Abstract
Background: Recurrent hepatocellular carcinoma (rHCC) patients with microvascular invasive (MVI) positive at first resection usually had poorly differentiated tumors and worse survivals. The optimal treatment for this population remains to be elucidated. Methods: We retrospectively analyzed 319 rHCC patients with MVI-positive at first resection from June, 2009 to June, 2017. Survival and costs between curative treatments [re-resection (RR) and radiofrequency ablation (RFA)] and transarterial chemoembolization (TACE) were compared. Subgroup comparisons were made in patients in Barcelona Clinic Liver Cancer (BCLC) stage 0-A and BCLC stage B-C, respectively. A one-to-one propensity score matching (PSM) was used to diminish bias. Results: In BCLC stage 0-A, 98 received RR/RFA, and 49 received TACE. The median overall survival (OS) of RR/RFA group was not reached, while the OS of TACE group was 26.3 months (P=0.001). After matching, the OS of the RR/RFA group was longer than that of the TACE group (39.5 vs. 26.3 months, P=0.045). In BCLC stage B-C, 137 patients received TACE, 11 received RR and 24 received RFA. The median OS was 29.8 months, 17.9 months and 11.1 months for RR, RFA and TACE group, respectively. No significant difference was found between RR and TACE (P=0.237) or RFA and TACE (P=0.484) after matching. Costs of the TACE group was significantly lower than that of the RR group but similar to that of the RFA group. Conclusion: RR/RFA provided better survival outcomes for rHCC patients with MVI-positive at first resection in selected BCLC stage 0-A. In selected BCLC stage B-C, TACE shared a similar efficacy with RR and RFA but a lower cost than RR.
Keywords: Hepatocellular carcinoma, recurrence, hepatectomy, therapeutic chemoembolization, catheter ablation
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer related death in the world [1]. Approximately 70% of HCC patients recur in 5 years after the first resection [2-4], 46.5-55% of whom recurred in the intermediate-advanced stage [5,6], significantly affecting the long-term survival of HCC patients. An optimal management of recurrent HCC (rHCC) treatment is urgent.
With limited data on rHCC management, no recognized guideline has been established [7]. The treatment algorithm of rHCC mostly followed that of primary HCC and clinical experience. Usually, only the status of rHCC was taken into account. In fact, several studies showed that the characteristics of rHCC were largely associated with those of primary HCC, indicating that the status of primary HCC should also be considered in the treatment of rHCC [6,8]. Particularly, the pathologic profile at the first resection of primary HCC, mainly referring to microvascular invasion (MVI), was reported to affect the characteristics and prognosis of rHCC. Accordingly, rHCC was more likely to have a poor differentiation if detected with MVI-positive at the first resection [9]. And MVI at the first resection had a significant negative impact on survival rate after recurrence [10].
Given the distinctive behavior, efforts have been made on searching the proper treatment for this particular population. Meniconi et al. compared early-stage rHCC patients treated by re-resection (RR)/radiofrequency ablation (RFA) and transarterial chemoembolization (TACE). It turned out that there was no significant difference of survival between these two groups in patients with MVI-positive at the first resection [10]. Hou et al. focused on recurrent patients who were MVI-positive and within Milan criteria at primary resection, and drew a conclusion that a second resection could largely improve survival compared with local therapy [9]. Another research constructed by Jin et al. came to a completely different result. They concluded that in rHCC patients within BCLC stage 0-A, TACE is more effective than curative treatments if the primary tumor is MVI-positive [11].
With scant evidence and various conclusions, the treatment for rHCC with MVI-positive at first resection remains to be elucidated. Previous studies on this topic share some common limitations. For one thing, early recurrent tumors were mainly discussed in these studies, while the tumors in intermediate and advanced stages were seldom referred. For another, the baselines between different groups were not strictly matched, making the results less convinced. Therefore, we retrospectively compared the curative treatments (RR and RFA) and TACE in rHCC patients with MVI-positive at the first resection. A propensity score matching (PSM) method was performed to adjust the baseline characteristics.
Methods
Study design
This is a retrospective study based on a prospectively collected database from the First Affiliated Hospital of Sun Yat-sen University and the Cancer Center of Sun Yat-sen University. From June, 2009 to June, 2017, 2137 HCC patients who initially underwent liver resection were consecutively collected. The inclusion criteria for the rHCC patients were as follows: a) first recurrence after the curative resection; b) MVI-positive at the first resection confirmed by pathology; c) Child-Pugh grade A-B at recurrence; d) ECOG performance status 0-1 at recurrence. The exclusion criteria were: a) patients received other treatments than RR, RFA or TACE for rHCC; b) lost to follow-up within 1 month after recurrence. At last, 319 patients were enrolled in this study.
HCC was diagnosed following the European Association for the Study of the Liver, European Organization for Research and Treatment of Cancer (EASL-EORTC) guideline [12]. MVI at the first resection was confirmed by two experienced pathologists in hepatology over 5 years. Evaluation of recurrence was performed at the first month after initial resection, and was repeated every 3 months for the first two years, 3-6 months thereafter. The modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria via contrastenhanced ultrasound (US) and dynamic contrast-enhanced liver computed tomography (CT) was used for recurrence evaluation. RHCC was also diagnosed following the guideline of EASL-EORTC. The follow-up schedule after the treatment of rHCC was the same to that of the initial resection. Overall survival (OS) was defined as the time interval from the date of diagnosis of rHCC to the date of death from any cause or to the date of the last follow-up visit.
