Abstract
Kidney injury molecule 1 (KIM-1) is a type I membrane protein, comprising an extracellular portion and a cytoplasmic portion. It is also named as HAVCR1 (Hepatitis A virus cellular receptor 1) or TIM1 (T-cell immunoglobulin mucin receptor 1), and is expressed in the kidney, liver, and spleen. KIM-1 plays different roles via various molecular targets in immune diseases and kidney injury. KIM-1 is involved in HAV infections, autoimmunity, immune tolerance, and atopic diseases. The urinary KIM-1 level is closely related to its tissue level, and correspondingly related to kidney tissue damage. KIM-1 is not only an early biomarker of acute kidney injury (AKI), but also has a potential role in predicting the long-term renal outcome. In this review, we provide a summary of KIM-1’s activities, focusing on the latest studies concerning the important roles of KIM-1 in the immune system and kidney diseases.
Keywords: KIM-1, immune, kidney diseases, acute kidney injury, chronic kidney disease
Introduction
The protein encoded by the KIM-1 gene is a membrane receptor for both human hepatitis A virus (HHAV) and T cell immunoglobulin and mucin domain containing 4 (TIMD4). Alternative splicing of this gene results in multiple transcript variants that are also known as HAVCR1 (Hepatitis A virus cellular receptor 1) and TIM1 (T-cell immunoglobulin mucin receptor 1) [1,2]. HAVCR1 was first reported by Kaplan [3], and recognized as the receptor for hepatitis A virus (HAV) on the surface of the monkey’s kidney that promotes cellular entry of the virus under certain conditions. TIM1 is a co-stimulator of T cell activation that regulates the innate and adaptive immune system via related molecular mechanisms [4]. Thus there is a potential link between HAVCR1/TIM1 and immune susceptibility.
Ichimura first reported that KIM-1, which is shed into urine after acute kidney damage, is a marker of renal tubular injury. Whereafter, their lab identified that KIM-1 overexpression also is a marker for the long-term prognosis of chronic kidney diseases [5-7].
Here, we first briefly review the comprehensive roles of HAVCR1/TIM1/KIM-1 in immune and kidney diseases. We then present a map of the potential relationships among them to aid future research.
The structure of KIM-1
The human KIM-1 gene maps to chromosome 5p33.3 and comprises 14 exons. The length of its mRNA is 1095 bp, encoding a 39 kDa type I membrane glycoprotein [7]. KIM-1 has signal peptide before the N-terminal domain, which may be directly responsible for KIM-1’s location on the cell surface. However, some studies showed that endogenous KIM-1 clusters mostly in the cytoplasm, and does not localize to the cell surface except under sustained cell activation. The cell-specific location of KIM-1 could affect its diverse cell functions [8,9]. For example, in kidney cells, KIM-1 trafficking to lysosomes can promote nuclear hormone receptor NUR/77 (NUR77) degradation. In lymphoid cells, KIM-1 in endosomes is required to sense enveloped viruses. Whereas in Jurkat T cells, intracellular KIM-1 may modulate antigen-driven immune responses by locating to the immune synapse rather than the cell surface (Figure 1).
Figure 1.
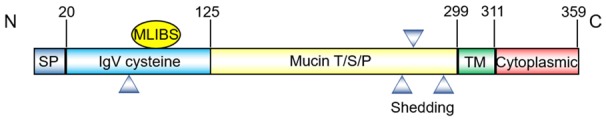
Structure of the Human KIM-1 protein. Schematic representation of KIM-1, showing the signal peptide, IgV, mucin, transmembrane, and cytoplasmic domains. The triangles are the predicted location of N-linked glycosylation sites. KIM-1, kidney injury molecule-1; MLIBS, metal ion-dependent ligand binding site; T/S/P, threonine/serine/proline; TM, transmembrane.
Its extracellular segment comprises a six-cysteine immunoglobulin variable (IgV) domain and a threonine/serine/proline (TSP) rich mucin region, which is characteristic of mucin-like glycosylated proteins [10,11]. Considering the many glycosylation sites on the extracellular domain, including one putative N-glycosylation in IgV region, along with three putative N-glycosylation and multiple O-glycosylation sites in mucin region, the molecular mass of the mature form of KIM-1 is 104 kDa, containing a 90 kDa soluble portion and a 14 kDa membrane-bound fragment [12]. The IgV domain has a unique metal ion-dependent ligand binding site (MILIBS). MILIBS can recognize phosphatidylserine (PtdSer) that is exposed on the outer leaflet of the apoptotic cell membrane [11]. Thus, cells expressing KIM-1 can engulf and eliminate apoptotic cells [13], which is an essential process for cell homeostasis and immune responses [14,15]. Recent data suggested that the mucin domain on the cell surface participates in intracellular calcium release [16]. During calcium stone formation, urinary mucin is decreased, which further enhances transient receptor potential cation channel subfamily V member 5 (TRPV5) channel activity to protect against kidney stones.
A short cytoplasmic tail with a conservative tyrosine phosphorylation motif follows the transmembrane segment. Tyrosine phosphorylation of this tail may be related to the activation of downstream signaling pathways by engaging several protein kinases [17]. The cytoplasmic portion has two splice variants, KIM-1a and KIM-1b. KIM-1a is mainly expressed in the liver and lacks the tyrosine kinase phosphorylation motif. KIM-1b, which contains two conserved tyrosine residues and a tyrosine kinase phosphorylation motif, is mainly expressed in the kidney [16].
