Abstract
Intraoperative auto-transfusion with the use of cell saver systems is routinely used to reduce the rate of packed red blood transfusion in major surgery. Nevertheless some concerns have been raised on possible risks of coagulation disorders. The aim of the study was to analyze the blood processed by the cell saver, ready to be re-infused to the patient, in order to individuate unexpected cellular components, that can favor coagulopathy. We tested the blood processed by the cell saver in thirteen patients undergoing coronary bypass surgery with Cellsearch®, ScreenCell®, Cytology and Immunofluorescence. Those four methods allowed us to look for the presence of unexpected cells, quantify and characterize them. Furthermore, the blood processed by the cell saver was mixed with the patient’s peripheral blood and analyzed with the ROTEM® thromboelastography. The Cellsearch® revealed and counted a mean number of 1241 unexpected cells/7.5 ml in the blood processed by the cell saver. The ScreenCell® and Cytology confirmed the presence of non-hematological cells. Immunofluorescence showed positivity for Calretinin and WT-1, confirming the mesothelial origin. Moreover we detected a peculiar arrangement of the platelets around the mesothelial cells in a “cloud” form, suggesting platelet activation. The ROTEM® analysis showed a significantly longer clot formation time, smaller clot amplitude and maximum clot firmness, compared to controls. In conclusion we demonstrated the presence of mesothelial cells in the cell saving blood, ready to be auto-transfused. This finding can contribute to develop a platelet depletion coagulopathy, with coagulation factors consumption.
Keywords: Mesothelial cells, cell saving system, circulating cells, platelet aggregation, coagulation, cardiac surgery
Introduction
The cell saving systems (CSS) are traditionally used in major surgery and routinely in cardiac surgery to increase the postoperative hemoglobin level and to reduce the rate of packed red blood transfusions. The rationale of the cell saving systems is “saving” the red blood cells when a surgical bleeding occurs, therefore, the blood is sucked from the surgical field, filtered, collected in a dedicated reservoir and eventually centrifuged separating the red blood cells from plasma, platelets and white blood cells. As a consequence, the re-infused blood actually lacks platelets and coagulation factors, a condition that may impair the coagulative system leading to a prolonged clot formation time [1] and a need of platelet transfusion [2].
These considerations suggested the need for in-depth evaluations of the cell saving process, analyzing specifically the cellular component, because the coagulative effects highlighted after the blood reinfusion may be caused by something more than just technical issues. During the cardiac surgical maneuvers, in fact, the pericardium and the pleurae are usually opened and the blood coming from the heart chambers and from the vessels comes in contact with the mesothelial surfaces. Moreover, mesothelial cells derived from the pericardium and the pleurae exfoliate during the surgical maneuvers, and may be eventually sucked with blood. We previously demonstrated the presence of mesothelial cells in patient peripheral blood during the cardiopulmonary bypass, after the suction of blood from the surgical field, and their interfering with coagulation [3].
Therefore, since the origin of re-infused blood from the cell saver is actually the blood from the surgical field, we verified the presence of mesothelial cells in the processed blood and their impact on the coagulation.
We applied multiple methods to demonstrate the presence of mesothelial cells in the blood ready to be transfused and the impact of this cell on coagulation.
Material and methods
After approval by the institutional ethical committee, a written informed consent was obtained by each patient. In thirteen coronary patients, undergoing an elective, off pump, coronary artery bypass graft procedure, we collected the processed blood from the cell saver (Sorin Xtra, LivaNova PLC, UK) system after the lavage.
Blood salvage systems, routinely used during the whole operation (from the cut skin to the closure) are exclusively managed by the institutional perfusionists. Standard set up of the machine have been used for the study: filter in the collection reservoir: 40 microns, pump fill rate: 300 ml/min, pump wash rate: 250 ml/min, empty fill rate: 250 ml/min, final hematocrit 55%, wash solution for every process: 900 ml.
