Abstract
Exosomes are extracellular vesicles that originate in the endosomal system. They perform important functions for cell-to-cell communication by transferring bioactive cargoes to recipient cells or activating signal transduction pathways in the target cells. Hypoxia is a severe cellular stress that can regulate the release of exosomes and change their contents. Exosomes have been investigated in different types of hypoxic diseases and found to have many effects from pathology to protection. Increasingly, studies have indicated that exosomes can reflect their cellular origin and disease state through the bioactive cargoes they carry, making exosomes useful as potential biomarkers for diagnosing or predicting hypoxic diseases. In this review, we summarize the effects and mechanisms of hypoxia on exosomes and introduce the basics of exosome production, release, and uptake. In addition, we also summarize current information on the involvement, diagnostic value, and therapeutic potential of exosomes in different types of hypoxic diseases, including myocardial infarction (MI), renal ischemia-reperfusion (IR) induced acute kidney injury (AKI) and hypoxic tumors.
Keywords: Exosomes, hypoxic diseases, hypoxia-inducible factor (HIF), MI, renal IR, hypoxic tumors
Introduction
Hypoxia is a physiological and pathological stress encountered during a variety of conditions, such as cancer, myocardial infarction (MI), and renal ischemic injury. With increasing research regarding the mechanism of hypoxia injury and its adaptation mechanism, it has been shown that hypoxia as a potential environmental death factor for cells by affecting the cell cycle, morphological structure, metabolism, proliferation, differentiation, autophagy, apoptosis and other aspects [1-3]. Recently, as an important means of intercellular communication, exosomes have been reported to be widely involved in the mechanism of hypoxic injury and its adaptation mechanism.
Exosomes are 30-100 nanometer-sized vesicles that are released by most types of cells and were initially considered to be carriers responsible for the removal of cellular debris [4,5]. However, after exosomes were discovered to stimulate immune responses, their role in cell-to-cell communication was valued. Exosomes can transfer different kinds of cargoes, including microRNAs (miRNAs), DNA, lipids, and proteins to recipient cells, regulating their basic functions as well as gene expression. Exosomes have been found in body fluids, such as serum, plasma, breast milk, saliva, urine, amniotic fluid, and cerebrospinal fluid [6-11]. They reach recipient cells by passing through these bodily fluids, fulfilling their functions as signal vehicles in physiology and pathology. Exosome cargo molecules vary considerably because of the difference in the donor cell’s type and health state, resulting in extremely different functional outcomes in target cells.
In recent years, increased levels of exosomes have been detected in different hypoxic experimental models and patients with hypoxia-related diseases. Furthermore, exosomes have been shown to extensively participate in the pathophysiological processes of hypoxic diseases by providing an effective pathway for hypoxia-specific information during adaption to cellular stress. However, the specific roles and mechanisms of exosomes in different types of hypoxic diseases are largely unclear, and the mechanisms the underlying exosome biogenesis and secretion remain elusive. In this review, we first investigate the effects and mechanisms of hypoxia on exosomes, and then introduce the basics of exosomes production, release, and uptake. Finally, we summarize current information on the involvement, diagnostic value, and therapeutic potential of exosomes in different types of hypoxic diseases.
Effects of hypoxia on exosomes
Hypoxia can stimulate the release of exosomes and change their contents
The difference between hypoxic exosomes and normoxic exosomes has been noted in a large number of studies indicating the association between hypoxia and exosomes. Interestingly, more and more studies have shown that hypoxia can stimulate cells to release more exosomes. For example, a study compared exosomes released from three kinds of breast cancer cell lines under normoxic, moderate (1% O2) hypoxic, and severe (0.1% O2) hypoxic conditions [12]. The results of this study showed that three kinds of breast cancer cell lines with moderate (1% O2) hypoxic and severe (0.1% O2) hypoxic conditions showed more significant exosomal release as determined by CD63 immunoblotting and nanoparticle tracking analysis (NTA) [12]. Aligned with these results, pulmonary artery endothelial cells (PAECs) [13], adipocyte [14], epithelial cells [15], and cardiac progenitor cells (CPCs) [16] were also found to release more exosomes under hypoxic conditions.
In addition to an increase in exosome release, the content of exosomes released by cells under hypoxic conditions also appears to undergo significant changes. A seminal study by Sano et al. [14] demonstrated that compared with that in normoxic exosomes, 75 proteins were upregulated, and 67 were downregulated in hypoxic adipocyte-derived exosomes. Interestingly, the authors showed that in these hypoxic exosomes, enzymes related to de novo lipogenesis, such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), and glucose-6-phosphate dehydrogenase (G6PD) were also selectively upregulated [14]. In addition to the analysis of exosomal proteins, a large number of studies also analyzed exosomal miRNAs. For example, Almendros et al. [17] explored miRNA contents in circulating exosomes isolated from an intermittent hypoxia (IH) group versus a group exposed to room air (RA), and 11 differentially expressed miRNAs were identified, which might be the key factors in their various effects on tumor cell functions. Similar results were reported by Khalyfa et al. [18], who showed that six differentially expressed miRNAs in IH versus RA circulating exosomes played important roles in the cardiovascular dysfunction associated with obstructive sleep apnea (OSA) by targeting specific effector pathways [18]. In summary, these findings provide evidence indicating that hypoxia not only increases the release of exosomes in various kind of cells but also can selectively change the cargoes in exosomes including selective proteins and miRNAs.
Relevant conditions under which hypoxia regulates the release of exosomes
In different hypoxic studies, it has been shown that the number and contents of exosomes released by different types of cells are significantly different, which might be related to the diversity of functions of exosome-secreting cells and the distinctions in the tolerance of different cells for hypoxic conditions. However, in some hypoxic studies, exosomes released by cells of the same type under different hypoxic conditions also showed significant differences. For example, the research by Tadokoro et al. [19] indicated that after exposure to hypoxia (1% O2) for 72 h, exosomes derived from K562 cells were different in nanoparticle size distribution from their normoxic controls. In contrast, after exposure to hypoxia (1% O2) for only 24 h, the exosomes were not significantly changed in size and concentration in comparison to their normoxic controls [19]. Their findings demonstrated that the effects of hypoxia on exosomes were related to the duration of hypoxia [19].
Moreover, a study by King et al. [12], indicated that breast cancer cells exposed to severe (0.1% O2) hypoxia released more exosomes than breast cells exposed to moderate (1% O2) hypoxia. Thus, their findings demonstrated that the effects of hypoxia on exosomes were related to the severity of hypoxia. Despite the differences in these study designs, these results appear to indicate that different cell types show various responses to hypoxia. In addition, the duration of hypoxic exposure, as well as the severity of hypoxia are of importance for the conditions under which hypoxia forms a component of the stimulus for exosome release.
