Abstract
Papillary thyroid cancer (PTC) is the most common malignancy of the thyroid carcinoma, despite ongoing advances, novel biomarkers are required for prognosis and diagnosis of PTC. Our previous research found that metallothionein 1M (MT1M) was a novel potential PTC associated gene in thyroid cancer. In the present study, the expression status and prognostic value of MT1M expression were investigated in thyroid cancer. Tissue samples from 60 patients with PTC were subjected to the quantitative real-time polymerase chain reaction and the relative expression of MT1M in the patient tissue was evaluated. The Cancer Genome Atlas (TCGA) RNA-seq database was downloaded to further explore the role of MT1M in PTC and its relationship with lymph node metastasis (LNM). Logistic analysis showed that reduced expression of MT1M, histological type, and clinical stage are independent high-risk factors for LNM in PTC. The biological function of MT1M was also researched by using the PTC cell lines TPC-1, KTC1 and BCPAP. In vitro experiments revealed that MT1M upregulation significantly inhibits the colony formation, proliferation, migration, and invasion of PTC cell lines. We also found that MT1M could modulate the expression of N-cadherin and vimentin. These results implied that MT1M involved in the progress of thyroid cancer and might act as a tumor suppressor gene. In this study, we identified, for the first time, MT1M was involved in thyroid carcinoma cell lines This study specified a potential new marker and a target for gene therapy in thyroid cancer treatment.
Keywords: MT1M, PTC
Introduction
Thyroid cancer is one of the most widespread endocrine malignancy with 56,870 newly estimated diagnosed cases and 2010 estimated deaths in the United States in 2017 [1,2]. Papillary thyroid carcinoma (PTC), the most common histologic type, is the majority of all types of thyroid cancers [3]. A research reported that its incidence increased at a scale of 4% yearly and expected to become the 4th leading cancer diagnosis by the year 2030 [4]. In China, nearly 90,000 new cases and 6,800 deaths from PTC occurred in 2015 [5]. Papillary thyroid cancer (PTC), is the highest common subtype of differentiated thyroid cancer (DTC), about reports for 80%-85% of all thyroid cancers types [6].
Due to the widespread practice of neck diagnostic sonography, patients with PTC can be diagnosed at an early stage, besides, after surgery and radio-iodinated treatment patient can have an overall ten years survival rate above 90% [7]. While patients with PTC tend to have a decent prognosis, a portion of patients die from cancer metastasis and proliferation in a short time [8]. A study of genomics developed, considerable studies have examined the gene mutations could lead to the tumorigenesis and progression of thyroid cancer over the past two decades [9,10]. However, the underlying mechanisms of thyroid carcinoma remain unclear.
There are several characteristic genetic alterations, such as RAS [11], PTEN [12], and TETR mutations [13] that also plays a vital role in thyroid cancer progression. Among those famous mutations, B-type Raf kinase (BRAF) V600E mutations might promote PTC tumorigenesis and progression by atypically triggering the mi-togen-activated pathway kinase (MAPK) pathway [14]. Many clinical reports have showed an involvement of BRAF (V600E) mutation with aggressive clinicopathologic characteristics and high tumor recurrence [15]. Despite the great improvement in genetic study, the underlying mechanisms of PTC remain to be totally elucidated.
Members of the metallothionein (MT) family are small cysteine-rich protein superfamily, which has described mostly linked with cellular metabolism of metal ions. And, it shows an important role in metal detoxification and protects cells from certain electrophilic carcinogens [16]. In humans being, MTs family contains four key isoforms (MT1, MT2, MT3 and MT4) which are encoded by genes located on chromosome 16q13. And it includes at least 11 functional members: MT1 (MT1A, MT1B, MT1E, MT1F, MT1G, MT1H, MT1M and MT1X; MT1C, MT1D, MT1I, MT1J and MT1L are pseudogenes that cannot encode MT proteins), MT2 (also known as MT2A), MT3, and MT4 [17]. Metallothionein 1M (MT1M), a member of Metallothioneins (MTs), had emerging evidence shown that it plays a pivotal part in the regulation of cellular or pathological processes including several cancers [18]. For instance, down-expression of MT1M is linked with poor prognosis in esophageal squamous cancer [19], large cell lung cancer [20], hepatocellular carcinoma [21]. Those findings have been revealed MT1M might be an effective tumor suppressor gene in human cancer.
In our previous unpublished study, 19 paired PTC tissue samples and adjacent noncancerous samples were subjected to whole transcriptome sequencing and bioinformatics analysis to assess the mRNA expression profiles. And, we firstly discovered MT1M was most significantly down-regulated in thyroid cancer. To further evaluate the expression of MT1M, 60 pairs of papillary thyroid carcinomas with matched adjacent noncancerous tissues were collected to conduct qRT-PCR to validate. Our study aimed to discover the role of MT1M in the metastasis of thyroid carcinoma and to analyze the relationship of MT1M expression through clinical and molecular features of PTC. We also estimate the expression of MT1M in different thyroid cell lines (HTORI3, TPC-1, KTC1, BCPAP). We established that MT1M overexpression effectively inhibits the proliferation and invasion of three PTC cell lines (TPC-1, KTC1, BCPAP) in vitro. Up-regulation of MT1M by pcDNA in those cell lines decreased the N-cadherin and vimentin.
