Abstract
Aim: Type III collagen abundantly exists in the cardiovascular system, including the aorta and heart. We prospectively investigated whether serum levels of aminoterminal propeptide of type III procollagen (PIIINP), a circulating biomarker of cardiovascular fibrosis, could predict cardiovascular events in patients undergoing hemodialysis.
Methods: Serum PIIINP concentrations were measured in 244 patients undergoing maintenance hemodialysis (men, 126; women, 118; mean age, 64 ± 11 years; dialysis duration, 11.5 ± 7.8 years) by immunoradiometric assay in February 2005. The endpoint was cardiovascular events, and the patients were followed up until the endpoint was reached, or until January 31, 2011.
Results: During the follow-up for 4.7 ± 1.8 years, cardiovascular events occurred in 78 (30.3%) of 244 patients. Stepwise Cox hazard analysis revealed that cardiovascular events were associated with increased serum PIIINP concentration (1 U/mL; hazard ratio, 1.616; P = 0.0001). The median serum PIIINP concentrations were higher in patients with cardiovascular events than in those without (2.30 ± 0.19 U/mL vs 1.30 ± 0.03 U/mL; P < 0.0001). When the patients were assigned to subgroups based on serum PIIINP cut-off value for cardiovascular events of 1.75 U/mL, defined by receiver operating characteristic analysis, cardiovascular event-free survival rates at 5 years were lower (P = 0.0001) in the subgroup of serum PIIINP ≥ 1.75 U/mL than in that of serum PIIINP < 1.75 U/mL (31.9% vs 88.2%).
Conclusions: Serum PIIINP could be a new biomarker for predicting the cardiovascular events in patients undergoing hemodialysis.
Keywords: Aortic stiffness, Cardiovascular fibrosis, Hemodialysis, Left ventricular hypertrophy, Procollagen
Introduction
Fibrillar collagen is synthesized in cardiac fibroblasts as a procollagen1). The myocardium and coronary artery comprise type I and III collagen as major fibrillar collagens. Increased collagen turnover plays an important role in determining the functional properties of the arterial vasculature and ventricular myocardium2), and that in the coronary artery contributes to coronary intimal thickening and progression of coronary plaque. In human aorta, type I, III, and V are the main collagens; particularly, more type III than type I collagen is found in the aortic media3). The aminoterminal propeptide of type III procollagen (PIIINP) is an extension peptide of type III procollagen, which is cleaved stoichiometrically during conversion from type III procollagen to type III collagen and liberated into the serum; serum PIIINP is thought to be a biomarker of collagen type III synthesis4). Among the many circulating molecules proposed as biomarkers of myocardial fibrosis in humans, only PIIINP and the carboxy-terminal propeptide of type I procollagen (CIPC), formed during the extracellular conversion of type I procollagen into mature fibril-forming type I collagen, have been shown to be associated with myocardial fibrosis5–8).
Aim
Left ventricular hypertrophy (LVH) and arterial stiffness, which significantly contribute to the occurrence of cardiovascular events, are the common findings in patients undergoing hemodialysis9, 10). Fibrosis in the cardiovascular system is involved in remodeling of the heart and vascular system, and it plays an important role in the genesis of LVH and arterial stiffness. Circulating PIIINP, a biomarker of type III collagen synthesis, has been shown to predict prognosis in patients with heart failure and ischemic heart disease11, 12) or in the general population with idiopathic dilated cardiomyopathy5, 6, 13). In a previous study, the extent of myocardial fibrosis was associated with mortality in patients with dilated cardiomyopathy and heart failure undergoing hemodialysis14). Because patients undergoing hemodialysis have higher cardiovascular risks such as LVH or aortic stiffness than the general population, increased fibrosis in the cardiovascular system may cause long-term cardiovascular events, even in the absence of heart failure or dilated cardiomyopathy. In the present study, we aimed to prospectively investigate whether the serum levels of PIIINP could predict the long-term cardiovascular events in patients without apparent heart failure or cardiomyopathy undergoing maintenance hemodialysis.
Methods
Patients
Fig. 1 is the flow diagram of subject recruitment. Patients with end-stage kidney disease undergoing maintenance hemodialysis for more than 1 year in Toujinkai Hospital, Japan, at the point of January 1, 2005, who met the inclusion criteria, were eligible for this study. Of the 384 patients undergoing hemodialysis, 302 met the eligibility criteria. Exclusion criteria were as follows: a history of coronary events and/or interventions; congestive heart failure of New York Heart Association grades III to IV; moderate or worse valvular heart disease (aortic or mitral valvular areas ≤ 1.5 cm2 for aortic or mitral stenosis, and Sellers grades III or IV for aortic or mitral regurgitation); permanent pacemaker implantation; idiopathic hypertrophic or dilated cardiomyopathy; chronic obstructive pulmonary disease; malignancy; and acute hepatitis, chronic hepatitis, liver cirrhosis, or carrier of hepatitis virus, or laboratory disorder of liver function by unknown origin. Of 302 patients who met the eligibility criteria, 58 were excluded based on the exclusion criteria. Consequently, 244 patients (men, 126; women, 118; mean age, 64 ± 11 years; dialysis duration, 11.5 ± 7.8 years) were enrolled in the study from January 1 to 31, 2005, and followed through January 31, 2011. Blood pressure was measured hourly during dialysis using a mercury sphygmomanometer; for study purposes, blood pressure was determined as the mean of the measurements obtained at the beginning of eight consecutive midweek hemodialysis sessions before enrollment. Histories of cigarette smoking and alcohol consumption were determined by a questionnaire. A smoking habit was defined as smoking 10 or more cigarettes per week. Alcohol consumption was defined as alcohol intake three times or more per week. The Ethics Committee for Human Research of Toujinkai Hospital approved this study. All patients provided written informed consent to all procedures associated with the study before participation. The study was performed in accordance with the principles of the Declaration of Helsinki and registered in the ClinicalTrials.gov (https://clinicaltrials.gov/): protocol identifier, NCT03391128.