Mechanisms of exosome release during hypoxia
General process and mechanism of exosome biogenesis and release
Exosome biogenesis begins within the endosome system [20]. Briefly, when plasma membrane receptors are marked for degradation or recycling by ubiquitination, endocytosis starts and primary endocytic vesicles are formed by internalization of the plasma membrane. Then, primary endocytic vesicles can fuse and form early endosomes (EEs). With the formation of internal vesicles in endosomes, EEs mature into late endosomes (LEs) or multivesicular bodies (MVBs). The internal vesicles are usually termed intraluminal vesicles (ILVs), which are formed by the inward sprouting of the limiting membrane of LEs or MVBs. Thus, their membrane orientation is the same as the extracellular facing side of the cellular plasma membrane [21]. MVBs can have different fates. They can fuse with lysosomes for degradation. Alternatively, they can fuse with the plasma membrane, and then release their ILVs as exosomes into the extracellular environment. The general process of exosomes biogenesis and release is shown schematically in Figure 1.
Figure 1.
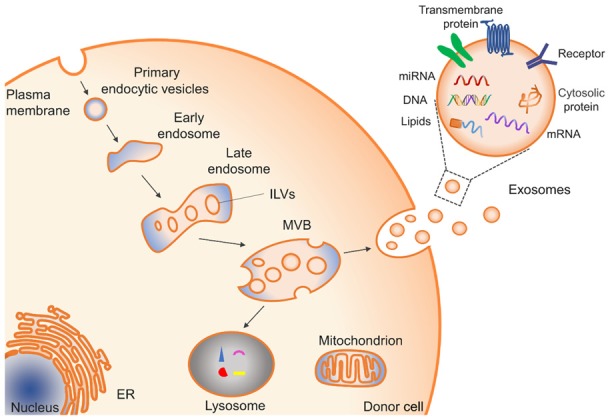
The process of exosome biogenesis and release. Exosomes are generated by inward budding from the endosomal membrane, which results in the generation of late endosomes and multivesicular bodies (MVBs). MVBs can have different fates. They can fuse with lysosomes for degradation; alternatively, MVBs can fuse with the plasma membrane leading to the release of exosomes. Exosomes have a complex composition, such as proteins, nucleic acids, lipids, and other metabolites.
Exosome secretion through exocytosis requires a two-step process involving the transportation and the fusion of MVBs with the plasma membrane. Transportation of MVBs to the plasma membrane depends on their interaction with actin and the microtubule cytoskeleton. Remarkably, several Rab guanosine triphosphatases (GTPases) have been proposed to play a role in regulating the transportation of MVBs to the site of the plasma membrane, although the exact mechanism for their role is not yet clear [22,23]. For example, in HeLa cells, knockdown of Rab27a leads to an increase in the size of MVBs, whereas the silence of Rab27a leads to the redistribution of MVBs to the perinuclear region rather than the plasma membrane [24]. Thereafter, once MVBs dock with the plasma membrane, the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and vesicle-associated membrane protein (VAMP) can directly or indirectly participate in docking and fusion of MVBs with the plasma membrane, and subsequently, their release in the extracellular environment [25]. Furthermore, exosome secretion can also be affected by factors such as p53 protein (activation of p53 can stimulate the release of exosomes), intracellular Ca2+ levels (increased Ca2+ levels result in increased exosome secretion) and extracellular and intracellular pH gradients [26-28].
Exosomes generated by a given cell type can have distinct composition and function, which is often attributed to the presence of multiple sorting machineries [29]. These machineries first separate some cargoes onto the microdomains of the limiting membranes of MVBs, which then inwardly bud and fission small membrane vesicles containing segregated cytosol. The endosomal sorting complex required for transport (ESCRT) machinery is the most important machinery consisting of ESCRT-0, -I, -II, -III and the associated AAA ATPase Vps4 complex [30]. The subunits of the ESCRT-0 complex are responsible for recruiting proteins for internalization [31]. ESCRT-I and ESCRT-II determine the initiation of the budding process and promote the de-ubiquitination of enzymes of the cargo proteins before the formation of ILVs. The ESCRT-III complex is responsible for driving the final stage of membrane invagination and segmentation [32]. Accessory proteins include ALG-2 interacting protein X (ALIX) participating in vesicle budding and VPS4 assisting in division [21,33].
Moreover, exosomes can also be formed in an ESCRT-independent manner [34]. It has been shown that the first ESCRT-independent mechanism of exosomes formation requires the production of ceramide via neutral type II sphingomyelinase, and the effect of ceramide on ILVs biogenesis is mediated by the local generation of its downstream metabolite sphingosine-1-phosphate (S1P) [35]. Subsequently, proteins of the tetraspanin family, such as CD63 [36], CD9 [37] and CD82 [37], have also been proposed to participate in the sorting of various cargoes into exosomes. Therefore, both ESCRT-dependent and ESCRT-independent mechanisms play a role in exosome biogenesis, and their effects can vary depending on the cargoes and the cell types.
HIF-α regulates the release of exosomes under hypoxia
We have shown that exosome secretion is increased under hypoxia. Interestingly, recent research showed that this increase appears to be HIF-1α-dependent. For example, a study by Yu et al. [38], indicated that the induction of cellular HIF-1α expression in cardiomyocytes contributed to the release of exosomes. Moreover, a study by King et al. [12], also showed that increased exosome release in breast cancer cells during hypoxia is HIF-1α-dependent as determined by reduced release upon HIF-1α knockdown prior to hypoxic treatment. Similarly, in rat renal proximal tubular cells (RPTC), HIF-1 induction by dimethyloxalylglycine (DMOG), a HIF hydroxylase inhibitor, significantly enhanced the release of exosomes, while the suppression of HIF-1 prevented the increased release of exosomes by hypoxia [39]. Therefore, the three studies discussed above fully demonstrated that HIF-1α plays a key role in the increased release of exosomes during hypoxia.