A total of 20 ml of blood was withdrawn from the reinfusion circuit (as recommended by the manufacturer and according to our internal guidelines, when we infuse any blood product we use a specific infusion set with built-in microaggregate filter) and divided in five samples to be analyzed in five different ways: Cellsearch®, ScreenCell®, Cytology, Immunofluorescence, and ROTEM®; test as summarized in Figure 1. The first three analyses were performed to achieve a direct observation of cells in the processed blood and to look for the presence of non-hematological cells eventually passed through the cell saver filters and ready to be transfused into patients. Immunofluorescence was performed in order to define the nature of the non-hematological cells. Finally the ROTEM® analysis, a qualitative and quantitative assessment of the hemostasis, was used to evaluate the effects of the re-infused blood on the coagulation competency.
Figure 1.
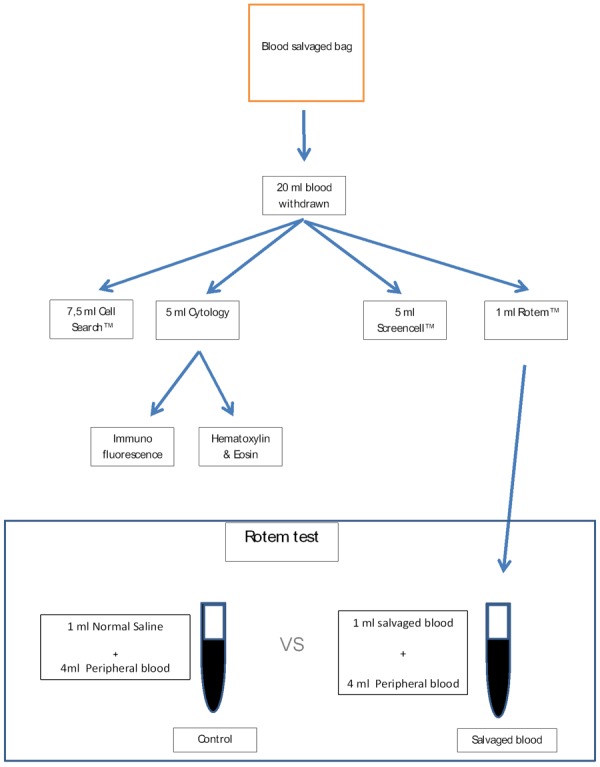
Flowchart highlighting steps in our analytic process: from the blood sampling taken from the blood salvage bag to the applied tests.
In our center, as a routine, the blood, sucked from the surgical field during the operation, is processed after the protamine administration and re-infused within 6 hours.
CELLSEARCH® Immunomagnetic enrichment and sample staining procedures of circulating epithelial cells
The CellSearch® system (Menarini, Silicon Biosystem) is a FDA-approved automated procedure originally developed to identify and count epithelial cells in blood samples, for diagnosis and follow-up of epithelial tumors [4-6]. We recently demonstrated the validity of this method to identify circulating non-hematological cells in cardiac procedures [3]. Therefore, we applied the same technique for the blood processed by the cell saver.
For each patient we collected a blood sample of 7.5 ml directly from the reinfusion bag. Since the hematocrit of the processed blood is generally around 60%, the sample was diluted with the diluent buffer, in order to make it suitable for the red cell detection control of the CellSearch® processing. Standard protocols and reagents were used. Reassuming, blood samples were collected into dedicated tubes and the CellSearch® Epithelial Cell kit (Menarini Silicon Biosystem) was applied for enrichment and enumeration. Epithelial non-hematological circulating cells were captured from blood by anti-EpCAM-antibody-bearing ferrofluid and subsequently identified by cytokeratin positivity, leukocyte common antigen CD45 negativity and 4’,6-diamidino-2-phenylindole (DAPI) staining to ensure the integrity of the nuclei.
Cytology
As previously reported we applied a cytologic method to collect and concentrate fluids containing a low number of cells [3]. In brief, blood samples from the reinfusion bag were collected in 5 ml ethylene diamine tetra acetic acid Vacutainer tubes, maintained at 4°C and processed within 2 hours after collection. A dilution with sterile phosphate-buffered saline and centrifugation at 800× g for 10 min, without brake, were performed. Subsequently, a red blood cell lysis buffer was used, followed by washing with phosphate buffered solution. After 10 min centrifugation, ≈100 μl of sediment samples were smeared on a slide, fixed with an aerosol preparation of a neutral pH-buffered saline that contains 4% w/v paraformaldehyde. Five slides for each case were collected and two of them were stained with hematoxylin and eosin for morphological evaluation at light microscope (Leica DMD108) by a team of pathologists (CM, AA, PZ).