Fig. 1.
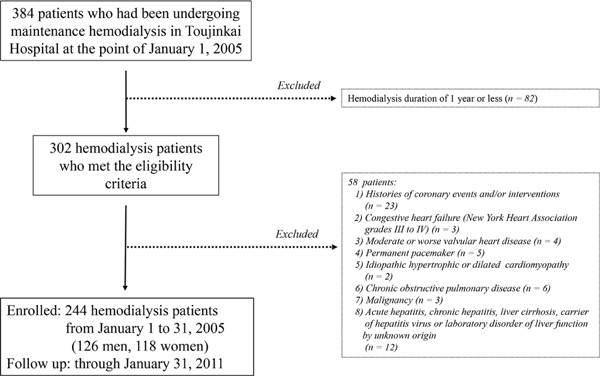
Flow diagram of subject recruitment.
Measurement of PIIINP
Blood was collected for determination of serum PIIINP concentration just before starting the first hemodialysis session of the week after the enrollment. Serum PIIINP was determined by immunoradiometric assay (RIA-gnost PIIIP c.t., CIS Bio International, Saclay, France). The intra- and interassay coefficients of variation for this assay were 1.4%–2.9% and 2.5%–3.1%, respectively.
Biochemical and Hematological Determinations
Other blood samples were collected at the same hemodialysis sessions for the sampling of PIIINP. We measured blood hemoglobin levels; serum concentrations of aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase, total cholesterol, high-density lipoprotein cholesterol, triglyceride, albumin, calcium, inorganic phosphorus, intact parathyroid hormone, β2 microglobulin, high-sensitivity C-reactive protein, or hemoglobin A1c; and plasma concentrations of B-type natriuretic peptide (BNP) or aldosterone. Plasma BNP and aldosterone concentrations were measured using commercially available immunoradiometric assay kits (Shionoria BNP kit, Shionogi, Osaka Japan; SPACS-S Aldosterone kit, Dai-ichi Radioisotope, Tokyo, Japan). The intra- and interassay coefficients of variation for determining BNP concentration were 5.3% and 5.9%, respectively.
Echocardiography
All patients underwent two-dimensionally guided echocardiography using a single ultrasonographic recorder (UF-8800, Fukuda Denshi, Tokyo, Japan) on a midweek non-dialysis day within 1 month after the enrollment. LV ejection fraction (LVEF) was quantified using the modified Simpson rule, and LV mass was normalized to body surface area, and is described herein as left ventricular mass index.
Endpoint
All 244 patients were followed up at Toujinkai Hospital. The endpoint was cardiovascular events: cardiovascular deaths, including death caused by acute myocardial infarction (AMI) or congestive heart failure, and sudden cardiac death (SCD); coronary interventions, including percutaneous coronary intervention or coronary artery bypass grafting; vasospastic angina identified by coronary angiography; malignant arrhythmias such as ventricular tachycardia or fibrillation; bradycardia needing permanent pacemaker implantation; congestive heart failure needing hospitalization; cardiac valvular disease needing operation; aortic aneurysm including rupture or dissection; or peripheral artery disease needing vascular bypass or leg amputation. SCD was defined as death within 24 h of the time that the victim was last seen alive in a normal state of health, and cardiac diseases such as malignant arrhythmias or acute coronary syndrome were considered the most frequent causes of death. Cerebrovascular accidents were ruled out by post-mortem examinations. Cardiologists in Toujinkai Hospital or Kyoto Second Red Cross Hospital diagnosed cardiac-derived death, and they did not know about the study protocol at the point of diagnosis.
Statistical Analysis
Continuous values were expressed as the median ± SE. Continuous variables were compared using Mann–Whitney U test. Categorical data were analyzed using the Chi-square test. Receiver operating characteristic analysis was performed to define the threshold of PIIINP for cardiovascular events; thresholds were obtained from minimal false positive and false negative results, that is, by minimizing the expression (1 − specificity)2 + (1−sensitivity)2. The associations of clinical factors with cardiovascular events were analyzed using Cox hazard model. Stepwise Cox hazard analysis was performed among significant (P < 0.05) factors in univariate analysis. Cardiovascular event-free survival rates were assessed using the Kaplan–Meier method and the log-rank test. A P value of < 0.05 was considered statistically significant. Individuals who were blinded to all personal information about the patients statistically analyzed all data. All statistical analyses were performed using IBM SPSS Statistics software, version 23.
Results
All 244 patients had been followed up in Toujinkai Hospital until the endpoint was reached, or until January 31, 2011 (end of the study). During follow-up for 4.7 ± 1.8 years, all-cause death occurred in 62 patients (25.4%), as follows: cardiovascular deaths (n = 20: 14 SCD, 3 AMI deaths, 2 heart failure deaths, and 1 rupture of dissecting aortic aneurysm), malignancy (n = 11), infection (n = 10), cerebrovascular accidents (n = 7:4 cerebral bleeding and 3 cerebral infarction), digestive system diseases (n = 6), respiratory failure (n = 4), liver failure (n = 1), leukemia (n = 1), multiple organ failure (n = 1), and senility (n = 1). Cardiovascular death was 31.7% of all-cause death. Cardiovascular events occurred in 78 (32%) of 244 patients, as follows: cardiac deaths (n = 19), non-fatal AMI (n = 1), obstructive coronary artery disease needing percutaneous coronary intervention (n = 22), vasospastic angina identified by angiography (n = 3), heart failure needing hospitalization (n = 21), bradycardia needing pacemaker implantation (n = 7:5 sick sinus syndrome and 2 complete atrioventricular block), dissecting aortic aneurysm (n = 2), aortic valvular stenosis needing valve replacement (n = 1), peripheral artery disease needing bypass surgery (n = 1), and leg amputation (n = 1). One of the two patients with dissecting aneurysm died immediately after the diagnosis.