HIF is a family of transcription factors that are responsive to hypoxia and primarily responsible for cell adaptation [40]. HIF is a heterodimeric transcription factor consisting of one of three different oxygen-sensitive HIF alpha subunits (HIF-1α, HIF-2α, and HIF-3α) and a common constitutive HIF beta subunit [41]. Under normoxic conditions, HIF-α is hydroxylated at the specific proline residues by prolyl hydroxylases, leading to its association with the E3 ligase VHL and consequent degradation of proteasomes. Under hypoxic conditions, proline hydroxylation in HIF-α is blocked, resulting in HIF-α accumulation and dimerization with HIF-β to form HIF, which is then translocated to the nucleus. HIF binds to target genes at the hypoxia response element (HRE) and recruits transcriptional coactivators. There are over 70 known HIF target genes, including many plasma membrane receptors such as the glucose transporter (GLUT-1), the transferrin receptor, and the Epidermal Growth Factor Receptor (EGFR). A study by Milane et al. [42] showed that hypoxia could increase the expression and nuclear translocation of HIF-1α and HIF-2α in cancer cell lines, leading to a subsequent increase in GLUT-1 and EGFR protein levels. Receptor expression changes (decreases or increases) can increase plasma membrane remodeling, and increased receptor expression can also directly increase receptor activation and internalization which consequently induces endocytosis [43]. Eventually, these changes can promote the release of exosomes.
Additionally, Wang et al. [44] reported that HIF increased the activity of Rab22a, a member of the Rab family, which was colocalized with budding microvesicles on the cell surface and was essential for vesicles formation, transport, and membrane fusion. Therefore, HIF may regulate vesicle formation, transport, and membrane fusion by increasing the activity of Rab22a during hypoxia [44]. Similarly, it was reported that HIF mediated the induction of Rab20 [45] and Rab27a [46], which may also be related to the formation and secretion of exosomes.
Exosome uptake and the role of exosomes in hypoxic diseases
Exosomes play a vital role in the process of intercellular communication, and released exosomes can target cells close to their donor cells as well as cells distant from their release sites. By providing an effective pathway for hypoxia-specific information during adaption to cell stress, exosomes have been widely implicated in the pathophysiological processes of hypoxia-related diseases, such as MI, renal ischemia-reperfusion (IR) induced acute kidney injury (AKI) and hypoxic tumors.
General process and mechanism of exosome uptake
Exosome uptake by recipient cells is cell-specific. Numerous studies have shown that target cell specificity can be determined by the interaction between proteins enriched on the surface of exosomes and receptors on the plasma membrane of the recipient cells. Several of these interacting mediators are known, including integrins, tetraspanins, phosphatidylserine (PS), and heparin sulfate proteoglycans (HSPGs). For example, exosomal integrins could interact with adhesion molecules such as intercellular adhesion molecules (ICAMs) at the surface of recipient cells [47]. Remarkably, a study indicated that the different integrin expression patterns of tumor-derived exosomes were vital causes of organotropism metastasis [48]. Tetraspanins on exosomes could also regulate cell-specific targeting. They were shown to interact with integrins and promote exosome docking as well as uptake by target cells [49,50]. Moreover, PS could recruit specific lipid-binding proteins, such as galectin 5, which could then induce docking of exosomes to the plasma membrane of the recipient cell [51]. HSPGs presented on the membranes of exosomes and contributed to the docking or attachment of exosomes to target cells [52].
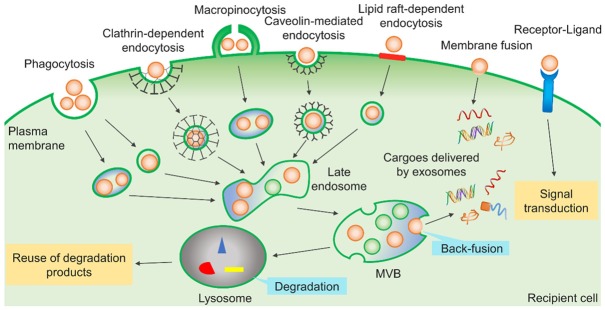
Once exosomes bind to recipient cells, they are likely to be taken up by recipient cells through the three potential pathways (Figure 2): First, numerous studies have shown that internalization is the main method of exosome uptake, and distinct internalization mechanisms have been described in different cell types [53]. For example, clathrin-dependent endocytosis or phagocytosis in neurons [54], macropinocytosis by macrophages [55], and microglia [56], receptor-mediated endocytosis by dendritic cells [57], caveolin-mediated endocytosis in epithelial cells [58], and cholesterol- and lipid raft-dependent endocytosis in endothelial and some tumor cells [59]. After internalization, exosomes follow the endocytic pathway and reach MVBs, which target lysosomes in most cases, leading to exosomal degradation and contents recycling to promote the metabolism of the recipient cells [60]. In some cases, the internalized exosomes can undergo back-fusion with the limiting membrane of the MVBs and release their cargoes into the cytoplasm of the recipient cells [56]. Secondly, the exosome membrane can also fuse with the plasma membrane, releasing their cargoes directly into the cytoplasm of the recipient cells [28]. Besides, cargoes delivered by exosomes, including proteins, miRNA [61], mRNA [61], lipids and so on, can activate various reactions and processes in recipient cells after internalization. Thirdly, exosome membrane surfaces can initiate intracellular signaling pathways by interacting with receptors and ligands on the recipient cell surface without exosomes entry as, for example, exosomes derived from dendritic cells and B cells that were able to present antigens to T cells and induce a specific antigenic response [62,63].
Figure 2.
Three pathways of exosome uptake by recipient cells. Exosomes can be internalized in a variety of ways, including phagocytosis, clathrin-dependent endocytosis, macropinocytosis, caveolin-mediated endocytosis, and lipid raft-dependent endocytosis. After internalization, exosomes follow the endocytic pathway and reach MVBs, which target lysosomes in most cases, leading to exosomal degradation and contents recycling to promote the metabolism of the recipient cells. Alternatively, internalized exosomes can undergo back-fusion with the limiting membrane of the MVBs and release their cargoes into the cytoplasm of the recipient cells. Secondly, the exosome membrane can also fuse with the plasma membrane, releasing its cargoes directly into the cytoplasm of the recipient cell. Thirdly, the exosome membrane surface can initiate intracellular signaling pathways by interacting with receptors/ligands on the recipient cell surface without exosome entry.
To conclude, recipient cells of different cell types can take up exosomes through various mechanisms leading to their functional responses and promoting phenotypic changes that affect their physiological or pathological status. Moreover, the diverse mechanisms of exosome uptake may be due to both differences in exosome composition and the specific structure of the plasma membrane of the recipient cells. As hypoxia can stimulate the release of exosomes and change the composition of their cargoes, the effect of exosomes on recipient cells also changes under hypoxia. Next, we will discuss the specific role and mechanism of exosomes in three specific types of hypoxia-related diseases below.