Kim-1 and immune diseases
Numerous studies have shown that KIM-1 is associated with control of viral infections, autoimmunity, immune tolerance, and atopic diseases [18-20]; which indicate that KIM-1 plays a major role in the immune system.
Initially, KIM-1 was identified as an entry receptor for HAV and is expressed on the surface of different epithelial cells. It also promotes the entry of a wide range of viruses such as Zaire Ebola virus (EBOV), Lake Victoria Marburg virus, Plasmodium berghei ANKA, Dengue virus (DV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) [21-25]. A recent report suggests that KIM-1 participates in the identification of alternative virus receptors. The interaction between a virus and KIM-1 indicates the entrance pathway for the virus [26]. Evidence for the HAV/KIM-1 interaction implies that both the IgV and mucin domains, especially the first N-glycosylation site, are required for HAV uncoating and subsequent cell infectivity [27,28]. Other studies revealed that KIM-1 is incorporated into HIV virions and retains particles on the cell plasma membrane by interacting with the virion-associated PtdSer [29,30]. KIM-1 acts as a dual-attachment receptor for EBOV by interacting directly with viral glycoprotein (GP) and PtdSer on the viral envelope [31], inducing a cytokine storm phenomenon [32].
Kim-1 is also linked with many immune dysfunction diseases, including allergies, asthma, ectopic dermatitis, rheumatoid arthritis, and systemic lupus erythematosus (SLE) [2,33-36].
KIM-1, previously named TIM-1, is expressed on CD4(+) T cells. In patients with SLE, the expression of interleukin (IL)10, which is a Th2 immuno-modulatory cytokine, correlated positively with the increase in KIM-1 expression [37]. In patients with allergic rhinitis, after stimulation by dust or lipopolysaccharide, The cells expressing KIM-1 differentiated into Th2 cells, suggesting that KIM-1 plays an important role in regulating the Th2 immune response [38-40].
In asthma, TIM4 binding to KIM-1 increases the expression of silent information regulator 1 (SIRT1) on CD4(+) T cells via the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT kinase (AKT) signaling pathway and pr- omotes the proliferation of Th2 cells [41]. Their combination could also trigger T cells activation via linker for activation of T cells (LAT), AKT, and extracellular regulated protein kinases (ERK1/2) signaling pathways and mediates T cell trafficking [40,42], which was predominant in inducing the Th2 immune response by increasing cell differentiation and decreasing apoptosis [11,43-45]. Some reports indicated that KIM-1 acts as a costimulatory molecule during antigen presentation, amplifying T cell receptor (TCR) signaling with TCR complex components zeta chain of T cell receptor associated protein kinase 70 (ZAP-70) and CD3 (T-cell receptor T3) [46]. KIM-1 combined with CD3 can be recruited to the TCR complex, thus increasing the activation signal of T cells, aided by activation of the PI3K pathway [47]. In a mouse transplantation experiment, KIM-1 regulated the endogenous Th2-immune response, which provided new insights for the clinical treatment of graft rejection [48]. These reports showed that KIM-1 is predominantly associated with the Th2 immune response. However, in asthma, KIM-1 also serves as a pattern recognition receptor on invariant natural killer cells (iNKTs), where it mediates cell activation when iNKT cells bind to PtdSer on the surface of cells undergoing apoptosis [49]. KIM-1 is also a marker of regulatory B cells [50] and its signaling is required for B cells to augment antibody or IL10 production by enhancing B cell proliferation and differentiation [51-53]. KIM-1 on immune cells may be a useful therapeutic target to treat immune diseases.
By contrast, in allergic inflammation, application of an anti-KIM-1 antibody induced T cells that markedly increased the production of pro-inflammatory IL4. This indicated that immunotherapies that regulate KIM-1 might inhibit immune tolerance [54]. KIM-1 on NKT cells and mast cells also enhanced Th2-type cytokine production of IL-4, IL-5, and IL-13 [37,55]. Moreover, in a mouse model of asthma, the production of IL4 induced by KIM-1 might have resulted from the elevation of Th2 transcription factor GATA binding protein 3 (GATA3) [39]. KIM-1 can promote macrophages to produce a dramatic increase of proinflammatory cytokines, including tumor necrosis factor alpha (TNFα) and IL6 [56]. KIM-1 is a major P-selectin ligand and is involved in a pivotal trafficking mechanism for Th1 and Th17 cells during inflammation, which are potent inducers of inflammation and autoimmunity. These observations suggested that interference with KIM-1 activity might provide a therapeutic approach in T cell-mediated diseases [57]. KIM-1 is also constitutively expressed on dendritic cells (DCs), where it confers pro-inflammatory properties [58]. In a DC-induced allergy model, disruption of the KIM-1/TIM4 interaction was promoted as a therapeutic strategy [59].
Patterns of variation in KIM-1 have been shaped by both positive and balancing natural selection in the course of primate evolution [60]. For example, the levels of polymorphisms in exon 4 of KIM-1 are unusually high in humans or among human, chimp, and gorilla, which represents evidence that natural selection may have applied to preserve functional variation in exon 4, suggesting that KIM-1 can adapt to a continually changing environment under long-range pressure. Several studies have shown that cells containing polymorphic KIM-1 are susceptible to HAV infection and immune diseases [34,35,61-65]. For example, a 6-amino-acid-encoding insertion in KIM-1 (157insMTTTVP) is associated with HAV-induced severe liver disease [61]. Moreover, KIM-1 gene polymorphisms (-416G>C and -1454G>A) were observed to be related to allergic rhinitis susceptibility in a Han Chinese population [63]. However, HAV infection induces the production of the short-form KIM-1 protein, which has the low efficiency to combine with HAV and limits its entry into cells [66,67]. In acquired immunodeficiency syndrome (AIDS), patients carrying the KIM-1 D3-A haplotype have lower expression levels of KIM-1, resulting in better survival rates compared with other patients because of their higher CD4 cell count [68]. These data prove a correlation between KIM-1 and immune-related diseases, confirming that both genetic and environmental factors play a role in such pathologies.
