Abstract
Osteolysis is a serious complication of several chronic inflammatory diseases and is closely associated with a local chronic inflammatory reaction with a variety of causes. Inflammatory factors and osteoclastogenesis can enhance bone erosion. Interleukin-27 (IL-27) is speculated to play an important role in the physiological immune response. However, there are few studies on its effects on osteoclastogenesis. In this study, IL-27 was shown to inhibit receptor activator nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis. The gene expression levels of osteoclast (OC)-specific genes, such as nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) and C-FOS, which are essential for OC differentiation and bone resorption, were significantly reduced. Further investigating the underlying mechanism, we found that IL-27 significantly reduced RANKL-induced osteoclastogenesis by inhibiting the phosphorylation of IκB and phosphorylation of nuclear factor κB (NF-κB) p65. Furthermore, IL-27 was shown to inhibit lipopolysaccharide (LPS)-induced osteolysis in vivo. Collectively, these results indicate that IL-27 may be a potential candidate for the treatment of osteolytic diseases.
Keywords: Interleukin-27, inflammatory osteolysis, lipopolysaccharide, osteoclast
Introduction
Osteolysis is a key pathological process in a variety of devastating skeletal disorders that can induce bone erosion, such as osteoporosis, arthritis, bone tumors, Paget’s disease, aseptic loosening and so on. The main causes of these conditions are inflammation and trauma [1]. These conditions have similarities in the mechanisms of bone lysis, which are related to the release of pro-inflammatory cytokines and the activation of osteoclasts (OCs). In addition, OC formation can be stimulated by proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [2]. The common factors of these inflammatory responses include local levels of inflammatory mediators that promote OC differentiation and bone resorption, leading to the loss of bone mass [3]. These proinflammatory factors activate two signaling pathways that are essential for OC differentiation, the NF-κB signaling pathway and OPG/RANKL/RANK signaling pathway. These two signaling pathways induce and activate NFATc1, which is the key factor of OC differentiation that promotes the formation of OC and upregulates OC bone resorption activity, leading to localized osteolysis in bone lysis diseases [4].
OCs belong to the monocyte/macrophage lineage, which are mainly differentiated from hematopoietic stem cells [5,6]. After the hematopoietic stem cells divide into precursors of pluripotent mononuclear/macrophage lineage cells, they are stimulated directly or indirectly by various gene regulatory cytokines and develop into mature OCs with multiple nuclei through proliferation, differentiation, survival, and fusion [7]. OCs are the only biological multinucleated giant cells that have the ability to absorb and reshape bone [8]. They play a key role in bone development, bone formation, bone resorption, and bone mass regulation. Osteoclastogenesis is effectively induced by the major osteoclastogenic cytokine RANKL and macrophage colony-stimulating factor (M-CSF). Binding of RANKL to its receptor (RANK) activates a broad range of signaling cascades, including canonical and noncanonical NF-κB pathways [9]. A series of molecular signals lead to the translocation of NF-κB p65 to the nucleus, followed by activation of the OC differentiation transcription factors C-FOS and NFATc1 to coordinate the transcription of OC-related genes, including tartrate-resistant acid phosphatase-5 (Acp5), matrix metallopeptidase-9 (MMP-9), and cathepsin K (CTSK), which eventually leads to the formation of OCs [10].
Drugs that inhibit the function of OCs are widely used to treat bone lysis diseases, but all have various side effects [11]. For example, long-term use of bisphosphonates is associated with atypical long bone fracture, mandibular osteonecrosis and potential esophageal cancer [12]. Therefore, it is very important to develop new specific and effective drugs for the treatment of osteolytic diseases.
IL-27 is a member of the IL-12 family. Numerous studies have confirmed that IL-27 mainly functions as an anti-inflammatory cytokine [13]. IL-27 is a heterodimeric cytokine composed of subunits p28 and EBI3 that binds to the WSX1/gp130 receptor and is mainly secreted by antigen-presenting cells [14]. The mechanism of IL-27 activity in bone includes its ability to compete with the common receptor subunit gp130 and naturally antagonize IL-6, which is mediated by reducing the receptor activator expressed by RANKL in OCs [15]. In addition, IL-27 has anti-inflammatory effects on bone marrow cells [16,17] and inhibits the secretion of a variety of pro-inflammatory factors, including IL-6, TNF-α, IL-1β, MMPs and GM-CSF [18].
In this study, we investigated the effect of IL-27 on OC differentiation and bone resorption function by observing changes in OC morphology, analyzing the expression of specific genes and exploring the role of the NF-κB signaling pathway in this process. Furthermore, a mouse model of skull inflammation was established to investigate whether IL-27 has a protective effect against osteolytic diseases in an inflammatory environment through micro-CT and histological analysis. We speculate that IL-27 has great potential for the treatment and prevention of osteolytic diseases.
Materials and methods
Materials and reagents
Recombinant mouse IL-27 protein (purity > 95%), recombinant mouse M-CSF and recombinant mouse RANKL were purchased from R&D Systems (Minneapolis, MN). Cell Counting Kit-8 (CCK8) was purchased from Solarbio (Beijing, China). A Tartrate-resistant acid phosphatase (TRAP) staining kit and LPS were purchased from Sigma-Aldrich (NY, USA). Actin cytoskeleton and focal adhesion (FAK) staining kits were purchased from Millipore (Darmstadt, Germany). Dulbecco’s-modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Life Technologies, USA). An Osteo Assay Stripwell Plate for bone resorption was purchased from Corning, Inc. (NY, USA). Antibodies against NFATc1 and C-FOS were purchased from Abcam (Cambridge, USA). Antibodies against phospho-NF-κB p65, NF-κB p65, phospho-IκBα, and IκBα were purchased from Cell Signaling Technologies (MA, USA). β-Actin polyclonal antibodies were purchased from Bioworld Technology, Inc. (MN, USA). Mouse IL-1β, IL-6, and TNF-α ELISA kits were purchased from Novus Biologicals (CO, USA).
Cell culture
The macrophage lineage cell line RAW264.7 was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM medium containing 10% FBS and antibiotics (1% penicillin and streptomycin) in an incubator with 5% CO2 at 37°C. The culture medium was replaced every other day and subcultured every three days.
Cytotoxicity assay
RAW264.7 cells (4×103 cells) were seeded in a 96-well plate for the cell viability assay in triplicate plates and cultured overnight at 37°C with 5% CO2. On the following day, the cells were treated with different concentrations of IL-27 (0, 50, 100, 200 ng/ml) for 24 h, 48 h and 72 h. Cell viability was assessed using the CCK-8 assay according to the manufacturer’s instructions. After the specified amount of time, the medium was removed. Then, fresh medium containing 1/10 CCK-8 reagent was added to each well and incubated for an additional 2 h at 37°C. The absorbance of all wells was measured at a wavelength of 450 nm using a 96-well plate reader (Bio Tek, Synergy, USA).
