Abstract
Temporomandibular joint (TMJ) arthritis causes severe debilitation and has few treatment options. Here, we found a small molecule, DNA methyltransferase 3B (Dnmt3b), as a putative therapeutic target, partially rescued osteoarthritic phenotype. Dnmt3b was detected differentially expressed in cell zones of mandibular condylar cartilage and the expression of Dnmt3b decreased in the progression of TMJ osteoarthritis. Dnmt3b deficiency using conditional knockout mice led to the onset of osteoarthritis-like conditions including cartilage clefts, cartilage matrix loss and premature chondrocyte hypertrophy, which suggested that Dnmt3b functioned as a osteoarthritis suppressor. Dnmt3b gain-of-function in TMJ stem/progenitor cells showed increases in collagen type II but decreases in collagen type X, whereas Dnmt3b knockdown had opposite effects with attenuated collagen type II but increased collagen type X. Dnmt3b acted via Wnt/β-catenin signaling and Dnmt3b regulated TMJ stem/progenitor cells differentiation by inducing their premature progression towards hypertrophic chondrocytes through β-catenin transnucleation and activation. Finally, local Dnmt3b delivery partially rescued cartilage degradation in experimentally induced osteoarthritis. Thus, novel molecules in articular cartilage, such as Dnmt3b, may have therapeutic effects for TMJ osteoarthritis.
Keywords: Temporomandibular joint, osteoarthritis, methyltransferase, hypertrophy
Introduction
The temporomandibular joint (TMJ) is a unique synovial articulation that controls jaw movements to enable us to masticate and speak. TMJ osteoarthritis (OA) is a common degenerative joint disease that causes orofacial pain and mandibular movement dysfunction, resulting in disability in normal daily activities [1]. The major characteristic of TMJ-OA is abnormal hypertrophic differentiation of articular chondrocytes, leading to progressive degeneration of cartilage and pathologic change of subchondral bone [2]. Although there are ongoing efforts to study the pathogenesis of TMJ-OA and to develop potential therapeutic intervention impeding TMJ-OA progression [3], the underlying cellular and molecular mechanisms of OA are still unclear.
Epigenetics, the study of changes in organisms caused by modification of gene expression without alteration in DNA sequence, is one of the fastest-growing fields in biomedical research [4,5]. DNA methylation is the first recognized and most well characterized epigenetic modification that plays crucial roles in multiple biological processes [6,7]. In mammalian cells, DNA methylation involves the addition of methyl group to DNA at the C5 position of CpG dinucleotides. Methylation of specific CpG sites in the promoter region of genes typically results in silencing of gene expression [8,9]. During development and differentiation, alterations in the DNA methylation that can switch genes on or off play a crucial role in the generation of tissue-specific gene expression profiles.
DNA methylation are controlled at several different levels and carried out by a family of enzymes called DNA methyltransferase (DNMTs), including Dnmt1, Dnmt3a and Dnmt3b [10]. DNMTs are required for maintenance and establishment of DNA methylation patterns and essential for development and homeostasis [11,12]. Aberrant DNA methylation causes multiple diseases including osteoarthritis [13]. Genome-wide DNA methylation studies have identified significant DNA methylation in osteoarthritic chondrocytes [14-16]. Despite their implications in osteoarthritis, the roles of DNMTs in the onset and development of osteoarthritis are elusive. Furthermore, little is known whether DNMTs may rescue osteoarthritis phenotype, given their involvement in the onset of the diseases. In the present study, we discovered that Dnmt3b differentially expressed during condylar cartilage chondrogenesis and expression of Dnmt3b decreased in the progression of TMJ osteoarthritis. We show that Dnmt3b not only plays crucial roles in the pathogenesis of osteoarthritis, but also partially rescues disease progression using multiple stem/progenitor cells and in vivo models.
Materials and methods
Mice
All animal handling and procedures in this study were performed in accordance with institutional guidelines, and approved by Columbia University Institutional Animal Care and Use Committee (IACUC). Postnatal-7-day and 12-week-old CD-1 mice were purchased from Charles River Laboratory. The conditional Dnmt3b knockout mice Agc1CreERT2; Dnmt3bf/f (Dnmt3b-/-) were given as generous gifts from the laboratory of Dr. Regis J O’Keefe. Dnmt3b gene knockout is chondrocyte specific and tamoxifen (TM)-inducible, generated by crossing the Agc1CreERT2 mice with the Dnmt3bf/f mice. Dnmt3bf/f mice and Cre-positive and negative control mice were administered TM (1 mg/10 g body weight, IP, daily for 5 days) at 2 month of age. Mice were killed 5 month after TM induction for histological analysis.
