Abstract
It has been shown that local anesthetics have potential neurotoxicity, but the exact mechanism remains unclear. In this study, PC12 cells were treated with different concentrations of ropivacaine mesylate (0.1, 0.5, 1, 2 and 4 mmol/L) for 24 h, the cell viability was assessed by CCK-8 assay. Then, cells in 0.5 mmol/L ropivacaine group, 2 mmol/L ropivacaine group and control group were subjected to morphological observation under a light microscope, assessment of cell necrosis by Hoechst33342/PI staining and apoptosis by Annexin V-FITC/PI staining, and the detection of Fas and FasL expression by qPCR, immunofluorescence and Western blot. Results showed that the cell viability decreased significantly (P<0.05), necrosis and apoptosis rate increased markedly (P<0.05), and the expression of Fas, FasL, caspase-3 and caspase-8 increased dramatically (P<0.05) with the increase in the concentration of ropivacaine mesylate. Therefore, ropivacaine mesylate may induce the apoptosis of PC12 cells, which may be related to the up-regulation of Fas/FasL.
Keywords: Ropivacaine mesylate, PC12 cells, apoptosis, Fas, FasL
Introduction
Local anesthetics are widely used to relieve acute or chronic pain by blocking the sodium channel and calcium channel with favorable safety [1]. However, in recent decades, some studies have suggested that most local anesthetics have neurotoxicity, especially when they are used at a high concentration and/or for a long time [2-6]. Ropivacaine mesylate (RM) is one of the most widely used local anesthetics in clinical anesthesia and pain management, because of its good separation of the sensory and motor nerve blocking, fewer systemic reactions and lower cardiac toxicity [7,8], but it may also cause nerve damage and abnormal sensations similar to other local anesthetics [9,10].
The exact mechanism underlying the neurotoxicity of local anesthetics is still unclear, and some studies have proposed cell apoptosis is an important mechanism [11,12]. Wen et al. found that ropivacaine hydrochloride was able to increase the expression of calcium/calmodulin-dependent protein kinase II (CaMK II) and the apoptosis rate of dorsal root ganglion neurons (DRG), resulting in nerve injury [13,14]. Lu et al. found that bupivacaine-induced reactive oxygen species (ROS) production results in an alteration in the permeability of the mitochondrial membranes and Cl- influx into mitochondria, which suggests that mitochondrial pathway is closely related to the neurotoxicity of local anesthetics [15]. However, it is not clear whether the death receptor pathway is involved in the neurotoxicity of local anesthetics.
Fas/Apo-1 is a type I transmembrane protein with three extracellular “cysteine-rich domains”, the hallmark of the tumor necrosis factor (TNF) receptor superfamily, and it is widely expressed on the immune cells and nerve cells. The Fas/FasL pathway is a typical death receptor pathway. Fas binds to FasL via the death domain (DD), Fas associated death domain (FADD), death effector domain (DED) and procaspase-8 to format a death-inducing signaling death inducing signal complex (DISC), which subsequently activates the caspase family members, especially caspase-3 and 8, eventually leading to apoptosis [16]. Whether the Fas/FasL pathway is associated with the neuronal apoptosis induced by local anesthetics is still poorly understood.
In this study, the toxic effects of ropivacaine mesylate at different concentrations on adrenal pheochromocytoma PC12 cells (PC12 cells) were evaluated, and then the expression of Fas, FasL, caspase-3 and caspase-8 was detected in order to reveal the role of Fas/FasL pathway in the neurotoxicity of RM in PC12 cells.