In a conclusion, we firstly demonstrated that MT1M plays a significant role in PTC progression and might become a potential target for biological implications.
Materials and methods
Patients and samples
We collected 60 fresh PTC tissue samples from the Department of Thyroid & Breast Surgery at The First Affiliated Hospital of Wenzhou Medical University between 2017-2018 and matched these with adjacent normal thyroid tissue samples as a validated cohort. None of the involved patients undertook preoperative managements, such as chemotherapy or radiotherapy. The samples were instantly achieved at the time of initial surgery and were frozen in liquid nitrogen instantly after lesion resection then stored at -80 Celsius before RNA extraction. All tumor tissues were histologically reviewed by two pathologists, and the cases were retrospectively reviewed by two senior pathologists to confirm the histological diagnosis. Informed consent for the scientific use of the biological material was obtained from each patient. All patient-derived information was recorded following the protocols approved by the ethical standards of the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (approval no. 2012-57).
The Cancer Genome Atlas (TCGA) data
RNA-Seq data of MT1M gene for PTC and related clinical information, including total 502 PTC samples and 58 non-cancerous thyroid tissues, were taken from TCGA databank. Data were taken from the TCGA data portal (https://tcgadata.nci.nih.gov/tcga/).
RNA extraction and quantitative RT-PCR (qRT-PCR)
The total RNA from the tissues was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol (Life Technologies, Carlsbad, CA). cDNA was prepared using a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan). qRT-PCR was performed in triplicate by utilizing Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) on the Applied Biosystems 7500 Real-time PCR system (Applied Biosystems, Foster City, CA) with. The GAPDH mRNA was used for normalization. The primer sequences for PCR are as follows: MT1M: forward primer 5’-AATAGAACAAGCTGCACAAC-3’; reverse primer: 5’-TGGCTCAGTATCGTATTGAA-3’; GAPDH: forward primer 5’-GGTCGGAGTCAACGGATTTG-3’; and reverse primer: 5’-ATGAGCCCCAGCCTTCTCCAT-3’.
Cell lines and cell culture
The human thyroid cancer cell lines TPC-1 and BCPAP were provided by Professor Mingzhao Xing of Johns Hopkins University School of Medicine (Baltimore, MA, USA). KTC-1 and HTORI3 cell lines were obtained from Stem Cell Bank, Chinese Academy of Sciences. Using the above cell lines were approved by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. These cell lines all were cultured in RPMI 1640 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 1 × MEM nonessential amino acids, and 1 × sodium pyruvate. All cells were maintained in a humidified incubator at 37°C with 5% CO2. TPC-1, KTC1, and BCPAP cells were plated onto six-well plates at concentrations of 4.5 × 105, 7.5 × 105 and 8 × 105 cells/well, respectively, and incubated for 24 hours in the growth medium.
Cell transfection
In order to overexpress MT1M, vectors which containing the full length of MT1M were assembled into CMV-MCS-EGFP-SV40-Neomycin. pcDNA for MT1M was purchased from Shanghai GeneChem (Shanghai, China). 4.5 × 105 (TPC-1), 7.5 × 105 (KTC1) and 8 × 105 (BCPAP) cells were plated in six-well plates overnight and transiently transfected using transfection reagent lipofectamine 3000 (Invitrogen).
Cell proliferation assay
Cell proliferation was assessed using the cell counting kit-8 (CCK-8, Beyotime, China) assay according to the manufacturer’s protocol. Concisely, 1500 cells/well for TPC-1 and KTC-1 cells and 2000 cells/well for BCPAP cells were plated into a 96 well plate. Following, in each well 10 ml CCK-8 solution was added. Subsequently after 4 hours of incubation time period at 37°C in the presence of 5% CO2, the incubation was terminated, after which the absorbance at 450 nm was measured with the help of a spectrophotometer at 24 hours, 48 hours, 72 hours, and 96 hours respectively after initial plating. For every group, data from 5 different wells were pooled. All the assays were performed in triplicate.
Colony formation assay
In the colony formation assay, the 3 treated and controlled cell lines (1.5 × 103 cells for TPC1 and KTC-1, 2 × 103 cells for BCPAP) were plated into 6-well plates, incubated for 8 to 14 days and later fixated with 4% PFA (paraformaldehyde; Sigma, USA) for 30 minutes. Afterwards, the cells were stained with 0.01% crystal violet for approximately 30 minutes. All the assays were performed in triplicate. Images were captured by a camera.