Expression and secretion of KIM-1 in the kidney
Normal kidney tissues rarely express KIM-1, except in the acute injury resulting from ischemia, hypoxia, toxicity, or some renal tubular interstitial, and polycystic kidney disease [69-71]. Under conditions of acute kidney injury, urinary and renal KIM-1 levels are significantly elevated in a short period of time, and correlate with the extent of kidney damage. KIM-1 is mainly expressed in differentiated proximal renal tubular epithelial cells, which can regenerate after injury, especially in the proximal tubule S3 outer medulla area because of its sensitivity to ischemia, hypoxia, and toxicity.
After injury to renal tubular cells, the extracellular section of KIM-1 is released into the renal tube cavity and is further shed into the urine, mediated by mitogen-activated protein kinase (MAPK) signaling pathways [72]. This shedding of KIM-1 is also based on the activation of type I and III membrane matrix metalloproteinases (MT-MMPS) [73,74] and a disintegrin and metalloprotease (ADAM) [75], which lead to its detection in urine. Measuring the expression level of KIM-1 in urine is sensitive for the early diagnosis of acute kidney disease (AKI) and chronic kidney diseases (CKD) [76], as well as useful to effectively assess renal pathological damage and disease progression [77].
KIM-1 and acute kidney injury
In patients with acute renal tubular injury, the KIM-1 expression level in the kidney is significantly elevated compare with that in the healthy population. The proposed mechanism is that acute renal damage initiates ERK1/2 and signal transducer and activator of transcription 3 (STAT3) phosphorylation. Then, nuclear STAT3 binds to the KIM-1 promoter and increases its mRNA and protein levels [78,79]. Shedding of the extracellular domain leads to greatly increased levels of KIM-1 in blood and urine, which can be used to diagnose acute renal tubular dysfunction after renal transplant [80]. The KIM-1 level is also significantly correlated with the decline of the estimated glomerular filtration rate (eGFR) and kidney damage [81].
Acute overexpression of KIM-1 in proximal renal tubular epithelial cells after ischemia, hypoxia, and toxicity promotes transformation of the cells into “semi-professional” phagocytic cells, with the help of KIM-1’s mucin domain. KIM-1 is a phosphatidylserine receptor on the surface of the liposome that can identify the apoptosis body and phosphatidylserine, which mediates further phagocytosis [82].
Thus, KIM-1 plays a role in the removal of apoptotic cells and necrotic tissue fragments. Furthermore, KIM-1 phosphorylation, and its interaction with p85, enhance cell autophagy to degrade KIM-1 phagosomes relying on Unc-51 like autophagy activating kinase 1 (ULK1, also known as ATG1) phosphorylation and maintain self-tolerance by the presentation of antigens on the proximal tubule cell [83]. In kidney diseases of protein overload, KIM-1 can be used to increase the phagocytosis of albumin by renal tubular epithelial cells, which alleviates the tubular damage [84].
In addition to its role in mediating phagocytosis, KIM-1 is also involved in the repair process after injury to renal tubular epithelial cells. In vitro, transient KIM-1 overexpression can promote the migration and proliferation of renal tubular epithelial cells by activation of the ERK/MAPK signaling pathway. KIM-1 serves as a therapeutic target to facilitate the renal repair process after AKI [85]. Other studies have reported that after the acute kidney ischemia injury in mice, KIM-1 and G Protein Alpha 12 (Gα12) interact directly. At the same time, Gα12 can inhibit the activation of downstream Ras homolog gene family, member A (RhoA). Through these signaling events, endocytosis is negatively regulated by Gα12 in acute renal ischemic injury [86]. Upregulation of KIM-1 can protect against kidney ischemia damage by suppressing Gα12 activation and blocking GTP loading [87]. Thus, accumulated evidence has demonstrated the beneficial effects of the KIM-1-related renal tubular protection mechanism in the early stages of kidney injury.
KIM-1 and chronic kidney disease
KIM-1 also is a sensitive biomarker for chronic proximal tubular injury [88]. Studies showed that in urine samples from adults and children, the level of KIM-1 correlates highly with the incidence and prognosis of CKD [89-91]. In the progression of IgA nephropathy, a higher level of KIM-1 in urine leads to more serious and rapid disease progression [92]. In protein-overload nephropathy, increasing KIM-1 levels are associated with inflammation of the renal tubules [93]. KIM-1 is the only effective clinical biomarker for CKD associated with hypertension [94]. In more severe CKD, KIM-1 is an independent risk factor for progression to end-stage renal disease (ESRD) [95].