Surgically induced TMJ-OA rabbit model
TMJ-OA was surgically induced in 3-4 month old New Zealand white rabbits as previously described [17]. Briefly, TMJ condyle and disc were exposed by surgical incision superior to the zygomatic process under general anaesthesia. A 2.5 mm defect was created using a punch biopsy in the TMJ disc. No disc attachments were severed and the surgical incision was closed with sutures. Sham operation was performed on the contralateral side, whereby the TMJ disc was accessed, but without any perforation. Rabbits were sacrificed at 4 and 8 weeks after surgery for immunohistochemistry.
Chemically induced TMJ-OA rat model
12-weeks-old Spargue-Dawley rats were deeply anesthetized with 3-5% isoflurane in 100% O2 (1 L/min) and randomly assigned to 3 groups (n = 6/group). The synovial compartments of bilateral TMJ were injected by 50 ul saline for sham group, or 0.5 mg monosodium iodoacetate (MIA, Sigma) dissolved in 50 ul saline for OA group, or 0.5 mg/50 ul monosodium iodoacetate and 100 ul Dnmt3b virus (a total of 1 × 106 infectious particles) for treatment group, using a 26-gauge 0.5-inch needle. Rats were sacrificed at 4 weeks postoperatively, and TMJ tissues were harvested and processed for histological analysis.
Histochemical analysis and quantificational evaluation
TMJ specimens from experimental animals were dissected, fixed overnight in 10% formalin, decalcified with EDTA, dehydrated and embedded in paraffin. 5 μm-thick tissue sections were cut and collected on slides. Sections were stained with HE and Safranin-O using standard protocol for histological observations. For immunohistochemistry, HRP-DAB Cell & Tissue Staining Kit (R&D Systems) was used according to the manufacturer’s instructions. Briefly, tissue sections were deparaffinized, rehydrated and heat-retrieved, followed by blocking with appropriate serum and incubation with primary antibodies: Dnmt1 (ab19905 1:200), Dnmt3a (ab23565, 1:200), Dnmt3b (ab2851 1:400), Ki67 (ab15580 1:200), Col II (ab34712 1:400), Col X (ab58632 1:200), β-catenin (ab16051 1:400) overnight at 4°C. After PBS wash, sections were incubated with HRP conjugated secondary antibodies. Immunoreactivity was detected with DAB followed by counterstaining with hematoxylin. Sections were mounted and visualized under microscope.
Quantitative histological assessments were done by Image J. All parameters were determined and averaged from three sections per mouse and five mice per group. Staining intensity of Dnmt3b was analyzed using color deconvolution feature of Image J. Safranin-O positive staining area was outlined and quantified on projected images of each histologic section to determine articular cartilage area and thickness. To analyze the proliferation of chondrocytes, the number of Ki67 immunopositeve cells was counted per unit area. To quantify changes in hypertrophic chondrocytes, the immunopositive area of Col II and Col X were measured from representative sections of each group.
Cell isolation and culture
Progenitor/stem cells were isolated from mandibular condylar cartilages of 10-week-old mice. Cartilage tissues were dissected with fine forceps under the microscope, and enzymatically digested with 3 mg/ml collagenase type I and 4 mg/ml dispase (Gibco) in 1X PBS for 3 hours at 37°C. The dissociated single cell suspension was filtered through a nylon mesh (70 mm pore size, BD Falcon) and cultured in basal medium consisting of a-MEM supplemented with 10% FBS and antibiotics. After one week, cells were passaged and maintained with medium change every 2-3 days.
Micromass culture
Cells were harvested and resuspended at a density of 2*107 cells/ml. 5 ul droplets of cell solution were seeded in the center of 12-well plate. Following incubation at 37°C for 2 h, micromass was generated and adhered to the culture surface. 1 ml chondrogenic differentiation media (Invitrogen) were then added and changed every 2-3 days. Micromasses were harvested at 1 w, 2 w and 3 w for Safranin O and Alcian Blue staining, gene expression analysis and protein detection.