Serum PIIINP
Serum PIIINP concentrations were distributed between 0.30 and 9.50 U/mL, with a median of 1.40 ± 0.08 U/mL for all subjects (Fig. 2). To evaluate reproducibility of serum PIIINP levels in this population, we remeasured the serum PIIINP concentrations after 1 month of the first measurement in 30 of the participants; serum PIIINP concentrations (mean ± SD) did not differ during 1 month: 1.83 ± 1.02 U/mL versus 1.85 ± 1.03 U/mL. In the Japanese population, the reference interval for PIIINP in this assay kit is 0.30–0.80 U/mL; the serum PIIINP concentrations of patients undergoing hemodialysis would be higher than those of normal population, as previously reported15). Serum PIIINP concentration was positively correlated with dialysis periods (r = 0.46, P < 0.001), serum calcium concentration (r = 0.24, P < 0.001), calcium-inorganic phosphate product (r = 0.15, P = 0.017) or cardiovascular events (r = 0.54, P < 0.001), and inversely correlated with diabetes mellitus (r = −0.17, P = 0.009) or diastolic blood pressure (r = −0.16, P = 0.014).
Fig. 2.
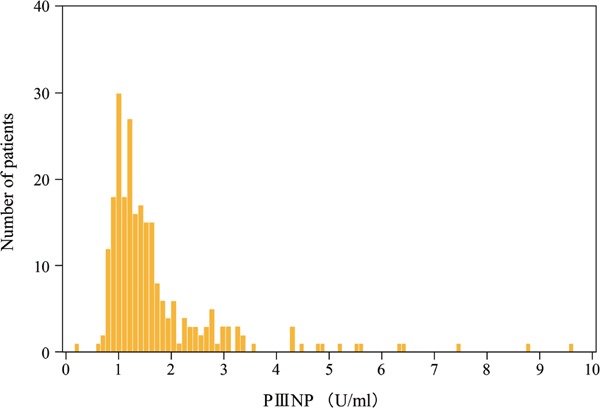
Histogram of serum PIIINP concentrations. Serum PIIINP concentrations distributed between 0.30 and 9.50 U/mL, and the median value was 1.40 ± 0.08 U/mL for all subjects. In the Japanese population, the reference interval for PIIINP in this assay kit is 0.30 to 0.80 U/mL; the serum PIIINP concentrations of patients undergoing hemodialysis would be higher than those of normal population.
Cardiovascular Events and PIIINP
Patients with cardiovascular events had longer dialysis duration; higher age or serum PIIINP concentration; and lower diastolic blood pressure, serum albumin, or intact parathyroid concentration, or administration ratio of antiplatelet drugs than those without (Table 1). In univariate Cox hazard analysis, cardiovascular events were positively associated with age, dialysis duration, cardiothoracic ratio, or serum PIIINP concentration, and inversely associated with diastolic blood pressure, LVEF, or serum albumin concentration (Table 2). Stepwise Cox hazard analysis among these factors revealed a positive association of cardiovascular events with age or serum PIIINP concentration, and an inverse association with diastolic blood pressure or LVEF (Table 3). Serum PIIINP concentration was also positively associated with cardiac death (hazard ratio, 1.32; 95% CI, 1.02–1.70; P = 0.032). In receiver operating characteristic analysis, the threshold of serum PIIINP concentration for cardiovascular events was determined as 1.75 U/mL (Fig. 3). When patients were assigned to subgroups based on this serum PIIINP cut-off value, cardiovascular event-free survival rates at 5 years were lower in the subgroup of serum PIIINP of 1.75 U/mL or more than in that of serum PIIINP below 1.75 U/mL (31.9% vs 88.2%) (Fig. 4).
Table 1. Clinical characteristics in the groups with or without cardiovascular events.