Materials and methods
Materials
The PC12 cell line was purchased from the Shanghai Institutes for Biological Sciences. RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Gibco (Gibco, USA). Rabbit anti-rat Fas polyclonal antibody, rabbit anti-rat FasL polyclonal antibody and rabbit anti-rat cleaved caspase-3 polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), RM was obtained from Cisen Pharmaceutical (Cisen, China). Primers of the β-actin, Fas, FasL, capase-3 and caspase-8 were supplied by Shanghai Sangong Company of China (Table 1). The cell counting kit-8 (CCK-8) and Hoechst33342/PI kit were purchased form Beyotime Biotechnology (Beyotime, China); Annexin V-FITC/PI kit was obtained from Beckman Coulter (Beckman Coulter, USA). The TRIzol reagent was obtained from Invitrogen (Invitrogen, USA), and the RT kits and real-time PCR kits were from Promega (Promega, USA). All reagents were from commercial suppliers and standard biochemical quality.
Table 1.
Sequences of primers used for qPCR
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| β actin | ||
| Forward | 5’-GATCAAGATCATTGCTCCTCCTGA-3’ | 170 |
| Reverse | 5’-CAGCTCAGTAACAGTCCGCC-3’ | |
| Fas | ||
| Forward | 5’-ATGAGATCGAGCACAACAGC-3’ | 105 |
| Reverse | 5’-TTAAAGCTTGACACGCACCA-3’ | |
| FasL | ||
| Forward | 5’-CACCAACCACAGCCTTAGAGTATCA-3’ | 171 |
| Reverse | 5’-ACTCCAGAGATCAAAGCAGTTCCA-3’ | |
| Caspase-3 | ||
| Forward | 5’-GAAAGCCGAAACTCTTCATCA-3’ | 155 |
| Reverse | 5’-ATAGTAACCGGGTGCGGTAT-3’ | |
| Caspase-8 | ||
| Forward | 5’-TCCGGTGTTTTATAGTTCCGCT-3’ | 186 |
| Reverse | 5’-GGGTAGGAGAGCTGTAACCTGT-3’ |
Cell culture
PC12 cells were cultured in the RPMI-1640 medium with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin in a humidified environment with 5% CO2 at 37°C. The medium was refreshed once every two or three days.
Cell viability detected with CCK-8 assay
The PC12 cells were seeded into 96-well plates at a density of 5×103 cells/well. When the cell confluence reached about 80%, cells were treated with RM at different concentrations (0.1, 0.5, 1, 2 and 4 mmol/L) for 24 h. In control group, cells were maintained in RPMI-1640 medium without RM treatment (n = 5 per group) for 24 h. Then, CCK-8 (10 µl) was added to each well, followed by incubation at 37°C for 1-4 h. The optical density (OD) was measured at 450 nm (OD450) and the cell viability was calculated. The cell viability in control group was set as 100%, to which the cell viability in other groups was normalized.
Cell processing and experiment protocol
According to CCK-8 assay, cells were randomly divided into 3 groups: 0.5 mmol/L RM group, 2 mmol/L RM group and control group. When the cell confluence reached about 80%, they were treated with 0.5 mmol/L RM or 2 mmol/L RM for 24 h. In the control group, cells were cultured without RM treatment for 24 h.
Observation of cell morphology
PC12 cells were seeded into 12-well plates, and then treated as described above. Cell morphology was observed under a light microscope (Olympus, IX73, Japan), and images were captured at a magnification of 200. At least 3 fields were randomly selected from each well.
Detection of cell necrosis by Hoechst/PI staining
PC12 cells were seeded into 6-well plates, and then treated as described above. The medium was removed, 1 ml of phosphate buffer saline (PBS) containing 5 µl of Hoechst33342 and 5 µl of propidium iodide (PI) was added to each well, followed by incubation for 15 min at 4°C. Thereafter, cells were washed with PBS, and observed under a fluorescence microscope (Olympus, IX73, Japan). Representative photographs were captured. The red fluorescence-positive cells were counted and the percentage of necrotic cells was calculated.
Detection of apoptosis rate by flow cytometry
Cells in above 3 groups were washed with ice-cold PBS twice, digested in trypsin (without EDTA) and then collected. After centrifugation at 2000 rpm for 5 min, the supernatant was removed, and cells were re-suspended with 1 binding buffer. After addition of 5 µl of Annexin V-FITC and 10 µl of PI, cells were incubated at 4°C in dark for 10-20 min, followed by flow cytometry. A total of 10000 cells were counted, and the cell apoptosis rate was calculated. At the same time, cells without incubation with Annexin V-FITC and PI served as a negative control.