Cell migration and invasion assays
In the migration assays, we used Transwell chambers (Corning Costar Corp, Cambridge, MA, USA). On 48 hours after transfection, treated PTC cells (3 × 105 cells/0.1 mL in serum-free medium for TPC1 and KTC-1 cells, 3.5 × 105 cells for BCPAP cells) were shifted into the upper chamber, and the lower chamber contained 0.6 mL of medium supplemented with 20% FBS. Following 24 hours, a cotton swab was used to remove the cells that adhered to the upper surface of the well, and migratory cells on the lower surface were secured and stained with 0.4% crystal violet. The pictures were captured by photomicroscope. Then, an image was measured and statistically studied. In the invasion assays, we used BioCoatTM Matrigel Invasion Chambers for 24-well plates (8.0 mm) (Corning, NY, USA). The same technique described for the migration assay was done with the invasion chambers.
Western blot analysis
The protein of transfected cells lysates was lysed in cell lysis buffer (Beyotime, China). Protein concentrations were measured using a bicinchoninic acid assay (BCA). By using SDS-PAGE on a 10% gel and electro transferred to PVDF membranes total proteins in the lysate were removed. Using 5 percent skim milk (BD, DifcoTM Skim Milk, 232100) the membranes were blocked and incubated by primary antibodies overnight at 4°C. Afterward, the membranes were rinsed by TBST (3 times, 10 min/time), incubated with secondary antibodies (goat anti-rabbit IgG conjugated through HRP, Abcam, San Francisco, USA) at room temperature for 1 hour, and further rinsed with TBST (for 3 times, 10 minutes/time). Decisively, an ECL chromogenic substrate alongside HRP was used to picture the protein bands. The band intensity was quantified using Image Lab software. Actin served as an internal control.
Statistical analysis
Data on normal distribution were expressed as the mean ± SD and evaluated by Student’s t-test. Categorical variables were stated as percentages and compared by Fisher’s exact test or by chi-square test. The univariate and multivariate logistic regression analyses were performed to evaluate the relationship among MT1M expression and LNM (lymph node metastasis) in PTC. Each P-values were two-sided, and P < 0.05 was considered statistically significant. All of the statistical analyses were achieved using SPSS Version 23.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism Version 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
MT1M was significantly downregulated in PTC
RNA sequencing results of MT1M for PTC from 502 PTC samples plus 58 noncancerous thyroid tissues were downloaded from the TCGA database to verify the role of MT1M. Results showed MT1M was significantly down-regulated in PTC (P < 0.001, Figure 1) and it showed that MT1M might act as a tumor suppressor gene in PTC. To further confirm our conjecture, we evaluated the mRNA expression of MT1M in 60 paired PTC tissues and adjacent noncancerous thyroid tissues by RT-qPCR analysis. Accordingly, our findings indicated that MT1M expression was significantly downregulated in PTC tissues compared with that in the adjacent noncancerous thyroid tissues (P < 0.001, Figure 2).
Figure 1.
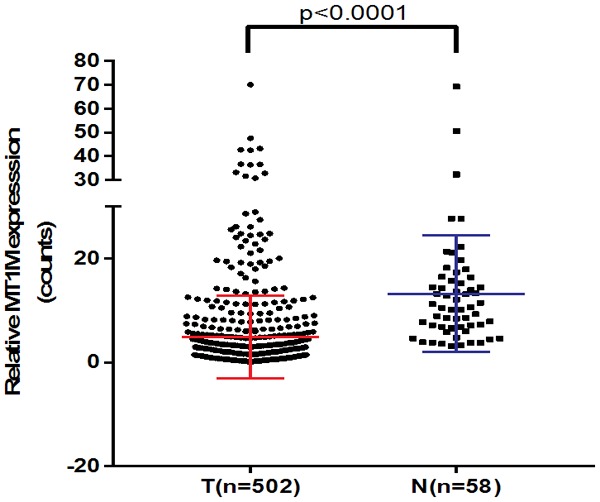
The mRNA expression of MT1M in the TCGA cohort. Including 502 PTC samples and 58 adjacent noncancerous thyroid samples, MT1M expression was also significantly downregulated in PTC in TCGA cohort (P < 0.001). Abbreviations: PTC, papillary thyroid carcinoma; TCGA, The Cancer Genome Atlas.
Figure 2.