Exosomes in MI
MI mostly occurs as a result of coronary atherosclerotic stenosis, primarily caused by plaque rupture or thrombus formation and subsequent coronary artery occlusion, leading to myocardial cell apoptosis and myocardial necrosis. Interruption of the coronary blood supply and the resultant ischemia/hypoxia prevent the process of oxidative phosphorylation for ATP production. In contrast, anaerobic glycolysis becomes the main mechanism for ATP production which leads to the production of lactic acid and a drop in intracellular pH [64]. Notably, cardiomyocytes originally thought to be a non-secretory cell type have now been proven to secrete exosomes [65-67], and the number and content of exosomes derived from the border zone of MI and normal myocardium are different. Reports have suggested that exosomes are important in the mechanism involved in heterologous cellular communication in adult hearts, and play a vital role in ischemic signal transduction [68-70]. For example, recent research demonstrated cross-talk between cardiac fibroblasts and cardiomyocytes [71]. Cardiac fibroblasts were shown to secrete exosomes enriched with miR-423-3p as a crucial paracrine signaling mediator under myocardial IR. miR-423-3p was shuttled to cardiomyocytes affecting its downstream effector RAP2C and mediated cardioprotective effects. These findings illustrate that exosome-mediated communication can exert beneficial effects during myocardial IR.
However, exosomes can also exert detrimental effects. For example, Yu et al. [38] indicated that cardiomyocytes could secrete exosomes enriched with a high level of tumor necrosis factor-α (TNF-α) under hypoxia. Excessive TNF-α expression is thought to be detrimental to coronary endothelial function by elevating the expression of arginase in endothelium [72]. Moreover, heat shock protein (HSP) 60 has been shown to be released from cardiomyocytes via exosomes [73], which could promote cardiomyocyte apoptosis and inflammation via activation of Toll-like receptor (TLR) 4 in ischemic myocardium [74-76]. These studies implicate a significant role for exosome-mediated communication in MI through a variety of mechanisms. More comprehensive information about specific effects and mechanisms of exosomes in MI is provided in Table 1.
Table 1.
Summary of the effects and mechanisms of different cell-derived exosomes in MI or MIRI
| Source of exosomes | Effects | Related cargoes | Reference |
|---|---|---|---|
| Cardiomyocytes | Endothelial dysfunction↑ | TNF-α | [38,72] |
| Cardiomyocyte apoptosis↑ | HSP60 | [73-75] | |
| Inflammation↑ | HSP60 | [76] | |
| Autophagy↓ | miR-30a | [135] | |
| Cardiac fibroblast | Cardiomyocyte hypertrophy↑ | miR-21* | [136] |
| Cardioprotection↑ | miR-423-3p | [71] | |
| Circulating plasma | Cardioprotection↑ | HSP70 | [137] |
| CPC | Cardiomyocyte apoptosis↓ | miR-210 | [90] |
| Angiogenesis↑ | miR-132 | [90] | |
| MSCs | Cardiomyocyte apoptosis↓ | Unknown | [94] |
| Angiogenesis↑ | Unknown | [138] | |
| Oxidative stress↓ | Unknown | [94] | |
| ESCs | CPC survival↑ | miR-294 | [97] |
| CPC proliferation↑ | miR-294 | [97] | |
| Cell cycle progression of CPC↑ | miR-294 | [97] | |
| CDCs | Cardiomyocyte apoptosis↓ | miR-146a | [139] |
| Cardiomyocyte proliferation↑ | miR-146a | [139] | |
| Angiogenesis↑ | miR-146a | [139] |
Note: MIRI: myocardial ischemia reperfusion injury; CDCs: Cardiosphere-derived cells. miR-21*: miR-21-3p; ↑: up-regulation; ↓: down-regulation.
In MI patients, cardiac and circulating muscle-specific miRNAs such as miR-1, miR-133a, miR-208, and miR-499 are dramatically increased, indicating myocardial damage [66,77]. Studies of acute myocardial infarction (AMI) have indicated that these specific miRNAs not only can be detected in the blood faster and earlier than cardiac troponin T [78,79], but also have better sensitivity and specificity than troponin T [80,81]. Moreover, recent research indicated that plasma miRNAs are temporally regulated and associated with left ventricular remodeling post-MI [82]. Collectively, the above evidence indicates that cardiac and circulating muscle-specific miRNAs are potential biomarkers for early diagnosis and assessment prognosis of AMI. Furthermore, exosomes derived from damaged cardiomyocytes have been shown to be enriched with these cardiac-specific miRNAs [66,83]. Other studies have reported a significant increase of exosome-derived miR-208 and miR-1 levels not only in the circulating blood of AMI rats but also in the urine of AMI patients [77]. Furthermore, the increase in serum exosome-derived miR-208a level is associated with a higher Killip class, higher cardiac troponin T peaks, higher creatine kinase isoenzyme (CK-MB) peaks, and reduced 1-year survival rate [84]. Thus, exosomes may serve as a valuable source of cardiac-specific miRNAs for early diagnosis and assessment of prognosis in MI.
Timely myocardial reperfusion using thrombolytic therapy, percutaneous coronary intervention (PCI), and coronary artery bypass grafting are the most effective strategies to reduce myocardial infarct size and improve clinical outcomes in treating patients with AMI. However, the process of reperfusion itself can induce further cardiomyocyte injury by activating inflammatory responses, elevating oxygen radical levels and damaging microvasculature. This ‘second hit’ phenomenon is referred to as ischemia-reperfusion injury (IRI) [64,85]. Recently, the addition of various forms of stem cell-based therapy has demonstrated cardioprotection and cardiovascular regeneration during MI and IRI [86,87]. Interestingly, stem cell-derived exosomes can replicate stem cell-induced therapeutic effects and blockade of exosome secretion alleviated the protective effects of stem cells on the infarcted mouse heart [88,89]. These data led to the hypothesis that transplanted stem cells may act in a paracrine manner through the release of exosomes. As a cell-free approach, exosomes can circumvent many of the limitations of cell transplantation. In addition, the therapeutic effect in MI varied among exosomes from different cell sources. Studies performed in experimental MI models demonstrated that exosomes secreted by human cardiac progenitor cells (CPCs) could inhibit cardiomyocyte apoptosis, stimulate cardiac angiogenesis and promote the recovery of cardiac function after MI [90]. Further study showed that the beneficial effects of these exosomes were due to the miRNAs they carry, such as miR-210 and miR-132 [90]. miR-210 down-regulated its known targets, ephrin A3 and protein tyrosine phosphatase-1b (PTP1b), inhibiting apoptosis in cardiomyocytes [90], whereas miR-132 down-regulated its target, RasGTPase activating protein (RasGAP)-p120, enhances tube formation in endothelial cells [90]. Moreover, the findings of another study confirmed that intramyocardial delivery of CPCs-derived exosomes could inhibit cardiomyocyte apoptosis in a murine model of acute myocardial IRI [91]. Of note, compared with exosomes from normoxic CPCs, injection of the myocardium with exosomes secreted by CPCs grown in hypoxic conditions produced increased angiogenic and antifibrotic potential in myocardial IRI models of rats, which indicated that hypoxia could modulate the therapeutic potential of exosomes [92].