Treatment selection
The treatment algorithm for rHCC in this study was similar to that of some early reports [8,13]. A multidisciplinary therapy discussion was conducted for each rHCC patient before treatment. Liver function, technical difficulty, tumor status and the general health status of patients were all taken into concern. After that, a final advice from experts of different departments (including the Department of Liver Surgery, the Department of Ultrasound Intervention and the Department of Oncology) was gave to the patient. Advices were mainly referred to the EASL-EORTC guideline. Possible efficacy, costs and the risks of complication during and after different treatments were also informed. Final decision was made by the patient. The cost for one patient was composed by the cost of surgery/RFA/TACE, treatment of complications and normal supportive care.
Re-resection procedure
Re-resection was performed under general anesthesia by surgeons with 10-40 years of experience. Type of surgery was decided according to a routine discussion for each patient in the Department of liver surgery. Anatomic or non-anatomic resection was decided according to the tumor burden and liver function of the patients. The surgical approach was chosen based on the liver remnant, tumor location and preference of the operator. Intraoperative US was used to assist in operative evaluation.
RFA procedure
RFA was performed with LeVeen electrodes (Boston Scientific, Natick, MA), Starburst XL electrodes (RITA Medical Systems, Mountain View, CA), or Cool-tip electrodes (Valleylab, Boulder, CO). The selection of device was based on the size and location of the tumor. The electrode was percutaneously inserted under the real-time ultrasound (US) guidance through the guiding needle. RFA was performed with the intent to completely eradicate the tumor with an ablative margin of 0.5 cm. Multiple overlapping ablations were performed for tumor larger than 3 cm. After RFA, the needle track was coagulated for reducing bleeding and tumor seeding.
TACE procedure
TACE was carried out by two radiologists with over 5 years of experience. A selective catheter was inserted into the tumor-feeding arteries after evaluating arterial blood supply of the liver and confirming patency of the portal vein by visceral angiography. Hepatic artery infusion chemotherapy was performed using carboplatin 300 mg (Bristol-Myers Squibb, New York, NY). Subsequently, chemolipiodolization was performed using epirubicin 50 mg (Pharmorubicin, Pfizer, Wuxi, China), and mitomycin C 8 mg (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, China) mixed with 5 mL of lipiodol (Lipiodol Ultra-Fluide; Andre’ Guerbet Laboratories, Aulnay-Sous-Bois, France). Embolization was finally performed with absorbable gelatin sponge particles (Gelfoam; Hanzhou Alc Ltd, China, 1-2 mm in diameter) or polyvinyl alcohol particles (Alicon Pharm SCT&TEC CO., LTD., Hangzhou, China, 350-560 μm in diameter).
Statistical analysis
Normal distribution test was performed for continuous variables. Continuous variables obey normal distribution were presented as means ± SD and others as median and quartile. Categorical variables were presented as numbers and percentages. Differences between the curative treatment group and TACE group were compared with the t-test for continuous variables and χ2 test for categorical variables. Survival curves were generated by the Kaplan-Meier method and compared by the log-rank test. The potential survival predictors were analyzed by univariate and multivariate Cox proportional hazard regression models. BCLC stage and Child-Pugh stage were not included in the multivariate Cox analysis since all factors defining these two stages were already included in the model.
Considering that the characteristics and prognosis vary a lot between BCLC stage 0-A and stage B-C, rHCC patients were divided into two groups. Comparisons of different treatment strategies were performed separately in BCLC stage 0-A and BCLC stage B-C. For patients in BCLC stage B-C, evidence comparing RR and RFA is limited. Therefore, outcomes of the RR group, the RFA group and the TACE group were compared separately.
In order to diminish the bias between different groups, PSM was performed in this study. Patients were matched based on logistic regression model. Propensity scores were estimated according to all baseline characteristics including age, gender, ECOG performance status, Child-Pugh grade, TMN stage, BCLC stage of the primary HCC, BCLC stage of the rHCC, tumor number, levels of AFP, platelet (PLT), hemoglobin (HB), albumin (ALB), total bilirubin (TB), alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), prothrombin time (PT), status of hepatitis B/C virus (HBV, HCV) and time to recurrence. One-to-one matching was performed using a 0.1 caliper.
Statistical significance was considered as a two-sided P value of less than 0.05. The above statistical analysis was performed with the STATA/MP 14.0.
Results
Patient characteristics
Table 1 summarized the baseline characteristics of rHCC patients in BCLC stage 0-A. Tables 2 and 3 summarized the baseline of patients in BCLC stage B-C before and after PSM, respectively. Among 147 patients recurred in BCLC stage 0-A, 98 received RR/RFA, and 49 received TACE. In the RR/RFA group, 73.5% patients recurred within 1 year. The percentage was significantly higher in the TACE group (95.9%, P=0.001). Also, age and PLT were significantly different be-tween the two groups. All characteristics were balanced after matching (Table 1). In BCLC stage B-C, 137 patients received TACE, 11 patients received RR and 24 patients received RFA. Patients received TACE were more likely to have multiple lesions than the RR group and the RFA group (P<0.001). After matching, there was no si-gnificant difference between RR and TACE, or between RFA and TACE.