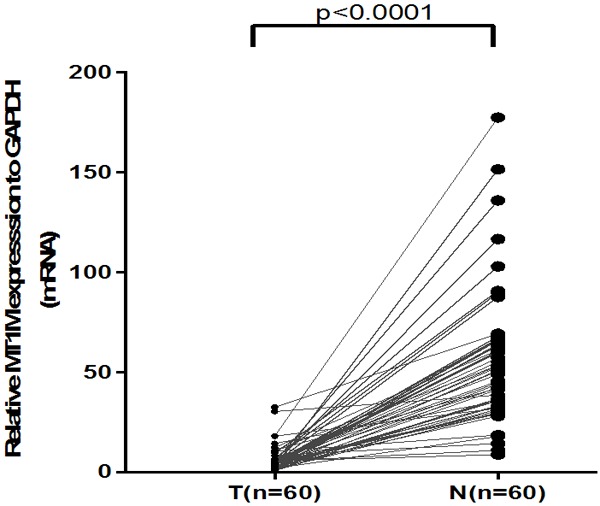
The mRNA expression of MT1M in our validated cohort (n = 60). MT1M expression was significantly downregulated in PTC tissues compared with the adjacent noncancerous thyroid tissues using qRT-PCR (P < 0.001).
MT1M expression was related to the clinicopathological features of PTC
In order to understand the association of MT1M in the progression of PTC, we researched the connection between the expression of MT1M and the clinicopathological features of PTC. As we divided TCGA cohort patients into lowest quartile MT1M expression (n = 125) and highest quartile MT1M expression (n = 125) groups according to MT1M expression. The decreased MT1M expression was associated with the histological type (P < 0.001), LNM (P < 0.001) (Table 1). No significant relations were found between MT1M expression and gender, age, tumor size, and multifocality. Accordingly, to our validated cohort, it also indicates that Lower MT1M expression was associated with the histological type (P = 0.045), LNM (P = 0.007) but disease stage (AJCC7) is not significant (P = 0.067) as shown in Table 2. Analyzing those results together, we considered that MT1M might serve as a tumor suppressor gene in PTC.
Table 1.
The Association between MT1M expression and clinicopathologic features in the TCGA cohort
| Clinicopathologic features | Lowest quartile (N = 125) | Highest quartile (N = 125) | X2 | P value |
|---|---|---|---|---|
| Gender | 0.02 | 0.886 | ||
| Female | 92 | 91 | ||
| Male | 33 | 34 | ||
| Age (years) | ||||
| Mean ± SD | 47.672 ± 15.184 | 48.264 ± 16.307 | 0.064 | 0.8 |
| ≤ 45 | 58 | 60 | ||
| > 45 | 67 | 65 | ||
| Histological type | 17.784 | < 0.001* | ||
| Classical | 101 | 70 | ||
| Other types | 24 | 55 | ||
| Multi-nodularity | 0.138 | 0.71 | ||
| Yes | 53 | 55 | ||
| No | 70 | 66 | ||
| Tumor size (mm) | 1.725 | 0.189 | ||
| ≥ 20 | 95 | 85 | ||
| < 20 | 30 | 39 | ||
| Lymph-node metastasis | 12.294 | < 0.001* | ||
| Yes | 43 | 77 | ||
| No | 68 | 48 | ||
| Distant metastasis | 0.095 | 0.758 | ||
| Yes | 6 | 5 | ||
| No | 119 | 120 | ||
| Disease stage (AJCC7) | 3.098 | 0.078 | ||
| I+II | 77 | 90 | ||
| III+IV | 47 | 34 |
*Chi-square test;
p value < 0.05.
Table 2.
The Association between MT1M expression and clinicopathologic features in the validated cohort
| Clinicopathologic features | Low Expression (N = 30) | High Expression (N = 30) | X2 | P value |
|---|---|---|---|---|
| Gender | 0.480 | 0.488 | ||
| Female | 24 | 26 | ||
| Male | 6 | 4 | ||
| Age (years) | ||||
| Mean ± SD | 48.53 ± 14.66 | 47.82 ± 14.98 | 0.635 | 0.426 |
| ≤ 45 | 13 | 10 | ||
| > 45 | 17 | 20 | ||
| Histological type | 4.022 | 0.045* | ||
| Classical | 25 | 18 | ||
| Other types | 5 | 12 | ||
| Multi-nodularity | 0.067 | 0.795 | ||
| Yes | 14 | 13 | ||
| No | 16 | 17 | ||
| Tumor size (mm) | 0.417 | 0.519 | ||
| ≥ 20 | 25 | 23 | ||
| < 20 | 5 | 7 | ||
| Lymph-node metastasis | 7.200 | 0.007* | ||
| Yes | 27 | 18 | ||
| No | 3 | 12 | ||
| Distant metastasis | 0.351 | 0.554 | ||
| Yes | 2 | 1 | ||
| No | 28 | 29 | ||
| Disease stage (AJCC7) | 3.354 | 0.067 | ||
| I+II | 26 | 20 | ||
| III+IV | 4 | 10 |
Chi-square test;
P value < 0.05.