In vivo animal models of unilateral ureteral obstruction (UUO) showed that continued chronic expression of KIM-1 in renal tubular promoted the secretion of monocyte chemotactic protein 1 (MCP-1), which enhanced macrophage chemotaxis, thus further promoting the occurrence of fibrosis [5]. In addition to these rodent models, the expression level of KIM-1 was also increased in human renal proximal tubule epithelial cells (HK2) under conditions of chronic hypoxia. This led to activation of mononuclear macrophages and the occurrence of renal tubule interstitial inflammation [96]. Moreover, KIM-1 plays a crucial role in macrophage activation via the MAPK signaling pathway in kidney disease, inducing macrophages to differentiate into the M1 type. The renal mRNA expression levels of the M1-dependent genes IFNG (interferon gamma) and INOS (nitric oxide synthase 2) markedly increased. This was consistent with the increases of proinflammatory macrophage cytokines in blood, such as TNF-α and IL-6. In contrast, the expression levels of M2-dependent genes (MR (mineralocorticoid receptor) and Arg1 (arginase 1)) and cytokines (IL-4 and IL-10) decreased [97].
In addition, when HK2 cells were cultured in high glucose, the expression levels of KIM-1 and LC3II (microtubule associated protein 1 light chain 3 alpha, a marker of autophagy) increased. Autophagy and apoptosis are initiated in high glucose at the same time, which leads to cell death. Meanwhile, silencing of KIM-1 resulted in the inhibition of the glucose-induced production of LC3II, autophagy, and apoptosis, followed by a reduction in cell death. This indicated that blocking KIM-1 in a high glucose environment helps to maintain cellular homeostasis via autophagy and apoptosis [98]. Observation of kidney pathological sections revealed a more severe extent of renal tubular injury, inflammation reaction, and fibrosis in the area where KIM-1 was markedly expressed [99]. These findings indicated that KIM-1 is involved in regulating the development of CKD and renal fibrosis.
In the early stage of diabetic kidney disease (DKD), the expression of KIM-1 in the glomeruli is significantly elevated, mainly in the proliferative parietal epithelium of the capsule. The expression of KIM-1 increases along with the development of the disease and correlates with decreased numbers of podocytes [100]. Moreover, glomerular KIM-1 expression was elevated in proportion to the extent of proteinuria and podocytopenia in diabetic animals; supporting the view that it could be used as a potential biomarker for glomerular injury in proteinuria kidney disease. In anti-neutrophil cytoplasmic antibodies (ANCA)-associated glomerulonephritis, the levels of both KIM-1 and MCP-1 in the urine can reflect inflammation and are related to prognosis evaluation of the glomeruli [101].
Conclusions
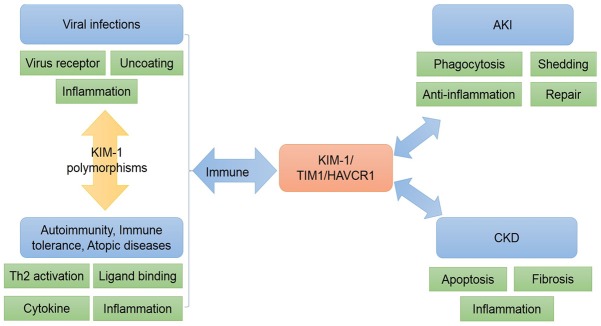
In summary, KIM-1 has a wide variety of physiological and pathological functions in different diseases (Figure 2). The earliest identified homolog of KIM-1, HAVCR1, which is the receptor for HAV, is involved in cell entry and pathogenesis of HAV, as well as other viruses. In immune system related diseases, TIM1 (a variant of KIM-1) is a co-stimulator of T cell activation and plays an important role in regulating the Th2 reaction. TIM4 and CD3 as ligands can promote the proliferation and activation of Th2 cells, as well as inducing the production of cytokines IL4, IL5, IL-10, and IL13, which play important roles in immune system activation. With the help of metalloproteinases, the ectodomain of KIM-1 is shed into the renal tube cavity and is excreted in the urine and blood, acting as an indicator of kidney injury. Furthermore, the function of KIM-1 in AKI and CKD is different. In the early stage of renal tubular damage, the increased expression of KIM-1 promotes cell phagocytosis, repairs tubular injury, and inhibits the renal inflammatory response. By contrast, the continuous increase in KIM-1 levels is not a protective factor in CKD, which in turn promotes the occurrence and development of renal fibrosis.
Figure 2.
The role of KIM-1 in different diseases. In the immune system, KIM-1 is related to viral infections and autoimmunity. In acute kidney injury (AKI), KIM-1 is involved in cell phagocytosis, repair processes, and anti-inflamation, and is shed into urine. However, KIM-1 can promote renal fibrosis, tubular apotosis, and inflammatory response in chronic kidney disease (CKD). KIM-1, kidney injury molecule-1; TIM-1, T-cell immunoglobulin mucin receptor 1.
In this review, we provided an overview of KIM-1. Further studies are required to determine its function to develop effective and suitable therapeutic methods to treat immune and renal diseases by directly targeting KIM-1.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (nos. 81600557, 81600532, 81600352, 81370802, 81300591, 81670647, and 81570616), the National Key Research and Development Program (no. 2016YFC0906103), and the Natural Science Foundation of Jiangsu Province (no. BK2012001, BK20160136, BK20160137).
Disclosure of conflict of interest
None.