| Events (−) | Events (+) | P value | |
|---|---|---|---|
| (n = 166) | (n = 78) | ||
| Male gender, n (%) | 82 (49.4) | 44 (56.4) | 0.367 |
| Age, years | 63.0 ± 0.8 | 66.0 ± 1.2 | 0.005 |
| Dialysis duration, months | 108.0 ± 6.4 | 147.0 ± 12.6 | 0.032 |
| Diabetes, n (%) | 51 (30.7) | 27 (34.6) | 0.543 |
| Body mass index, kg/m2 | 18.3 ± 0.3 | 18.9 ± 0.5 | 0.882 |
| Smoking, n (%) | 54 (32.5) | 25 (32.1) | 0.941 |
| Alcohol, n (%) | 80 (48.2) | 32 (41.0%) | 0.295 |
| Systolic blood pressure before dialysis, mmHg | 145.0 ± 1.3 | 142.0 ± 1.8 | 0.102 |
| Diastolic blood pressure before dialysis, mmHg | 79.0 ± 0.8 | 73.0 ± 1.4 | < 0.001 |
| Cardiothoracic ratio, % | 51.0 ± 0.3 | 52.0 ± 0.6 | 0.077 |
| Left ventricular ejection fraction, % | 69.6 ± 0.9 | 68.4 ± 1.6 | 0.109 |
| Left ventricular mass index, g/m2 | 116.3 ± 4.4 | 118.1 ± 4.5 | 0.670 |
| Serum aspartate aminotransferase, IU/L | 17.0 ± 0.2 | 17.0 ± 0.5 | 0.926 |
| Serum alanine aminotransferase, IU/L | 19.0 ± 0.2 | 18.0 ± 0.6 | 0.529 |
| Serum γ-glutamyl transferase, IU/L | 22.0 ± 0.2 | 22.0 ± 0.5 | 0.796 |
| Blood hemoglobin, g/dL | 10.0 ± 0.1 | 10.3 ± 0.1 | 0.219 |
| Serum albumin, g/dL | 4.0 ± 0.03 | 3.8 ± 0.05 | 0.003 |
| Serum calcium, mg/dL | 8.9 ± 0.1 | 9.0 ± 0.1 | 0.075 |
| Serum inorganic phosphorus, mg/dL | 5.3 ± 0.1 | 5.6 ± 0.1 | 0.497 |
| Serum intact parathyroid hormone, pg/mL | 210.0 ± 13.3 | 140.0 ± 28.0 | 0.046 |
| Serum C-reactive protein, mg/L | 2.9 ± 0.2 | 3.9 ± 0.4 | 0.098 |
| Serum hemoglobin A1c, % | 6.1 ± 0.1 (n = 51) | 5.6 ± 0.2 (n = 27) | 0.440 |
| Serum total cholesterol, mg/dL | 170.0 ± 3.1 | 163.0 ± 4.2 | 0.299 |
| Serum high-density lipoprotein cholesterol, mg/dL | 35.0 ± 0.6 | 36.0 ± 0.7 | 0.911 |
| Serum triglyceride, mg/dL | 126.0 ± 5.2 | 132.0 ± 6.7 | 0.942 |
| Serum β2 microglobulin, ng/mL | 41.8 ± 0.4 | 40.7 ± 0.4 | 0.580 |
| Serum PIIINP, U/mL | 1.30 ± 0.03 | 2.30 ± 0.19 | < 0.001 |
| Plasma B-type natriuretic peptide, pg/mL | 283.0 ± 29.5 | 276.0 ± 34.5 | 0.655 |
| Plasma aldosterone, pg/mL | 121.0 ± 28.8 | 116.0 ± 15.7 | 0.272 |
| Medications | |||
| α1 blockers, n (%) | 25 (15.1) | 6 (7.7) | 0.107 |
| β blockers, n (%) | 35 (21.1) | 16 (20.5) | 0.918 |
| Calcium channel blockers, n (%) | 42 (25.3) | 17 (21.8) | 0.551 |
| ACE inhibitors, n (%) | 21 (12.7) | 7 (9.0) | 0.401 |
| ARB, n (%) | 34 (20.5) | 16 (20.5) | 0.996 |
| Nitrates, n (%) | 7 (4.2) | 4 (5.1) | 0.748 |
| Antiplatelet drugs, n (%) | 54 (32.5) | 15 (19.2) | 0.032 |
| Anticoagulants (%) | 4 (2.4) | 1 (1.3) | 0.562 |
| Statins, n (%) | 10 (6.0) | 4 (5.1) | 0.779 |
| Vitamin D, (%) | 90 (54.2) | 44 (56.4) | 0.509 |
PIIINP, aminoterminal propeptide of type III procollagen; ACE, angiotensin I converting enzyme; ARB, angiotensin II type-1 receptor blocker.
Table 2. Univariate Cox-hazard analysis for cardiovascular events.
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Male gender (0 = female; 1 = male) | 1.42 | 0.91–2.23 | 0.122 |
| Age (1 year) | 1.04 | 1.02–1.06 | < 0.001 |
| Dialysis duration (1 month) | 1.00 | 1.00–1.01 | 0.002 |
| Diabetes mellitus (0 = no; 1 = yes) | 1.21 | 0.76–1.93 | 0.424 |
| Body mass index (1 kg/m2) | 0.98 | 0.93–1.04 | 0.554 |