Low MT1M expression was correlated with a high risk of LNM in PTC
Through logistic regression analysis, the association between MT1M expression and LNM was further evaluated. Univariate logistic regression analysis showed that MT1M expression (odds ratio [OR] = 0.421, 95% CI = 0.289-0.614, P < 0.001), Histological Type ([OR] = 2.383, 95% CI = 1.544-3.680, P < 0.001), Tumor size ([OR] = 2.525, 95% CI = 1.652-3.858, P < 0.001), Age ([OR] = 0.62, 95% CI = 0.427-0.899, P = 0.012), Gender ([OR] = 1.551, 95% CI = 1.022-2.353, P = 0.039) Disease stage (AJCC7) ([OR] = 3.493, 95% CI = 2.316-5.268, P < 0.001) were significantly related to LNM (Table 3). Multivariate logistic regression analysis validated that MT1M expression (OR = 0.489, 95% CI = 0.312-0.796, P = 0.002), Histological Type ([OR] = 2.351, 95% CI = 1.406-3.932, P = 0.001), Age ([OR] = 0.29, 95% CI = 0.09-0.097, P < 0.001) Disease stage (AJCC7) ([OR] = 58.103, 95% CI = 17.059-197.896, P < 0.001) were independent high risk factors of LNM (Table 4). Therefore, the low expression level of MT1M could promote the risk of LNM in PTC.
Table 3.
Univariate logistic regression analysis for the risk of lymph node metastasis
| Factor | OR | 95% CI | P-value |
|---|---|---|---|
| MT1M expression (high vs. low) | 0.421 | 0.289-0.614 | < 0.001* |
| Histological Type | 2.383 | 1.544-3.680 | < 0.001* |
| Tumor size (mm) | 2.525 | 1.652-3.858 | < 0.001* |
| Multi-nodularity | 1.446 | 0.994-2.103 | 0.054 |
| Age, years (≤ 45 vs. > 45) | 0.62 | 0.427-0.899 | 0.012* |
| Gender (male vs. female) | 1.551 | 1.022-2.353 | 0.039* |
| Disease stage (AJCC7) | 3.493 | 2.316-5.268 | < 0.001* |
P value < 0.05.
Table 4.
Multivariate logistic regression analysis for risk of lymph node metastasis
| Factor | OR | 95% CI | P-value |
|---|---|---|---|
| MT1M expression (high vs. low) | 0.489 | 0.312-0.796 | 0.002* |
| Histological Type | 2.351 | 1.406-3.932 | 0.001* |
| Tumor size (mm) | 1.321 | 0.776-2.250 | 0.305 |
| Age, years (≤ 45 vs. > 45) | 0.29 | 0.09-0.097 | < 0.001* |
| Gender (male vs. female) | 1.422 | 0.858-2.356 | 0.172 |
| Disease stage (AJCC7) | 58.103 | 17.059-197.896 | < 0.001* |
P value < 0.05.
MT1M regulates cell proliferation of PTC lines in vitro
To approve the function of MT1M in thyroid cancer, we evaluated thyroid expression level in several thyroid cancer cell lines and normal thyroid cell lines by using RT-qPCR. We discovered that the MT1M gene is expressed at higher levels in HTORI3 (normal thyroid cell line) than TPC-1, KTC1, and BCPAP (Figure 3A). So, we selected papillary thyroid cancer cell line as our experimental cell line. For additional analysis whether MT1M functions in thyroid cancer progression, we overexpressed MT1M expression in TPC-1, KTC1, and BCPAP cell lines using a pcDNA. As can be seen in Figure 3B, we have shown that the mRNA levels of MT1M were significantly overexpressed. We found that upregulated MT1M effectively inhibited TPC-1, KTC1and BCPAP cell line proliferation and colony formation (Figure 3C-F) compared with the control group.
Figure 3.
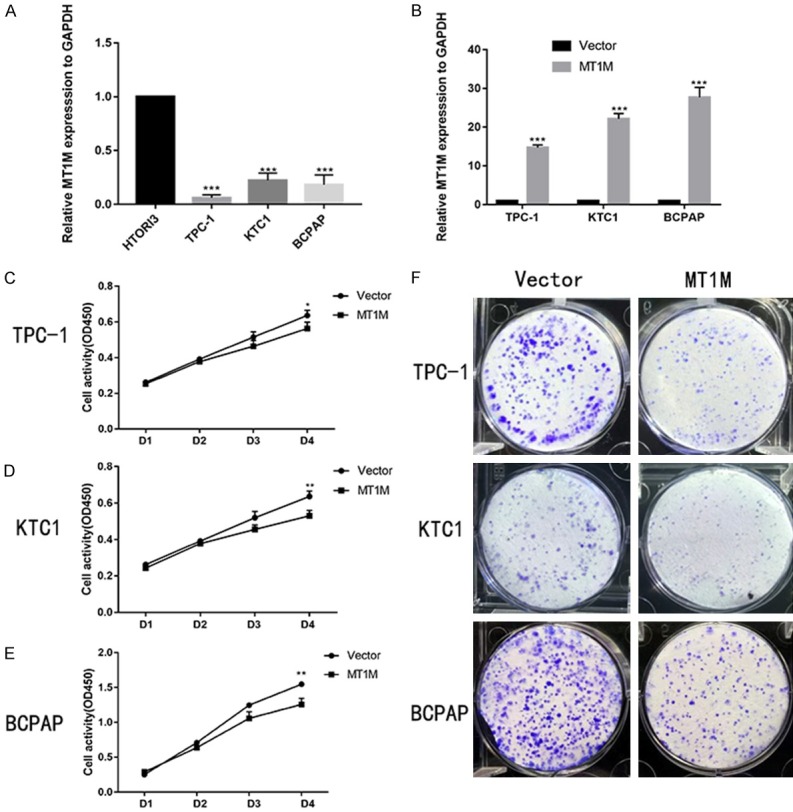
MT1M regulates cell proliferation of PTC lines in vitro. The relative expression of MT1M (compared with the GAPDH gene) was examined via RT-qPCR. MT1M gene is expressed at higher levels in HTORI3 (normal thyroid cell line) than TPC-1, KTC and BCPAP (A). The relative expression of MT1M (compared with the GAPDH gene) in TPC-1 KTC1 and BCPAP. Compared with the corresponding control group, the expression of MT1M in up-regulation group was higher (B). Up-regulation MT1M gene expression in PTC cell lines transfected with Vector or MT1M were cultured in 96-well plates for 1-4 days and using CCK-8 measured cell proliferation (C-E). Colony formation of Vector and MT1M Cells (F). *P < 0.05; **P < 0.01; ***P < 0.001 in comparison with the NC group using Student’s t-test.