In vitro osteoclastogenesis assay
RAW264.7 cells (4×103 cells) were seeded in a 96-well plate. After 24 h of culture, RANKL (50 ng/ml), M-CSF (25 ng/ml) and different concentrations of IL-27 (0, 50, 100, 200 ng/ml) were added and further cultured for 72 h to generate multinucleated OCs. The medium was changed every three days. After the specified amount of time, the cells were washed twice with PBS, fixed with 4% paraformaldehyde for 15 min, and stained with TRAP staining solution according to the manufacturer’s directions. TRAP-positive multinucleated cells containing three or more nuclei were considered OCs and were counted under an optical microscope (Leica, DMI 6000b, Germany).
The formation of OCs from RAW264.7 cells was induced as described previously. FAK staining was performed on OCs induced by RANKL according to the instructions of the FAK kit.
To investigate the effects of IL-27 on OC differentiation under LPS-induced inflammatory conditions, RAW264.7 cells were seeded in a 96-well plate at a density of 4×103 cells per well and pretreated with RANKL (50 ng/ml) and M-CSF (25 ng/ml) for 24 h. Then, the medium was replaced with fresh medium containing LPS (100 ng/ml) either with or without different concentrations of IL-27 (0, 50, 100, 200 ng/ml) for an additional 48 h for differentiation into OCs. The medium was changed every three days. After 72 h, the cells were stained for TRAP as previously described. TRAP-positive multinucleated cells containing three or more nuclei were considered OCs and were counted under an optical microscope.
Pit formation assay
RAW264.7 cells (4×103 cells) were seeded in an Osteo Assay Surface Plate (Corning Osteo Assay Surface) and cultured with RANKL (50 ng/ml), M-CSF (25 ng/ml) and different concentrations of IL-27 (0, 50, 100, 200 ng/ml) for 7 days to generate multinucleated OCs. On day 7, the cells were lysed with sodium hypochlorite for 5 min at room temperature, followed by washing twice with distilled water. Individual pits or multiple pit clusters were observed using a microscope at 40× magnification, and the absorption area was analyzed by ImageJ (NIH, Bethesda, MD, USA).
RNA extraction and analysis by real-time quantitative PCR (qRT-PCR)
RAW264.7 cells (4×103 cells) were seeded in a 12-well plate and induced with M-CSF (25 ng/ml), RANKL (50 ng/ml) and different concentrations of IL-27 (0, 100, 200 ng/ml) for 72 h. RAW264.7 cells were also seeded in a 12-well plate and pretreated with RANKL (50 ng/ml) and M-CSF (25 ng/ml) for 24 h. Then, the cells were stimulated with LPS (100 ng/ml) and different concentrations of IL-27 (0, 100, 200 ng/ml) for an additional 48 h.
Total RNA was isolated with TRIzol (Life Technologies) and used to synthesize cDNA using a reverse transcription kit (TaKaRa Bio, Inc., Japan). qRT-PCR was conducted to analyze the indicated gene expression using SYBR Premix Ex Taq II (TaKaRa Bio, Inc., Japan) and a PCR detection system (Bio-Rad, Hercules, CA, USA), and GAPDH was used as an internal control. The primer sequences are shown in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Genes | Forward | Reverse |
|---|---|---|
| NFATc1 | 5’-GACCCGGAGTTCGACTTCG-3’ | 5’-TGACACTAGGGGACACATAACTG-3’ |
| C-FOS | 5’-CGGGTTTCAACGCCGACTA-3’ | 5’-TTGGCACTAGAGACGGACAGA-3’ |
| CTSK | 5’-GAAGAAGACTCACCAGAAGCAG-3’ | 5’-TCCAGGTTATGGGCAGAGATT-3’ |
| TRAP | 5’-CACTCCCACCCTGAGATTTGT-3’ | 5’-CATCGTCTGCACGGTTCTG-3’ |
| DC-STAMP | 5’-CTAGCTGGCTGGACTTCATCC-3’ | 5’-TCATGCTGTCTAGGAGACCTC-3’ |
| OC-STAMP | 5’-GGGCTACTGGCATTGCTCTTAGT-3’ | 5’-CCAGAACCTTATATGAGGCGTCA-3’ |
| CTR | 5’-CGCATCCGCTTGAATGTG-3’ | 5’-TC TGTCTTTCCCCAGGAAATGA-3’ |
| MMP9 | 5’-CTGGACAGCCAGACACTAAAG-3’ | 5’-CTCGCGGCAAGTCTTCAGAG-3’ |
| ATP6V0d2 | 5’-GATGGAGAAGCTGATGGCTTGG-3’ | 5’-TTCTTCACCTCGCCTGTCTTGC-3’ |
| IL-1β | 5’-CTCAACTGTGAAATGCCACC-3’ | 5’-TGTCCTCATCCTGGAAGGT-z’ |
| IL-6 | 5’-TGGGAAATCGTGGAAATGAGA-3’ | 5’-ACTCTGGCTTTGTCTTTCTTGT-3’ |
| TNF-α | 5’-AGGCGGTGCTTGTTCCTCA-3’ | 5’-AGGCGAGAAGATGATCTGACTGC-3’ |
| GAPDH | 5’-AAATGGTGAAGGTCGGTGTG-3’ | 5’-TGAAGGGGTCGTTGATGG-3’ |
Note. NFATc1: nuclear factor of activated T cells; CTSK: cathepsin K; TRAP: tartrate-resistant acid phosphatase; DC-STAMP: dendritic cell-specific transmembrane protein; CTR: calcitonin receptor; MMP9: matrix metalloproteinase-9; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α, tumor necrosis factor-α; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; qRT-PCR, quantitative real-time polymerase chain reaction.
Western blot analysis
RAW264.7 cells were seeded in 6-well plates and treated with RANKL (50 ng/ml) and M-CSF (25 ng/ml) to generate OCs with or without IL-27 (200 ng/ml) for 72 h. By contrast, RAW264.7 cells were seeded in 6-well plates and pretreated with or without IL-27 (200 ng/ml) for 2 h in the presence of M-CSF (25 ng/ml) before RANKL (100 ng/ml) stimulation for 0, 15, 30 and 60 min. Subsequently, the cells were washed twice with PBS and lysed in lysis buffer (10 mM Tris, pH 7.2, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% Triton X-100 and 1% deoxycholic acid) supplemented with a protease inhibitors or phosphatase inhibitors. Final protein concentrations were determined using a BCA protein assay kit. Protein samples (30 µg) were run on 8-10% SDS-PAGE gels and transferred by electroblotting onto polyvinylidene difluoride membranes. Then, the membranes were blocked with 5% nonfat dry milk for 2 h and incubated with rabbit primary antibodies (C-FOS 1:1000, NFATc1 1:1000, NF-κB p65 1:1000, p-NF-κB p65 1:1000, IκBα 1:000, p-IκBα 1:1000, and β-actin 1:5000) overnight at 4°C. Next, the membranes were washed with TBST for 10 min and incubated with secondary antibodies (1:2000) at room temperature for 1.5 h. Antibody reactivity was analyzed by exposure to a ChemiDoc XRS+ imaging system (Bio-Rad, Hercules, USA). β-actin was used as an internal control.