The remaining slides were collected for Immunofluorescence.
ScreenCell® Cyto-kit, filtration system
ScreenCell® is method based on size-exclusion filtering, for isolation of circulating rare cells. It is a filtration mini-device, composed of 8 micron microporous membrane filter, in order to exclude red blood cells and to isolate cells over 8 micron. Hematoxilin and eosin staining of the filters allows the morphologic observation of the cells and their measurement.
All blood samples were collected in 3 ml ethylene diamine tetraacetic acid Vacutainer tubes, maintained at 4°C and processed within 2 hours after the collection, as recommended by the manufacturer (ScreenCell® Cyto kit, Paris, France). Blood was diluted with FC2 buffer (ScreenCellTM Cyto kit, Paris, France) and incubated for 8 min at room temperature, shaking gently after 2 and 6 min. Thereafter the filters were rinsed with 1,6 ml of sterile phosphate buffered solution 1X, pH 7.4, collected from the device, air-dried and immediately stained for 1 min with Hematoxylin Solution S (Merck, Darmstadt, Germany) and then for 20 seconds with Shandon Eosin Y Aqueous Solution (Thermo Fisher Scientific Inc., Waltham, MA, USA), washed in distilled water, air dried and mounted on a glass slide for cytological evaluation by certified pathologists (CM, AA, PZ).
Immunofluorescence
For each patient we prepared slides dedicated to immunofluorescent analysis (Superfrost, Leica Microsystems, Milan, Italy), as previously reported.
Briefly, fixed cells were permeabilized with 1% saponin, and blocked with 10% bovine serum albumin (BSA) in phosphate buffered solution 1X for 30 min at 37°C. Cells were then stained overnight with anti-Calretinin (mouse, 1:100; cod. NCL-L-CALRETININ, clone 5A5; Leica Microsystems, Germany) and anti WT-1 (rabbit, 1:1500; cod. PA5-16879; Life Technologies, California, United States) in 1% BSA, followed by incubation with an anti-mouse FITC-conjugated secondary antibody (1:400; Santa Cruz Biotechnology, Heidelberg, Germany) and anti-rabbit Alexa Fluor 546-conjugated secondary antibody (1:400; Santa Cruz Biotechnology, Heidelberg, Germany) for 60 min at room temperature. The cells were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (2 g/ml; Santa Cruz Biotechnology, Heidelberg, Germany), mounted using an antifade mounting medium (Life Technologies, Monza, Italy).
All nucleated cells in each slide were imaged in 2 fluorescent channels. Calretinin and WT-1 positive cells containing intact DAPI nuclei were identified as mesothelial cells. As positive controls we used cytological slides obtained from pleural washing. Negative controls were also carried out. Microscopic observation was performed at a Laser Microscope (Leica DM2500 Leica Microsystems, Milan, Italy) by three pathologists (CM, AA, PZ).
ROTEM® delta test
To have a qualitative in vitro controlled assessment of the effect of intraoperative salvage blood on the coagulation, we used the ROTEM® Delta test (Tem International GmbH) [7,8]. Briefly, the ROTEM® Delta Test measures kinetic changes of the clot elasticity of whole blood samples, testing both the intrinsic and the extrinsic pathway. It gives qualitative and quantitative evaluation of the clot formation, providing important information on the hemostasis competency.
For each patient we collected two samples of 4 ml of peripheral blood immediately after the skin closure. These specimens were mixed respectively with 1 ml of normal saline (control group), and 1 ml of intraoperative saved blood to simulate a reinfusion of 20% of total circulating blood (reinfusion group). The two samples have been tested with the ROTEM analysis for the INTEM and the EXTEM assay (respectively testing the thromboelastography via the intrinsic or the extrinsic pathway). The assay tested the clotting time (CT), clot formation time (CFT), the alfa angle, the amplitude of the clot 5 and 10 min after the clotting time (A5 and A10), the maximum clot firmness (MCF). An impaired activation of the coagulation is expressed by a prolonged CT; a polymerization disorder is associated to prolonged CFT and/or reduced MCF; on the other hand a reduced MCF with a normal CFT rather indicates a deficiency of clottable substrate (fibrinogen and/or platelets).