Overexpression and knockdown of Dnmt3b in progenitor/stem cells
For Dnmt3b overexpression, pWPI lentiviral vector (Plasmid #12254, Addgene) encoding Dnmt3b gene was constructed. pWPI or pWPI-Dnmt3b, pMD2.G (Plasmid #12259, Addgene) and psPAX2 (Plasmid #12260, Addgene) vectors were co-transfected into 293T cells using Calcium Phosphate Transfection Kit per manufacturer’s protocol (Invitrogen). Viral harvest was collected 48-72 h post-transfection and filtered with 0.45 μm membrane. TMJ progenitor/stem cells were infected either with pWPI lentivirus (Control group) or pWPI-Dnmt3b lentivirus (Dnmt3b overexpression group), with addition of 8 ug/ml polybrene to increase the infection efficiency. Infected cells were sorted by FACS based on GFP reporter gene expression and were further passaged for following experiments. For knocking-down Dnmt3b, TMJ progenitor/stem cells were transfected by adding the Dnmt3b shRNA lentiviral particles following manufacturer’s protocol (Santa Cruz). Cells stably express the shRNA of Dnmt3b (Dnmt3b knockdown group) were sorted by addition of 6 ug/ml puromycin after 3-5 days and expanded for further experiments.
Real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen) and converted to cDNA with iScriptTM cDNA Synthesis Kit (Bio-Rad) according to manufacturer’s protocol. Primers were designed as follows: Col II, forward 5’-AGCAAGAGCAAGGAAAAGAAA-3’, reverse 5’-GTGGACAGTAGACGGAGGAAA-3’; Col X, forward 5’-TTTCTGCTGCTAATGTTCTTG-3’, reverse 5’-AATCCCTTTACTCTTTATGGC-3’; Dnmt3b, forward 5’-CTAAGAGCGTCAGTACCCCATC-3’, reverse 5’-CACGAGGTCACCTATTCCAAA-3’; Gapdh, forward 5’-AATGGATTTGGACGCATTGGT-3’, reverse 5’-TTTGCACTGGTACGTGTTGAT-3’. Real-time PCR was performed using SYBR method by a 7500 Real-Time PCR system (Applied Biosystems). Gene expressions were normalized to Gapdh and analyzed by 2ΔΔCt method. The experiments were repeated at least three times.
Western blot
Total protein was extracted in RIPA Lysis Buffer (Thermo Scientific) supplied with Protease/Phosphatase Inhibitor Cocktail (Cell Signaling Technology). Whole lysate protein concentrations were determined with BCA Protein Assay Reagent (Thermo Fisher Scientific). Proteins were separated by a NuPAGE Novex 4-12% Bis-Tris Protein Gel (Invitrogen), transferred onto nitrocellulose membrane (Bio-Rad) and immunoblotted with anti-Dnmt3b (Abcam 1:200) antibody. Bound antibody detection was visualized by IR fluorescence & Odyssey using corresponding secondary antibody (LI-COR).
Luciferase reporter assay
A total of 105 cells were cotransfected with 3 ug Firefly luciferase reporter plasmid Topflash and 10 ng Renilla luciferase control plasmid by Lipofectamine 2000 (Invitrogen). After 48 h transfection, cells were lysed and the transcriptional activity of β-catenin was measured by using Dual-Luciferase® Reporter Assay System (Promega) per manufacturer’s instructions. Relative luciferase activity was indicated by normalizing Firefly luciferase activity against Renilla luciferase activity.
Immunocytofluorescence
Cells grown on coverslips were fixed with 4% paraformaldehyde for 15 min, treated with 0.5% Triton X-100, and blocked with blocking buffer for 1 h, followed by incubation with β-catenin antibody (Abcam 1:200) overnight at 4°C. After PBS washing, cells were incubated with FITC-conjugated secondary antibody (Invitrogen) for 30 min. Coverslips were mounted with mounting medium containing DAPI (Abcam) and fluorescent images were observed by fluorescence light microscope (Olympus).
Statistical analysis
All experiments were performed at least three times. All quantitative data presented as mean ± SD were statistically analyzed by Student’s t-test or one-way ANOVA using SPSS software. Any p values <0.05 were considered statistically significant.