LPS-induced skull osteolysis mouse model
Twenty healthy 6-week-old C57/BL6 female mouse were obtained from the animal center of the Army Medical University. The study followed the guidelines of the Army Medical University animal care and use committee. The mice were divided evenly into four groups as follows (5 mice in each group): sham group (PBS, control), LPS group (LPS, 5 mg/kg body weight), low-dose IL-27 group (LPS, 5 mg/kg with IL-27, 10 μg/kg) and high-dose IL-27 group (LPS, 5 mg/kg with IL-27, 20 μg/kg). The mice received subcutaneous injections over the sagittal midline suture of the skull under light anesthesia every other day over a 14-day period. Finally, micro-CT analysis (QuantumFX CT, Perkin Elmer) of the mice was performed under light anesthesia. Subsequently, mouse blood was obtained by excising the eyeball under deep anesthesia for ELISA analysis. Next, all mice were sacrificed, and the skulls were separated for histological analysis. The skull samples were fixed with 4% paraformaldehyde and decalcified with 10% EDTA for 2 weeks. Then, all skull samples were cut into 5-μm-thick sections for H&E staining. The obtained results were used to describe inflammatory osteolysis in vivo.
Enzyme-linked immunosorbent assay (ELISA)
Serum IL-1β, IL-6, and TNF-α levels were analyzed using mouse IL-1β, IL-6, and TNF-α ELISA kits according to the manufacturer’s protocol. Serum was obtained from blood after 30 min at room temperature and centrifugation at 3000 g for 10 min at 4°C. The absorbance of each standard and sample was measured at a wavelength of 450 nm. A standard concentration gradient was used as a standard curve.
Statistics
All data are expressed as the mean ± SEM. Each experiment was repeated at least three times separately, and the results were analyzed with Prism 7 (GraphPad Software, La Jolla, CA, USA). A two-tailed, unpaired Student’s T-test was used for comparisons between two groups. One-way analysis of variance was used to analyze differences in multiple comparisons. P-values of *(P < 0.05), **(P < 0.01), and ***(P < 0.001) were considered statistically significant.
Results
The effect of IL-27 on RAW264.7 cell viability
RAW264.7 cells were treated with IL-27 at different concentrations (0, 50, 100, 200 ng/ml) for 24 h to 72 h, followed by analysis using CCK-8 assays. The results showed that IL-27 concentrations of less than 200 ng/ml did not affect cell proliferation after 24 h to 72 h (Figure 1A-C). Therefore, IL-27 concentrations of 100 ng/ml and 200 ng/ml were used for subsequent studies.
Figure 1.
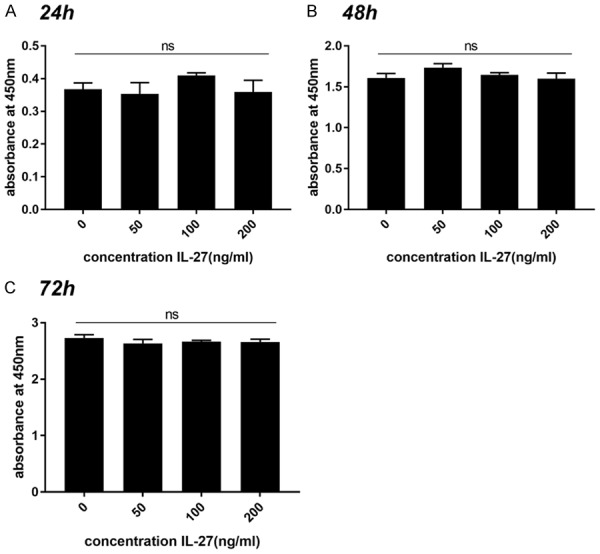
IL-27 concentrations less than 200 ng/ml did not affect cell proliferation after 24 h, 48 h and 72 h. RAW264.7 cells were treated with different concentrations of IL-27 (0, 50, 100, 200 ng/ml) for 24 h, 48 h and 72 h. Cell viability was assessed using a Cell Counting Kit-8 (CCK-8) assay. (*P < 0.05, **P < 0.01).
IL-27 inhibits RANKL-induced osteoclast differentiation and formation
RAW264.7 cells were induced with RANKL (50 ng/ml), M-CSF (25 ng/ml) and IL-27 (0, 100, 200 ng/ml) to generate OCs. The effect of IL-27 on the differentiation of OCs induced by RANKL was analyzed by TRAP staining. TRAP-positive OCs (nuclei ≥ 3) in each well (96-well plate) were quantitatively analyzed. We observed that IL-27 significantly inhibited RANKL-induced osteoclastogenesis in a concentration-dependent manner and that IL-27 markedly suppressed the number of TRAP-positive multinucleated cells (Figure 2A, 2B). Furthermore, FAK staining was used to analyze the formation of the F-actin ring structure in OCs, which is essential for bone resorption. The results showed that at an IL-27 concentration of 200 ng/ml, the size of the F-actin ring structure decreased significantly (Figure 2C-E). These data suggested that IL-27 effectively inhibited OC differentiation and formation.
Figure 2.