Statistical analysis
All statistical analysis were performed using R 3.3 for Windows (R Core Team, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/andSAS 9.4 software, by SAS Institute Inc., Cary, NC, USA).
Continuous variables were reported as mean+/- standard deviation. Categorical variables were expressed as percentages. Comparisons of mean values for the ROTEM tests (CT, CFT, alfa angle, A5, A10, MCF) were performed with Paired T-Test, a P < 0.05 was considered significant.
Results
Patient clinical features are summarized in Table 1. Mean age of the patients recruited was 69.3 ± 7.1 years, nine were male.
Table 1.
Characteristics of the patients
| Patient n° | Age | Sex | History of smoke | Diabetes | EF (%) | Recent STEMI | Previous PTCA | Number of grafts | ITA use | Bilateral ITA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Male | Yes | Yes | 45 | No | No | 3 | Y | No |
| 2 | 68 | Male | Yes | No | 50 | No | Yes | 2 | Y | Yes |
| 3 | 64 | Female | Yes | Yes | 38 | Yes | No | 3 | Y | Yes |
| 4 | 81 | Female | No | Yes | 40 | No | Yes | 2 | Y | No |
| 5 | 74 | Male | Yes | No | 45 | Yes | No | 1 | Y | No |
| 6 | 56 | Male | Yes | Yes | 55 | No | No | 4 | Y | Yes |
| 7 | 63 | Female | No | Yes | 55 | No | No | 3 | Y | Yes |
| 8 | 74 | Male | Yes | No | 40 | Yes | Yes | 2 | Y | Yes |
| 9 | 77 | Male | No | No | 50 | No | No | 2 | Y | No |
| 10 | 61 | Male | Yes | Yes | 45 | No | No | 3 | Y | Yes |
| 11 | 65 | Female | Yes | Yes | 50 | Yes | Yes | 2 | Y | Yes |
| 12 | 74 | Male | No | Yes | 40 | No | Yes | 3 | Y | No |
| 13 | 70 | Male | Yes | No | 55 | Yes | Yes | 3 | Y | No |
EF, Ejection fraction; STEMI, ST elevation myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; ITA, internal thoracic artery.
The Cellsearch® automatically counted a mean number of 1241.4 ± 379.5 non-hematological cells per 7.5 ml, results are showed in Table 2. The identified cells were single and arranged in cellular micro-aggregates (clusters) as showed in Figure 2.
Table 2.
Number of mesothelial cells automatically counted by the CellSearch® in 7.5 ml of saved blood
| N° cells counted per 7.5 ml | |
|---|---|
| Patient 1 | 906 |
| Patient 2 | 1044 |
| Patient 3 | 1740 |
| Patient 4 | 850 |
| Patient 5 | 1532 |
| Patient 6 | 1630 |
| Patient 7 | 1952 |
| Patient 8 | 856 |
| Patient 9 | 1247 |
| Patient 10 | 986 |
| Patient 11 | 1025 |
| Patient 12 | 896 |
| Patient 13 | 1475 |
| Mean ± sd. dev | 1241.1 ± 379.5 |
Figure 2.
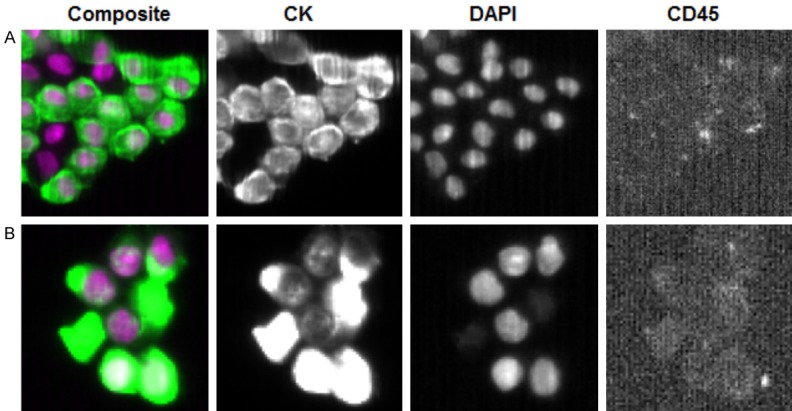
CellSearch® system. Representative images of mesothelial cells macroemboli (A) and microemboli (B) detected by CellSearchTM technologies. Images are automatically taken by the instrument and analyzed by pathologists. Mesothelial cells were recognized as nucleated cells (DAPI, purple staining) positive for cytokeratin 8-18 and 19 (CK, green staining) and negative for CD45, as leucocyte marker. (Magnification 630×).