In addition to CPC-derived exosomes, the therapeutic effects of exosomes derived from mesenchymal stem cells (MSCs) and embryonic stem cells (ESCs) in cardiac repair have recently been studied. Several experimental studies have demonstrated that intramyocardial injection of MSC-derived exosomes could reduce infarct size and improve contractile performance in a rat AMI model [92,93]. These beneficial effects were reported to be associated with increased reductive potential [94], reduced oxidative stress [94], reduced autophagic flux [95] and increased angiogenesis [96]. Moreover, studies with intramuscular injection of ESC-derived exosomes showed improved function in MI mouse heart, associated with increased angiogenesis, enhanced cardiomyocyte survival, and reduced cardiac fibrosis [97]. miRNA arrays revealed a significant enrichment of miR-294 in ESC-derived exosomes, suggesting that the beneficial effect of mouse ESC-derived exosomes may be due to the delivery of miR-294 to cardiac cells [97]. Taken together, these findings suggest that multifunctional processes involving metabolic alterations, antifibrotic mechanisms, angiogenesis-promoting activities, and anti-cardiomyocyte apoptosis mechanisms may all contribute to the protective role of stem progenitor cells derived exosomes in MI.
Exosomes in renal IR induced AKI
AKI is a clinical syndrome characterized by a rapid loss of renal excretory function [98]. It is usually diagnosed by the accumulation of nitrogen metabolism (urea and creatinine) end-products, reduced urine volume, or both [98]. The kidney is highly sensitive to blood flow shortages and hypoxia because of the high metabolism and vascular anatomy of the kidney. In the kidney, IR contributes to the pathological state of AKI and is a common cause of AKI [99]. The pathophysiology of IRI in the kidney is very complex, and associated with tubular cell necrosis, endothelial cell dysfunction, and inflammation. In response to renal IR, exosomes and their contents released by various cells in the kidney also change. Studies have shown that due to the mechanical and charge barriers of the glomerulus, circulating exosomes in the serum cannot pass through the nephron, at least under physiological conditions, indicating that urinary exosomes are primarily derived from renal cells [9,100]. Therefore, exosomal proteins might become biomarkers for AKI. In this regard, Aquaporin-1 (AQP1) and fetuin-A proteins in urine exosomes have been identified as potential biomarkers for AKI. The level of AQP1 proteins in urine exosomes was significantly decreased during IR in rat kidneys [101]. Remarkably, similar reductions were observed postoperatively in renal transplant recipients, indicating that AQP1 proteins in urinary exosomes might be a biomarker for renal IR induced AKI.
Moreover, in both animal models and human patients of AKI, fetuin-A was markedly increased in urinary exosomes, and its elevation occurred before serum creatinine elevation [102]. Additionally, in mouse models of IR-induced AKI, a study detected increased activating transcription factor 3 (ATF3) in urine exosomes, and this increase also preceded serum creatinine, suggesting that ATF3 might be a biomarker for early diagnosis of AKI [103]. To support this possibility, one clinical study indicated that urine exosomes containing ATF3 mRNA were 60 times higher in AKI patients than in normal controls [104]. Therefore, urinary exosomes may be a potential source of sensitive biomarkers for early detection of renal IR-induced AKI.
In the clinic, current treatment of AKI is still limited to supportive care and dialysis. It is necessary to develop new treatment strategies that effectively reduce renal cell death. Regarding this issue, emerging evidence has suggested that exosomes derived from differentiable cells such as MSCs and ECFCs can attenuate renal injury and promote kidney repair in animal models of IR-induced AKI. These observations indicate that exosomes are promising therapeutic remedies for IR-induced AKI. In addition, exosomes released by different types of differentiable cells produce different biological effects in IR-induced AKI (Table 2), which is related to the special biologically active substances carried by exosomes. For example, studies performed in experimental ischemic AKI mice models demonstrated that administration of exosomes from human ECFCs at reperfusion significantly attenuated increases in plasma creatinine, renal tubular necrosis, apoptosis, and oxidative stress in kidney tissues [105,106]. In cell experiments, studies indicated ECFCs derived exosomes inhibited apoptosis in cultured endothelial cells exposed to hypoxia/reoxygenation (HR) [106].
Table 2.
Summary of the effects and mechanisms of different cell-derived exosomes in AKI or renal IRI
| Source of exosomes | Effects | Related cargoes | Reference |
|---|---|---|---|
| RTC | Renal function↑ | Unknown | [140] |
| Renal tubular damage↓ | Unknown | [140] | |
| Neutrophil infiltration↓ | Unknown | [140] | |
| Renal fibrosis↓ | Unknown | [140] | |
| MSC | Inflammatory reaction↓ | CCR2 | [109] |
| Tubular regeneration↑ | Unknown | [107] | |
| Renal fibrosis↓ | Unknown | [107] | |
| Apoptosis↓ | Unknown | [141] | |
| ECFCs | Endothelial cell apoptosis↓ | miR-486-5p | [105] |
| Proinflammatory responses↓ | Unknown | [106] | |
| Endothelial proliferation↑ | Unknown | [106] | |
| RAPC | Endothelial migration↑ | miR-218 | [142] |
Note: renal IRI: renal ischaemia-reperfusion injury; RTC: Renal Tubular Cell; ECFCs: Endothelial Colony-Forming Cells; RAPC: renal artery derived vascular progenitor cells; CCR2: C-C motif chemokine receptor 2. ↑: up-regulation; ↓: down-regulation.