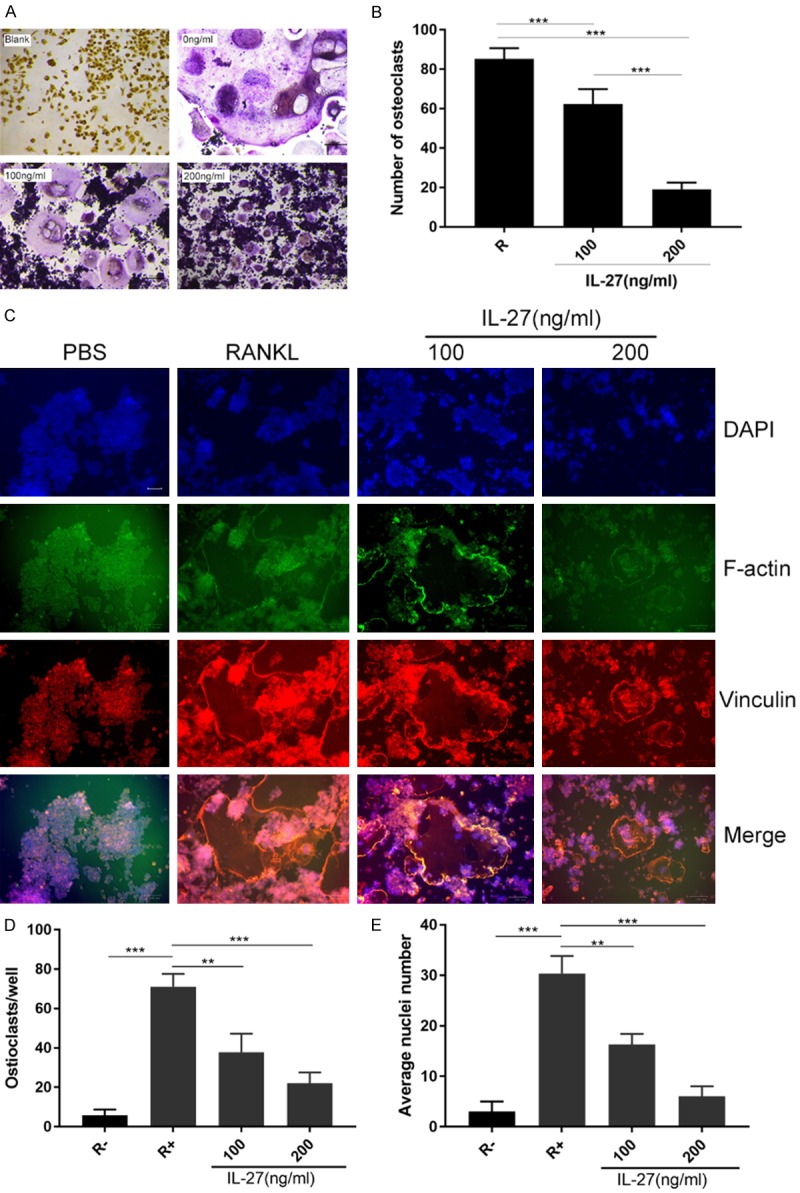
IL-27 inhibits RANKL-induced osteoclast differentiation and formation. A. The effect of IL-27 on the differentiation of OCs induced by RANKL was analyzed by TRAP staining. B. TRAP-positive osteoclasts (nuclei ≥ 3) in each well (96-well plate) were quantitatively analyzed. C. FAK staining was used to analyze the formation of the F-actin ring structure in OCs. D. The number of OCs in each well was quantitatively analyzed. E. The average nuclei number of OCs in each well was quantitatively analyzed. (*P < 0.05, **P < 0.01).
IL-27 attenuates bone resorption area in vitro
RAW264.7 cells were seeded in a 96-well plate (Corning Osteo Assay Surface) and treated with RANKL (100 ng/ml), M-CSF (25 ng/ml) and different concentrations of IL-27 (0, 100, 200 ng/ml) for 7 days. RAW264.7 cells were also seeded in another 96-well plate (Corning Osteo Assay Surface) and induced into OCs as describe for 3 days. Next, IL-27 was added in different concentrations (0, 100, 200 ng/ml) for another 4 days. According to the result, we can conclude that IL-27 doesn’t affect bone resorption activity of OC but reduced the bone resorption area by inhibiting the OC differentiation (Figure 3A-D).
Figure 3.
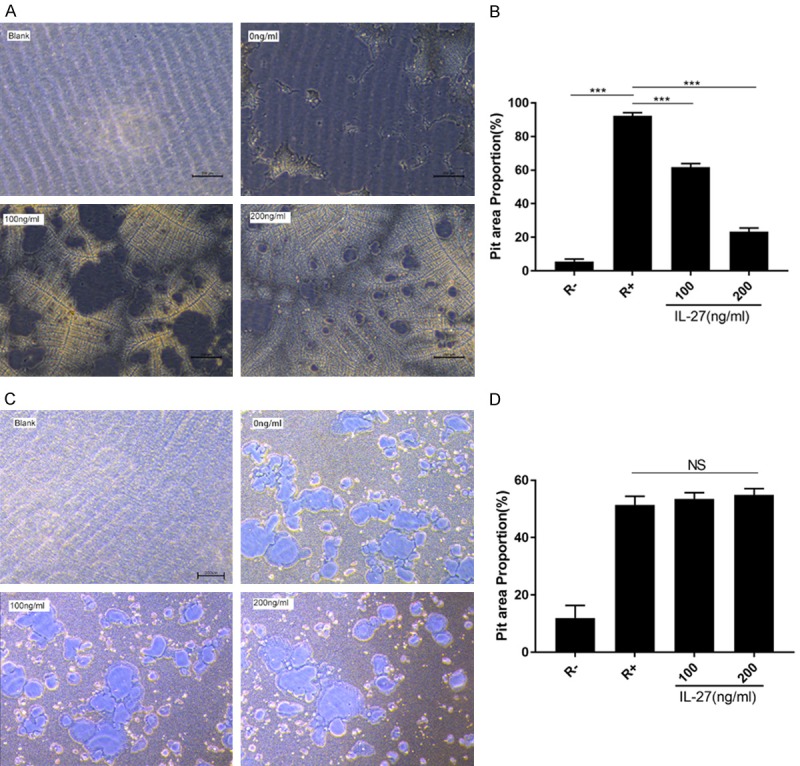
IL-27 attenuates bone resorption arear. A. RAW264.7 cells were plated on the Osteo Assay Surface and cultured with RANKL (50 ng/ml) and M-CSF (25 ng/ml) for 7 days in the presence or absence of IL-27 (200 ng/ml). B. Quantification of the bone resorption area on the Osteo Assay Surface. C. RAW264.7 cells were seeded in 96-well plate (Corning Osteo Assay Surface) and induced into OCs for 3 days and IL-27 was added for another 4 days. D. Quantification of the bone resorption area on the Osteo Assay Surface. (*P < 0.05, **P < 0.01).
IL-27 suppresses osteoclast differentiation by inhibiting the phosphorylation of the NF-κB signaling pathway
RANKL-induced NF-κB signaling pathway activation is necessary for OC differentiation and function. To investigate the mechanism of IL-27 in osteoclastogenesis, RAW264.7 cells were treated with RANKL (50 ng/ml) and M-CSF (25 ng/ml) in the presence or absence of IL-27 (200 ng/ml) for 0, 15, 30 and 60 min and analyzed by Western blot. We found that the activation of the NF-κB signaling pathway was inhibited in the presence of IL-27. The phosphorylation of NF-κB p65 was downregulated by IL-27 treatment. We further observed that the phosphorylation and degradation of IκB-α were also significantly inhibited by IL-27 (Figure 4A). Overall, these results indicated that IL-27 inhibited RANKL-induced OC differentiation and function by attenuating the activities of the NF-κB signaling pathway.
Figure 4.
