Abstract
We evaluated the relationship between IL-17 expression and tumor budding in the oral squamous cell carcinoma (OSCC) tumor invasion front (TIF). We also analyzed the correlation between OSCC prognosis and the co-expression of IL-17 and tumor budding. We obtained tumor tissues, tumor margins, and adjacent normal tissues from a total of 80 patients with OSCC. We measured IL-17 expression by ELISA and performed hematoxylin and eosin (H&E) and immunohistochemical staining to evaluate the presentation of tumor budding and IL-17 expression in the TIF. IL-17 expression was significantly increased in the tumor tissues and margins compared with that in the normal tissues, which was confirmed by immunohistochemical staining. There were correlations between IL-17-positive tumor budding and T classification, lymph node metastasis, distant metastasis, clinical stage, and recurrence. In a multivariate survival analysis, the IL-17-positive tumor budding, besides tumor budding alone, was highly correlated with prognosis. Our results demonstrate a positive relationship between IL-17 expression and tumor budding in the OSCC TIF. IL-17 combined with tumor budding can serve as a valuable predictor of prognosis for patients with OSCC.
Keywords: IL-17, tumor budding, oral squamous cell carcinoma, tumor invasion front, prognosis
Introduction
Worldwide, head and neck squamous cell carcinoma is the sixth most common malignant tumor, affecting more than 500,000 patients annually [1]. Oral squamous cell carcinoma (OSCC) accounts for 95% of all modalities of head and neck squamous cell carcinoma [2]. Despite advances in prevention and multimodal therapies, OSCC is still associated with an unfavorable prognosis, with a 5-year survival rate around 50% [3]. Loco-regional relapse, cervical metastasis, and advanced clinical stage are leading causes of the unfavorable prognosis [4,5]. Although a growing body of literature has revealed factors that drive OSCC invasion and metastasis, the mechanisms underlying OSCC progression are not fully understood [6]. Therefore, it has been difficult to identify biological markers with prognostic value in OSCC.
The tumor invasion front (TIF) is defined as the region located in the tumor border near the stroma, which directly reflects the interaction between the tumor and the tumor microenvironment. The TIF determines the invasive potential of the tumor and can be evaluated on the basis of several parameters, such as the depth of tumor invasion, the mode of invasion, and tumor budding [7]. Tumor budding is characterized by the existence of insulated single or small clusters of cancer cells (no more than five cells) and indicates the loss of cellular cohesion and the presence of active invasive movement [8]. For those reasons, tumor budding has been proposed as a promising prognostic feature in many neoplasms [9-11]. Tumor budding at the TIF of OSCC is related to local metastasis and poor prognosis [12]; however, the underlying molecular mechanisms of that relation are still obscure [13].
In addition to the histopathologic status of the TIF, inflammatory factors in the tumor microenvironment also play an essential role in tumor pathogenesis, invasion, and metastasis [14]. Overall, the tumor microenvironment is a chronic inflammatory stroma composed of different types of cells (e.g., fibroblasts and immune cells) and extracellular elements (e.g., chemokines, cytokines, and extracellular matrix) [15]. Interleukin 17 (IL-17, also known as IL-17A or CTLA-8) is a proinflammatory cytokine that is primarily secreted by T-helper type 17 cells and neutrophils. IL-17 has been identified in various carcinomas and is closely correlated with poor prognosis in breast cancer [16], ovarian cancer [17], hepatocellular cancer [18], and gastric cancer [19]. Increased IL-17 expression has also been observed in OSCC [20]. Additionally, IL-17 overexpression in tongue cancer has been shown to be correlated with cancer progression [21]. IL-17 promotes tumor dissemination through its effects on matrix metalloproteinases (MMPs) and angiogenesis [22], but the roles of IL-17 in OSCC still need to be explored.
We hypothesized that IL-17 expression is related to tumor budding in the OSCC TIF and that both of those factors may be correlated with prognosis. To test that hypothesis, we conducted immunohistochemistry (IHC) staining of IL-17 in tumor and normal tissues and analyzed the relationships between IL-17 expression and clinicopathologic features and survival.
Materials and methods
Patients and samples
The Ethics Committee of Shanghai Jiao Tong University approved this study. The inclusion criteria were as follows: patients from 35 to 55 years of age who were pathologically diagnosed with OSCC at Shanghai Ninth People’s Hospital from October 2015 to May 2018. Patients with malignancies in other organs and those who had received any anti-cancer therapy were excluded. All of the enrolled patients gave written informed consent for inclusion in the study.
Eighty patients met the inclusion criteria and were included in the study. We collected surgically resected primary tumor tissues, tumor margins, and adjacent normal tissues (more than 3 cm away from the tumor margins) from all of the participants. All samples were stored at -80°C. Some of the tissues were embedded in paraffin for hematoxylin and eosin (H&E) and IHC staining. We categorized the tumor histological grades and clinical stages according to the 8th edition of the TNM classification system of the American Joint Committee on Cancer [23]. Thus, we classified 13, 17, 31, and 19 tumors as pathological stages I, II, III, and IV, respectively. We conducted retrospective follow up for 20 to 61 months after diagnosis. The average length of survival was 42.76 months.