Detection of Fas, FasL, caspase-3 and caspasse-8 mRNA expression by quality real-time PCR (qPCR)
PC12 cells were lysed with TRIzol (Invitrogen, USA) following a standard protocol. According to the protocol provided by the manufacturer (Promega, USA), total RNA (2 µg) was reverse-transcribed for 60 min at 42. The PC12 cells were collected with TRIzol (Invitrogen, USA) following a standard protocol, and the concentrations of RNA in each group are shown in Table 2. qPCR was performed using SYBR Green fluorescent dye (GoTaq® qPCR Master Mix, Promega, USA; Applied Biosystems, USA). β-actin was used as an internal control. Relative mRNA expression was calculated based on the cycle threshold values corrected for β-actin expression. Briefly, a 20-µl reaction mixture containing 4 µl of cDNA template, 10 µl of GoTaq® qPCR Master Mix, 5.2 µl of nuclease-free water, and 0.40 µl of each primer was amplified under following conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The qPCR primers for Fas, FasL, caspase-3, caspase-8, and βactin are shown in Table 1. Fluorescence signals were measured, and the Ct value were determined. 2-ΔΔCT method was employed to calculate the relative quantity of target gene expression.
Table 2.
The concentrations of RNA
| Group | A260/280 | Concentration (ng/µl) |
|---|---|---|
| 0 mM | 1.82 | 736.8 |
| 1.85 | 753.4 | |
| 1.75 | 689.6 | |
| 0.5 mM | 1.78 | 702.1 |
| 1.79 | 713.7 | |
| 1.83 | 769.3 | |
| 2 mM | 1.88 | 801.6 |
| 1.73 | 689.4 | |
| 1.84 | 784.2 |
Detection of Fas, FasL and cleaved caspase-3 expression by western blotting
Total protein was harvested from PC12 cells with lysis buffer, and protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Beyotime, China). Then, the protein extract was boiled for 5 minutes. Subsequently, 50 µg of protein was separated in each sample by sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis, and then electrotransferred onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) and then immunoblotted with anti-Fas (1:1000), anti-FasL (1:1000), anti-cleaved caspase-3 (1:1000), or anti-αtubulin antibody (1:1000, as an internal control) overnight at 4°C. After rinsing, the PVDF membranes were incubated with horseradish peroxidase (HRP) conjugated anti-rabbit immunoglobulin (1:1000) for 1 h. Specific proteins were detected by enhanced chemiluminescence (Bio-rad, USA), and the protein bands were quantified with Image J software. The expression of Fas, FasL, and cleaved caspase-3 was normalized to that of α tubulin.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Comparisons among groups were performed using one-way analysis of variance (ANOVA). All the experiments were repeated at least 3 times, and a value of P<0.05 was considered statistically significant.
Results
Cell viability of PC12 cells after RM treatment
The viability of untreated PC12 cells was regarded as 100%. After treatment with 0.1 mM, 0.5 mM, 1 mM, 2 mM and 4 mM RM for 24 h, the cell viability decreased to 91.90 ± 1.75%, 87.03 ± 1.70%, 75.50 ± 2.31%, 54.18 ± 2.64% and 38.44 ± 1.99%, respectively. Moreover, the reduction of cell viability after treatment with RM was dependent on the concentration of RM (Figure 1).
Figure 1.

Cell viability of PC12 cells treated with different concentrations of ropivacaine mesylate. aDenotes P<0.05 vs control group, bdenotes P<0.05 vs 0.1 mM RM group, cdenotes P<0.05 vs 0.5 mM RM group, ddenotes P<0.05 vs 1 mM RM group, edenotes P<0.05 vs 2 mM RM group.