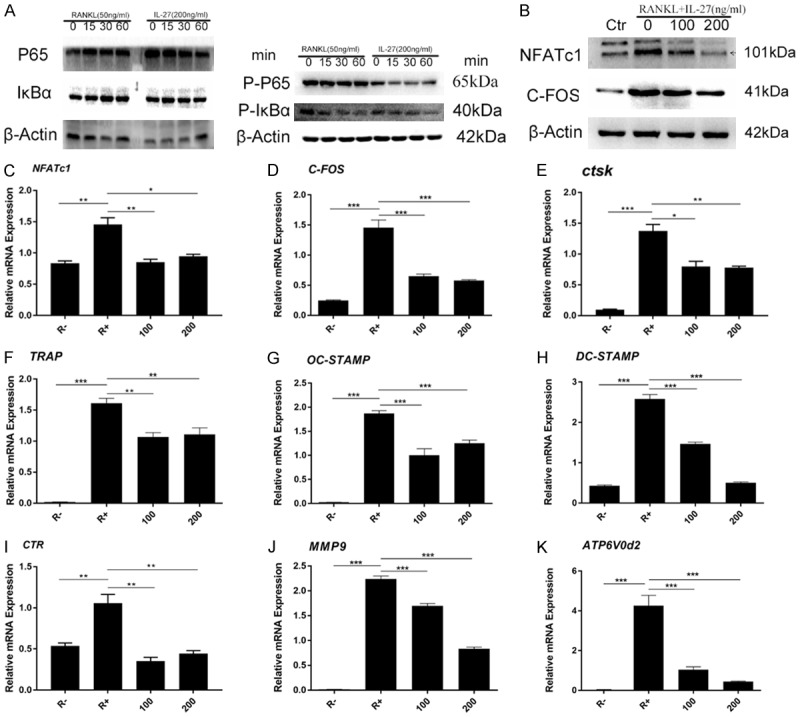
IL-27 suppresses osteoclast differentiation and marker gene expression by inhibiting the NF-κB signaling pathway. A. RAW264.7 cells were pretreated with or without IL-27 for 2 h in the presence of M-CSF (25 ng/ml) before RANKL (100 ng/ml) stimulation for the indicated time. B. Cells were lysed for Western blot analyses using antibodies against NFATc1, C-FOS, and β-actin. C-K. The expression of genes associated with osteoclast differentiation, fusion, and function were detected by qRT-PCR. (*P < 0.05, **P < 0.01).
IL-27 downregulates the expression of osteoclast marker genes
To further clarify the effect of IL-27 on OC differentiation, the protein expression of NFATc1 and C-FOS, key factors of OC differentiation that are generally upregulated during the process of osteoclastogenesis, were analyzed by Western blot. The results indicated that the expression levels of these two proteins were significantly upregulated by RANKL and suppressed by IL-27 in a concentration-dependent manner (Figure 4B). Furthermore, the expression of messenger RNA (mRNA) levels of OC-specific genes, which are generally upregulated during the process of osteoclastogenesis, was analyzed by qRT-PCR. The results showed that the mRNA expression levels of OC-specific genes, including NFATc1, C-FOS, CTSK, TRAP, OC-STAMP, and DC-STAMP, were all dramatically upregulated by RANKL during the OC differentiation process. However, IL-27 inhibited the elevated mRNA expression levels of these genes in a concentration-dependent manner (Figure 4C-K). This result was consistent with the results obtained by Western blot. In summary, these data demonstrated that IL-27 inhibited RANKL-induced OC-related gene expression in vitro.
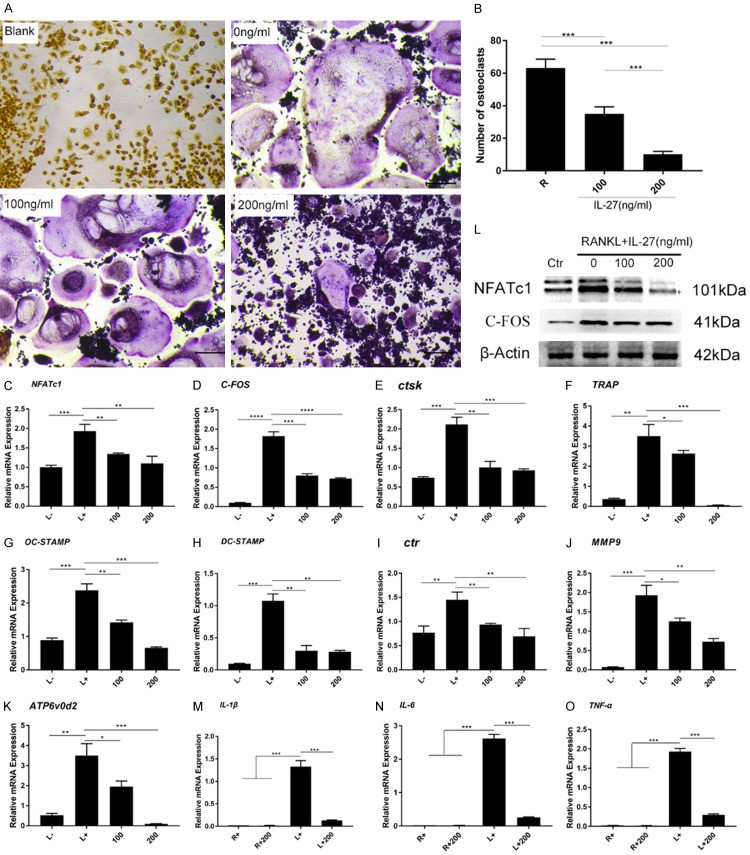
To investigate the effects of IL-27 on osteoclastogenesis under LPS-induced inflammatory conditions, RAW264.7 cells were pretreated with RANKL (50 ng/ml) and M-CSF (25 ng/ml) for 24 h. Then, the cells were incubated with LPS (100 ng/ml) and IL-27 (0, 100, 200 ng/ml) for an additional 48 h to generate multinucleated OCs, and TRAP staining was used to analyze LPS-induced OC differentiation. We observed that TRAP-positive multinucleated OCs were significantly reduced in a concentration-dependent manner (Figure 5A, 5B). To further study the effect of IL-27 on LPS-induced OC differentiation, a variety of OC marker genes were analyzed by qRT-PCR. As shown in Figure 5, IL-27 clearly downregulated the expression of these marker genes, consistent with the TRAP staining results (Figure 5C-K). Similarly, Western blot was used to analyze the protein expression of NFATc1 and C-FOS induced by LPS. Similar results were obtained, and the protein expression of NFATc1 and C-FOS was effectively reduced (Figure 5L). These results indicated that IL-27 has a negative effect on osteoclastogenesis under LPS-induced inflammatory conditions.
Figure 5.
IL-27 downregulates LPS-induced osteoclastogenesis and reduces the expression of osteoclast marker genes by reducing pro-inflammatory cytokines. A. TRAP staining was used to analyze LPS-induced osteoclast differentiation. B. TRAP-positive osteoclasts (nuclei ≥ 3) in each well were quantitatively analyzed. C-K. The expression of genes associated with osteoclast differentiation, fusion, and function was detected by qRT-PCR. L. Cells were lysed for Western blot analyses using antibodies against NFATc1, C-FOS, and β-actin. M-O. The mRNA expression of TNF-α, IL-1β, and IL-6 was detected by qRT-PCR. (*P < 0.05, **P < 0.01).