Table 1.
Baseline characteristics of patients in BCLC stage 0-A
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| RR/RFA (98) | TACE (49) | P | RR/RFA (49) | TACE (49) | P | |
| Age (years) | 0.370 | 0.812 | ||||
| ≤60 | 67 (68.4) | 37 (75.5) | 38 (77.6) | 37 (75.5) | ||
| >60 | 31 (31.6) | 12 (24.5) | 11 (22.4) | 12 (24.5) | ||
| Gender | 0.145 | 0.294 | ||||
| Male | 84 (85.7) | 46 (93.9) | 43 (87.7) | 46 (93.9) | ||
| Female | 14 (14.3) | 3 (6.1) | 6 (12.2) | 3 (6.1) | ||
| Hemoglobin (g/L) | 0.748 | 0.337 | ||||
| ≤120 | 16 (16.3) | 7 (14.3) | 4 (8.2) | 7 (14.3) | ||
| >120 | 82 (83.7) | 42 (85.7) | 45 (91.8) | 42 (85.7) | ||
| Albumin (g/L) | 0.100 | 0.182 | ||||
| ≤35 | 6 (6.1) | 7 (14.3) | 3 (6.1) | 7 (14.3) | ||
| >35 | 92 (93.9) | 42 (85.7) | 46 (93.9) | 42 (85.7) | ||
| Total bilirubin (mmol/L) | 0.615 | 0.315 | ||||
| ≤34.2 | 97 (99.0) | 48 (98.0) | 49 (100.0) | 48 (98.0) | ||
| >34.2 | 1 (1.0) | 1 (2.0) | 0 (0.0) | 1 (2.0) | ||
| ALT (U/L) | 0.675 | 1.000 | ||||
| ≤40 | 77 (78.6) | 37 (75.5) | 37 (75.5) | 37 (75.5) | ||
| >40 | 21 (21.4) | 12 (24.5) | 12 (24.5) | 12 (24.5) | ||
| AST (U/L) | 0.883 | 0.602 | ||||
| ≤40 | 79 (80.6) | 39 (79.6) | 41 (83.7) | 39 (79.6) | ||
| >40 | 19 (19.4) | 10 (20.4) | 8 (16.3) | 10 (20.4) | ||
| GGT (U/L) | 0.159 | 1.000 | ||||
| ≤50 | 58 (59.2) | 23 (46.9) | 23 (46.9) | 23 (46.9) | ||
| >50 | 40 (40.8) | 26 (53.1) | 26 (53.1) | 26 (53.1) | ||
| PT (s) | 0.247 | 0.092 | ||||
| ≤14 | 93 (94.9) | 44 (89.8) | 48 (98.0) | 44 (89.8) | ||
| >14 | 5 (5.1) | 5 (10.2) | 1 (2.0) | 5 (10.2) | ||
| HBV positive | 87 (88.8) | 47 (95.9) | 0.150 | 45 (91.8) | 47 (95.9) | 0.399 |
| HCV positive | 2 (2.0) | 0 (0.0) | 0.314 | 1 (2.0) | 0 (0.0) | 0.315 |
| BCLC stage of primary tumor | 0.157 | 0.544 | ||||
| 0-A | 60 (61.2) | 24 (49.0) | 27 (55.1) | 24 (49.0) | ||
| B-C | 38 (38.8) | 25 (51.0) | 22 (44.9) | 25 (51.0) | ||
| Platelet (×109/L) | 0.036 | 1.000 | ||||
| ≤100 | 27 (27.6) | 6 (12.2) | 6 (12.2) | 6 (12.2) | ||
| >100 | 71 (72.4) | 43 (87.8) | 43 (87.8) | 43 (87.8) | ||
| AFP (ug/L) | 0.322 | 0.527 | ||||
| ≤200 | 68 (69.4) | 30 (61.2) | 33 (67.3) | 30 (61.2) | ||
| >200 | 30 (30.6) | 19 (38.8) | 16 (32.7) | 19 (38.8) | ||
| Child-Pugh classification | 0.615 | 0.315 | ||||
| Child-Pugh A | 97 (99.0) | 48 (98.0) | 49 (100.0) | 48 (98.0) | ||
| Child-Pugh B | 1 (1.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | ||
| Tumor size (cm) | 0.199 | 1.000 | ||||
| ≤5 | 91 (92.9) | 48 (98.0) | 48 (98.0) | 48 (98.0) | ||
| >5 | 7 (7.1) | 1 (2.0) | 1 (2.0) | 1 (2.0) | ||
| TTR | 0.001 | 0.558 | ||||
| <1 year | 72 (73.5) | 47 (95.9) | 48 (98.0) | 47 (95.9) | ||
| ≥1 year | 26 (26.5) | 2 (4.1) | 1 (2.0) | 2 (4.1) | ||
Abbreviations: PSM (propensity score matching), ALT (alanine transaminase), AST (aspartate aminotransferase), GGT (glutamyl transpeptidase), PT (prothrombin time), HBV (hepatitis B virus), AFP (alpha fetal protein), TTR (time to recurrence).