Morphology of PC12 cells
Figure 2 shows the morphology of PC12 cells after treated with different concentrations of RM. After RM treatment, PC12 cells became round and shrunken with the disappearance of neurites. Moreover, most cells treated with RM lost their cellular integrity as compared to control group.
Figure 2.
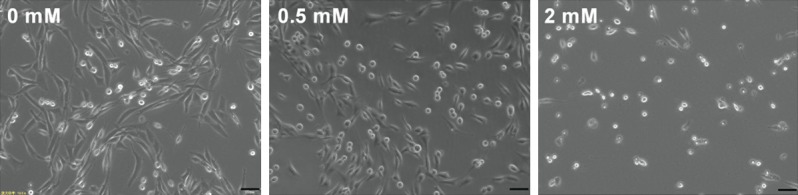
The morphology of PC12 cells after treatment with different concentrations of ropivacaine mesylate. After RM treatment, PC12 cells became round and shrunken with the disappearance of neurites. Magnification: 200×, Scale = 50 µm.
Cell necrosis
As shown in Figure 3, with the increase in the concentration of RM, the necrotic cells increased. Compared with control group, the necrotic cells increased in the 0.5 mM RM group and 2 mM RM group markedly (P<0.05). Compared with 0.5 mM RM group, the necrotic cells increased in the 2 mM RM group significantly (P<0.05).
Figure 3.

The cell necrosis. With the increase in the concentration of RM, the necrotic cells increased. A. The necrotic cells in different groups were detected after Hoechst33342/PI staining (200×, scale = 50 µm). PI: necrotic cells; Hoechst33342: nucleus. B. Average fluorescence intensity (mean ± SD), a P<0.05 vs control group, b P<0.05 vs 0.5 mM group.
Apoptosis rate
RM induced the apoptosis of PC12 cells. The apoptosis rate of PC12 cells in control group was 3.06 ± 0.48%. After treatment with 0.5 mM RM and 2 mM RM for 24 h, the apoptosis rate increased to 6.82 ± 0.59% and 10.8 ± 0.69%, respectively (Figure 4).
Figure 4.
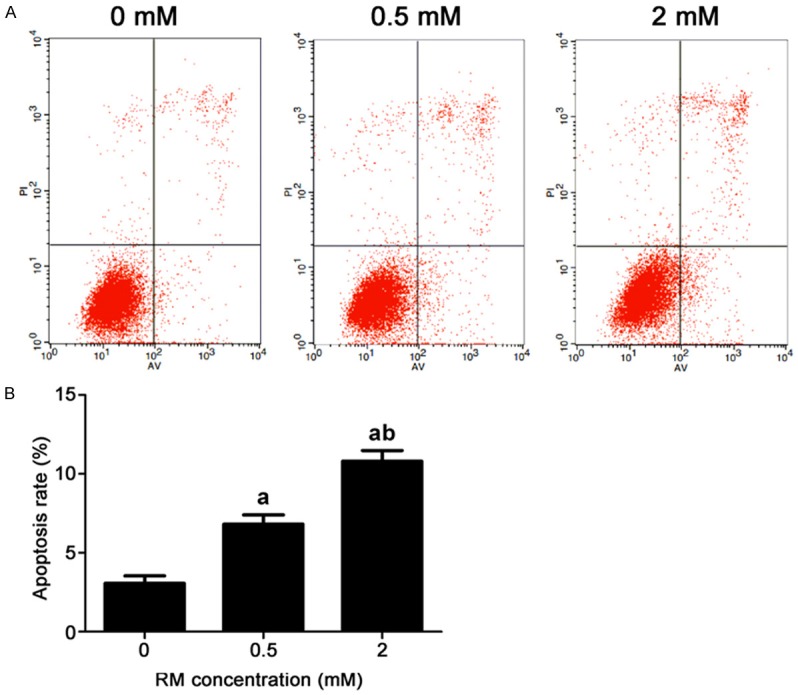
Cell apoptosis rate in different groups (mean ± SD). RM induced the apoptosis of PC12 cells. The apoptosis rate of PC12 cells in control group was 3.06 ± 0.48%. After treatment with 0.5 mM RM and 2 mM RM for 24 h, the apoptosis rate increased to 6.82 ± 0.59% and 10.8 ± 0.69%, respectively. A. The apoptotic cells detected by flow cytometry. B. The apoptosis rate in each group, a P<0.05 vs control group, b P<0.05 vs 0.5 mM group.