References
- 1.Vila MR, Kaplan GG, Feigelstock D, Nadal M, Morote J, Porta R, Bellmunt J, Meseguer A. Hepatitis a virus receptor blocks cell differentiation and is overexpressed in clear cell renal cell carcinoma. Kidney Int. 2004;65:1761–1773. doi: 10.1111/j.1523-1755.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Chang YJ, Chuang YT, Lee HH, Kasahara DI, Martin T, Hsu JT, Savage PB, Shore SA, Freeman GJ, Dekruyff RH, Umetsu DT. T-cell immunoglobulin and mucin domain 1 deficiency eliminates airway hyperreactivity triggered by the recognition of airway cell death. J Allergy Clin Immunol. 2013;132:414–425. doi: 10.1016/j.jaci.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone SM. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010;16:556–61. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- 7.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 8.Echbarthi M, Zonca M, Mellwig R, Schwab Y, Kaplan G, DeKruyff RH, Roda-Navarro P, Casasnovas JM. Distinct trafficking of cell surface and endosomal TIM-1 to the immune synapse. Traffic. 2015;16:1193–1207. doi: 10.1111/tra.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasubramanian S, Kota SK, Kuchroo VK, Humphreys BD, Strom TB. TIM family proteins promote the lysosomal degradation of the nuclear receptor NUR77. Sci Signal. 2012;5:ra90. doi: 10.1126/scisignal.2003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, Bonventre JV. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–48. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 13.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong K, Valdez PA, Tan C, Yeh S, Hongo JA, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci U S A. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci U S A. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie M, Bal MS, Yang Z, Liu J, Rivera C, Wenzel A, Beck BB, Sakhaee K, Marciano DK, Wolf MT. Mucin-1 increases renal TRPV5 activity in vitro, and urinary level associates with calcium nephrolithiasis in patients. J Am Soc Nephrol. 2016;27:3447–3458. doi: 10.1681/ASN.2015101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane LP. T cell Ig and mucin domain proteins and immunity. J Immunol. 2010;184:2743–2749. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manangeeswaran M, Jacques J, Tami C, Konduru K, Amharref N, Perrella O, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ, Perrella A, Kaplan GG. Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans. Gastroenterology. 2012;142:1516–1525. doi: 10.1053/j.gastro.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto W, Nishikori M, Arima H, Miyoshi H, Sasaki Y, Kitawaki T, Shirakawa K, Kato T, Imaizumi Y, Ishikawa T, Ohno H, Haga H, Ohshima K, Takaori-Kondo A. Expression of Tim-1 in primary CNS lymphoma. Cancer Med. 2016;5:3235–3245. doi: 10.1002/cam4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtiss M, Colgan J. The role of the T-cell costimulatory molecule Tim-1 in the immune response. Immunol Res. 2007;39:52–61. doi: 10.1007/s12026-007-0063-6. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Liu M, Huang S, Wu B, Guo H, Su XZ, Lu F. Expression of Tim-1 and Tim-3 in plasmodium berghei ANKA infection. Parasitol Res. 2013;112:2713–2719. doi: 10.1007/s00436-013-3442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Qiao L, Hou Z, Luo G. TIM-1 promotes hepatitis C virus cell attachment and infection. J Virol. 2017;91 doi: 10.1128/JVI.01583-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB Jr, Chiorini J, Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011;108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costafreda MI, Kaplan G. HAVCR1 (CD365) and its mouse ortholog are functional hepatitis A virus (HAV) cellular receptors that mediate HAV infection. J Virol. 2018;92 doi: 10.1128/JVI.02065-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson P, Lu J, Kaplan GG. The Cys-rich region of hepatitis A virus cellular receptor 1 is required for binding of hepatitis A virus and protective monoclonal antibody 190/4. J Virol. 1998;72:3751–3761. doi: 10.1128/jvi.72.5.3751-3761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silberstein E, Xing L, van de Beek W, Lu J, Cheng H, Kaplan GG. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin-and mucin-like regions. J Virol. 2003;77:8765–8774. doi: 10.1128/JVI.77.16.8765-8774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moller-Tank S, Albritton LM, Rennert PD, Maury W. Characterizing functional domains for TIM-mediated enveloped virus entry. J Virol. 2014;88:6702–6713. doi: 10.1128/JVI.00300-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Ablan SD, Miao C, Zheng YM, Fuller MS, Rennert PD, Maury W, Johnson MC, Freed EO, Liu SL. TIM-family proteins inhibit HIV-1 release. Proc Natl Acad Sci U S A. 2014;111:E3699–3707. doi: 10.1073/pnas.1404851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan S, Cao L, Ling H, Dang M, Sun Y, Zhang X, Chen Y, Zhang L, Su D, Wang X, Rao Z. TIM-1 acts a dual-attachment receptor for Ebolavirus by interacting directly with viral GP and the PS on the viral envelope. Protein Cell. 2015;6:814–824. doi: 10.1007/s13238-015-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younan P, Iampietro M, Nishida A, Ramanathan P, Santos RI, Dutta M, Lubaki NM, Koup RA, Katze MG, Bukreyev A. Ebola virus binding to tim-1 on T lymphocytes induces a cytokine storm. MBio. 2017;8 doi: 10.1128/mBio.00845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 34.Page NS, Jones G, Stewart GJ. Genetic association studies between the T cell immunoglobulin mucin (TIM) gene locus and childhood atopic dermatitis. Int Arch Allergy Immunol. 2006;141:331–336. doi: 10.1159/000095459. [DOI] [PubMed] [Google Scholar]