Because IL-27 inhibits LPS-induced OC formation, the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, was analyzed under LPS stimulation, which was determined to promote OC formation and ultimately lead to destructive bone loss. qRT-PCR was used to analyze the expression of these genes at the mRNA level. The results showed that IL-27 significantly inhibited the expression of these genes stimulated by LPS (Figure 5M-O). In summary, IL-27 can indirectly negatively regulate the formation of OCs induced by LPS by reducing the levels of pro-inflammatory cytokines.
IL-27 prevents LPS-induced inflammatory osteolysis in vivo
Since IL-27 inhibited the differentiation of OCs in vitro, we further investigated whether potential protective effects could be observed in an LPS-induced skull inflammation mouse model. LPS (5 mg/kg) was injected into a sagittal suture in the mouse skull every 2 days in the presence or absence of IL-27. The expression levels of TNF-α, IL-1β, and IL-6 in mouse serum were quantitatively analyzed using an ELISA kit. The results showed that IL-27 clearly inhibited the production of these pro-inflammatory cytokines (Figure 6A-C). Micro-CT scanning and 3D reconstruction showed that bone resorption in the LPS treatment group significantly increased compared with that in the sham group (PBS injection). However, the IL-27 treatment group (10 µg/kg group and 20 µg/kg group) showed substantial inhibition of LPS-induced inflammatory osteolysis in a concentration-dependent manner. Quantitative analysis of bone parameters further indicated that compared with the LPS group, the IL-27 treatment group exhibited significantly increased bone volume/total volume (BV/TV) and percentage of resorption area (%) in a concentration-dependent manner (Figure 6D-F). Furthermore, histological analysis (H&E staining) confirmed that IL-27 prevented LPS-induced osteolysis (Figure 6G), which is consistent with the imaged bone parameters. In conclusion, our data showed that IL-27 inhibits LPS-induced bone loss in vivo.
Figure 6.
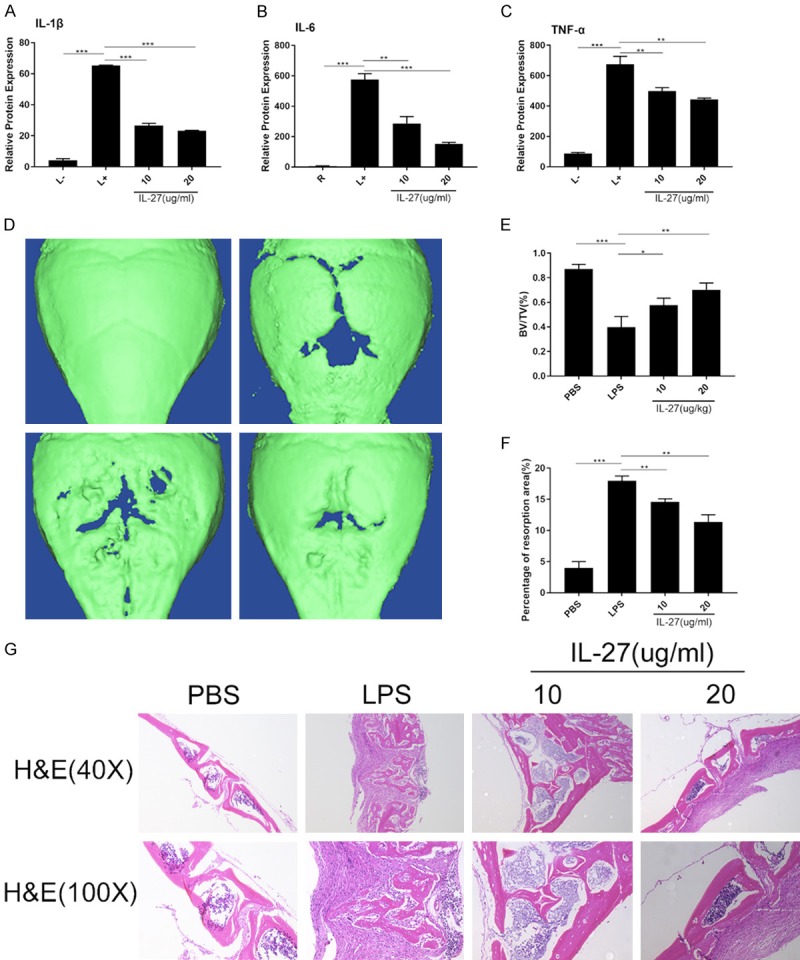
IL-27 prevents LPS-induced inflammatory osteolysis in vivo. A-C. The expression levels of TNF-α, IL-1β, and IL-6 in mouse serum were analyzed using an ELISA kit. D. Three-dimensional images of whole skulls were reconstructed using a micro-CT scanner. E, F. Quantitative analysis of bone volume/total volume (BV/TV) and percentage of resorption area (%). G. H&E staining of sections of skull samples from 6-week-old C57BL/6 mice treated with PBS, LPS, and LPS + IL-27. (*P < 0.05, **P < 0.01).
Discussion
Bone remodeling is characterized by successive bone resorption and bone formation at the same site, and the cells involved in bone remodeling are strictly coordinated with respect to time, site and functional activities. Therefore, bone reconstruction is an ordered and coupled process of bone resorption and bone formation that can be divided into initiation, transition and termination stages [19]. Recruitment, differentiation, activation and bone resorption of OC precursors occur at the initiation stage. At the transition stage, bone resorption activities are gradually inhibited, and OCs form new bone by feedback through the cell death regulator/cysteine aspartate protease-3 axis or through Fas/FasL-regulated programmed cell death. Finally, bone resorption is completely repaired at the termination stage, and local bone reconstruction activities enter the static stage, while excessive OC formation and bone resorption are the main mechanisms of many osteolytic bone diseases [20]. In the complex microenvironment of bone repair, the relationship between immune cells and bone-related cells is crucial for bone repair and reconstruction [21]. Studies have confirmed that products of staphylococcus aureus, such as staphylococcal protein A and staphylococcal lipoteichoic acid, can affect the differentiation of OCs and change the normal process of bone repair [22,23]. Moreover, bacteria cause osteolysis not only by directly killing osteoblasts but also by influencing OC formation and bone resorption through pathogen-induced inflammatory responses [24]. Bone infection is a response of inflammatory cells to repair damaged tissue. After bone infection, macrophages secrete various cytokines and chemokines to recruit inflammatory cells, promote neovascularization, regulate the migration and differentiation of mesenchymal stem cells (MSCs), and mediate bone remodeling [25]. Among the many cytokines secreted, the most typical include IL-1β, IL-6, and TNF-α. These cytokines promote bone resorption by enhancing OC differentiation and activity [26].
The NF-κB signaling pathway is a representative transcription factor that regulates the inflammatory response. In the skeletal system, the NF-κB signaling pathway directly participates in the regulation of OC differentiation and bone resorption [27]. Inflammatory factors including IL-1β, IL-6, and TNF-α cooperate with the RANKL signaling pathway to activate the NF-κB signaling pathway, thereby promoting OC differentiation [28]. Since the NF-κB signaling pathway is involved in both inflammation and osteoclastogenesis, we believe that it may be a potential therapeutic target for inflammatory osteolysis.