| Smoking habit (0 = no; 1 = yes) | 1.02 | 0.63–1.64 | 0.941 |
| Alcohol consumption (0 = no; 1 = yes) | 0.78 | 0.50–1.23 | 0.288 |
| Systolic blood pressure before dialysis (1 mmHg) | 0.99 | 0.97–1.00 | 0.055 |
| Diastolic blood pressure before dialysis (1 mmHg) | 0.96 | 0.95–0.98 | < 0.001 |
| Cardiothoracic ratio (1%) | 1.05 | 1.00–1.11 | 0.040 |
| Left ventricular ejection fraction (1%) | 0.98 | 0.96–1.00 | 0.010 |
| Left ventricular mass index (1 g/m2) | 1.00 | 0.99–1.00 | 0.342 |
| Serum aspartate aminotransferase (1 IU/L) | 0.94 | 0.86–1.03 | 0.172 |
| Serum alanine aminotransferase (1 IU/L) | 0.97 | 0.90–1.05 | 0.433 |
| Serum γ-glutamyl transferase (1 IU/L) | 1.01 | 0.93–1.10 | 0.859 |
| Blood hemoglobin (1 g/dL) | 1.13 | 0.84–1.36 | 0.191 |
| Serum albumin (1 g/dL) | 0.37 | 0.21–0.64 | < 0.001 |
| Serum calcium (1 mg/dL) | 1.18 | 0.90–1.54 | 0.233 |
| Serum inorganic phosphorus (1 mg/dL) | 1.04 | 0.85–1.26 | 0.714 |
| Serum intact parathyroid hormone (1 pg/mL) | 1.00 | 1.00–1.00 | 0.849 |
| Serum C-reactive protein (1 mg/L) | 1.08 | 0.99–1.18 | 0.097 |
| Serum hemoglobin A1c (1%) | 1.15 | 0.83–1.64 | 0.425 |
| Serum total cholesterol (1 mg/dL) | 1.00 | 0.99–1.00 | 0.099 |
| Serum high-density lipoprotein cholesterol (1 mg/dL) | 0.99 | 0.96–1.03 | 0.700 |
| Serum triglyceride (1 mg/dL) | 1.00 | 1.00–1.00 | 0.783 |
| Serum β2 microglobulin (1 ng/mL) | 0.99 | 0.94–1.03 | 0.560 |
| Plasma B-type natriuretic peptide (1 pg/mL) | 1.00 | 1.00–1.00 | 0.667 |
| Plasma aldosterone (1 pg/mL) | 1.00 | 1.00–1.00 | 0.138 |
| Serum PIIINP (1 U/mL) | 1.53 | 1.40–1.68 | < 0.001 |
| Medications | |||
| α1 blockers (0 = no; 1 = yes) | 0.52 | 0.23–1.19 | 0.123 |
| β blockers (0 = no; 1 = yes) | 0.99 | 0.57–1.72 | 0.984 |
| Calcium channel blockers (0 = no; 1 = yes) | 0.81 | 0.47–1.38 | 0.437 |
| ACE inhibitors (0 = no; 1 = yes) | 0.74 | 0.34–1.61 | 0.446 |
| ARB (0 = no; 1 = yes) | 0.99 | 0.57–1.71 | 0.965 |
| Nitrates (0 = no; 1 = yes) | 1.22 | 0.45–3.33 | 0.701 |
| Antiplatelet drugs (0 = no; 1 = yes) | 0.60 | 0.34–1.06 | 0.079 |
| Anticoagulation drugs (0 = no; 1 = yes) | 0.70 | 0.10–5.02 | 0.721 |
| Statins (0 = no; 1 = yes) | 0.82 | 0.30–2.25 | 0.704 |
| Vitamin D (0 = no; 1 = yes) | 1.17 | 0.75–1.84 | 0.485 |
PIIINP, aminoterminal propeptide of type III procollagen; ACE, angiotensin I converting enzyme; ARB, angiotensin II type-1 receptor blocker.
Table 3. Multivariate Cox-hazard analysis for cardiovascular events.
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Age (1 year) | 1.05 | 1.02–1.07 | < 0.001 |
| Serum PIIINP (1 U/mL) | 1.59 | 1.43–1.77 | < 0.001 |
| Diastolic blood pressure before dialysis (1 mmHg) | 0.98 | 0.96–1.00 | 0.034 |
| Left ventricular ejection fraction (1%) | 0.98 | 0.96–1.00 | 0.024 |
PIIINP, aminoterminal propeptide of type III procollagen.
Fig. 3.
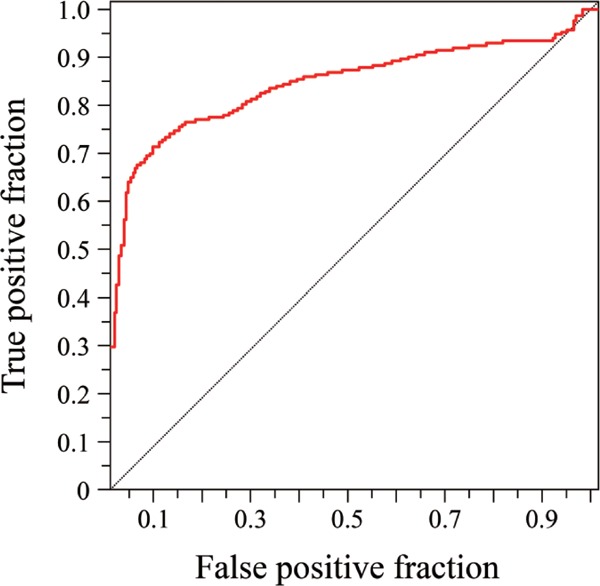
Receiver operating characteristic curves to determine the threshold of serum PIIINP concentration for cardiovascular events. The area under the curve was 0.842.
Fig. 4.
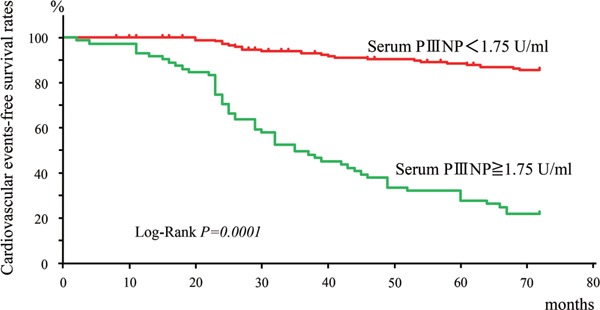
Kaplan–Meier analysis of cardiovascular event-free survival rates differentiated by the threshold of serum PIIINP concentration (1.75 U/mL).