Table 2.
Baseline characteristics of patients in BCLC stage B-C
| Variable | RR (11) | RFA (24) | TACE (137) | P |
|---|---|---|---|---|
| Age (years) | 0.619 | |||
| ≤60 | 8 (72.7) | 19 (79.2) | 112 (81.8) | |
| >60 | 3 (27.3) | 5 (20.8) | 25 (18.2) | |
| Gender | 0.062 | |||
| Male | 10 (90.9) | 18 (75.0) | 125 (91.2) | |
| Female | 1 (9.1) | 6 (25.0) | 12 (8.8) | |
| Hemoglobin (g/L) | 0.313 | |||
| ≤120 | 0 (0.0) | 5 (20.8) | 26 (19.0) | |
| >120 | 11 (100.0) | 19 (79.2) | 111 (81.0) | |
| Albumin (g/L) | 0.163 | |||
| ≤35 | 2 (18.2) | 1 (4.2) | 26 (19.0) | |
| >35 | 9 (81.8) | 23 (95.8) | 111 (81.0) | |
| Total bilirubin (mmol/L) | 0.601 | |||
| ≤34.2 | 11 (100.0) | 23 (95.8) | 134 (97.8) | |
| >34.2 | 0 (0.0) | 1 (4.2) | 3 (2.2) | |
| ALT (U/L) | 0.904 | |||
| ≤40 | 8 (72.7) | 18 (75.0) | 95 (69.3) | |
| >40 | 3 (27.3) | 6 (25.0) | 42 (30.7) | |
| AST (U/L) | 0.537 | |||
| ≤40 | 7 (63.6) | 17 (70.8) | 80 (58.4) | |
| >40 | 4 (36.4) | 7 (29.2) | 57 (41.6) | |
| GGT (U/L) | 0.234 | |||
| ≤50 | 7 (63.6) | 12 (50.0) | 55 (40.1) | |
| >50 | 4 (36.4) | 12 (50.0) | 82 (59.9) | |
| PT (s) | 0.175 | |||
| ≤14 | 10 (90.9) | 24 (100.0) | 120 (87.6) | |
| >14 | 1 (9.1) | 0 (0.0) | 17 (12.4) | |
| HBV positive | 11 (100.0) | 21 (87.5) | 123 (89.8) | 0.623 |
| HCV positive | 0 (0.0) | 0 (0.0) | 5 (3.6) | 1.000 |
| BCLC stage of primary tumor | 0.669 | |||
| 0-A | 5 (45.5) | 11 (45.8) | 52 (38.0) | |
| B-C | 6 (54.5) | 13 (54.2) | 85 (62.0) | |
| Platelet (×109/L) | 0.132 | |||
| ≤100 | 0 (0.0) | 2 (8.3) | 28 (20.4) | |
| >100 | 11 (100.0) | 22 (91.7) | 109 (79.6) | |
| AFP (ug/L) | 0.800 | |||
| ≤200 | 6 (54.5) | 16 (66.7) | 84 (61.3) | |
| >200 | 5 (45.5) | 8 (33.3) | 53 (38.7) | |
| Child-Pugh classification | 0.513 | |||
| Child-Pugh A | 11 (100.0) | 24 (100.0) | 128 (93.4) | |
| Child-Pugh B | 0 (0.0) | 0 (0.0) | 9 (6.6) | |
| Tumor size (cm) | 0.101 | |||
| ≤5 | 8 (72.7) | 23 (95.8) | 111 (81.0) | |
| >5 | 3 (27.3) | 1 (4.2) | 26 (19.0) | |
| Tumor number | <0.001 | |||
| 1 | 5 (45.5) | 11 (45.8) | 16 (11.7) | |
| >1 | 6 (54.5) | 13 (54.2) | 121 (88.3) | |
| Macrovascular invasion | 3 (27.3) | 2 (8.3) | 33 (24.1) | 0.217 |
| TTR | 0.112 | |||
| <1 year | 8 (72.7) | 23 (95.8) | 126 (92.0) | |
| ≥1 year | 3 (27.3) | 1 (4.2) | 11 (8.0) |
Abbreviations: PSM (propensity score matching), ALT (alanine transaminase), AST (aspartate aminotransferase), GGT (glutamyl transpeptidase), PT (prothrombin time), HBV (hepatitis B virus), AFP (alpha fetal protein), TTR (time to recurrence).
Table 3.