Expression mRNA of Fas, FasL, caspase-3 and caspase-8
As shown in Figure 5, compared with control group, the mRNA expression of Fas, FasL, caspase-3 and caspase-8 increased significantly in the 0.5 mM RM group and 2 mM group (P<0.05). Compared with 0.5 mM RM group, the mRNA expression of Fas, FasL, caspase-3 and caspase-8 increased markedly in 2 mM RM group (P<0.05). These indicate that RM can up-regulate the mRNA expression of Fas, FasL, caspase-3 and caspase-8.
Figure 5.
The mRNA expression of Fas, FasL, caspase-3 and caspase-8 detected by qPCR (mean ± SD). RM can up-regulate the mRNA expression of Fas, FasL, caspase-3 and caspase-8. A. Fas mRNA expression. B. FasL mRNA expression. C. Caspase-3 mRNA expression. D. Caspase-8 mRNA expression. a P<0.05 vs control group, b P<0.05 vs 0.5 mM RM group.
Protein expression of Fas, FasL and cleaved caspase-3
As shown in Figure 6, compared with control group, the protein expression of Fas, FasL, and cleaved caspase-3 increased significantly in the 0.5 mM RM group and 2 mM RM group (P<0.05). Compared with 0.5 mM group, the protein expression of Fas, FasL, and cleaved caspase-3 increased markedly in the 2 mM RM group (P<0.05). These indicate that caspase-3 is activated, suggesting that RM may induce the apoptosis of PC12 cells by up-regulating the expression of Fas/FasL.
Figure 6.

Fas, FasL, and cleaved caspase-3 protein expression detected by Western blotting (mean ± SD). Caspase-3 is activated, suggesting that RM may induce the apoptosis of PC12 cells by up-regulating the expression of Fas/FasL. A. Protein band of Fas, FasL, cleaved caspase-3 and α-tubulin. B. Fas protein expression. C. FasL protein expression. D. Cleaved caspase-3 protein expression. a P<0.05 vs control group, b P<0.05 vs 0.5 mM group.
Discussion
With wide use of local anesthesia, the mechanism and prevention of neurotoxicity of local anesthetics have become a focus in current researches [4,17]. In the present study, neurotoxicity of RM was investigated in the adrenal pheochromocytoma PC12 cells. The morphological, physiological, and biochemical functions of PC12 cells are similar to those of normal neurons, and they have been widely used in neurobiological and neurotoxicological studies [18,19].
RM is usually used local anesthesia and pain management. In our study, the cell viability of PC12 cell treated with 2 mM RM for 24 h was 54.18 ± 2.64%. This indicates that 2 mM RM is able to significantly cause damage to PC12 cells with acceptable cell necrosis. Thus, in the following experiment, the highest concentration of RM was 2 mM. Our results showed that RM induced PC12 cell injury and apoptosis in a concentration dependent manner. The apoptosis rate and mRNA/protein expression of Fas and FasL increased after treatment with 0.5 and 2 mmol/L RM for 24 h; at the same time, the mRNA expression of caspase-3 and caspase-8 and the protein expression of cleaved caspase-3 increased, which indicates that RM can increase the expression of Fas/FasL, and then activate the caspase family to induce the apoptosis of neurons, leading to nerve damage.