Discussion
Various studies have indicated that circulating PIIINP levels are associated with LV remodeling induced by hypertension, myocardial infarction, heart failure4, 5, 7, 13, 16–18), or aortic stiffness18, 19) and prognosis including mortality6, 11, 12, 20, 21). However, there are few studies showing the relationship between circulating PIIINP and cardiovascular events in patients undergoing hemodialysis. To the best of our knowledge, this study is the first to show the independent association of serum PIIINP concentration with cardiovascular event occurrence in patients undergoing hemodialysis. Because serum PIIINP levels are reportedly elevated in acute or chronic liver diseases and correlate positively with serum aminotransferase or bilirubin levels22), patients with liver disease were excluded from this study. In addition, laboratory data of serum aminotransferases were not related with serum PIIINP levels in the participants of our study. Because serum PIIINP concentrations did not change during 1 month in our study and did not vary throughout the day in the study by Saggese et al.23), it may be concluded that serum PIIINP concentrations remain stable in patients undergoing hemodialysis. The molecular weight of PIIINP is 42000 Da; PIIINP would not be removed by hemodialysis, but partly removed by hemodialysis filtration, although we had not evaluated the changes of serum PIIINP before and after dialysis. Because serum PIIINP is reportedly cleared from the blood via hepatobiliary elimination and does not depend on renal function24, 25), predialysis serum PIIINP concentrations are thought to directly indicate PIIINP turnover. In addition, because collagen type III abundantly exists in the cardiovascular system2, 3), increased serum PIIINP levels indicating advanced turnover of collagen type III may be a risk factor for cardiovascular events in this population.
Myocardial fibrosis is of two types: focal and diffuse26). Focal fibrosis replaces dead cardiomyocytes and forms scars. Diffuse fibrosis occurs in the interstitial and perivascular space, and it is involved in the various pathophysiological and clinical features of chronic cardiac diseases: increased LV stiffness and diastolic dysfunction, impaired LV systolic dysfunction, arrhythmias, and impaired coronary flow reserve27–30). In addition, increased collagen turnover is associated with the functional properties of the arterial vasculature. Increased arterial stiffness due to medial fibrosis contributes to LVH, myocardial ischemia, and microcirculatory disturbance in the heart31). Diffuse fibrosis in the heart and vasculature affect each other to promote the occurrence of cardiovascular events.
In the present study, SCD was the main cause of cardiac death. Sudden death occurs more frequently in patients undergoing hemodialysis compared with general population: SCD accounts for approximately one-fourth of all-cause mortality in patients undergoing dialysis32, 33). The main cause of SCD is believed to be hemodynamic collapse due to malignant arrhythmia such as ventricular fibrillation in the setting of structural heart disease34). Arrhythmias were reportedly responsible for 78% of all cardiac deaths or 29% of allcause mortality in patients undergoing hemodialysis35). A triggering event or condition interacts with the underlying structural heart disease to produce fatal arrhythmia. LVH, the most frequent cardiac abnormality in patients undergoing dialysis36), causes electrical remodeling of the heart37), and ventricular arrhythmia is increased in patients with echocardiographically identified LVH compared with those without38). Advanced myocardial fibrosis, an important component of LVH, might be associated with fatal arrhythmias such as ventricular tachycardia/fibrillation via the mechanism of localized conduction disturbance and spiral reentry39).
In addition, obstructive coronary artery disease accounted for a large percentage of cardiovascular events. Because the main fibrillar collagen in coronary plaque is type I collagen, type III collagen is thought not to be involved in the progression of coronary plaque3). On the contrary, type III collagen is the most abundant form of collagen in the aortic wall3); increased collagen turnover of medial layers of the aorta decreases vascular distensibility, leading to increased arterial stiffness, as described earlier. Increased arterial stiffness contributes to causing LVH, including myocardial fibrosis27, 28); it easily leads to impaired coronary blood flow30). In pathological studies, intramyocardial arteriolar thickening, reduced capillary density, and myocardial fibrosis are unique findings in the heart of patients undergoing hemodialysis40, 41); these myocardial abnormalities can potentially cause myocardial microcirculatory disturbance. These characteristics in patients undergoing hemodialysis result in increased susceptibility of myocardial cells to reduced myocardial blood supply in patients undergoing hemodialysis than in non-dialysis patients. Advanced collagen turnover in the aorta may be involved in the early detection of myocardial ischemia based on obstructive coronary artery disease.
Not only LVH but also LV systolic function is deeply involved in the prognosis of patients undergoing hemodialysis. Previous studies have indicated that LVEF examined by echocardiography could detect the high-risk group of hemodialysis population42, 43). Myocardial fibrosis and myocardial ischemia are likely involved in reduced LV systolic function, as described earlier. In the present study, LVEF and serum PIIINP were independently associated with the occurrence of cardiovascular events. We may have to pay attention to protecting patients undergoing hemodialysis with low LVEF and high serum PIIINP concentration of 1.75 U/mL or more from future cardiovascular events.
This study has several limitations. The long observation duration (mean period: 4.7 years) could have introduced various biases on the results. We considered 19 sudden deaths as cardiac death, although a coronary origin was not clearly determined. We could not completely eliminate the possibility of hyperkalemia or some other cause for these deaths. The tissue and organ origins of circulating PIIINP have not been identified yet. It is well known that circulating BNP is a useful biomarker for predicting cardiovascular events; however, plasma BNP was not associated with cardiovascular events in this study. Plasma BNP concentrations ranged from 23 to 3930 pg/mL, and the coefficient of variation of plasma BNP was 102%; the extraordinarily wide distribution of plasma BNP concentrations might be involved with no relationship between plasma BNP and cardiovascular events. Because BNP samples were obtained just before starting the first hemodialysis session of the week after the enrollment, and not on the non-dialysis day like echocardiography, the temporary increase in volume load in some patients might have contributed to the wide distribution of plasma BNP concentrations and lack of association between plasma BNP and cardiovascular events. Finally, the predictive value of serum PIIINP concentration could not be defined because of the relatively small sample size. A larger patient population is needed to establish the clinical implications and prognostic value of this method.