- 35.Chae SC, Park YR, Song JH, Shim SC, Yoon KS, Chung HT. The polymorphisms of Tim-1 promoter region are associated with rheumatoid arthritis in a Korean population. Immunogenetics. 2005;56:696–701. doi: 10.1007/s00251-004-0743-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Meng J, Wang X, Liu S, Shu Q, Gao L, Ju Y, Zhang L, Sun W, Ma C. Expression of human TIM-1 and TIM-3 on lymphocytes from systemic lupus erythematosus patients. Scand J Immunol. 2008;67:63–70. doi: 10.1111/j.1365-3083.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundrud MS, Grill SM, Ni D, Nagata K, Alkan SS, Subramaniam A, Unutmaz D. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J Immunol. 2003;171:3542–3549. doi: 10.4049/jimmunol.171.7.3542. [DOI] [PubMed] [Google Scholar]
- 39.Xu G, Cheng L, Lu L, Zhu Y, Xu R, Yao X, Li H. Expression of T-cell immunoglobulin- and mucin-domain-containing molecule-1 (TIM-1) is increased in a mouse model of asthma and relationship to GATA-3. Life Sci. 2008;82:663–669. doi: 10.1016/j.lfs.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Khademi M, Illés Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallström E. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 41.Hu T, Fan X, Ma L, Liu J, Chang Y, Yang P, Qiu S, Chen T, Yang L, Liu Z. TIM4-TIM1 interaction modulates Th2 pattern inflammation through enhancing SIRT1 expression. Int J Mol Med. 2017;40:1504–1510. doi: 10.3892/ijmm.2017.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, Anderson AC, Sobel RA, Hafler DA, Strom TB, Kuchroo VK. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 46.Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J Immunol. 2007;178:4342–50. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 47.de Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol. 2008;180:6518–6526. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, Popoola J, Wang Y, Yagita H, Akiba H, Ansari MJ, Yang J, Turka LA, Rothstein DM, Padera RF, Najafian N, Sayegh MH. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HH, Meyer EH, Goya S, Pichavant M, Kim HY, Bu X, Umetsu SE, Jones JC, Savage PB, Iwakura Y, Casasnovas JM, Kaplan G, Freeman GJ, DeKruyff RH, Umetsu DT. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. J Immunol. 2010;185:5225–5235. doi: 10.4049/jimmunol.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol. 2015;194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, Ueno T, Padera RF, Kuchroo VK, Najafian N, Rothstein DM. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am J Transplant. 2015;15:942–953. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J, Usui Y, Takeda K, Harada N, Yagita H, Okumura K, Akiba H. TIM-1 signaling in B cells regulates antibody production. Biochem Biophys Res Commun. 2011;406:223–228. doi: 10.1016/j.bbrc.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Miles K, Simpson J, Brown S, Cowan G, Gray D, Gray M. Immune tolerance to apoptotic self is mediated primarily by regulatory B1a cells. Front Immunol. 2017;8:1952. doi: 10.3389/fimmu.2017.01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HS, Kim HS, Lee CW, Chung DH. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol. 2010;184:4095–4106. doi: 10.4049/jimmunol.0901991. [DOI] [PubMed] [Google Scholar]
- 56.Hein RM, Woods ML. TIM-1 regulates macrophage cytokine production and B7 family member expression. Immunol Lett. 2007;108:103–108. doi: 10.1016/j.imlet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, Della Bianca V, Toffali L, Piacentino G, Budui S, Rennert P, Xiao S, Laudanna C, Casasnovas JM, Kuchroo VK, Constantin G. TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity. Immunity. 2014;40:542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao S, Zhu B, Jin H, Zhu C, Umetsu DT, DeKruyff RH, Kuchroo VK. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur J Immunol. 2011;41:1539–1549. doi: 10.1002/eji.201040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng BS, Chen X, He SH, Zheng PY, Foster J, Xing Z, Bienenstock J, Yang PC. Disruption of T-cell immunoglobulin and mucin domain molecule (TIM)-1/TIM4 interaction as a therapeutic strategy in a dendritic cell-induced peanut allergy model. J Allergy Clin Immunol. 2008;122:55–61. 61.e1–7. doi: 10.1016/j.jaci.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 60.Nakajima T, Wooding S, Satta Y, Jinnai N, Goto S, Hayasaka I, Saitou N, Guan-Jun J, Tokunaga K, Jorde LB, Emi M, Inoue I. Evidence for natural selection in the HAVCR1 gene: high degree of amino-acid variability in the mucin domain of human HAVCR1 protein. Genes Immun. 2005;6:398–406. doi: 10.1038/sj.gene.6364215. [DOI] [PubMed] [Google Scholar]