Enzyme linked immunosorbent assays (ELISAs)
ELISAs were performed according to the manufacturer’s instructions (Abcam, Cambridge, MA, USA). Briefly, the standards for IL-17 were prepared with a two-fold dilution series (2000 pg/ml to 31.25 pg/ml). Wash buffer and substrate solutions were prepared according to the manufacturer’s instructions. Assay diluent for the samples and standards to each well and incubated the plates for 3 h at room temperature (RT). Each well was then aspirated to eliminate any unbound IL-17 and washed three times. Afterwards, the IL-17 antibody was added to each well, the mixture was incubated for 1 h at RT, and the wells were washed three times. The substrate solution was then added to each well and incubated for 30 min at RT in the dark. Finally, the stop solution was added, and the absorbance was read by a BioTek synergy 2 plate reader at 450 nm and 540 nm (540 nm used for correlation). The standard curve of known concentrations was used for reference. We performed all assays in duplicate for each sample.
Tissue sections and staining
We cut the tissue samples into 4-μm sections. Some of the sections were stained with H&E for diagnosis and histopathological examination, while the remaining sections were prepared for IHC studies.
Immunohistochemistry
For the IHC studies, the tissue samples were first dehydrated and embedded in paraffin using routine procedures and subsequently deparaffinized and rehydrated. Antigen retrieval was carried out by pressure cooking in EDTA for 3 min at 100°C. The sections were blocked with 3% peroxidemethanol for endogenous peroxidase ablation at RT, followed by blocking of non-specific binding sites at 37°C with a 1:100 dilution of goat serum in phosphate buffered saline. Subsequently, the sections were incubated with anti-pan-cytokeratin and IL-17 antibodies (Abcam, Cambridge, MA, USA) overnight at 4°C. After incubation with a biotinylated secondary antibody and repeat washing, the reactions for pan-cytokeratin and IL-17 were visualized using 3,3-diaminobenzidine.
Evaluation of tumor budding and IL-17 expression
Two head and neck pathologists who were blind to the clinical data reviewed and scored the intensity of budding on the tissue sections. Following previous studies [7,12], we defined tumor budding as the presence of a single cell or a cluster of no more than five cancer cells at the TIF. Initially, the sections were scanned with a 4× objective lens to choose the fields with the highest density of budding. Then, the number of budding sites was counted under a 20× objective lens. If the two pathologists counted different numbers of budding sites in a given field, they examined the specimen together and reached a consensus. In accordance with previous reports [9], we set the tumor budding grading cut-off at five and categorized the specimens into two groups: low-grade tumor budding (0-4 budding sites per field) and high-grade tumor budding (≥ 5 budding sites per field).
The evaluation of IL-17 expression was semi-quantitative. Five fields from each sample were randomly selected for evaluation using the Aperio ImageScope software (Leica Biosystems Inc., IL, USA). We used five grades to score the immunoreactive intensity: 0 = negative; 1 = weak; 2 = moderate; 3 = strong; 4 = very strong. In addition, we separated the proportions of positive cells into five grades: 0: < 5%; 1: 6-25%; 2: 26-50%; 3: 51-75%; 4: > 75%. We considered the patients with an overall IL-17 expression score ≤ 6 to have low IL-17 expression and those with an overall IL-17 expression score > 6 to have high IL-17 expression. Finally, we classified the patients with high-grade tumor budding and high IL-17 expression as high IL-17 with tumor budding group, and all the rest cases were defined as the low group.
Statistical analysis
We evaluated the correlation between IL-17 expression and tumor budding by Spearman correlation analysis. The Fisher’s exact and chi-square test were used to estimate the correlations among gender, age, T classification, lymph node metastasis, distant metastasis, clinical stage, recurrence, tumor budding, and IL-17-positive tumor budding. We obtained survival curves using the Kaplan-Meier method and the log-rank test. We examined the relationships between clinicopathological features and survival by multivariate analysis using a Cox proportional hazard regression model. SPSS software (version 23.0, IBM, Chicago, IL, USA) was used for all statistical analyses, and a p value < 0.05 indicated statistical significance.
Results
IL-17 expression differed between tumor and normal tissues
The IHC results showed that IL-17 expression in the OSCC tumors and margins was significantly higher than that in the normal control tissues, (P = 0.023 and P = 0.015, respectively; Figure 1A-D).
Figure 1.