Interleukin-27 (IL-27) is a multifunctional cytokine an isomer of the IL-12 family. It is produced by monocytes and dendritic cells in respond to bacterial antigens, participates in the immune response of the body, and plays an anti-inflammatory and protective role in local inflammatory regions [29,30]. Accordingly, we hypothesized that IL-27’s inhibitory effect on inflammation helps protect against inflammatory osteolysis.
In the process of promoting the differentiation and function of OCs, RANKL is a key factor that promotes osteoclastogenesis. RANKL induces OCs from the mononuclear macrophage lineages and plays a unique role in hematopoietic cell differentiation [31]. The terminal differentiation of cells to OCs induced by RANKL involves the following steps: (1) activation of TRAP, which participates in the expression of markers of bone resorption and OCs; (2) the formation of multinucleated OCs by TRAP-positive cells with actin rings [32]. Furthermore, as noted in many studies, RANKL binds to RANK in OC precursors, which triggers the cascading activation of a series of downstream signaling pathways, including the RANKL-mediated NF-κB signaling pathway. Typically, NF-κB is retained in the cytoplasm in an inactive form coupled with IκB-α, an inhibitory subunit [33]. When RANKL binds to RANK through ubiquitin-dependent phosphorylation and degradation of IκB-α, this binding leads to the translocation of p65 into the nucleus from the cytosol, which activates transcription of the target genes NFATc1 and C-FOS and triggers transcriptional activation of several osteoblast-related genes, which is necessary for OC differentiation and function [33,34]. However, IL-27 attenuates these changes. Therefore, inhibition of the nuclear translocation of p65, which is an indispensable step in the activation of NF-κB, could be considered an essential mechanism for IL-27 participation in the inhibition of OC differentiation (Figure 7).
Figure 7.
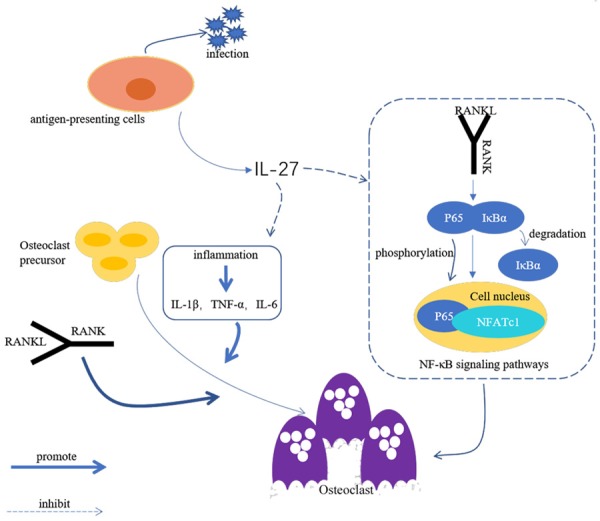
Schematic diagram of inhibition of osteoclast differentiation and formation by IL-27.
Another indispensable transcription factor, NFATc1, which is a regulator of OC formation and a common target gene for the essential transcription factors NF-κB and C-FOS, also plays an important role in regulating the expression of OC-specific genes in the RANKL signaling pathway [35]. In the early stage of OC differentiation, NFATc1 expression is enhanced by binding with transcription factors [36,37]. Therefore, TRAP, CTSK, MMP-9 and other OC-related genes regulated by NFATc1 are highly expressed at the final differentiation stage induced by RANKL, thereby promoting bone resorption [38,39]. Our data suggest that RANKL-induced NFATc1 expression is effectively inhibited by IL-27 in a concentration-dependent manner. IL-27 also reduces the expression levels of most OC-related marker genes. Although it is not clear whether IL-27 directly or indirectly inhibits the expression of NFATc1 through the NF-κB signaling pathway or whether NF-κB signaling pathway plays a direct role in the regulation of NFATc1 expression, our results suggest that IL-27 inhibition of the expression of NFATc1 and inhibition of the activity of NF-κB signaling pathway might play crucial roles in inhibiting OC differentiation and bone resorption function.
LPS is an amphiphilic molecule that is present in the outer membrane of Gram-negative bacteria and can activate the immune system, recruit immune cells, including monocytes and macrophages, and stimulate immune cells to produce pro-OC cytokines and promote the formation and activation of OCs, thus leading to bone resorption. Various studies have shown that TNF-α, IL-1β and IL-6 function as pro-inflammatory cytokines and change the nature of leukocyte infiltration to eventually transform acute inflammation to chronic inflammation [40]. Therefore, chronic inflammatory diseases and LPS-induced mouse models show decreased bone density, increased bone fragility and pro-inflammatory cytokine activity. Therefore, we investigated the use of IL-27 to prevent LPS-induced bone loss. As evidenced by the in vivo results, IL-27 reduces OC activity and bone resorption induced by LPS, prevents inflammatory osteolysis and effectively reduces pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. These results are consistent with the in vitro results. However, we cannot rule out the possibility that IL-27 may affect the formation of osteoblasts; therefore, further studies are needed to investigate the effect of IL-27 on the function of osteoblasts.
Conclusions
We demonstrated that IL-27 can effectively inhibit OC differentiation and bone resorption area by inhibiting the NF-κB signaling pathway. Specifically, IL-27 was shown to significantly reduce the expression levels of various genes and proteins that play an important role in OC differentiation, including NFATc1 and C-FOS. We also found that IL-27 inhibited the activity of the NF-κB signaling pathway through the inhibition of the phosphorylation of NF-κB P65 and phosphorylation of IκB-α, which is believed to be the mechanism by which IL-27 participates in the inhibition of OC differentiation. Furthermore, IL-27 has been shown to be effective in inhibiting LPS-induced OC differentiation and reducing proinflammatory cytokine levels while preventing inflammatory osteolysis in mice. Therefore, our results suggest that IL-27 may be a therapeutic candidate for osteolytic-related diseases.
Acknowledgements
I would like to thank Professor Shiwu Dong of the Teaching and Research Office of Biomedical Materials at the Third Military Medical University for his careful guidance and the help of all the teachers, students and staff. This study was funded by the National Natural Science Foundation of China (No. 81560356), the Science Foundation of Guizhou Province (No. (2015) 3044) and the Department of Science and Technology of Guizhou Province (No. [2017] 5724).
Disclosure of conflict of interest
None.