Baseline characteristics of patients in BCLC stage B-C after PSM
| Variable | RR (11) | TACE (11) | P | RFA (24) | TACE (24) | P |
|---|---|---|---|---|---|---|
| Age (years) | 0.647 | 0.439 | ||||
| ≤60 | 8 (72.7) | 7 (63.6) | 19 (79.2) | 21 (87.5) | ||
| >60 | 3 (27.3) | 4 (36.4) | 5 (20.8) | 3 (12.5) | ||
| Gender | 1.000 | 0.477 | ||||
| Male | 10 (90.9) | 10 (90.9) | 18 (75.0) | 20 (83.3) | ||
| Female | 1 (9.1) | 1 (9.1) | 6 (25.0) | 4 (16.7) | ||
| Hemoglobin (g/L) | 1.000 | 0.439 | ||||
| ≤120 | 0 (0.0) | 0 (0.0) | 5 (20.8) | 3 (12.5) | ||
| >120 | 11(100.0) | 11(100.0) | 19 (79.2) | 21 (87.5) | ||
| Albumin (g/L) | 1.000 | 0.298 | ||||
| ≤35 | 2 (18.2) | 2 (18.2) | 1 (4.2) | 3 (12.5) | ||
| >35 | 9 (81.8) | 9 (81.8) | 23 (95.8) | 21 (87.5) | ||
| Total bilirubin (mmol/L) | 1.000 | 1.000 | ||||
| ≤34.2 | 11(100.0) | 11(100.0) | 23 (95.8) | 23 (95.8) | ||
| >34.2 | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (4.2) | ||
| ALT (U/L) | 1.000 | 0.731 | ||||
| ≤40 | 8 (72.7) | 8 (72.7) | 18 (75.0) | 19 (79.2) | ||
| >40 | 3 (27.3) | 3 (27.3) | 6 (25.0) | 5 (20.8) | ||
| AST (U/L) | 0.647 | 0.745 | ||||
| ≤40 | 7 (63.6) | 8 (72.7) | 17 (70.8) | 18 (75.0) | ||
| >40 | 4 (36.4) | 3 (27.3) | 7 (29.2) | 6 (25.0) | ||
| GGT (U/L) | 1.000 | 0.074 | ||||
| ≤50 | 7 (63.6) | 7 (63.6) | 12 (50.0) | 18 (75.0) | ||
| >50 | 4 (36.4) | 4 (36.4) | 12 (50.0) | 6 (25.0) | ||
| PT (s) | 1.000 | 1.000 | ||||
| ≤14 | 10 (90.9) | 10 (90.9) | 24 (100.0) | 24 (100.0) | ||
| >14 | 1 (9.1) | 1 (9.1) | 0 (0.0) | 0 (0.0) | ||
| HBV positive | 11(100.0) | 9 (81.8) | 0.138 | 21 (87.5) | 22 (91.7) | 0.637 |
| HCV positive | 0 (0.0) | 0 (0.0) | 1.000 | 0 (0.0) | 2 (8.3) | 0.149 |
| BCLC stage of primary tumor | 0.665 | 0.773 | ||||
| 0-A | 5 (45.5) | 4 (36.4) | 11 (45.8) | 12 (50.0) | ||
| B-C | 6 (54.5) | 7 (63.6) | 13 (54.2) | 12 (50.0) | ||
| Platelet (×109/L) | 1.000 | 0.637 | ||||
| ≤100 | 0 (0.0) | 0 (0.0) | 2 (8.3) | 3 (12.5) | ||
| >100 | 11(100.0) | 11(100.0) | 2 (91.7) | 21 (87.5) | ||
| AFP (ug/L) | 1.000 | 0.525 | ||||
| ≤200 | 6 (54.5) | 6 (54.5) | 16 (66.7) | 18 (75.0) | ||
| >200 | 5 (45.5) | 5 (45.5) | 8 (33.3) | 6 (25.0) | ||
| Child-Pugh classification | 1.000 | 1.000 | ||||
| Child-Pugh A | 11(100.0) | 11(100.0) | 24 (100.0) | 24 (100.0) | ||
| Child-Pugh B | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Tumor size (cm) | 0.647 | 1.000 | ||||
| ≤5 | 8 (72.7) | 7 (63.6) | 23 (95.8) | 23 (95.8) | ||
| >5 | 3 (27.3) | 4 (36.4) | 1 (4.2) | 1 (4.2) | ||
| Macrovascular invasion | 3 (27.3) | 5 (45.5) | 0.375 | 2 (8.3) | 2 (8.3) | 1.000 |
| TTR | 0.611 | 0.551 | ||||
| <1 year | 8 (72.7) | 9 (81.8) | 23 (95.8) | 22 (91.7) | ||
| ≥1 year | 3 (27.3) | 2 (18.2) | 1 (4.2) | 2 (8.3) |
Abbreviations: PSM (propensity score matching), ALT (alanine transaminase), AST (aspartate aminotransferase), GGT (glutamyl transpeptidase), PT (prothrombin time), HBV (hepatitis B virus), AFP (alpha fetal protein), TTR (time to recurrence).
Survival outcomes
Median overall survival (OS) for all rHCC patients was 25.9 months. The OS in patients with BCLC stage 0-A was significantly better than that in patients with stage B-C (49.2 vs. 14.4 months, P<0.001).
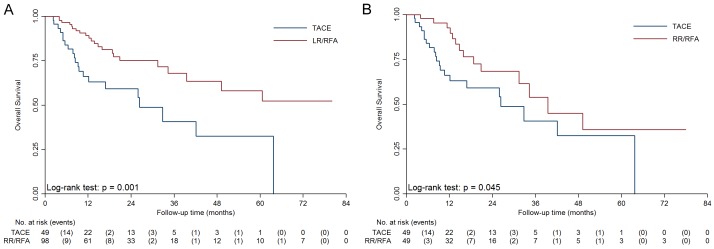
For patients in BCLC stage 0-A, median OS of the TACE group was 26.3 months, while median OS of the RR/RFA group was not reached (Figure 1A, P=0.001). After matching, as showed in Figure 1B, the OS in the RR/RFA group was superior to that in the TACE group (39.5 vs. 26.3 months, P=0.045).