- 61.Kim HY, Eyheramonho MB, Pichavant M, Gonzalez Cambaceres C, Matangkasombut P, Cervio G, Kuperman S, Moreiro R, Konduru K, Manangeeswaran M, Freeman GJ, Kaplan GG, DeKruyff RH, Umetsu DT, Rosenzweig SD. A polymorphism in TIM1 is associated with susceptibility to severe hepatitis A virus infection in humans. J Clin Invest. 2011;121:1111–1118. doi: 10.1172/JCI44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biasin M, Sironi M, Saulle I, Pontremoli C, Garziano M, Cagliani R, Trabattoni D, Lo Caputo S, Vichi F, Mazzotta F, Forni D, Riva S, Aguilar-Jimenez W, Cedeno S, Sanchez J, Brander C, Zapata W, Rugeles MT, Clerici M. A 6-amino acid insertion/deletion polymorphism in the mucin domain of TIM-1 confers protections against HIV-1 infection. Microbes Infect. 2017;19:69–74. doi: 10.1016/j.micinf.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Mou Z, Shi J, Tan Y, Xu R, Zhao Z, Xu G, Li H. Association between TIM-1 gene polymorphisms and allergic rhinitis in a Han Chinese population. J Investig Allergol Clin Immunol. 2010;20:3–8. [PubMed] [Google Scholar]
- 64.Mete F, Ozkaya E, Aras S, Koksal V, Etlik O, Baris I. Association between gene polymorphisms in TIM1, TSLP, IL18R1 and childhood asthma in Turkish population. Int J Clin Exp Med. 2014;7:1071–1077. [PMC free article] [PubMed] [Google Scholar]
- 65.García-Lozano JR, Abad C, Escalera A, Torres B, Fernández O, García A, Sánchez-Román J, Sabio JM, Ortego-Centeno N, Raya-Alvarez E, Núñez-Roldán A, Martín J, González-Escribano MF. Identification of HAVCR1 gene haplotypes associated with mRNA expression levels and susceptibility to autoimmune diseases. Hum Genet. 2010;128:221–229. doi: 10.1007/s00439-010-0844-1. [DOI] [PubMed] [Google Scholar]
- 66.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, Hallmayer JF, Underhill PA, Risch NJ, Freeman GJ, DeKruyff RH, Umetsu DT. Immunology: hepatitis A virus link to atopic disease. Nature. 2003;425:576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 67.Chatenoud L, Bach JF. Genetic control of hepatitis A severity and susceptibility to allergy. J Clin Invest. 2011;121:848–850. doi: 10.1172/JCI46418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wichukchinda N, Nakajima T, Saipradit N, Nakayama EE, Ohtani H, Rojanawiwat A, Pathipvanich P, Ariyoshi K, Sawanpanyalert P, Shioda T, Kimura A. TIM1 haplotype may control the disease progression to AIDS in a HIV-1-infected female cohort in Thailand. AIDS. 2010;24:1625–1631. doi: 10.1097/QAD.0b013e32833a8e6d. [DOI] [PubMed] [Google Scholar]
- 69.Han WK, Alinani A, Wu CL, Michaelson D, Loda M, McGovern FJ, Thadhani R, Bonventre JV. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16:1126–1134. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu M, Chen W, Yu X, Ding D, Zhang W, Hua H, Xu M, Meng X, Zhang X, Zhang Y, Zhang A, Jia Z, Huang S. Celastrol aggravates LPS-induced inflammation and injuries of liver and kidney in mice. Am J Transl Res. 2018;10:2078–2086. [PMC free article] [PubMed] [Google Scholar]
- 71.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Humphreys BD, Bonventre JV. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol. 2007;18:2704–14. doi: 10.1681/ASN.2007030325. [DOI] [PubMed] [Google Scholar]
- 73.Guo L, Takino T, Endo Y, Domoto T, Sato H. Shedding of kidney injury molecule-1 by membrane-type 1 matrix metalloproteinase. J Biochem. 2012;152:425–432. doi: 10.1093/jb/mvs082. [DOI] [PubMed] [Google Scholar]
- 74.Lim AI, Chan LY, Lai KN, Tang SC, Chow CW, Lam MF, Leung JC. Distinct role of matrix metalloproteinase-3 in kidney injury molecule-1 shedding by kidney proximal tubular epithelial cells. Int J Biochem Cell Biol. 2012;44:1040–1050. doi: 10.1016/j.biocel.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Schweigert O, Dewitz C, Moller-Hackbarth K, Trad A, Garbers C, Rose-John S, Scheller J. Soluble T cell immunoglobulin and mucin domain (TIM)-1 and -4 generated by A Disintegrin And Metalloprotease (ADAM)-10 and -17 bind to phosphatidylserine. Biochim Biophys Acta. 2014;1843:275–287. doi: 10.1016/j.bbamcr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Jin Y, Shao X, Sun B, Miao C, Li Z, Shi Y. Urinary kidney injury molecule1 as an early diagnostic biomarker of obstructive acute kidney injury and development of a rapid detection method. Mol Med Rep. 2017;15:1229–1235. doi: 10.3892/mmr.2017.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y, Li A, Wen J, Zhen J, Hao Q, Zhang Y, Hu Z, Xiao X. Kidney injury molecule-1 level is associated with the severity of renal interstitial injury and prognosis in adult henoch-schonlein purpura nephritis. Arch Med Res. 2017;48:449–458. doi: 10.1016/j.arcmed.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS. A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol. 2014;25:105–18. doi: 10.1681/ASN.2013020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collier JB, Schnellmann RG. Extracellular signal-regulated kinase 1/2 regulates kidney injury molecule-1 expression physiologically and following ischemic and septic renal injury. J Pharmacol Exp Ther. 2017;363:419–427. doi: 10.1124/jpet.117.244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shahbaz SK, Pourrezagholi F, Barabadi M, Foroughi F, Hosseinzadeh M, Ahmadpoor P, Nafar M, Yekaninejad MS, Amirzargar A. High expression of TIM-3 and KIM-1 in blood and urine of renal allograft rejection patients. Transpl Immunol. 