Results
Dnmt3b expression patterns and potential roles in experimentally induced osteoarthritic
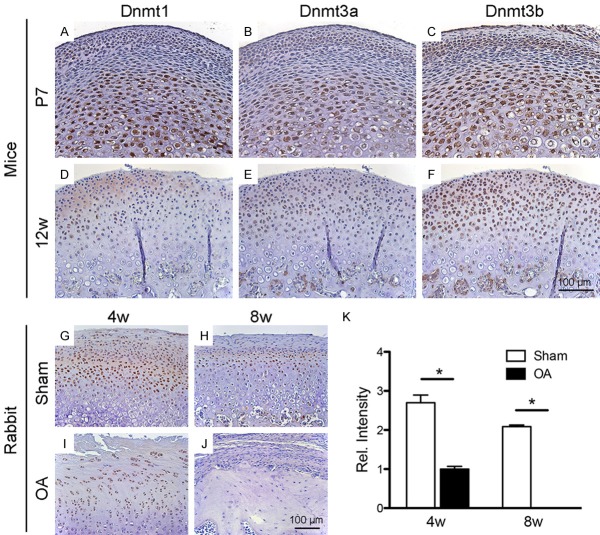
In neonatal stage at P7, Dnmt1, Dnmt3a and Dnmt3b were detected in articular and hypertrophic zones of wild-type condyles, but absent in the mature zone (Figure 1A-C). Dnmt3b showed particular robust expression (Figure 1C). In the adult of 12-week-old mice, Dnmts were expressed throughout cartilage, again with Dnmt3b robustly expressed (Figure 1F), whereas Dnmt1 and Dnmt3a only modestly expressed (Figure 1D, 1E). Given Dnmt3b’s robust expression in both neonatal and adult stages (Figure 1C, 1F), we further interrogated its expression in our existing induced osteoarthritis rabbit model in vivo by surgical perforation of the articular disc dinucleotides [6,7]. Strikingly, Dnmt3b diminished postoperatively in 4- and 8-wks, coinciding with development of osteoarthritis phenotype (Figure 1I, 1J) in comparison to controls compared to the sham group (Figure 1G, 1H). Quantitatively, Dnmt3b expression showed significantly decreases by 4 wks following experimentally induced OA and diminished by 8 wks (Figure 1K). Thus, Dnmt3b likely plays significant roles in cartilage development and OA onset.
Figure 1.
Dnmt3b expression patterns in normal and osteoarthritic TMJ condyle. A-F. Immunohistochemistry of Dnmts (Dnmt1, Dnmt3a, Dnmt3b) showed similar expression pattern but different expression intensity in TMJ condyle of postnatal-day-7 and 12-week-old mice. In P7 mice, Dnmts were expressed in articular and hypertrophic zones during condylar cartilage development. In 12-week-old mice, Dnmts were expressed throughout all the condylar cartilage. Dnmt3b was strongly expressed, while Dnmt1 and Dnmt3a were moderately expressed. G-J. Immunohistochemistry of Dnmt3b in 3-4 month old rabbit TMJ condyle at 4 weeks and 8 weeks after TMJ-OA or Sham operated. Dnmt3b expression was decreased during TMJ-OA cartilage degeneration. K. Quantification of Dnmt3b expression. Scale bar = 100 µm.
Dnmt3b deficiency induced osteoarthritis phenotype
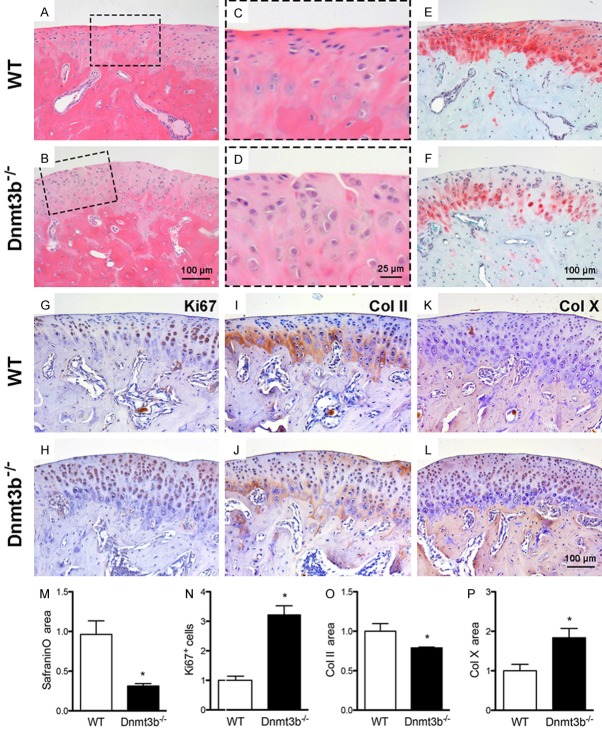
We then adopted Agc1-Cre-ERT2; Dnmt3bf/f mice (Dnmt3b-/-) to appreciate Dnmt3b’s roles in cartilage degeneration. At 5 months after TM induction, osteoarthritic phenotype was readily observed in TMJ condylar cartilage, including surface fibrillation and vertical clefts in Dnmt3b-/- mice (Figure 2A-D). Loss of glycosaminoglycans was observed in Dnmt3b-/- condyles relative to controls (Figure 2E, 2F), and quantitatively verified in Figure 2M. Ki67 positive cells showed marked increases in Dnmt3b-/- condyles, relative to the control (Figure 2G, 2H), as quantitatively verified in Figure 2N. Proteoglycan loss is associated with decreased Col II (Figure 2I, 2J) as quantitatively verified in Figure 2O, but increased Col X (Figure 2K, 2L), as quantitatively verified in Figure 2P.