Figure 1.
Kaplan-Meier survival curves of the RR/RFA and TACE group in BCLC stage 0-A before and after PSM. A. Median overall survival of the RR/RFA group was significantly longer than the TACE group (P=0.001). B. After matching, median overall survival of the RR/RFA group was still longer than the TACE group (P=0.045).
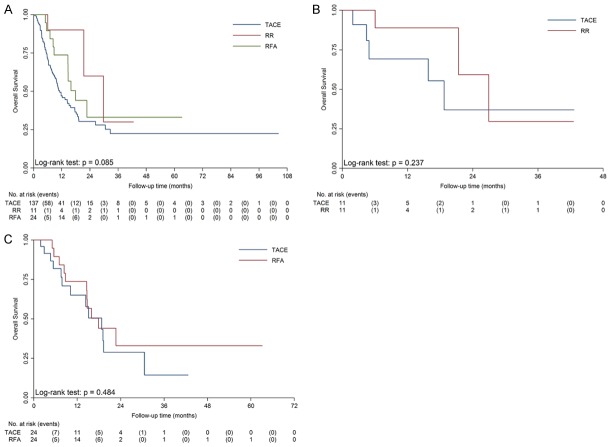
For patients in BCLC stage B-C, median OS were 11.1 months for the TACE group, 29.7 months for the RR group and 17.9 months for the RFA group, respectively (Figure 2, P=0.085). PSM were performed in the comparisons between RR and TACE, and between RFA and TACE. After matching, there was no significant difference between RR and TACE (P=0.237) or between RFA and TACE (P=0.484).
Figure 2.
Kaplan-Meier survival curves of the RR group, the RFA group and the TACE group in BCLC stage B-C. A. There was no significant difference between the overall survival of RR, RFA and TACE (P=0.085). B. After matching, there was no significant difference between the overall survival of RR and TACE (26.9 vs. 18.7 months, P=0.237). C. After matching, there was no significant difference between the overall survival of RFA and TACE (17.9 vs. 18.7 months, P=0.484).
Univariate and multivariate analysis
According to the univariate analysis, HB, ALB, ALT, AST, GGT, PT, BCLC stage of primary tumor, BCLC stage of rHCC, treatment strategies, AFP, tumor size, tumor number, macrovascular invasion in rHCC, Child-Pugh stage of rHCC and time to recurrence were correlated to OS after recurrence. In the multivariate analysis, HB [Hazard Ratio (HR): 0.50, P=0.002], AST (HR: 1.69, P=0.007), GGT (HR: 1.54, P=0.028), PT (HR: 1.18, P=0.009), rHCC in BCLC stage B-C (HR: 1.57, P=0.038), macrovascular invasion in rHCC (HR: 1.66, P=0.048), treated by RR/RFA (HR: 0.60, P=0.022) and time to recurrence >1 year (HR: 0.28, P=0.003) were independent prognostic factors of OS. Details were showed in Table 4.
Table 4.
Variables associated with overall survival according to the cox proportional hazards model
| Variable | Univariable Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (>60 years) | 0.80 | 0.52, 1.23 | 0.302 | |||
| Gender (Female) | 0.82 | 0.46, 1.46 | 0.508 | |||
| Hemoglobin (>120 g/L) | 0.54 | 0.36, 0.82 | 0.003 | 0.50 | 0.33, 0.78 | 0.002 |
| Albumin (>35 g/L) | 0.38 | 0.25, 0.60 | <0.001 | |||
| Total bilirubin (>34.2 mmol/L) | 2.50 | 0.96, 7.03 | 0.061 | |||
| ALT (>40 U/L) | 1.82 | 1.27, 2.60 | 0.001 | |||
| AST (>40 U/L) | 2.38 | 1.67, 3.38 | <0.001 | 1.69 | 1.15, 2.47 | 0.007 |
| GGT (>50 U/L) | 2.20 | 1.54, 3.15 | <0.001 | 1.54 | 1.05, 2.27 | 0.028 |
| PT (>14 s) | 2.23 | 1.37, 3.62 | 0.001 | 1.95 | 1.18, 3.22 | 0.009 |
| HBV positive | 1.51 | 0.76, 2.97 | 0.236 | |||
| HCV positive | 1.66 | 0.61, 4.50 | 0.322 | |||
| BCLC stage of primary tumor (B-C) | 1.74 | 1.23, 2.48 | 0.002 | |||
| BCLC stage of recurrent tumor (B-C) | 2.66 | 1.84, 3.85 | <0.001 | 1.57 | 1.02, 2.41 | 0.038 |
| Macrovascular invasion | 2.94 | 1.85, 4.66 | <0.001 | 1.66 | 1.00, 2.75 | 0.048 |
| Treatment for rHCC | ||||||
| TACE | 1.00 | |||||
| RR/RFA | 0.35 | 0.24, 0.52 | <0.001 | 0.60 | 0.38, 0.93 | 0.022 |
| Platelet (>100×109/L) | 1.28 | 0.81, 2.01 | 0.287 | |||
| AFP (>200 ug/L) | 1.79 | 1.27, 2.53 | 0.001 | |||
| Child-Pugh classification B | 4.14 | 2.09, 8.19 | <0.001 | |||
| Tumor size (>5 cm) | 1.91 | 1.17, 3.12 | 0.009 | |||
| Tumor number (>1) | 2.09 | 1.45, 3.02 | <0.001 | |||
| TTR (≥1 year) | 0.23 | 0.10, 0.51 | <0.001 | 0.28 | 0.12, 0.66 | 0.003 |
Abbreviations: ALT (alanine transaminase), AST (aspartate aminotransferase), GGT (glutamyl transpeptidase), PT (prothrombin time), HBV (hepatitis B virus), AFP (alpha fetal protein), TTR (time to recurrence).