The neurotoxicity of local anesthetics is related to multiple factors, which has a complex system. The mechanisms underlying the neurotoxicity of local anesthetics vary among anesthetics, but most are related to cell apoptosis [11,12]. Generally, apoptosis may execute in two pathways: the death receptor pathway on cell membrane and the mitochondrial pathway in cells. The Fas/FasL pathway belongs to the death receptor pathway. Some investigators have shown that local anesthetics can induce the release of cytochrome c and ROS from mitochondria, and then activate caspase-9 and caspase-3, leading to neuronal apoptosis [15,20]. Zheng et al. [21] found that a caspase-independent apoptosis parthanatos was involved in the ropivacaine induced SH-SY5Y cell injury, accompanied by the activation of poly ADP-ribose polymerase-1 (PARP-1), the release of apoptosis-inducing factor (AIF) and the consumption of nicotinamide adenine dinucleotide (NAD+). Besides, CaMK II [13,14], Cav3.1 T-type calcium and Cav3.3 T-type calcium [22,23], mitogen activated protein kinase (MAPK) [24,25], phosphatidyl-3-kinase (PI3K) [26], serine-threonine protein kinase B (PKB/Akt) [27], adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) [28-30], and autophagy [26,31] are also involved in the neurotoxicity of local anesthetics.
Of note, there were limitations in this study. This was an in vitro study, and only the influence of RM was investigated in PC12 cells. However, the in vivo environment of human neurons is very complex, and Fas/FasL pathway is not blocked; the role of Fas/FasL up-regulation in the neurotoxicity of RM is still unclear. Studies have shown that p38 MAPK inhibitor SB202190 is able to suppress the expression of Fas and FasL in human neuroblastoma SK-N-SH cells, indicating that p38 MAPK may be important to up-regulate the expression of Fas/FasL [32,33]. Thus, whether the increased Fas/FasL expression and neuronal apoptosis after RM treatment is related to p38 MAPK is unknown.
In summary, the present study suggests that the neurotoxicity of RM is concentration dependent. In addition, RM is able to up-regulate Fas/FasL mRNA and protein expression in PC12 cells. These results provide new evidence for further exploration of the mechanisms underlying the neurotoxicity of local anesthetics, and for the prevention of nerve complications caused by local anesthetics.
Acknowledgements
The study was supported by project of Health Commission of Hubei Province (WJ2019F074).
Disclosure of conflict of interest
None.
References
- 1.Eng HC, Ghosh SM, Chin KJ. Practical use of local anesthetics in regional anesthesia. Curr Opin Anaesthesiol. 2014;27:382–387. doi: 10.1097/ACO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 2.Cai XY, Xiong LM, Yang SH, Shao ZW, Xie M, Gao F, Ding F. Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro. Spine J. 2014;14:483–490. doi: 10.1016/j.spinee.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 3.El-Boghdadly K, Chin KJ. Local anesthetic systemic toxicity: continuing professional development. Can J Anaesth. 2016;63:330–349. doi: 10.1007/s12630-015-0564-z. [DOI] [PubMed] [Google Scholar]
- 4.Farber SJ, Saheb-Al-Zamani M, Zieske L, Laurido-Soto O, Bery A, Hunter D, Johnson P, Mackinnon SE. Peripheral nerve injury after local anesthetic injection. Anesth Analg. 2013;117:731–739. doi: 10.1213/ANE.0b013e3182a00767. [DOI] [PubMed] [Google Scholar]