Further research showed that ECFCs exosomes were enriched with miR-486-5p, which could directly target endothelial cell PTEN leading to enhanced Akt phosphorylation and inhibited apoptosis [105]. Similarly, studies demonstrated the ability of exosomes derived from adipose-derived mesenchymal stem cells (ADMSC) to improve AKI induced by acute IRI in rats [107,108]. Furthermore, these exosomes could promote tubular regeneration and mitigate renal fibrosis through tubular epithelial cell-dependent activation of Sox9 [107]. In addition, inhibition of inflammatory reactions is considered to be pivotal to protecting the kidney from IRI. A study found that CCR2 was enriched in exosomes of mouse bone marrow MSCs [109]. In a mouse model of IR-induced renal injury, these CCR2-positive exosomes could strongly bind and reduce the concentration of extracellular CCL2, thereby inhibiting the recruitment and activation of peripheral monocytes or macrophages and reduce inflammation in the kidney [109]. Together, these findings suggest that versatile processes involving inhibition of apoptosis, reduction of inflammatory reactions, promotion of endothelial proliferation, and antifibrotic mechanisms may all contribute to the protective effects of exosomes in IR-induced AKI.
Exosomes in hypoxic tumors
In the process of tumor formation, the vasculature often fails to meet the oxygen demand of rapidly proliferating tumor cells, resulting in the presence of hypoxic regions in the tumor. In these regions, hypoxic tumor cells remodel their local and distant tumor microenvironment for tumor growth and metastatic dissemination, which usually cause more aggressive cancer phenotypes and worse prognoses [110,111]. Under hypoxic conditions, tumor cell-derived exosomes, as one of the key mechanisms of interaction between the tumor microenvironment and cancer cells, are actively released into the tumor microenvironment with pleiotropic roles in tumor growth and metastasis [112-115]. For example, a study showed that exosomes isolated from hypoxic glioblastoma multiforme (GBM) cells promoted in vitro and in vivo angiogenesis and tumor development more efficiently than their normoxic counterparts, thereby emphasizing exosomes as mediators of communication with an important role in the hypoxic tumor microenvironment [116].
In recent years, the effects of hypoxia on the content and function of exosomes have been studied in different tumor models. Research has shown that the hypoxic tumor microenvironment affects the content of tumor-derived exosomes resulting in the loading of unique cargoes that reflect the hypoxic state of tumor cells. For example, studies have found that higher levels of lactic acid are loaded in exosomes secreted by tumor cells under hypoxia, indicating that tumor cells secrete more exosomes as a survival mechanism to remove metabolic waste [117]. Moreover, numerous studies have shown that hypoxic features carried by tumor-derived exosomes can enhance the invasiveness and the stemness of adjacent tumor cells, thereby promoting tumor aggressiveness and metastasis [118-120]. Interestingly, RNAs are reported to be the predominant molecular cargoes of tumor cell-derived exosomes and many species of noncoding RNAs including miRNAs and long non-coding RNAs (lncRNAs) are significantly upregulated in exosomes secreted by hypoxic tumor cells [121-123]. Angiogenesis has a well-established role in tumor progression, and some of the noncoding RNAs can be transferred to cells in the tumor microenvironment by exosomes contribute to angiogenesis through different mechanisms. For example, a study by Jung et al. [124] indicated that miR-210 was significantly upregulated in exosomes secreted by hypoxic tumor cells. Thus, miR-210 could be transferred to adjacent cells by exosomes, which increased VEGF to promote angiogenesis by down-regulating the expression of vascular remodeling-related genes such as Ephrin A3 and PTP1b [124]. Similarly, miR-23a was significantly up-regulated in exosomes secreted by hypoxic lung cancer, which could directly inhibit its target PHD1 and PHD2, resulting in the accumulation of HIF-1α in endothelial cells and enhanced angiogenesis [125]. Notably, this study also showed that exosome miR-23a could inhibit tight junction protein ZO-1, thereby increasing vascular permeability and cancer trans-endothelial migration [125].
Moreover, it was reported that HIF-1α was detected in the exosomes of nasopharyngeal tumor cells. The active form of HIF-1α could be transferred to recipient cells by exosomes and could regulate pro-metastatic effects by changing the expression of E-cadherins and N-cadherins associated with EMT [126]. Furthermore, studies have shown that hypoxic tumor cell-derived exosomes could arbitrate the generation of an immunosuppressive environment by blunting the response of immune effector cells and triggering the expansion of immune suppressor cells [121,127]. Collectively, these studies indicate that exosomes released by hypoxic tumor cells have an important and fundamental role in many steps leading to tumor progression (Table 3).
Table 3.
Summary of the effects and mechanisms of different cell-derived exosomes in hypoxic tumors
| Source of exosomes | Effects | Related cargoes | Reference |
|---|---|---|---|
| GBM | Angiogenesis↑ | Unknown | [116] |
| Hypoxic tumor cells | Angiogenesis↑ | miR-210 | [124] |
| HR-MM cells | Angiogenesis↑ | miR-135b | [143] |
| Hypoxic lung cancer cells | Angiogenesis↑ | miR-23a | [125] |
| Vascular permeability↑ | miR-23a | [125] | |
| Bladder tumor | Tumor progression↑ | lncRNAs | [123] |
| Hypoxic PCA cells | Invasiveness of PCA cells↑ | Unique proteins | [118] |
| Nasopharyngeal tumor cells | EMT↑ | HIF-1α | [126] |
| Pancreatic cancer | Metastasis↑ | miR-301a | [122] |
| Glioma | Immunosuppressive effects↑ | miR-10a; miR-21 | [127] |
Note: HR-MM cells: hypoxia-resistant multiple myeloma cells; FIH-1: hypoxia-inducible factor 1; PCA: prostate cancer; EMT: epithelial-mesenchymal transition. ↑: up-regulation; ↓: down-regulation.
Similar to circulating tumor cells, exosomes derived by tumor cells can reflect their cellular origin and disease state through proteins, lipids, and nucleic acids they carry [128]. Therefore, the isolation of tumor-derived exosomes in body fluids and analysis of the specific biomarkers carried in these exosomes are expected to be a non-invasive method for diagnosis and monitoring of tumors. In this regard, detection of exosomes in the serum of glioblastoma [129], prostate cancer [130], nasopharyngeal carcinoma [131], colorectal cancer [132], and ovarian cancer patients [133] have emphasized the potential utility of exosomes and their contents as biomarkers for cancer. Moreover, Studies have shown that exosomes released by hypoxic cancer cells can be taken up more avidly by hypoxic cancer cells, making it possible for hypoxic exosomes to be used as ideal carriers to provide anticancer drugs and radiosensitizers for various hypoxic cancers [134].