Figure 2.
Dnmt3b deficiency induced osteoarthritis phenotype. A-F. Cartilage degeneration including cartilage cleft (dotted frame), loss of proteoglycan were observed in Dnmt3b-/- mice, indicated by HE and Safranin-O staining. G-L. Immunohistochemistry of Ki67, Col II and Col X in TMJ condyle from WT and Dnmt3b-/- mice. M-P. Quantification assay compared between WT and Dnmt3b-/- mice. Safranin-O positive area decreased 60%, Ki67 expression increased 3 fold, Col II ex pression decreased 20% and Col X expression increased 1.8 fold in Dnmt3b-/- mice compared to the age-matched WT mice. Scale bar = 100 µm.
Dnmt3b inhibited chondrogenic differentiation of TMJ progenitor/stem cells
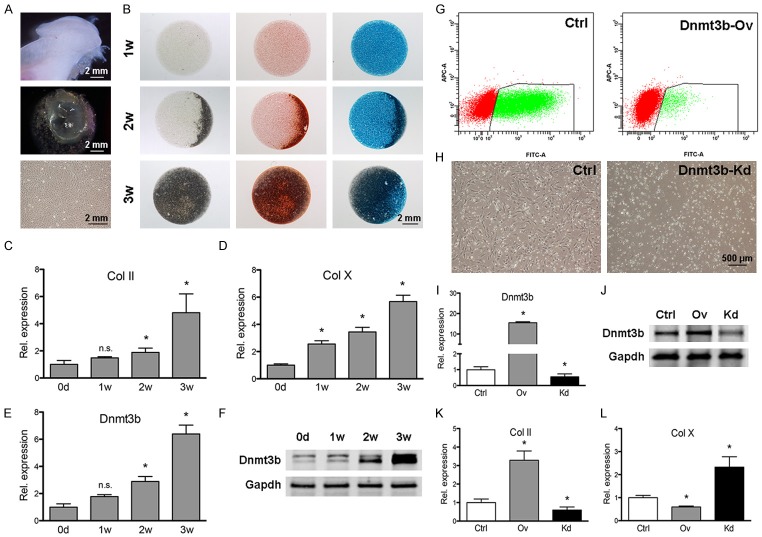
We then isolated stem/progenitor cells from TMJ cartilage of 10-week-old mice [18-20] and manipulated Dnmt3b expression to appreciate how it may mediate cell behavior. We induced TMJ stem/progenitor cells to undergo chondrogenesis in micromass culture system (Figure 3A). TMJ progenitor/stem cells formed a cartilage-like sphere enriched with abundant cartilage matrix (Figure 3B) and the mRNA and protein expressions of Col II and Col X were significantly upregulated during chondrogenic differentiation (Figure 3C-F). Following confirmation of Dnmt3b overexpressed and knockdown by qPCR and Western-blot analyses (Figure 3G, 3H), we first found that Dnmt3b overexpression upregulated Col II but attenuated Col X (Figure 3I, 3J). Conversely, Dnmt3b knockdown enhanced chondrogenic hypertrophy by downregulation of Col II but upregulation of Col X (Figure 3K, 3L).
Figure 3.
Dnmt3b inhibited TMJ progenitor/stem cell differentiation. A. TMJ progenitor/stem cells were isolated from mandibular condylar cartilages of 10-week-old mice and cultured in vitro. B. TMJ progenitor/stem cells were micromass cultured followed by chondrogenic differentiation, assayed by Safranin-O and Alcin blue staining at 1 w, 2 w and 3 w. Scale bar = 2 mm. C-F. Col II, Col X and Dnmt3b mRNA expression and Dnmt3b protein expression were upregulated during chondrogenic differentiation, detected by qRT-PCR and western blot analysis. G, H. TMJ progenitor/stem cells were infected with control, Dnmt3b overexpression (Dnmt3b-Ov) or Dnmt3b shRNA (Dnmt3b-Kd) lentivirus, followed by cell line selection using FACS or puromycin method. Scale bar = 500 µm. I, J. Dnmt3b overexpression and knockdown efficiency were determined at the mRNA and protein levels. K, L. Dnmt3b knockdown enhanced chondrogenic hypertrophy, by downregulation of Col II and upregulation of Col X, while Dnmt3b overexpression had the opposite effects.