- 5.Hampl K, Steinfeldt T, Wulf H. Spinal anesthesia revisited: toxicity of new and old drugs and compounds. Curr Opin Anaesthesiol. 2014;27:549–555. doi: 10.1097/ACO.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Zhang QG, Lai LY, Wen XJ, Zheng T, Cheung CW, Zhou SQ, Xu SY. Neuroprotective effect of ginkgolide B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid Med Cell Longev. 2013;2013:159864. doi: 10.1155/2013/159864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Wan L, Mei W, Tian Y. Update on the clinical utility and practical use of ropivacaine in Chinese patients. Drug Des Devel Ther. 2014;8:1269–1276. doi: 10.2147/DDDT.S57258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian X, Yang P, Su T, Yu J, Zhao S, Xiang G, Yu D, Zhang W, Manyande A, Gao F, Tian Y, Yang H. Intraperitoneal ropivacaine and early postoperative pain and postsurgical outcomes after laparoscopic herniorrhaphy in toddlers: a randomized clinical trial. Paediatr Anaesth. 2016;26:891–898. doi: 10.1111/pan.12953. [DOI] [PubMed] [Google Scholar]
- 9.Rodola F, Anastasi F, Vergari A. Ropivacaine induced acute neurotoxicity after epidural injection. Eur Rev Med Pharmacol Sci. 2007;11:133–135. [PubMed] [Google Scholar]
- 10.Zhai MZ, Wu HH, Yin JB, Cui YY, Mei XP, Zhang H, Zhu X, Shen XF, Kaye AD, Chen GZ. Dexmedetomidine dose-dependently attenuates ropivacaine-induced seizures and negative emotions via inhibiting phosphorylation of amygdala extracellular signal-regulated kinase in mice. Mol Neurobiol. 2016;53:2636–2646. doi: 10.1007/s12035-015-9276-1. [DOI] [PubMed] [Google Scholar]
- 11.Mete M, Aydemir I, Tuglu IM, Selcuki M. Neurotoxic effects of local anesthetics on the mouse neuroblastoma NB2a cell line. Biotech Histochem. 2015;90:216–222. doi: 10.3109/10520295.2014.979439. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Xu S, Liu H, Zhang Q, Liang H, Yang C, Wang H. Neurotoxicity induced by bupivacaine via T-type calcium channels in SH-SY5Y cells. PLoS One. 2013;8:e62942. doi: 10.1371/journal.pone.0062942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Wen X, Lai X, Li X, Zhang T, Liang H. The effects of ropivacaine hydrochloride on the expression of CaMK II mRNA in the dorsal root ganglion neurons. Biomed Pharmacother. 2016;84:2014–2019. doi: 10.1016/j.biopha.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Wen XJ, Li XH, Li H, Liang H, Yang CX, Wang HB. CaMK II gamma down regulation protects dorsal root ganglion neurons from ropivacaine hydrochloride neurotoxicity. Sci Rep. 2017;7:5262. doi: 10.1038/s41598-017-05678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J, Xu SY, Zhang QG, Xu R, Lei HY. Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol. 2011;657:51–58. doi: 10.1016/j.ejphar.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003;14:53–66. doi: 10.1016/s1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 17.Moon YE, Choi JH, Park HJ, Park JH, Kim JH. Ultrasound-guided nerve block with botulinum toxin type a for intractable neuropathic pain. Toxins (Basel) 2016;8 doi: 10.3390/toxins8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Y, Wang Q, Zhao B, She Y, Bi X. GNB2 is a mediator of lidocaine-induced apoptosis in rat pheochromocytoma PC12 cells. Neurotoxicology. 2016;54:53–64. doi: 10.1016/j.neuro.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, She Y, Bi X, Zhao B, Ruan X, Tan Y. Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity through downregulation of COL3A1 mediated by miR-let-7b. DNA Cell Biol. 2017;36:518–528. doi: 10.1089/dna.2016.3623. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Ji J, Lin Y, He Y, Liu J. The ganglioside GM-1 inhibits bupivacaine-induced neurotoxicity in mouse neuroblastoma neuro2a cells. Cell Biochem Funct. 2016;34:455–462. doi: 10.1002/cbf.3208. [DOI] [PubMed] [Google Scholar]