Conclusion
Higher levels of circulating PIIINP may be associated with increased collagen turnover or fibrosis of the cardiovascular tissues, leading to sclerosis of the vascular systems and myocardial remodeling or overload. In the EPHESUS study of patients with congestive heart failure after AMI, circulating PIIINP levels did not correlate with cardiovascular events or mortality for a mean follow-up of 16 months12). On the contrary, high basal values of PIIINP were correlated with cardiovascular mortality in patients with chronic heart failure in the RALES study10). Serum PIIINP concentrations may be able to predict more accurately longterm cardiovascular events in chronic conditions such as heart failure or end-stage kidney disease than early-phase events such as AMI.
Acknowledgments
The authors deeply appreciate the staff of the Kyoto Red Cross Hospital, Kyoto Medical Center, and Takeda General Hospital for coronary angiography, cardiac disease assessment, and coronary intervention.
Disclosures
No specific grant from funding agencies in the public, commercial, or not-for-profit sectors was received as support for this research.
COI
Masato Nishimura reports honoraria from Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Takeda Pharmaceutical Company Limited, Otsuka Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd., and Bayer Yakuhin, Ltd. Other authors declare that they have no conflicts of interest.
References
- 1). Bishop JE, Laurent GJ: Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J, 1995; 16 (Suppl C): 38-44 [DOI] [PubMed] [Google Scholar]
- 2). González A, López B, Querejeta R, Díez J: Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol, 2002; 34: 1585-1593 [DOI] [PubMed] [Google Scholar]
- 3). McCullagh KG, Duance VC, Bishop KA: The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol, 1980; 130: 45-55 [DOI] [PubMed] [Google Scholar]
- 4). Poulsen SH, Høst NB, Jensen SE, Egstrup K: Relationship between serum amino-terminal propeptide of type III procollagen and changes of left ventricular function after acute myocardial infarction. Circulation, 2000; 101: 1527-1532 [DOI] [PubMed] [Google Scholar]
- 5). Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M: Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation, 2005; 112: 2940-2945 [DOI] [PubMed] [Google Scholar]
- 6). Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG, Glogar HD: Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol, 1995; 75: 913-918 [DOI] [PubMed] [Google Scholar]
- 7). López B, González A, Querejeta R, Zubilaga E, Larman M, Díez J: Galectin-3 and histological, molecular and biochemical aspects of myocardial fibrosis in heart failure of hypertensive origin. Eur J Heart Fail, 2015; 17: 385-392 [DOI] [PubMed] [Google Scholar]
- 8). López B, González A, Ravassa S, Beaumont J, Moreno MU, San José G, Querejeta R, Díez J: Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J Am Coll Cardiol, 2015; 65: 2449-2456 [DOI] [PubMed] [Google Scholar]
- 9). Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant, 1996; 11: 1277-1285 [PubMed] [Google Scholar]
- 10). Vlachopoulos C, Aznaouridis K, Stefanadis C: Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol, 2010; 55: 1318-1327 [DOI] [PubMed] [Google Scholar]
- 11). Zannad F, Alla F, Dousset B, Perez A, Pitt B: Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation, 2000; 102: 2700-2706 [DOI] [PubMed] [Google Scholar]
- 12). Iraqi W, Rossignol P, Angioi M, Fay R, Nuée J, Ketelslegers JM, Vincent J, Pitt B, Zannad F: Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation, 2009; 119: 2471-2479 [DOI] [PubMed] [Google Scholar]
- 13). Sato Y, Kataoka K, Matsumori A, Sasayama S, Yamada T, Ito H, Takatsu Y: Measuring serum aminoterminal type III procollagen peptide, 7S domain of type IV collagen, and cardiac troponin T in patients with idiopathic dilated cardiomyopathy and secondary cardiomyopathy. Heart, 1997; 78: 505-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K: Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialsysi patients. Kidney Int, 2005; 67: 333-3 [DOI] [PubMed] [Google Scholar]
- 15). Honkanen E, Fröseth B, Grönhagen-Riska C: Serum hyaluronic acid and procollagen III amino terminal propeptide in chronic renal failure. Am J Nephrol, 1991; 11: 201-206 [DOI] [PubMed] [Google Scholar]
- 16). Díez J, Laviades C, Mayor G, Gil MJ, Monreal I: Increased serum concentrations of procollagen peptides in essential hypertension. Relation to cardiac alterations. Circulation, 1995; 91: 1450-1456 [DOI] [PubMed] [Google Scholar]
- 17). Uusimaa P, Risteli J, Niemelä M, Lumme J, Ikäheimo M, Jounela A, Peuhkurinen K: Collagen scar formation after acute myocardial infarction: relationships to infarct size, left ventricular function, and coronary artery patency. Circulation, 1997; 96: 2565-2572 [DOI] [PubMed] [Google Scholar]
- 18). Bonapace S, Rossi A, Cicoira M, Golia G, Zanolla L, Franceschini L, Conte L, Marino P, Zardini P, Vassanelli C: Aortic stiffness correlates with an increased extracellular matrix turnover in patients with dilated cardiomyopathy. Am Heart J, 2006; 152: 93.e1-e6 [DOI] [PubMed] [Google Scholar]