Dnmt3b regulated chondrogenic differentiation through Wnt/β-catenin signaling pathway
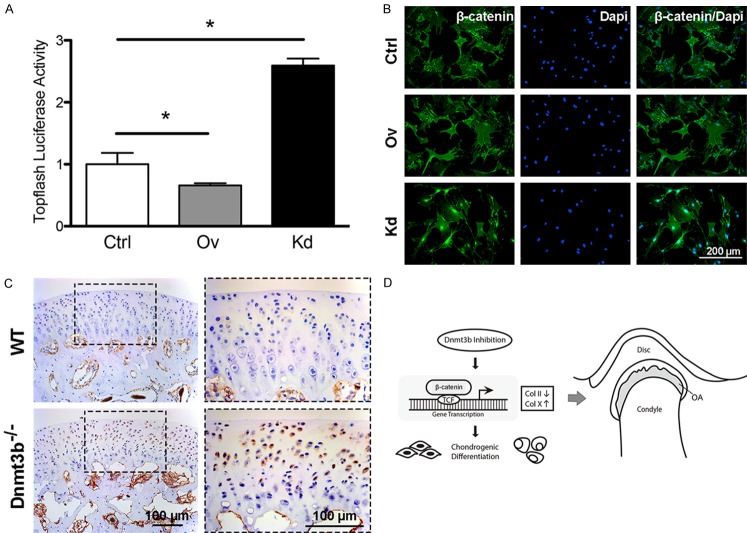
Given the importance of Wnt signaling in chondrogenic differentiation and osteoarthritis [21], we probed Wnt/β-catenin and surprisingly found that Dnmt3b overexpression decreased Topflash luciferase activity and suppressed β-catenin nuclear translocation, whereas Dnmt3b knockdown increased Topflash luciferase activity and β-catenin nuclear translocation relative to controls (Figure 4A, 4B). Additionally, β-catenin expression was enhanced in Dnmt3b-/- mice compared to controls (Figure 4C). Accordingly, we proposed a model in which Dnmt3b attenuation induced premature chondrogenic hypertrophy through Wnt/β-catenin activation and onset of TMJ osteoarthritis (Figure 4D).
Figure 4.
Dnmt3b regulated chondrogenic differentiation through Wnt/β-catenin signaling pathway. A. Dnmt3b overexpression in TMJ progenitor/stem cells led to decreased Topfalsh luciferase activity while Dnmt3b knockdown showed significantly stronger Topfalsh luciferase activity compared to the control group. B. β-catenin nuclear translocalization was suppressed by Dnmt3b overexpression and activated by Dnmt3b knockdown, detected by immunocytofluorescence. C. β-catenin signal was much stronger in condylar cartilage of Dnmt3b-/- mice than WT mice. D. A proposed diagram for the role of Dnmt3b in TMJ-OA development. Inhibition of Dnmt3b induced TMJ-OA by enhancing chondrogenic hypertrophy through activation of β-catenin signaling pathway. Scale bar = 100 µm.
Local Dnmt3b delivery partially rescued experimentally induced osteoarthritis
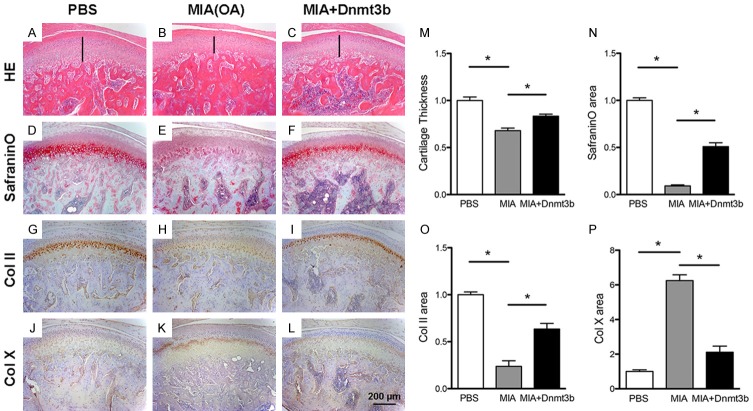
To appreciate Dnmt3b putative roles in osteoarthritis, we adopted an existing chemically induced experimental rat OA model by intra-articular monosodium iodoacetate (MIA) injection [22], with intra-articular PBS injection as a control (Figure 5A, 5B). Indeed, intra-articular MIA injection led to chemically induced OA with cartilage degeneration in 4 wks, characterized by reduced cartilage thickness compared to the sham (Figure 5B, 5M). Contrastingly, local virus delivery with Dnmt3b partially rescued cartilage thickness (Figure 5C), as quantitatively verified in Figure 5M. Safranin-O positive proteoglycans were present in PBS injected control cartilage (Figure 5D), markedly lost in MIA injected OA cartilage (Figure 5E), and partially restored in Dnmt3b injected sample (Figure 5F), as quantitatively verified in Figure 5N. In parallel, Type II collagen was abundant in PBS injected control cartilage (Figure 5G), markedly lost in MIA injected OA cartilage (Figure 5H), and partially restored in Dnmt3b injected sample (Figure 5I), as quantitatively verified in Figure 5O. Conversely, Type X collagen was scarce in PBS injected control cartilage (Figure 5J), markedly enhanced in MIA injected OA cartilage (Figure 5K), and only modestly expressed in Dnmt3b injected sample (Figure 5L), as quantitatively verified in Figure 5P.