- 21.Zheng T, Zheng CY, Zheng XC, Zhao RG, Chen YQ. Effect of parthanatos on ropivacaine-induced damage in SH-SY5Y cells. Clin Exp Pharmacol Physiol. 2017;44:586–594. doi: 10.1111/1440-1681.12730. [DOI] [PubMed] [Google Scholar]
- 22.Gong Q, Wen X, Li H, He J, Wang Y, Wu H, Wang H, Wang X. Up-regulation of Cav3.1 expression in SH-SY5Y cells induced by lidocaine hydrochloride. Artif Cells Nanomed Biotechnol. 2018;12:1–8. doi: 10.1080/21691401.2018.1425697. [DOI] [PubMed] [Google Scholar]
- 23.Wen X, Liang H, Li H, Ou W, Wang HB, Liu H, Li S. In vitro neurotoxicity by ropivacaine is reduced by silencing Cav3.3 T-type calcium subunits in neonatal rat sensory neurons. Artif Cells Nanomed Biotechnol. 2018;46:1617–1624. doi: 10.1080/21691401.2017.1384386. [DOI] [PubMed] [Google Scholar]
- 24.Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, Gerner P. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg. 2008;106:1456–1464. doi: 10.1213/ane.0b013e318168514b. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Tan Y, Zhang N, Xu Y, Wei W, She Y, Bi X, Zhao B, Ruan X. Dexmedetomidine inhibits activation of the MAPK pathway and protects PC12 and NG108-15 cells from lidocaine-induced cytotoxicity at its maximum safe dose. Biomed Pharmacother. 2017;91:162–166. doi: 10.1016/j.biopha.2017.04.084. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Liu Z, Yu X, Lai L, Li H, Liu Z, Li L, Jiang S, Xia Z, Xu SY. iTRAQ proteomics analysis reveals that PI3K is highly associated with bupivacaine-induced neurotoxicity pathways. Proteomics. 2016;16:564–575. doi: 10.1002/pmic.201500202. [DOI] [PubMed] [Google Scholar]
- 27.Fan YL, Li HC, Zhao W, Peng HH, Huang F, Jiang WH, Xu SY. Curcumin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via activation of the Akt signaling pathway. Neurochem Res. 2016;41:2425–2432. doi: 10.1007/s11064-016-1955-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Kondo F, Gosho M, Feng GG, Harato M, Xia ZY, Ishikawa N, Fujiwara Y, Okada S. Enhanced expression of WD repeat-containing protein 35 via CaMKK/AMPK activation in bupivacaine-treated Neuro2a cells. PLoS One. 2014;9:e98185. doi: 10.1371/journal.pone.0098185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Xu SY, Zhang QG, Lei HY. Bupivacaine induces reactive oxygen species production via activation of the AMP-activated protein kinase-dependent pathway. Pharmacology. 2011;87:121–129. doi: 10.1159/000323402. [DOI] [PubMed] [Google Scholar]
- 30.Niu X, Chen J, Wang P, Zhou H, Li S, Zhang M. The effects of hispidulin on bupivacaine-induced neurotoxicity: role of AMPK signaling pathway. Cell Biochem Biophys. 2014;70:241–249. doi: 10.1007/s12013-014-9888-5. [DOI] [PubMed] [Google Scholar]
- 31.Xiong J, Kong Q, Dai L, Ma H, Cao X, Liu L, Ding Z. Autophagy activated by tuberin/mTOR/p70S6K suppression is a protective mechanism against local anaesthetics neurotoxicity. J Cell Mol Med. 2017;21:579–587. doi: 10.1111/jcmm.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KC, Chang LS. Notexin upregulates Fas and FasL protein expression of human neuroblastoma SK-N-SH cells through p38 MAPK/ATF-2 and JNK/c-Jun pathways. Toxicon. 2010;55:754–761. doi: 10.1016/j.toxicon.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Chen KC, Kao PH, Lin SR, Chang LS. Upregulation of Fas and FasL in Taiwan cobra phospholipase A2-treated human neuroblastoma SK-N-SH cells through ROS- and Ca2+-mediated p38 MAPK activation. J Cell Biochem. 2009;106:93–102. doi: 10.1002/jcb.21979. [DOI] [PubMed] [Google Scholar]