ScreenCell® filtration system confirmed the presence of non-hematological cells in all samples (Figure 3, arrows). Moreover, multiple platelet aggregates were observed on the microporous filter surface (Figure 3, arrows head).
Figure 3.
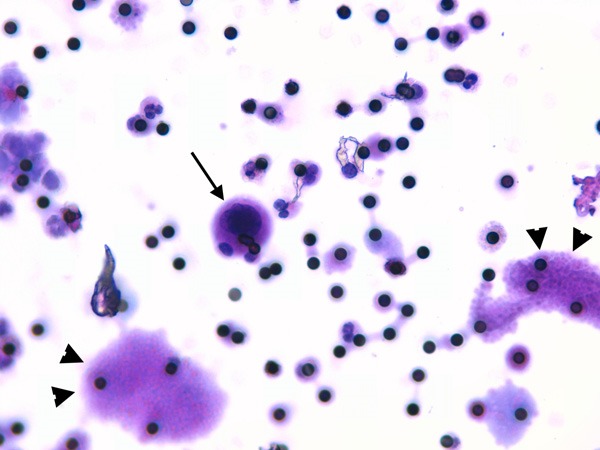
ScreenCell® filtration system. ScreenCell® microporous filter stained with hematoxylin and eosin; the black dots are the pores of the filters (8 μm in diameter). Please note an epithelial cell indicated by an arrow, whereas arrows head indicates platelet aggregates. (Magnification 400×).
Cytology showed single cells with a mean size of 30 µm, and clusters with a mean size of 70 µm, surrounded by platelets in a cloud form (Figure 4A). In addition, we revealed the presence of platelet aggregation in a clot-like organization (Figure 4B).
Figure 4.
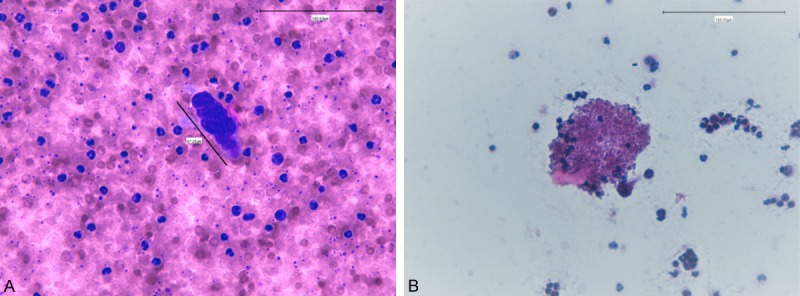
Cytology. Hematoxylin and eosin staining of a cytology smear. A. In the center is present a 67, 25 m macro-embolus (cluster), surrounded by platelets organized in a “cloud form”. (Magnification 400×). Hematoxylin and eosin staining of a cytology smear. B. Platelet aggregation in a clot-like organization is showed. (Magnification 200×).
Immunofluorescence analysis highlighted the presence of double-positive cells for Calretinin and WT-1, confirming the mesothelial origin of the detected cells (Figure 5).
Figure 5.