References
- 1.Zhang X, Li X, Fang J, Hou X, Fang H, Guo F, Li F, Chen A, Huang S. (2R,3R)Dihydromyricetin inhibits osteoclastogenesis and bone loss through scavenging LPS-induced oxidative stress and NF-kappaB and MAPKs pathways activating. J Cell Biochem. 2018;119:8981–8995. doi: 10.1002/jcb.27154. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Guo Q, Yang J, Ni B. Tumor necrosis factor alpha promotes osteoclast formation via PI3K/Akt pathway-mediated blimp1 expression upregulation. J Cell Biochem. 2017;118:1308–1315. doi: 10.1002/jcb.25672. [DOI] [PubMed] [Google Scholar]
- 3.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotti TN, Dharmapatni AA, Alias E, Haynes DR. Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J Immunol Res. 2015;2015:281287. doi: 10.1155/2015/281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muruganandan S, Dranse HJ, Rourke JL, McMullen NM, Sinal CJ. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells. 2013;31:2172–2182. doi: 10.1002/stem.1450. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Chim SM, Kuek V, Lim BS, Chow ST, Zhao J, Yang S, Rosen V, Tickner J, Xu J. HtrA1 is upregulated during RANKL-induced osteoclastogenesis, and negatively regulates osteoblast differentiation and BMP2-induced Smad1/5/8, ERK and p38 phosphorylation. FEBS Lett. 2014;588:143–150. doi: 10.1016/j.febslet.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T, Takayanagi H. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97:1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 8.Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10:109–117. doi: 10.1007/s11914-012-0096-1. [DOI] [PubMed] [Google Scholar]
- 9.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Kim N. Signaling pathways in osteoclast differentiation. Chonnam Med J. 2016;52:12–17. doi: 10.4068/cmj.2016.52.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss PJ, Steybe D, Poxleitner P, Schmelzeisen R, Munzenmayer C, Fuellgraf H, Stricker A, Semper-Hogg W. Osteonecrosis of the jaw in patients transitioning from bisphosphonates to denosumab treatment for osteoporosis. Odontology. 2018;106:469–480. doi: 10.1007/s10266-018-0362-5. [DOI] [PubMed] [Google Scholar]
- 12.Feehan AG, Zacharin MR, Lim AS, Simm PJ. A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone. 2018;113:137–143. doi: 10.1016/j.bone.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla P, Mansoori MN, Kakaji M, Shukla M, Gupta SK, Singh D. Interleukin 27 (IL-27) alleviates bone loss in estrogen-deficient conditions by induction of early growth response-2 gene. J Biol Chem. 2017;292:4686–4699. doi: 10.1074/jbc.M116.764779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 16.Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J Immunol. 2010;185:6599–6607. doi: 10.4049/jimmunol.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–413. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zolochevska O, Diaz-Quinones AO, Ellis J, Figueiredo ML. Interleukin-27 expression modifies prostate cancer cell crosstalk with bone and immune cells in vitro. J Cell Physiol. 2013;228:1127–1136. doi: 10.1002/jcp.24265. [DOI] [PubMed] [Google Scholar]
- 19.Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124:991–998. doi: 10.1242/jcs.063032. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura I, Takahashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012;22:167–177. doi: 10.1007/s10165-011-0530-8. [DOI] [PubMed] [Google Scholar]
- 21.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17:393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Wang Y, Cao Z, Dou C, Bai Y, Liu C, Dong S, Fei J. Staphylococcal lipoteichoic acid promotes osteogenic differentiation of mouse mesenchymal stem cells by increasing autophagic activity. Biochem Biophys Res Commun. 2017;485:421–426. doi: 10.1016/j.bbrc.2017.02.062. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu X, Dou C, Cao Z, Liu C, Dong S, Fei J. Staphylococcal protein a promotes osteoclastogenesis through MAPK signaling during bone infection. J Cell Physiol. 2017;232:2396–2406. doi: 10.1002/jcp.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang R, Yi J, Yang J, Chen Y, Luo W, Dong S, Fei J. Interleukin-37 inhibits osteoclastogenesis and alleviates inflammatory bone destruction. J Cell Physiol. 2019;234:7645–7658. doi: 10.1002/jcp.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavagis A, Takamori E, Granjeiro J, Oliveira R, Ferreira C, Peppelenbosch M, Zambuzzi W. TNFalpha contributes for attenuating both Y397FAK and Y416Src phosphorylations in osteoblasts. Oral Dis. 2014;20:780–786. doi: 10.1111/odi.12202. [DOI] [PubMed] [Google Scholar]
- 26.Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D, Gabet Y. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. 2018;9:2963. doi: 10.3389/fimmu.2018.02963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin TH, Pajarinen J, Lu L, Nabeshima A, Cordova LA, Yao Z, Goodman SB. NF-kappaB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol. 2017;107:117–154. doi: 10.1016/bs.apcsb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medic A, Jukic T, Matas A, Vukojevic K, Sapunar A, Znaor L. Effect of preoperative topical diclofenac on intraocular interleukin-12 concentration and macular edema after cataract surgery in patients with diabetic retinopathy: a randomized controlled trial. Croat Med J. 2017;58:49–55. doi: 10.3325/cmj.2017.58.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, Yui K. Interleukin-27-producing CD4(+) T cells regulate protective immunity during malaria parasite infection. Immunity. 2016;44:672–682. doi: 10.1016/j.immuni.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–341. doi: 10.1007/s00418-018-1636-2. [DOI] [PubMed] [Google Scholar]
- 32.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40:706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soysa NS, Alles N. Osteoclast function and bone-resorbing activity: an overview. Biochem Biophys Res Commun. 2016;476:115–120. doi: 10.1016/j.bbrc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Song D, Meng T, Xu W, Hou T, Lin Z, Yin H, Li B, Zhou L, Wang T, Han S, Fan T, Miao W, Liu M, Luo J, Zhou W, Li Z, Xiao J. 5-Fluoruracil blocked giant cell tumor progression by suppressing osteoclastogenesis through NF-kappaB signals and blocking angiogenesis. Bone. 2015;78:46–54. doi: 10.1016/j.bone.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Honma M, Ikebuchi Y, Kariya Y, Suzuki H. Regulatory mechanisms of RANKL presentation to osteoclast precursors. Curr Osteoporos Rep. 2014;12:115–120. doi: 10.1007/s11914-014-0189-0. [DOI] [PubMed] [Google Scholar]
- 36.Bi H, Chen X, Gao S, Yu X, Xiao J, Zhang B, Liu X, Dai M. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: a review. Front Med (Lausanne) 2017;4:234. doi: 10.3389/fmed.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda Y, Matsuo K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J Orthop. 2012;3:167–174. doi: 10.5312/wjo.v3.i11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins JA, Diekman BO, Loeser RF. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol. 2018;30:101–107. doi: 10.1097/BOR.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Park C, Kim GY, Park EK, Jeon YJ, Kim S, Hwang HJ, Choi YH. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-kappaB and activation of the Nrf2/HO-1 signaling pathway. Biosci Trends. 2018;12:257–265. doi: 10.5582/bst.2018.01107. [DOI] [PubMed] [Google Scholar]
- 40.Sapkota M, Li L, Kim SW, Soh Y. Thymol inhibits RANKL-induced osteoclastogenesis in RAW264.7 and BMM cells and LPS-induced bone loss in mice. Food Chem Toxicol. 2018;120:418–429. doi: 10.1016/j.fct.2018.07.032. [DOI] [PubMed] [Google Scholar]