MT1M regulates migratory and invasive capacities of PTC cell lines in vitro
For additional investigation in PTC function of MT1M, we investigated the function of MT1M in the migratory and invasive abilities of various thyroid cancer cell lines. As expected in three PTC cell lines, the migratory ability of MT1M cells decreased compared with that of control cells. Likewise, the invasive ability of the MT1M PTC cell lines was lower than that of the corresponding control PTC cell lines (Figure 4A-C). Above data suggest that MT1M plays a significant role in the migratory and invasive abilities of the PTC cell lines.
Figure 4.
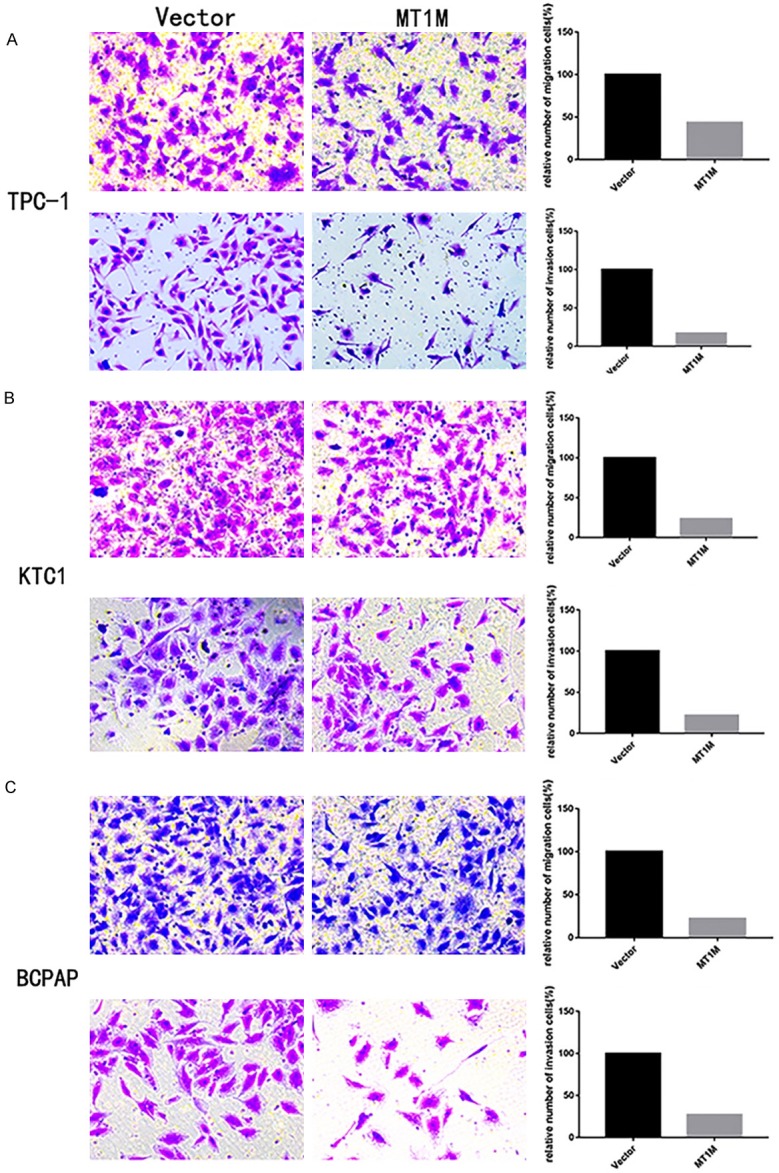
Up-regulation MT1M gene expression in TPC-1, KTC and BCPAP cell inhibiting migration and invasion. Transwell migration and invasion assays in Up-regulation MT1M cells and their corresponding control cells. Quantitative results of migration and invasion assays. The stained cells were manually counted from 5 randomly selected fields and normalized with cell proliferation (A-C). *P < 0.05; **P < 0.01; ***P < 0.001 in comparison with the vector group using Student’s t-test.