2017;43-44:11–20. doi: 10.1016/j.trim.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brooks CR, Yeung MY, Brooks YS, Chen H, Ichimura T, Henderson JM, Bonventre JV. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015;34:2441–64. doi: 10.15252/embj.201489838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao X, Jiang C, Olufade R, Liu D, Emmett N. Kidney injury molecule-1 enhances endocytosis of albumin in renal proximal tubular cells. J Cell Physiol. 2016;231:896–907. doi: 10.1002/jcp.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, Cai CX. Kidney injury molecule-1 (KIM-1) mediates renal epithelial cell repair via ERK MAPK signaling pathway. Mol Cell Biochem. 2016;416:109–16. doi: 10.1007/s11010-016-2700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ismail OZ, Zhang X, Bonventre JV, Gunaratnam L. G protein α12 (Gα12) is a negative regulator of kidney injury molecule-1-mediated efferocytosis. Am J Physiol Renal Physiol. 2016;310:607–620. doi: 10.1152/ajprenal.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ismail OZ, Zhang X, Wei J, Haig A, Denker BM, Suri RS, Sener A, Gunaratnam L. Kidney injury molecule-1 protects against Gα12 activation and tissue damage in renal ischemia-reperfusion injury. Am J Pathol. 2015;185:1207–15. doi: 10.1016/j.ajpath.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardiner L, Akintola A, Chen G, Catania JM, Vaidya V, Burghardt RC, Bonventre JV, Trzeciakowski J, Parrish AR. Structural equation modeling highlights the potential of Kim-1 as a biomarker for chronic kidney disease. Am J Nephrol. 2012;35:152–163. doi: 10.1159/000335579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waikar SS, Sabbisetti V, Arnlov J, Carlsson AC, Coresh J, Feldman HI, Foster MC, Fufaa GD, Helmersson-Karlqvist J, Hsu CY, Kimmel PL, Larsson A, Liu Y, Lind L, Liu KD, Mifflin TE, Nelson RG, Riserus U, Vasan RS, Xie D, Zhang X, Bonventre JV Chronic Kidney Disease Biomarkers Consortium Investigators. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant. 2016;31:1460–1470. doi: 10.1093/ndt/gfw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castillo-Rodriguez E, Fernandez-Prado R, Martin-Cleary C, Pizarro-Sanchez MS, Sanchez-Nino MD, Sanz AB, Fernandez-Fernandez B, Ortiz A. Kidney injury marker 1 and neutrophil gelatinase-associated lipocalin in chronic kidney disease. Nephron. 2017;136:263–267. doi: 10.1159/000447649. [DOI] [PubMed] [Google Scholar]
- 91.Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, Lamb EJ. Biological variation of plasma and urinary markers of acute kidney injury in patients with CKD. Clin Chem. 2016;62:876–83. doi: 10.1373/clinchem.2015.250993. [DOI] [PubMed] [Google Scholar]
- 92.Kwon SH, Park MY, Jeon JS, Noh H, Choi SJ, Kim JK, Hwang SD, Jin SY, Han DC. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clin Exp Nephrol. 2013;17:359–364. doi: 10.1007/s10157-012-0707-2. [DOI] [PubMed] [Google Scholar]
- 93.van Timmeren MM, Bakker SJ, Vaidya VS, Bailly V, Schuurs TA, Damman J, Stegeman CA, Bonventre JV, van Goor H. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol. 2006;291:F456–464. doi: 10.1152/ajprenal.00403.2005. [DOI] [PubMed] [Google Scholar]
- 94.Hosohata K. Biomarkers for chronic kidney disease associated with high salt intake. Int J Mol Sci. 2017;18:2080. doi: 10.3390/ijms18102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alderson HV, Ritchie JP, Pagano S, Middleton RJ, Pruijm M, Vuilleumier N, Kalra PA. The associations of blood kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin with progression from CKD to ESRD. Clin J Am Soc Nephrol. 2016;11:2141–2149. doi: 10.2215/CJN.02670316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Q, Chen Y, Lv J, Zhang H, Tang J, Gunaratnam L, Li X, Yang L. Kidney injury molecule-1 expression in IgA nephropathy and its correlation with hypoxia and tubulointerstitial inflammation. Am J Physiol Renal Physiol. 2014;306:F885–95. doi: 10.1152/ajprenal.00331.2013. [DOI] [PubMed] [Google Scholar]
- 97.Tian L, Shao X, Xie Y, Wang Q, Che X, Zhang M, Xu W, Xu Y, Mou S, Ni Z. Kidney injury molecule-1 is elevated in nephropathy and mediates macrophage activation via the mapk signalling pathway. Cell Physiol Biochem. 2017;41:769–783. doi: 10.1159/000458737. [DOI] [PubMed] [Google Scholar]
- 98.Gou R, Chen J, Sheng S, Wang R, Fang Y, Yang Z, Wang L, Tang L. KIM-1 mediates high glucose-induced autophagy and apoptosis in renal tubular epithelial cells. Cell Physiol Biochem. 2016;38:2479–2488. doi: 10.1159/000445598. [DOI] [PubMed] [Google Scholar]
- 99.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 100.Zhao X, Zhang Y, Li L, Mann D, Imig JD, Emmett N, Gibbons G, Jin LM. Glomerular expression of kidney injury molecule-1 and podocytopenia in diabetic glomerulopathy. Am J Nephrol. 2011;34:268–80. doi: 10.1159/000330187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bulanov NM, Serova AG, Kuznetsova EI, Bulanova ML, Novikov PI, Kozlovskaya LV, Moiseev SV. Kidney injury molecules (KIM-1, MCP-1) and type IV collagen in the assessment of activity of antineutrophil cytoplasmic antibody-associated glomerulonephritis. Ter Arkh. 2017;89:48–55. doi: 10.17116/terarkh201789648-55. [DOI] [PubMed] [Google Scholar]