Costs
Median cost of patients in the RR/RFA group was $4367.11 (3149.86, 5687.28), significantly higher than that of the TACE group [$3939.60 (3067.39, 4865.84), P=0.04]. When separately compared the curative treatments to TACE, cost of RR was significantly higher [$7537.48 (6418.66, 8825.05), P<0.001], while RFA [$3964.15 (2938.22, 4774.56)] shared a similar cost with TACE (P=0.65). Results were analogic in subgroup analysis. The median costs of TACE, RR and RFA were $3623.13 (3060.14, 4572.56), $6679.22 (5668.36, 8091.37) and $3740.51 (2894.04, 4461.06) for patients in BCLC stage 0-A. The corresponding figures were $4105.70 (3114.55, 4986.66), $7828.03 (7380.71, 10229.83) and $4592.30 (3419.15, 5408.70) in patients with BCLC stage B-C. Significant differences were found between RR and TACE (P<0.001 for both BCLC stage 0-A and B-C), but not between RFA and TACE (P=0.98 for BCLC stage 0-A, P=0.22 for BCLC stage B-C).
Discussion
Our study showed that, for rHCC with MVI at the first resection, the independent risk factors for OS after the rHCC treatment were treatment allocation, macrovascular invasion and time to recurrence. Curative treatments had a better efficacy in selected patients with BCLC stage 0-A, while shared a similar efficacy with TACE in selected patients with BCLC stage B-C. Patients in the TACE group had a significant lower cost than that of the RR group, while no significant difference was found between RFA and TACE.
MVI is one of the most important prognostic factors for not only recurrence of primary HCC but also overall survival of rHCC [14,15]. In this study, 87.8% of the MVI-positive patients recurred within 1 year after the initial resection. This result once again demonstrated the specialty and essentiality of management of MVI-positive patients. Expanding the resection margin is one way to prolong the RFS for MVI-positive patients [16]. But clinically, it is not practical since there is no way to diagnose MVI before surgery [17], and expanding surgical margin would cause more patients lose the chance to get curative surgery due to the insufficient remnant liver. Another way is the adjuvant therapy after surgery. A large multicenter RCT, the STORM study, focused on this issue but failed to get a positive result [18]. The negative result might partly due to the lack of subgroup analysis on finding the precise candidates for adjuvant therapy. Therefore, with aforementioned strategies not applicable, the management of recurrence for MVI-positive patients is of vital importance.
Currently, the management of rHCC is mostly based on that of primary HCC and clinical experience. However, given the specialty of MVI-positive patients, a more precisely defined management of this particular population could be necessary. As mentioned before, although several studies focused on this issue, subjects were limited to early stage rHCC patients. This article is the first to compare different treatment strategies in both early stage and intermediate-advanced stage rHCC patients to our knowledge.
Considering that the normally applied treatment and the tumor status were quite different between early and intermediate-advanced HCC patients, we separately compared the two treatment groups in BCLC stage 0-A and BCLC stage B-C. The RR/RFA group had a significant better survival for early stage rHCC patients. Resection and RFA are thought to be curative and are the first-line treatments recommended for BCLC stage 0-A in primary HCC [19]. Situations seem to be the same in rHCC. Previously reported 5-year survival rates after repeat resection for selected patients were 37-70% [8,20,21], indicating that patients recurrence in BCLC stage 0-A may have quite a chance of being cured with a second curative treatment. According to our results, this conclusion could also be applied to patients who were MVI-positive at initial resection. Nevertheless, opposite results were concluded by other studies. Jin et al. showed that TACE had better efficacy in rHCC patients with MVI-positive at first resection if rHCC recurred within 1 year. But with only 4 patients in one of the subgroups, the results need to be validated. Study constructed by Meniconi et al. had the same limitation, enrolling only 8 patients in the RR/RFA group. Therefore, we prefer curative treatment could be a better choice for rHCC patients in BCLC stage 0-A with MVI-positive at first resection.