Conclusions
In this review, we have summarized the distinctions between hypoxic exosomes and normoxic exosomes, the basics of exosomes and current information on the involvement, diagnostic value, and therapeutic potential of exosomes in different types of hypoxic diseases. Hypoxia can regulate the release of exosomes and change their contents, which is closely related to cell specificity, the duration of hypoxic exposure, and the severity of hypoxia. Hypoxia-stimulated exosome release appears to be HIF-α-dependent. However, HIF-α regulation of exosome function is a new area of study. The direct mechanisms of exosome induction by HIF-α and the influence of HIF-α in the regulation of exosome formation, content selection, transport, and release have yet to be determined. Moreover, by providing an effective pathway for hypoxia-specific information during adaptation to cellular stress, exosomes have been widely implicated in the pathogenesis and progression of hypoxia-related diseases such as MI, renal IR-induced AKI, and hypoxic tumors. A variety of cell-derived exosomes play a protective and therapeutic role in different kinds of hypoxic diseases through multifunctional processes involving the reduction of oxidative stress, antifibrotic mechanisms, promotion of angiogenesis, and inhibition of apoptosis. In addition, numerous studies also indicate that exosomes in body fluids of patients with hypoxic diseases can reflect their cellular origin and disease state through the specific proteins, lipids, and nucleic acids they carry, which may provide powerful tools for monitoring diagnosis, prognosis, and drug treatment. However, at present, our understanding of the contribution of exosomes to the pathophysiological processes and underlying mechanisms of hypoxic diseases, as well as the clinical application of exosomes in the diagnosis and treatment of hypoxic diseases remains limited. Therefore, efforts to further explore the role of exosomes in the pathophysiology, diagnosis, and treatment of hypoxic diseases are needed in the future. In conclusion, exosomes are a promising factor that may encompass a new strategy for the diagnosis and treatment of hypoxic diseases.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81670086), International S&T Cooperation Program of China (ISTCP, 2015DFA50310) and Chronic disease prevention and control of major projects of science and technology in Tianjin (NO. 17ZXMFSY00080).
Disclosure of conflict of interest
None.
References
- 1.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 2.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorlach A, Dimova EY, Petry A, Martinez-Ruiz A, Hernansanz-Agustin P, Rolo AP, Palmeira CM, Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poe AJ, Knowlton AA. Exosomes as agents of change in the cardiovascular system. J Mol Cell Cardiol. 2017;111:40–50. doi: 10.1016/j.yjmcc.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 6.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 7.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzi P, Rizzari S, Maugeri M, Scollo P, Tatone C, Valadi H, Purrello M, Di Pietro C. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102:1751–1761. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson O, Rodosthenous RS, Jara C, Brennan KJ, Wright RO, Baccarelli AA, Wright RJ. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: implications for early child development. Epigenetics. 2016;11:721–729. doi: 10.1080/15592294.2016.1216285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Luo H, Li X, Li T, He J, Qi Q, Liu Y, Yu Z. Exosomes derived from human pulmonary artery endothelial cells shift the balance between proliferation and apoptosis of smooth muscle cells. Cardiology. 2017;137:43–53. doi: 10.1159/000453544. [DOI] [PubMed] [Google Scholar]
- 14.Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, Iwao H, Yoshiyama M. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 15.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH, LeBleu VS, Kalluri R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME. Experimental, systems, and computational approaches to understanding the MicroRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res. 2017;120:701–712. doi: 10.1161/CIRCRESAHA.116.309935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almendros I, Khalyfa A, Trzepizur W, Gileles-Hillel A, Huang L, Akbarpour M, Andrade J, Farre R, Gozal D. Tumor cell malignant properties are enhanced by circulating exosomes in sleep apnea. Chest. 2016;150:1030–1041. doi: 10.1016/j.chest.2016.08.1438. [DOI] [PubMed] [Google Scholar]
- 18.Khalyfa A, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Andrade J, Hanly PJ, Poulin MJ, Gozal D. Effect on intermittent hypoxia on plasma exosomal Micro RNA signature and endothelial function in healthy adults. Sleep. 2016;39:2077–2090. doi: 10.5665/sleep.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010;12:3–4. doi: 10.1038/ncb0110-3. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C, Morohashi Y, Yoshimura SI, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 25.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 27.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 28.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5:a016766. doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 32.Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2017;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Stoorvogel W. Resolving sorting mechanisms into exosomes. Cell Res. 2015;25:531–532. doi: 10.1038/cr.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 35.Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz SN, Conlon MM, Rider MA, Brownstein NC, Meckes DG Jr. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J Extracell Vesicles. 2016;5:31295. doi: 10.3402/jev.v5.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of TNF-alpha autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1alpha, presented by exosomes. J Mol Cell Cardiol. 2012;53:848–857. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313:F906–F913. doi: 10.1152/ajprenal.00178.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 42.Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011;11:3. doi: 10.1186/1475-2867-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234–3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackenbeck T, Huber R, Schietke R, Knaup KX, Monti J, Wu X, Klanke B, Frey B, Gaipl U, Wullich B, Ferbus D, Goubin G, Warnecke C, Eckardt KU, Wiesener MS. The GTPase RAB20 is a HIF target with mitochondrial localization mediating apoptosis in hypoxia. Biochim Biophys Acta. 2011;1813:1–13. doi: 10.1016/j.bbamcr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA, Cohn DE, Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rana S, Yue S, Stadel D, Zoller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Rana S, Claas C, Kretz CC, Nazarenko I, Zoeller M. Activation-induced internalization differs for the tetraspanins CD9 and Tspan8: impact on tumor cell motility. Int J Biochem Cell Biol. 2011;43:106–119. doi: 10.1016/j.biocel.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 52.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 56.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 57.Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 58.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Biol Chem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 61.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 62.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 64.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304:H954–965. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 67.Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barile L, Gherghiceanu M, Popescu LM, Moccetti T, Vassalli G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol. 2012;2012:354605. doi: 10.1155/2012/354605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manole CG, Cismasiu V, Gherghiceanu M, Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–2296. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo H, Li X, Li T, Zhao L, He J, Zha L, Qi Q, Yu Z. Exosomes/microvesicles microRNA-423-3p derived from cardiac fibroblasts mediates the cardioprotective effects of ischemic postconditioning. Cardiovasc Res. 2018 doi: 10.1093/cvr/cvy231. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Zhang C, Wu J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 74.Heiserman JP, Chen L, Kim BS, Kim SC, Tran AL, Siebenborn N, Knowlton AA. TLR4 mutation and HSP60-induced cell death in adult mouse cardiac myocytes. Cell Stress Chaperones. 2015;20:527–535. doi: 10.1007/s12192-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105:1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, Knowlton AA, Yuan WJ, Lin L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc Res. 2013;98:391–401. doi: 10.1093/cvr/cvt047. [DOI] [PubMed] [Google Scholar]