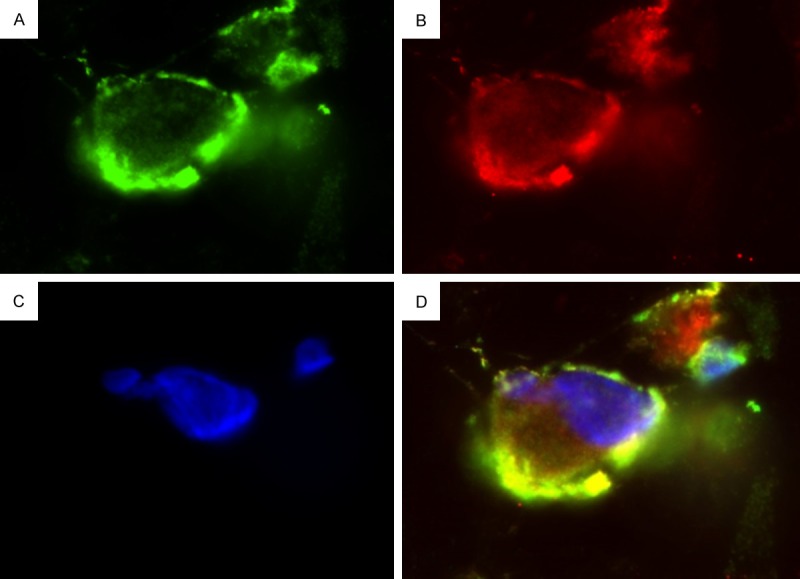
Immunofluorescence analysis. Immunofluorescence images demonstrating Calretinin and WT-1 double positive cells. Please note the specific and strong cytoplasmic staining for Calretinin (shown in green - A), and for WT-1 (shown in red - B). Nuclear counterstaining: 4’,6-diamidino-2-phenylindole (shown in blue - C). A yellow signal by the merging of both channels demonstrating the double positivity of the cells (D). (Magnification 630×).
ROTEM® analysis showed an altered coagulation process in the reinfusion samples, indeed both the INTEM and EXTEM tests were deranged. The clot formation time was significantly longer in the reinfusion samples than in the controls. Similarly the A5 and A10, the amplitude of the clot at 5 and 10 min respectively, were significantly smaller in the reinfusion compared to controls; as a consequence, the MCF resulted smaller as well (Table 3).
Table 3.
ROTEM®results of the blood re-infused after the cell saving process compared with the controls
| INTEM | EXTEM | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Reinfusion | P value | Control | Reinfusion | P value | |
| CT (s) | 206.9 ± 23.1 | 207.2 ± 16.9 | 0.96 | 73.9 ± 15.9 | 68.9 ± 12.1 | 0.08 |
| CFT (s) | 81.1 ± 21.4 | 119.5 ± 34.7 | 0.005 | 110.3 ± 16.7 | 142.9 ± 22.7 | < 0.001 |
| A5 (mm) | 46.6 ± 7.3 | 38.4 ± 5.9 | 0.003 | 44.4 ± 5.1 | 37.0 ± 6.4 | < 0.001 |
| A10 (mm) | 55.9 ± 8.4 | 48.6 ± 5.5 | 0.006 | 55.2 ± 5.1 | 48.0 ± 5.7 | < 0.001 |
| MCF (mm) | 64.4 ± 7.9 | 57.6 ± 3.8 | 0.008 | 63.2 ± 4.4 | 57.1 ± 5.0 | < 0.001 |
| Alfa (°) | 74.9 ± 1.9 | 67.1 ± 6.3 | < 0.001 | 68.7 ± 5.6 | 67.1 ± 4.1 | 0.008 |
CT, clotting time, CFT: clot formation time, A5 and A10: amplitude of the clot 5 and 10 minutes after the clotting time; MCF: maximum clot firmness; Alfa: the alfa angle.
Discussion
Cell salvage (the autotransfusion of shed blood) was first proposed in 1818 and first mentioned in the literature in 1885 [9]. Nowadays, the use of cell saver is largely adopted with the intent of reducing allogeneic blood transfusion and did not appear to impact adversely on clinical outcomes in cardiac surgery [10].
In our study, anyway, we demonstrated for the first time the presence of a considerable number of mesothelial cells, single and micro-aggregates (clusters) in the reinfusion bag of the cell saver, via the application of Cellsearch® technology. The presence of cells was also confirmed by ScreenCell® filtration system and Cytology. Immunofluorescence demonstrated the mesothelial origin of the identified cells. Moreover, cytological analysis highlighted the presence of platelets aggregation around the mesothelial cells and clusters, suggesting that platelet activation occurs early in the reinfusion bag. This new finding demonstrated that the processed blood, ready to be infused, contains isolated mesothelial cells, clusters of aggregated mesothelial cells and activated platelets. It is unclear if the cell aggregates develop when the blood remains in the reservoir or during the different phases of the procedure, anyway they are present in the blood ready for infusion into patient.