Figure 5.
Local Dnmt3b delivery partially rescued experimentally induced osteoarthritis. A-L. Representative staining of rat condylar cartilage with HE, Safranin-O and immunostaining of Col II and Col X. Rats were treated with PBS (Sham group), MIA (OA group), or MIA+Dnmt3b (Treatment group) in the synovial compartment of TMJ. MIA induced OA phenotype with thinner cartilage layer, loss of proteoglycan, downregulated Col II and upregulated Col X, which was attenuated by treatment of Dnmt3b. Scale bar = 200 µm. M-P. Quantification assay of cartilage thickness, Safranin-O staining and immunostaining of Col II and Col X.
Discussion
Osteoarthritis (OA) is a degenerative joint disease characterized by progressive loss of cartilage matrix, cartilage clefts and subchondral bone breakdown. TMJ arthritis remains a poorly understood cluster of diseases, and hence, clinical management is palliative. Epigenetic changes occur during chondrogenesis and in osteoarhritis [23]. In the present study, we identified DNA methyltransferase 3B (Dnmt3b) as key regulator in chondrogenic differentiation and TMJ OA patheogenesis. We showed that Dnmt3b deficiency enhanced chondrocyte proliferation and premature hypertrophy of TMJ stem/progenitor cells and induced the onset of TMJ OA, indicating that Dnmt3b maintains chondrocyte homeostasis and inhibits pathological hypertrophic differentiation. We also showed for the first time that DNA methyltransferase 3B (Dnmt3b), as a putative therapeutic target, partially rescued osteoarthritic phenotype.
Wnt/β-catenin signaling is critically involved in cartilage homeostasis and associated with osteoarthritis pathogenesis [24,25]. Upregulation of Wnt/β-catenin signaling in articular chondrocytes of knee joint or TMJ enhanced hypertrophic differentiation and induced the onset of OA [26,27]. It was reported that Dnmt3b interacted with Wnt/β-catenin signaling in hematopoietic stem cells [28], providing us new insights into a possible mechanism behind the relationship between Dnmt3b and Wnt/β-catenin signaling. In the present study, we found that Dnmt3b attenuation activated β-catenin nuclear translocation both in vitro and in vivo, providing clues to the importance of Wnt signaling in TMJ arthritis. Further investigations are warranted to unveil the DNA methylation of Wnt/β-catenin signalling pathway related genes in our further prospective studies.
TMJ-OA animal models are well accepted in investigating the pathophysiology during the progression of TMJ-OA [3,22,29]. In this study, we generated biochemically induced TMJ-OA model and treated by local delivery with Dnmt3b. We found that Dnmt3b prevented TMJ-OA progression by repressing chondrocyte hypertrophic differentiation, suggesting that Dnmt3b played a protective role in cartilage maintenance and promoted cartilage repair during TMJ-OA development. Currently, there are several studies of treatment strategies for TMJ-OA. For example, intra-articular injection of TGF-β1 was reported to increase the cartilage matrix and protect subchondral bone form damage in TMJ-OA [30]. A comparative study demonstrated that intra-articular injection of NEL-like molecule-1 (Nell-1) into the TMJ had chondroprotective effects by upregulation of type II collagen and aggrecan [31]. Our study provided new insight into Dnmt3b as an alternative therapeutic intervention for TMJ-OA and the benefits of this treatment remain to be further evaluated.
In summary, Dnmt3b appears to play important roles in cartilage homeostasis and the onset and progression of osteoarthritis. Dnmt3b, as a putative therapeutic target, partially rescued osteoarthritic phenotype.
Acknowledgements
The work is supported by NIH grants R01DE025643, R01DE023112, R01AR065023 and R34DE026645 to J. J. Mao, National Natural Science Foundation of China grants 81371136 to X. Zhou, and National Natural Science Foundation of Tongji University affiliated stomatological hospital grants 20183004 to Y. Zhou. We acknowledge Dr. Mildred Embree for technical support and providing the rabbit TMJ OA samples.