Things were different when it comes to patients in BCLC stage B-C. A meta-analysis for all rHCC patients in BCLC stage B-C, regardless of the status of MVI at primary resection, showed that re-resection was a better choice than TACE as long as the tumors were resectable [22]. The result was similar to that of primary HCC, where two meta-analyses comparing TACE and resection drew the same result that resection showed survival benefits in BCLC stage B-C [23,24]. However, rHCC patients with MVI showed different conclusion according to our results. Although assigned with more multifocal patients, TACE still shared a similar efficacy with RR and RFA in BCLC stage B-C patients. This could be explained by that MVI-positive at initial resection indicated an aggressive behavior of HCC, and was a marker of potential microintrahepatic spread [25,26]. Therefore, recurrence of MVI-positive patients might have a higher degree of malignancy. With similar efficacy, RFA and TACE has the advantages of lower cost than RR. However, RFA had a limited efficacy for large tumors and major portal invasion, leaving a rather narrow range of candidates for RFA in BCLC stage B-C. We therefore recommend TACE for rHCC patients in BCLC stage B-C with MVI-positive at initial resection, if the patient was not candidate to RFA.
Several limitations should be considered in this article. First, with BCLC stage C included, this article did not make a comparison with Sorafenib treatment. Sorafenib is of high cost and is not included in national medical insurance. The proportion of patients receiving Sorafenib is quite low due to the limited cost-effectiveness, making the retrospective data of Sorafenib treatment very finite. Second, patients with MVI-negative at first resection were not included and compared in this study. As previous reported, positive rates for MVI varies from 15% to 74.4% in different researches [17,27]. The detection of MVI relies on the number and location of pathological sections. And with no guideline or common instruction on biopsy for MVI, false MVI-negative was unavoidable and therefore could cause severe bias. Last but not least, indications of TACE and curative treatments are quite different. Especially in BCLC stage B-C, the characteristics of patients might be diverse even between RR and RFA. We tried to diminish the bias by separating RR and RFA, and applying PSM analysis for comparison. Yet the bias of treatment assignment is hard to dispel even after PSM. Thus, conclusions of this article need further validation by prospective studies. Despite these limitations, this article does provide valuable evidence for the management on rHCC with MVI-positive at first resection.
In summary, for rHCC with MVI-positive at first resection in BCLC stage 0-A, curative treatments had a better efficacy. While in BCLC stage B-C, TACE shared a similar efficacy with the curative treatments but lower cost.
Acknowledgements
This work is supported by the Science and Technology Program of Guangzhou, China (201704020215), the Science and Technology Program of Guangzhou, China (201704020099), the National Natural Science Foundation of China (NSFC, No. 81770608), and the Kelin Outstanding Young Scientist of the First Affiliated Hospital, Sun Yat-sen University (2017). All co-authors have seen and agreed with the content of the manuscript and there is no financial interest to report. We thank the Clinical Trials Unit, the First Affiliated Hospital of Sun Yat-sen University for providing statistical support for this study.
Disclosure of conflict of interest
None.
References
- 1.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Furst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topor-Madry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJ, Naghavi M, Fitzmaurice C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J, Zou R, Shen J, Li B, Yuan Y. Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin Gastroenterol Hepatol. 2018;16:756–764. e710. doi: 10.1016/j.cgh.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 7.Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther. 2016;16:1063–1072. doi: 10.1080/14737140.2016.1226136. [DOI] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou YF, Li B, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LV, Chen KF. Second hepatectomy improves survival in patients with microvascular invasive hepatocellular carcinoma meeting the milan criteria. Medicine. 2015;94:e2070–2078. doi: 10.1097/MD.0000000000002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meniconi RL, Komatsu S, Perdigao F, Boëlle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454–462. doi: 10.1016/j.surg.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, Shin WY. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056–1064. doi: 10.1111/jgh.12507. [DOI] [PubMed] [Google Scholar]
- 12.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, Schirmacher P, Vilgrain V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018:1–55. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Joliat GR, Allemann P, Labgaa I, Demartines N, Halkic N. Treatment and outcomes of recurrent hepatocellular carcinomas. Langenbecks Arch Surg. 2017;402:1–8. doi: 10.1007/s00423-017-1582-9. [DOI] [PubMed] [Google Scholar]
- 14.Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, Lin XJ, Lau WY. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol. 2015;63:122–130. doi: 10.1016/j.jhep.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44:846–853. doi: 10.1111/hepr.12196. [DOI] [PubMed] [Google Scholar]
- 17.Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suenaga M, Sugiura H, Kokuba Y, Uehara S, Kurumiya T. Repeated hepatic resection for recurrent hepatocellular carcinoma in eighteen cases. Surgery. 1994;115:452–457. [PubMed] [Google Scholar]
- 22.Wang DY, Liu L, Qi XS, Su CP, Chen X, Liu X, Chen J, Li HY, Guo XZ. Hepatic re-resection versus transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma after initial resection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16:5573–5578. doi: 10.7314/apjcp.2015.16.13.5573. [DOI] [PubMed] [Google Scholar]
- 23.Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68:977–993. doi: 10.1002/hep.29883. [DOI] [PubMed] [Google Scholar]
- 24.Qi X, Wang D, Su C, Li H, Guo X. Hepatic resection versus transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2015;6:18715–18733. doi: 10.18632/oncotarget.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, Qian CN, Zheng L. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878–4889. doi: 10.1002/cncr.26137. [DOI] [PubMed] [Google Scholar]
- 26.Hou YF, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LN, Li B, Chen KF. Microvascular invasion patterns affect survival in hepatocellular carcinoma patients after second hepatectomy. J Surg Res. 2016;200:82–90. doi: 10.1016/j.jss.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]