MT1M regulates migration and invasion by modulating N-cadherin and vimentin
Many reports have confirmed that a cancer cell’s abilities of migration and invasion were connected with tumor progression [22-24]. As showed above, the MT1M gene in the TCGA cohort was significantly associated with LNM. Hence, we researched the potential mechanism by which this gene provides to PTC metastasis. We found the protein expression of Epithelial-to-mesenchymal transition (EMT)-related molecules by Western blot. After upregulated MT1M in the PTC cell lines (TPC1, KTC-1, and BCPAP), the MT1M cell group showed lower Vimentin, N-cadherin expression compared with the corresponding control group (Figure 5A-C).
Figure 5.
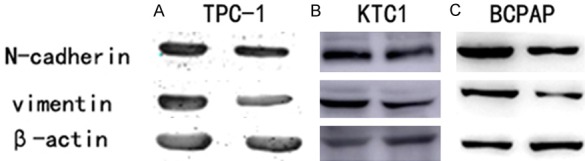
MT1M regulates migration and invasion by modulating N-cadherin and Vimentin. A. The influence on MT1M expression on N-cadherin and Vimentin in TPC-1 by western blot. B. The influence of MT1M expression on N-cadherin and Vimentin in KTC1 by western blot. C. The influence of MT1M expression on N-cadherin and Vimentin in BCPAP by western blot.
Discussion
The number of thyroid cancer has constantly increased for the past decades at a rate of 4% per year and some articles indicate that thyroid cancer would become the 4th dominant cancer diagnosis by 2030 [1,25,26]. Although distinct development in medical research has been made, the underlying molecular mechanisms of PTC remain unidentified. Thus, is necessary for doctors to develop molecular and genetic markers which play a vital role in thyroid cancer progression. These markers might help surgeons and endocrinologists to formulate an inclusive regimen, including thyroidectomy, possible lymphadenectomy, and postoperative radioactive iodine administration [27]. However, the current management concerning treatment and prognosis for thyroid cancer patients almost relies on the clinical and pathologic limitations.
Facing this challenge, we should further investigate PTC tumorigenesis to establish potential molecular biomarkers that can predict PTC progression and evaluate the risk for PTC. Earlier, 19 pairs of PTC tumors and adjacent normal tissues were subjected to whole transcriptome resequencing, and we discovered that MT1M is differentially expressed. Our investigation showed that MT1M gene likely played a vital tumor suppressor in PTC.
Metallothioneins (MTs), a unique class of metalloproteins, is vital for cell proliferation and differentiation, besides act as antioxidants to protect cells against oxygen radical and plays a protective role against DNA damage and apoptosis [28-30]. Metallothionein 1M (MT1M), a member of MTs, is a highly conserved, low-molecular-weight (6-7 kDa), and cysteine residues-rich protein [31]. Gathering proof elaborated that it plays a pivotal role in the course of carcinogenesis and play vital roles in tumor growth, progression, metastasis, and drug resistance [32]. Numerous proof showed that the down-expression of MT1M is closely associated with poor prognosis in many cancers. Oka D et al [19] found the loss of MT1M expression can be correlated with smoking duration; da Motta et al [20] discovered that the downregulated expression of the MT1M protein may cause aggressiveness in lung large-cell carcinoma. Ding J [21] confirmed that low MT1M expression is correlated with poor hepatocellular carcinoma prognosis following curative resection. However, the little is known about the MT1M in human cancers, particularly thyroid cancer.
The present report aimed to study the role of MT1M in thyroid carcinoma. First, we found that MT1M expression was significantly downregulated in PTC. Second, we analyzed The TCGA database to investigate the role of MT1M in PTC and its relationship with LNM. This finding is reliable with maximum studies concerning human cancer and indicated that MT1M plays different roles in different kinds of human cancer.
Our results showed that MT1M upregulation could suppress the cell proliferation and colony formation of PTC cells. Overexpression of MT1M expression compromised the migration and invasion capacity of PTC cells. Our report also showed that MT1M overexpress remarkably blocked the activity of Vimentin, N-cadherin in PTC cells. Our study is the first to present the significant role of MT1M in PTC.
Despite our findings, some limitations do exist. Initial, the relationship between MT1M expression and clinical factors should be further studied upon complete data collection. Additionally, the exact mechanism involved in the tumorigenic role of MT1M in PTC should be further investigated.