Disclosure of conflict of interest
None.
References
- 1.Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 2.Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis Cartilage. 2005;13:817–827. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94:666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 4.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 7.Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2:607–617. doi: 10.1177/1947601910393957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 9.Siegfried Z, Eden S, Mendelsohn M, Feng X, Tsuberi BZ, Cedar H. DNA methylation represses transcription in vivo. Nat Genet. 1999;22:203–206. doi: 10.1038/9727. [DOI] [PubMed] [Google Scholar]
- 10.Hermann A, Gowher H, Jeltsch A. Biochemistry and biology of mammalian DNA methyltransferases. Cell Mol Life Sci. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PubMed] [Google Scholar]
- 11.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 12.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann P, Boeuf S, Dickhut A, Boehmer S, Olek S, Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008;58:2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Tajes J, Soto-Hermida A, Vazquez-Mosquera ME, Cortes-Pereira E, Mosquera A, Fernandez-Moreno M, Oreiro N, Fernandez-Lopez C, Fernandez JL, Rego-Perez I, Blanco FJ. Genome-wide DNA methylation analysis of articular chondrocytes reveals a cluster of osteoarthritic patients. Ann Rheum Dis. 2014;73:668–677. doi: 10.1136/annrheumdis-2012-202783. [DOI] [PubMed] [Google Scholar]
- 15.Jeffries MA, Donica M, Baker LW, Stevenson ME, Annan AC, Humphrey MB, James JA, Sawalha AH. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheumatol. 2014;66:2804–2815. doi: 10.1002/art.38762. [DOI] [PubMed] [Google Scholar]
- 16.Herlofsen SR, Bryne JC, Hoiby T, Wang L, Issner R, Zhang X, Coyne MJ, Boyle P, Gu H, Meza-Zepeda LA, Collas P, Mikkelsen TS, Brinchmann JE. Genome-wide map of quantified epigenetic changes during in vitro chondrogenic differentiation of primary human mesenchymal stem cells. BMC Genomics. 2013;14:105. doi: 10.1186/1471-2164-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embree MC, Iwaoka GM, Kong D, Martin BN, Patel RK, Lee AH, Nathan JM, Eisig SB, Safarov A, Koslovsky DA, Koch A, Romanov A, Mao JJ. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthritis Cartilage. 2015;23:629–639. doi: 10.1016/j.joca.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinton RJ. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev Dyn. 2014;243:864–874. doi: 10.1002/dvdy.24130. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–177. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, Koyama E. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka C, Yagi T. Electron microscopic observations on the fate of hypertrophic chondrocytes in condylar cartilage of rat mandible. J Craniofac Genet Dev Biol. 1988;8:253–264. [PubMed] [Google Scholar]
- 22.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Abu-Amer Y, O’Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58:49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi H. Regulation of osteoarthritis development by Wnt-beta-catenin signaling through the endochondral ossification process. J Bone Miner Res. 2009;24:8–11. doi: 10.1359/jbmr.081115. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Zhu M, Rosier RN, Zuscik MJ, O’Keefe RJ, Chen D. Beta-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010;1192:344–350. doi: 10.1111/j.1749-6632.2009.05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Li S, Xie W, Shen J, Im HJ, Holz JD, Wang M, Diekwisch TG, Chen D. Activation of beta-catenin signalling leads to temporomandibular joint defects. Eur Cell Mater. 2014;28:223–235. doi: 10.22203/ecm.v028a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challen GA, Sun D, Mayle A, Jeong M, Luo M, Rodriguez B, Mallaney C, Celik H, Yang L, Xia Z, Cullen S, Berg J, Zheng Y, Darlington GJ, Li W, Goodell MA. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell. 2014;15:350–364. doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 2003;3:391–394. discussion 406-397. [PMC free article] [PubMed] [Google Scholar]
- 30.Ying B, Chen K, Hu J, Man C, Feng G, Zhang B, Zhu S. Effect of different doses of transforming growth factor-beta(1) on cartilage and subchondral bone in osteoarthritic temporomandibular joints. Br J Oral Maxillofac Surg. 2013;51:241–246. doi: 10.1016/j.bjoms.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Xiao D, Hu J, Chen K, Man C, Zhu S. Protection of articular cartilage by intra-articular injection of NEL-like molecule 1 in temporomandibular joint osteoarthritis. J Craniofac Surg. 2012;23:e55–58. doi: 10.1097/SCS.0b013e3182418d02. [DOI] [PubMed] [Google Scholar]