As already shown in our previous study, the surgical maneuvers may lead to exfoliation of mesothelial cells from pericardium and pleura and consequent mixing with the sucked blood [3]; moreover, we demonstrated that, when a sample of mesothelial cells come in contact with the own patient’s blood, it cause deranging of the clotting competency [3]. This result is not really surprising, since activated mesothelial cells express the Tissue Factor, therefore they can activate the clotting cascade [11-14]. The morphological manifestation of this occurrence was, probably, the presence of platelet aggregation, also around the cell clusters.
Our observations raised an important question about the use of Cell Saver. How much the re-infused blood can trigger the inflammatory reactions and coagulopathy?
We tried to answer to this question with the ROTEM analysis. This analysis allowed us to evaluate the in vitro effects of the re-infused blood on the coagulation, and showed that both INTEM and EXTEM test, in the cell saver samples had significantly longer CFT and smaller MCF, A5 and A10 than controls, suggesting a platelet and fibrinogen depletion. In this scenario, the coagulopathy appeared as a consequence of platelets activation and aggregation around the mesothelial cells rather than a simple dilution of coagulation factors, since the control samples were actually diluted with normal saline.
The recent literature data are difficult to compare to our study due to the different setting of the published studies and the effects of cardiopulmonary bypass on the results: anyway, the processing of the shed blood from pericardium is widely accepted and recommended [15], with several evidences showing positive effects of the processing of cardiotomy blood [16,17].
Nevertheless, other authors showed a deranged thromboelastographic profile similar to our results [18], and further evidences on high risk patients showed an impaired blood coagulative function associated with the cell saver use [19]. Moreover, Westerberg and colleagues [20] showed that avoiding cell saver and cardiotomy suction leads to lower activation of tumor necrosis factor alfa (TNFα), interleukin-6 (IL-6) and complement factor C3.
The aim of our study was to analyze specifically the blood processed by the cell saver system, avoiding the detrimental effect of the cardiopulmonary bypass. For this purpose we tested a group of patients undergoing off pump coronary bypass, in order to obtain more reliable results.
Moreover, most of the studies are focused on in vivo evaluations, based on clinical outcome of patients re-transfused with saved blood; this experimental approach implies several possible confounding factors, such as the blood losses in the chest drains, transfusions, and the different management of the patients. Our controlled test, on the contrary, supported by the direct microscopic observation of mesothelial cells and activated platelets, was designed to show an impact of the reinfusion on the thromboelastographic profile of the blood of the same patient independently by the perioperative management, since the simulation of the reinfusion has been made in vitro.
Some words of caution are necessary. We tested only one cell saving system (Sorin Xtra, LivaNova PLC, UK), nevertheless, since the centrifugal system is similar, we may expect the same results. It would be advisable to test other machines in the future.
We simulated a reinfusion of 20% of circulating blood, therefore we can only suppose the effects of a different rate of reinfusion and the possible therapeutic options (platelet, fibrinogen and coagulation factor transfusion). Finally, our findings have been obtained on a cardiac setting, where large mesothelial surfaces are clearly open and exposed to surgical maneuvers: it would be interesting to verify whether other surgical environments (liver transplant, orthopedic surgery) with different mesothelial surfaces would lead to similar results.
Conclusions
Intraoperative blood salvage is an important resource in cardiac surgery and in major surgery.
We demonstrated for the time the presence of mesothelial cells in the blood ready to be infused into patient. In addition, we observed activated platelets and alteration of ROTEM® test, highlighting a detrimental impact on coagulative competence. These findings open a new scenario on autotrasfusion procedures. Further studies will be needed to better understand the effect of re-infused blood by cell saving systems and consequently ameliorating the existing devices, to improve the perioperative outcome of patients.
Acknowledgements
The authors thank Federica Figuccia and Teresa Mancuso for the valuable technical help.
Disclosure of conflict of interest
None.
