | 95% confidence intervals | P-value |
|---|---|---|---|
| Age, years | |||
| ≥ 65 vs. < 65 | 1.50 | 1.39–1.61 | < 0.001 |
| Female vs. male | 0.71 | 0.65–0.78 | < 0.001 |
| Vascular risk factors | |||
| Stroke | 1.08 | 1.00–1.17 | 0.046 |
| Hypertension | 0.86 | 0.79–0.93 | < 0.001 |
| Dyslipidemia | 0.99 | 0.89–1.10 | 0.80 |
| Diabetes mellitus | 0.81 | 0.74–0.89 | < 0.001 |
| Coronary heart disease | 1.08 | 0.97–1.20 | 0.18 |
| Current or previous smoking | 0.93 | 0.85–1.01 | 0.10 |
| Moderate or heavy drinkers | 1.02 | 0.94–1.11 | 0.66 |
| Body mass index | 0.99 | 0.98–1.00 | 0.17 |
| Mean time to blood collection, days | 0.96 | 0.94–0.98 | < 0.001 |
| Admission NIHSS score | 1.03 | 1.02–1.04 | < 0.001 |
Ordinal logistic regression model was used to evaluate association of INR with baseline patient characteristics. INR was divided into three categories: low INR (< 0.9), medium INR group (0.9–1.1), high INR (>1.1). Abbreviations: INR, international normalized ratio; NIHSS, National Institutes of Health Stroke Scale.
It was also found a high risk of all-cause death and combined end point in patients with INR grading category > 1.05, with 0.05 increments, as compared with those with INR from 0.95 to 1.00. This result supports the feasibility of considering INR, divided in three groups, for patients with ischemic cerebrovascular events. Yet, no significant association between INR value and stroke recurrence or poor functional outcome was observed. This could be explained by the relative few events occurring in each group, which could enlarge the confidence interval.
In addition, a low INR had a minor risk reduction in all stroke outcomes. As compared with INR of 0.95–1.00, none of low INR grading categories was meaningfully associated with any stroke outcomes. The reason for this phenomenon is that the baseline INR was barely increased, because the activation of anticoagulation system was low after minor ischemic cerebrovascular events onset. However, another unknown mechanism cannot be excluded to interpret this result.
It should be recognized that this study had some limitations, which are listed in the following. First, it was an observational study, which limited the possibility to make causal inferences. Secondly, the diffusion-weighted imaging lesion volume measurements were not performed to determine the relationship between INR and brain infarct size. Thus, the association between INR and cerebral infarct size was not considered in this study. Thirdly, the association between INR and outcomes based on the subtypes of ischemic stroke was not evaluated, because there are no data on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtypes or other classification of ischemic stroke subtypes. Furthermore, the patients with cardioembolic stroke were excluded from the current study due to the treatment with anticoagulant drugs. Finally, our cohort included exclusively Chinese patients. Hence, the results may not be generalized to other ethnic populations with cerebrovascular events.
Conclusions
In patients with acute ischemic stroke, increased baseline INR in the absence of anticoagulation was an independent predictor of 1-year all-cause mortality, stroke recurrence, combined end point, and poor functional outcome. These findings might suggest the importance of INR evaluation in clinical practice in acute ischemic stroke patients both with and without preadimission anticoagulant use. Nevertheless, further investigation would be necessary to verify and confirm the relationship between INR and clinical outcomes of stroke.
Acknowledgments
We thank all participating hospitals, their physicians and nurses, and the CNSR II Steering Committee members.
Funding
Funding for this study was provided by theNational Key R&D Program of China (2016YFC 1307300, 2016YFC1307301, 2017YFC1310900 and 2017YFC1310901).
Contributors
Yilong Wang, Yongjun Wang, and Xingquan Zhao conceived and designed the study. Xuewei Xie, Xianwei Wang, and Yilong Wang interpreted the data and prepared the report. Yongjun Wang, Liping Liu, and Zhongrong Miao contributed to the comments on the draft manuscript and revised the report. Zixiao Li and Xia Meng coordinated the study. Hao Li and Xiangwei Wang conducted the statistical analysis.