Conclusion
In summary, MT1M plays a significant role in PTC progression. MT1M over-expression inhibits thyroid tumorigenesis by impairing cell proliferation, colony formation, migration, and invasion. Our report showed that MT1M indicates potential for biological implications and is worthy of further study.
Acknowledgements
The authors would like to thank all the doctors of Department of Thyroid and Breast Surgery, The First Affiliated Hospital of Wenzhou Medical University, (Wenzhou, China) for providing all the necessary information required for this study. This study was funded by National Natural Science Foundation of China (NO. 81572291) and Natural Science Foundation of Zhejiang province (LY17H160053, LGF18H160031, GF18H160071, and LGF18H160032) and the Medical and Health Technology Projects of Zhejiang province (NO. 2017187475) and the Science and Technology Project of Wenzhou (Y20170030).
Written informed consent was issued by the patients for the publication of this research and accompanying images. A copy of the written consent is ready for review by the Editor in Chief of this journal.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Burns WR, Zeiger MA. Differentiated thyroid cancer. Semin Oncol. 2010;37:557–566. doi: 10.1053/j.seminoncol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 6.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 8.Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decaussin-Petrucci M, Descotes F, Depaepe L, Lapras V, Denier ML, Borson-Chazot F, Lifante JC, Lopez J. Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology. 2017;28:482–487. doi: 10.1111/cyt.12493. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson S, Zbuk KM, Scacheri C, Eng C. Cowden syndrome. Semin Oncol. 2007;34:428–434. doi: 10.1053/j.seminoncol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Zhou X, Cai Y, Chen E, Zhang X, Wang O, Wang Q, Liu H. TEKT4 promotes papillary thyroid cancer cell proliferation, colony formation, and metastasis through activating PI3K/Akt pathway. Endocr Pathol. 2018;29:310–316. doi: 10.1007/s12022-018-9549-0. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 15.Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010;73:113–128. doi: 10.1016/S1726-4901(10)70025-3. [DOI] [PubMed] [Google Scholar]
- 16.Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Metallothionein expression in human neoplasia. Histopathology. 2004;45:103–118. doi: 10.1111/j.1365-2559.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 17.Si M, Lang J. The roles of metallothioneins in carcinogenesis. J Hematol Oncol. 2018;11:107. doi: 10.1186/s13045-018-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi S. Positive and negative regulators of the metallothionein gene (review) Mol Med Rep. 2015;12:795–799. doi: 10.3892/mmr.2015.3459. [DOI] [PubMed] [Google Scholar]
- 19.Oka D, Yamashita S, Tomioka T, Nakanishi Y, Kato H, Kaminishi M, Ushijima T. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history: a target for risk diagnosis and prevention of esophageal cancers. Cancer. 2009;115:3412–3426. doi: 10.1002/cncr.24394. [DOI] [PubMed] [Google Scholar]
- 20.da Motta LL, De Bastiani MA, Stapenhorst F, Klamt F. Oxidative stress associates with aggressiveness in lung large-cell carcinoma. Tumour Biol. 2015;36:4681–4688. doi: 10.1007/s13277-015-3116-9. [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Lu SC. Low metallothionein 1M expression association with poor hepatocellular carcinoma prognosis after curative resection. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15048735. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari A, Xia E, Zhou Y, Guan Y, Xiang J, Kong L, Wang Y, Yang F, Wang O, Zhang X. ITGA7 functions as a tumor suppressor and regulates migration and invasion in breast cancer. Cancer Manag Res. 2018;10:969–976. doi: 10.2147/CMAR.S160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brzozowa M, Wyrobiec G, Kolodziej I, Sitarski M, Matysiak N, Reichman-Warmusz E, Zaba M, Wojnicz R. The aberrant overexpression of vimentin is linked to a more aggressive status in tumours of the gastrointestinal tract. Prz Gastroenterol. 2015;10:7–11. doi: 10.5114/pg.2014.47502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari A, Shen Y, Sindan N, Xia E, Gautam B, Lv S, Zhang X. MAL2 promotes proliferation, migration, and invasion through regulating epithelial-mesenchymal transition in breast cancer cell lines. Biochem Biophys Res Commun. 2018;504:434–439. doi: 10.1016/j.bbrc.2018.08.187. [DOI] [PubMed] [Google Scholar]
- 25.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 27.Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. 2012;19:1874–1880. doi: 10.1245/s10434-011-2129-x. [DOI] [PubMed] [Google Scholar]
- 28.Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krezel A, Maret W. The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimoda R, Achanzar WE, Qu W, Nagamine T, Takagi H, Mori M, Waalkes MP. Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci. 2003;73:294–300. doi: 10.1093/toxsci/kfg095. [DOI] [PubMed] [Google Scholar]
- 31.Romero-Isart N, Vasak M. Advances in the structure and chemistry of metallothioneins. J Inorg Biochem. 2002;88:388–396. doi: 10.1016/s0162-0134(01)00347-6. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]