- 77.Cheng Y, Wang X, Yang J, Duan X, Yao Y, Shi X, Chen Z, Fan Z, Liu X, Qin S, Tang X, Zhang C. A translational study of urine miRNAs in acute myocardial infarction. J Mol Cell Cardiol. 2012;53:668–676. doi: 10.1016/j.yjmcc.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Zhang L, Su T, Li H, Huang Q, Wu D, Yang C, Han Z. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J Thorac Dis. 2015;7:890–896. doi: 10.3978/j.issn.2072-1439.2014.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olivieri F, Antonicelli R, Lorenzi M, D’Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La SL, Galeazzi R, Recchioni R, Testa R, Pompilio G, Capogrossi MC, Procopio AD. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol. 2013;167:531–536. doi: 10.1016/j.ijcard.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 81.Sayed AS, Xia K, Yang TL, Peng J. Circulating microRNAs: a potential role in diagnosis and prognosis of acute myocardial infarction. Dis Markers. 2013;35:561–566. doi: 10.1155/2013/217948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Danielson KM, Shah R, Yeri A, Liu X, Camacho Garcia F, Silverman M, Tanriverdi K, Das A, Xiao C, Jerosch-Herold M, Heydari B, Abbasi S, Van Keuren-Jensen K, Freedman JE, Wang YE, Rosenzweig A, Kwong RY, Das S. Plasma circulating extracellular RNAs in left ventricular remodeling post-myocardial infarction. EBioMedicine. 2018;32:172–181. doi: 10.1016/j.ebiom.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li C, Pei F, Zhu X, Duan DD, Zeng C. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin Biochem. 2012;45:727–732. doi: 10.1016/j.clinbiochem.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bi S, Wang C, Jin Y, Lv Z, Xing X, Lu Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int J Clin Exp Med. 2015;8:4275–4280. [PMC free article] [PubMed] [Google Scholar]
- 85.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 86.Liao SY, Tse HF. Multipotent (adult) and pluripotent stem cells for heart regeneration: what are the pros and cons? Stem Cell Res Ther. 2013;4:151. doi: 10.1186/scrt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hulot JS, Stillitano F, Salem JE, Kovacic JC, Fuster V, Hajjar RJ. Considerations for pre-clinical models and clinical trials of pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2014;5:1. doi: 10.1186/scrt390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Yuan MJ, Maghsoudi T, Wang T. Exosomes mediate the intercellular communication after myocardial infarction. Int J Med Sci. 2016;13:113–116. doi: 10.7150/ijms.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 91.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 94.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, Zhong Z, Zhao J, Li Q, Zhu D, Ke C, Zhong S, Wu X, Yu H, Zhu W, Chen J, Zhang J, Wang JA, Hu X. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of mir-125b. Circ Res. 2018;123:564–578. doi: 10.1161/CIRCRESAHA.118.312758. [DOI] [PubMed] [Google Scholar]
- 96.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 97.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 99.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gildea JJ, Seaton JE, Victor KG, Reyes CM, Bigler Wang D, Pettigrew AC, Courtner CE, Shah N, Tran HT, Van Sciver RE, Carlson JM, Felder RA. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem. 2014;47:89–94. doi: 10.1016/j.clinbiochem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y, Yoshinaga K, Uchida K, Ueda Y, Kimiya K, Uezono S, Ueda A, Ito K, Ikeda M. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1006–1016. doi: 10.1152/ajprenal.00200.2009. [DOI] [PubMed] [Google Scholar]
- 102.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA 3rd, Yuen PS, Star RA. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen HH, Lai PF, Lan YF, Cheng CF, Zhong WB, Lin YF, Chen TW, Lin H. Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion. J Cell Physiol. 2014;229:1202–1211. doi: 10.1002/jcp.24554. [DOI] [PubMed] [Google Scholar]
- 105.Vinas JL, Burger D, Zimpelmann J, Haneef R, Knoll W, Campbell P, Gutsol A, Carter A, Allan DS, Burns KD. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016;90:1238–1250. doi: 10.1016/j.kint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 106.Burger D, Vinas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol. 2015;185:2309–2323. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 107.Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8:70707–70726. doi: 10.18632/oncotarget.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–185. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 109.Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, Fan Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;2016:1240301. doi: 10.1155/2016/1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 111.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 114.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu C, Chen M, Jiang R, Guo Y, Wu M, Zhang X. Exosome-related tumor microenvironment. J Cancer. 2018;9:3084–3092. doi: 10.7150/jca.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, Abd Elmageed ZY, Deep G. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep. 2018;8:3853. doi: 10.1038/s41598-018-22068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R, Deep G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2015;54:554–565. doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci. 2016;37:606–617. doi: 10.1016/j.tips.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 120.Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, Li X, Chen J, Liu K, Li C, Zhu G. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76:1770–1780. doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- 121.Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 123.Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, Pang H, An H, Wang X, Hou H, Li X. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16:143. doi: 10.1186/s12943-017-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8:9899–9910. doi: 10.18632/oncotarget.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 126.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H, Li G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37:4239–4259. doi: 10.1038/s41388-018-0261-9. [DOI] [PubMed] [Google Scholar]
- 128.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768–774. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ye SB, Zhang H, Cai TT, Liu YN, Ni JJ, He J, Peng JY, Chen QY, Mo HY, Jun C, Zhang XS, Zeng YX, Li J. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 132.Cheshomi H, Matin MM. Exosomes and their importance in metastasis, diagnosis, and therapy of colorectal cancer. J Cell Biochem. 2018;120:2671–2686. doi: 10.1002/jcb.27582. [DOI] [PubMed] [Google Scholar]
- 133.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 134.Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139–148. doi: 10.1016/j.biomaterials.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med. 2016;94:711–724. doi: 10.1007/s00109-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 136.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 138.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtio J, Nolta JA. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dominguez JH, Liu Y, Gao H, Dominguez JM 2nd, Xie D, Kelly KJ. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J Am Soc Nephrol. 2017;28:3533–3544. doi: 10.1681/ASN.2016121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang R, Lin M, Li L, Li L, Qi G, Rong R, Xu M, Zhu T. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi. 2014;94:3298–3303. [PubMed] [Google Scholar]
- 142.Pang P, Abbott M, Chang SL, Abdi M, Chauhan N, Mistri M, Ghofrani J, Fucci QA, Walker C, Leonardi C, Grady S, Halim A, Hoffman R, Lu T, Cao H, Tullius SG, Malek S, Kumar S, Steele G, Kibel A, Freedman BS, Waikar SS, Siedlecki AM. Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney Int. 2017;91:129–143. doi: 10.1016/j.kint.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]