- 19). Dellegrottaglie S, Sands RL, Gillespie BW, Gnanasekaran G, Zannad F, Sengstock D, Finkelstein F, Kiser M, Eisele G, Hinderliter AL, Levin NW, Cattan V, Saran R, Rajagopalan S: Association between markers of collagen turnover, arterial stiffness and left ventricular hypertrophy in chronic kidney disease (CKD): the Renal Research Institute (RRI)-CKD study. Nephrol Dial Transplant, 2011; 26: 2891-2898 [DOI] [PubMed] [Google Scholar]
- 20). Cicoira M, Rossi A, Bonapace S, Zanolla L, Golia G, Franceschini L, Caruso B, Marino PN, Zardini P: Independent and additional prognostic value of aminoterminal propeptide of type III procollagen circulating levels in patients with chronic heart failure. J Card Fail, 2004; 10: 403-411 [DOI] [PubMed] [Google Scholar]
- 21). Velagaleti RS, Gona P, Sundström J, Larson MG, Siwik D, Colucci WS, Benjamin EJ, Vasan RS: Relations of biomarkers of extracellular matrix remodeling to incident cardiovascular events and mortality. Arterioscler Thromb Vasc Biol, 2010; 30: 2283-2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Montalto G, Soresi M, Aragona F, Tripi S, Carroccio A, Anastasi G, Magliarisi C, Barresi E, Notarbartolo A: Procollagen III and laminin in chronic viral hepatopathies. Presse Med, 1996; 25: 59-62 [PubMed] [Google Scholar]
- 23). Saggese G, Baroncelli GI, Bertelloni S, Cinquanta L, DiNero G: Twenty-four-hour osteocalcin, carboxyterminal propeptide of type I procollagen, and aminoterminal propeptide of type III procollagen rhythms in normal and growth-retarded children. Pediatr Res, 1994; 35: 409-415 [PubMed] [Google Scholar]
- 24). Raedsch R, Stiehl A, Sieg A, Walker S, Kommerell B: Biliary excretion of procollagen type III peptide in healthy humans and in patients with alcoholic cirrhosis of the liver. Gastroenterology, 1983; 85: 1265-1270 [PubMed] [Google Scholar]
- 25). Keller F, Rehbein C, Scwarz A, Fleck M, Hayasaka A, Schuppan D, Offermann G, Hahn EG: Increased procollagen III production in patients with kidney disease. Nephron, 1998; 50: 332-333 [DOI] [PubMed] [Google Scholar]
- 26). Weber KT, Pick R, Jalil JE, Janicki JS, Caroll EP: Patterns of myocardial fibrosis. J Mol Cell Cardiol, 1989; 21(Suppl 5): 121-131 [DOI] [PubMed] [Google Scholar]
- 27). Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM: Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation, 2015; 131: 1247-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J: Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation, 2004; 110: 1263-1268 [DOI] [PubMed] [Google Scholar]
- 29). Takarada A, Yokota Y, Fukuzaki H: Analysis of ventricular arrhythmias in patients with dilated cardiomyopathy--relationship between the effects of antiarrhythmic agents and severity of myocardial lesions. Jpn Circ J, 1990; 54: 260-271 [DOI] [PubMed] [Google Scholar]
- 30). Dai Z, Aoki T, Fukumoto Y, Shimokawa H: Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol, 2012; 60: 416-421 [DOI] [PubMed] [Google Scholar]
- 31). O'Rourke MF, Hashimoto J: Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol, 2007; 50: 1-3 [DOI] [PubMed] [Google Scholar]
- 32). Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS: Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int, 2004; 65: 2380-2389 [DOI] [PubMed] [Google Scholar]
- 33). Wanner C, Krane V, Marz W: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med, 2005; 353: 238-248 [DOI] [PubMed] [Google Scholar]
- 34). Demirovic J, Myerburg RJ: Epidemiology of sudden coronary death: an overview. Prog Cardiovasc Dis, 1994; 37: 39-48 [DOI] [PubMed] [Google Scholar]
- 35). US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis, 2017; 69(Supple 1): S7-S8 [DOI] [PubMed] [Google Scholar]
- 36). Foley RN, Parfrey PS, Harnett JD: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int, 1995; 47: 186-192 [DOI] [PubMed] [Google Scholar]
- 37). Swynghedauw B, Chevalier B, Charlemagne D, Mansier P, Carré F: Cardiac hypertrophy, arrhythmogenicity and the new myocardial phenotype. II. The cellular adaptational process. Cardiovasc Res, 1997; 35: 6-12 [DOI] [PubMed] [Google Scholar]
- 38). Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP: Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Study. Am J Cardiol, 1987; 60: 560-565 [DOI] [PubMed] [Google Scholar]
- 39). Jarife J: Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol, 2000; 62: 25-50 [DOI] [PubMed] [Google Scholar]
- 40). Barenbrock M, Spieker C, Laske V, Heidenreich S, Hohage H, Bachmann J, Hoeks AP, Rahn KH: Studies of vessel wall properties in hemodialysis patients. Kidney Int, 1994; 45: 1397-1400 [DOI] [PubMed] [Google Scholar]
- 41). Amann K, Breitbach M, Ritz E, Mall G: Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol, 1998; 9: 1018-1022 [DOI] [PubMed] [Google Scholar]
- 42). Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS: Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol, 2004; 15: 1029-1037 [DOI] [PubMed] [Google Scholar]
- 43). Yamada S, Ishii H, Takahashi H, Aoyama T, Morita Y, Kasuga H, Kimura K, Ito Y, Takahashi R, Toriyama T, Yasuda Y, Hayashi M, Kamiya H, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T: Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol, 2010; 5: 1793-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]