References
- 1.Campbell J, Holland C, Richens D, Skinner H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: a pilot study. Perfusion. 2012;27:221–224. doi: 10.1177/0267659111432567. [DOI] [PubMed] [Google Scholar]
- 2.Al-Riyami AZ, Al-Khabori M, Baskaran B, Siddiqi M, Al-Sabti H. Intra-operative cell salvage in cardiac surgery may increase platelet transfusion requirements: a cohort study. Vox Sang. 2015;109:280–286. doi: 10.1111/vox.12280. [DOI] [PubMed] [Google Scholar]
- 3.Santise G, Marinaro C, Maselli D, Dominici C, Di Vito A, Donato G, Camastra C, Zeppa P, Barni T, Rizzuto A, Viglietto G, Mignogna C. Circulating non-hematological cells during cardiopulmonary bypass: new findings in cardiac surgery procedures. Perfusion. 2016;31:584–592. doi: 10.1177/0267659116638916. [DOI] [PubMed] [Google Scholar]
- 4.Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, Madhavan D, Sprick MR, Schott S, Domschke C, Baccelli I, Schönfisch B, Burwinkel B, Marmé F, Heil J, Sohn C, Pantel K, Trumpp A, Schneeweiss A. Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer. 2014;14:512. doi: 10.1186/1471-2407-14-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 6.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the cell search system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 7.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–232. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 8.Peng HT, Grodecki R, Rizoli S, Shek PN. A comparative study of tissue factor and kaolin on blood coagulation assays using rotational thromboelastometry and thromboelastography. Blood Coagul Fibrinolysis. 2016;27:31–41. doi: 10.1097/MBC.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 9.Baker RA, Merry AF. Cell salvage is beneficial for all cardiac surgical patients: arguments for and against. J Extra Corpor Technol. 2012;44:38–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;14:CD001888. doi: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonalanti-human tissue factor antibody. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 12.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 13.Pronk A, de Groot PG, Hoynck van Papendrecht AA, Verbrugh HA, Leguit P, van Vroonhoven TJ, Sixma JJ. Thrombogenicity and procoagulant activity of human mesothelial cells. Arterioscler Thromb. 1992;12:1428–1436. doi: 10.1161/01.atv.12.12.1428. [DOI] [PubMed] [Google Scholar]
- 14.Bottles KD, Laszik Z, Morrissey JH, Kinasewitz GT. Tissue factor expression in mesothelial cells: induction both in vivo and in vitro. Am J Respir Cell Mol Biol. 1997;17:164–172. doi: 10.1165/ajrcmb.17.2.2438. [DOI] [PubMed] [Google Scholar]
- 15.Shann KG, Likosky DS, Murkin JM, Baker RA, Baribeau YR, DeFoe GR, Dickinson TA, Gardner TJ, Grocott HP, O’Connor GT, Rosinski DJ, Sellke FW, Willcox TW. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. doi: 10.1016/j.jtcvs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Ji B, Zhang Y, Zhu X, Liu J, Long C, Zheng Z. Comparison of the effects of three cell saver devices on erythrocyte function during cardiopulmonary bypass procedure - a pilot study. Artif Organs. 2012;36:931–935. doi: 10.1111/j.1525-1594.2012.01494.x. [DOI] [PubMed] [Google Scholar]
- 17.Engels GE, van Klarenbosch J, Gu YJ, van Oeveren W, de Vries AJ. Intraoperative cell salvage during cardiac surgery is associated with reduced postoperative lung injury. Interact Cardiovasc Thorac Surg. 2016;22:298–304. doi: 10.1093/icvts/ivv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell J, Holland C, Richens D, Skinner H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: a pilot study. Perfusion. 2012;27:221–4. doi: 10.1177/0267659111432567. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Shen S, Zhang J, Wang W, Zheng J. The efficacy, safety and cost-effectiveness of intra-operative cell salvage in high-bleeding-risk cardiac surgery with cardiopulmonary bypass: a prospective randomized and controlled trial. Int J Med Sci. 2015;12:322–328. doi: 10.7150/ijms.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg. 2004;78:54–59. doi: 10.1016/j.athoracsur.2003.12.029. [DOI] [PubMed] [Google Scholar]