Disclosure
The authors report no disclosures relevant to the manuscript.
References
- 1). Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg, 2011; 76: S85-90 [DOI] [PubMed] [Google Scholar]
- 2). Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke, 2004; 35: 731-735 [DOI] [PubMed] [Google Scholar]
- 3). Ignjatovic V. Prothrombin time/international normalized ratio. Methods Mol Biol, 2013; 992: 121-129 [DOI] [PubMed] [Google Scholar]
- 4). Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA, 2003; 290: 2685-2692 [DOI] [PubMed] [Google Scholar]
- 5). Wazni OM, Beheiry S, Fahmy T, Barrett C, Hao S, Patel D, Di Biase L, Martin DO, Kanj M, Arruda M, Cummings J, Schweikert R, Saliba W, Natale A. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: comparison of strategies of anticoagulation management in the periprocedural period. Circulation, 2007; 116: 2531-2534 [DOI] [PubMed] [Google Scholar]
- 6). Yasaka M, Minematsu K, Yamaguchi T. Optimal intensity of international normalized ratio in warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. Intern Med, 2001; 40: 1183-1188 [DOI] [PubMed] [Google Scholar]
- 7). Fletcher AP, Alkjaersig N, Davies A, Lewis M, Brooks J, Hardin W, Landau W, Raichle ME. Blood coagulation and plasma fibrinolytic enzyme system pathophysiology in stroke. Stroke, 1976; 7: 337-348 [DOI] [PubMed] [Google Scholar]
- 8). Pikija S, Trkulja V, Mutzenbach JS, McCoy MR, Ganger P, Sellner J. Fibrinogen consumption is related to intracranial clot burden in acute ischemic stroke: a retrospective hyperdense artery study. J Transl Med, 2016; 14: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Verma A, Kole T. International normalized ratio as a predictor of mortality in trauma patients in India. World J Emerg Med, 2014; 5: 192-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Okada A, Sugano Y, Nagai T, Takashio S, Honda S, Asaumi Y, Aiba T, Noguchi T, Kusano KF, Ogawa H, Yasuda S, Anzai T, Na DEFI. Prognostic Value of Prothrombin Time International Normalized Ratio in Acute Decompensated Heart Failure- A Combined Marker of Hepatic Insufficiency and Hemostatic Abnormality. Circ J, 2016; 80: 913-923 [DOI] [PubMed] [Google Scholar]
- 11). Kiris T, Yazici S, Durmus G, Canga Y, Karaca M, Nazli C, Dogan A. The relation between international normalized ratio and mortality in acute pulmonary embolism: A retrospective study. J Clin Lab Anal, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Ay H, Arsava EM, Gungor L, Greer D, Singhal AB, Furie KL, Koroshetz WJ, Sorensen AG. Admission international normalized ratio and acute infarct volume in ischemic stroke. Ann Neurol, 2008; 64: 499-506 [DOI] [PubMed] [Google Scholar]
- 13). Bettger JP, Li Z, Xian Y, Liu L, Zhao X, Li H, Wang C, Wang C, Meng X, Wang A, Pan Y, Peterson ED, Wang Y, Wang Y, investigators CI Assessment and provision of rehabilitation among patients hospitalized with acute ischemic stroke in China: Findings from the China National Stroke Registry II. Int J Stroke, 2017; 12: 254-263 [DOI] [PubMed] [Google Scholar]
- 14). Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, Zhou Y, Zhao X, Wang C, Liu L, Nguyen-Huynh MN, Claiborne Johnston S, Wong L, Li H, China National Stroke Registry I The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke, 2011; 6: 355-361 [DOI] [PubMed] [Google Scholar]
- 15). Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr., Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H, American Heart Association Stroke C, Council on Cardiovascular N, Council on Peripheral Vascular D and Council on Clinical C Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2013; 44: 870-947 [DOI] [PubMed] [Google Scholar]
- 16). Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation, 2004; 109: 2698-2704 [DOI] [PubMed] [Google Scholar]