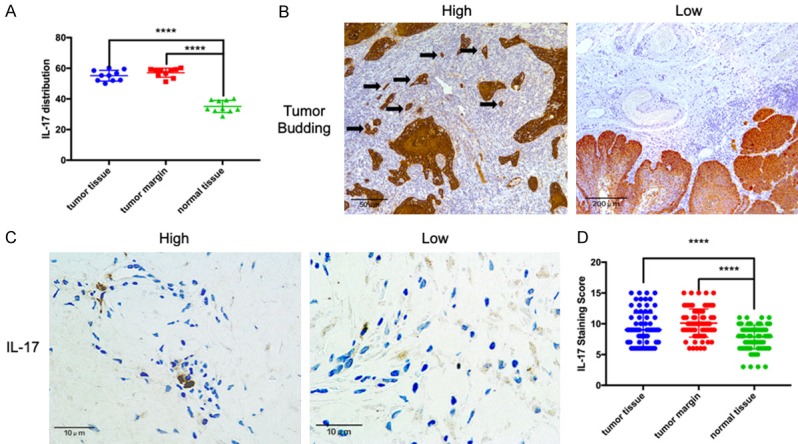
IL-17 and pan-cytokeratin expression in oral squamous cell carcinoma (OSCC) tissues. A. IL-17 levels in tumor tissues, tumor margins, and normal tissues evaluated by ELISA. B. Left: Immunohistochemistry staining of pan-cytokeratin in high-grade tumor budding; black arrows indicate tumor budding. Right: Low-grade tumor budding. C. Immunohistochemistry staining of IL-17 showing high and low expression levels in the tumor invasion front. D. The IL-17 expression was significantly elevated in tumor tissues and tumor margins compared with that in normal tissues. ****P < 0.001.
The intensity of IL-17 expression and tumor budding correlated with clinicopathologic features
Among the 80 patients, 39 (48.75%) exhibited low-grade tumor budding and 41 (51.25%) exhibited high-grade tumor budding (Figure 1B). There was a positive correlation between the degree of IL-17 expression and the grade of tumor budding (R = 0.462; P < 0.001). Forty-seven (58.75%) of the patients had low IL-17 expression, while the other 33 patients (41.25%) had high IL-17 expression (Figure 1D). The associations of clinicopathologic features with tumor budding and IL-17 expression are summarized in Table 1. There were no effects of gender or age on the grade of tumor budding or on IL-17 combined with tumor budding. However, the grade of tumor budding was associated with T classification (P = 0.043), lymph node metastasis (P = 0.012), distant metastasis (P = 0.024), clinical stage (P = 0.013), and recurrence (P = 0.036). Similarly, IL-17 with tumor budding was significantly associated with T classification (P = 0.049), lymph node metastasis (P = 0.006), distant metastasis (P = 0.013), clinical stage (P < 0.001), and recurrence (P = 0.026).
Table 1.
Correlations between tumor budding, IL-17 expression, and clinical variables
| Characteristics | Total | Tumor budding (n) | IL-17 with tumor budding (n) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low | High | p | Low | High | p | ||
| Sex | |||||||
| Male | 62 | 29 | 33 | 0.512 | 35 | 27 | 0.438 |
| Female | 18 | 10 | 8 | 12 | 6 | ||
| Age | |||||||
| ≥ 48 | 45 | 23 | 22 | 0.632 | 29 | 16 | 0.241 |
| < 48 | 35 | 16 | 19 | 18 | 17 | ||
| T classification | |||||||
| T1-2 | 42 | 25 | 17 | 0.043* | 29 | 13 | 0.049* |
| T3-4 | 38 | 14 | 24 | 18 | 20 | ||
| Lymph node metastasis | |||||||
| N0-1 | 46 | 28 | 18 | 0.012* | 33 | 13 | 0.006* |
| N2-3 | 34 | 11 | 23 | 14 | 20 | ||
| Distant metastasis | |||||||
| No | 66 | 36 | 30 | 0.024* | 43 | 23 | 0.013* |
| Yes | 14 | 3 | 11 | 4 | 10 | ||
| Clinical stage | |||||||
| I-II | 44 | 27 | 17 | 0.013* | 36 | 8 | < 0.001* |
| III-IV | 36 | 12 | 24 | 11 | 25 | ||
| Recurrence | |||||||
| No | 48 | 28 | 20 | 0.036* | 33 | 15 | 0.026* |
| Yes | 32 | 11 | 21 | 14 | 18 | ||
P < 0.05.
IL-17 combined with tumor budding was associated with poor prognosis
To further estimate the prognostic value of IL-17-positive tumor budding in OSCC, we surveyed overall survival (OS) among all the participants. The 5-year OS rate was higher (P = 0.001) among the patients with low-grade tumor budding (92.3%) than among the patients with high-grade tumor budding (51.2%; Figure 2). The 5-year OS rate was 93.6% among the patients in low IL-17 with tumor budding group, as compared with 36.4% among the patients in high group (Figure 2). Furthermore, the mean OS duration among the patients without high IL-17 with tumor budding was 58.35 months (95% CI: 55.49-61.20), while that among the patients in high group was 35.62 months (95% CI: 31.90-39.33; P = 0.008). The multivariate analysis using the Cox proportional hazard regression model showed that IL-17-positive tumor budding was associated with poor prognosis (P = 0.02). In contrast, other clinicopathologic variates such as gender, age, T classification, and lymph node metastasis were not associated with OS (P ≥ 0.05; Table 2).
Figure 2.
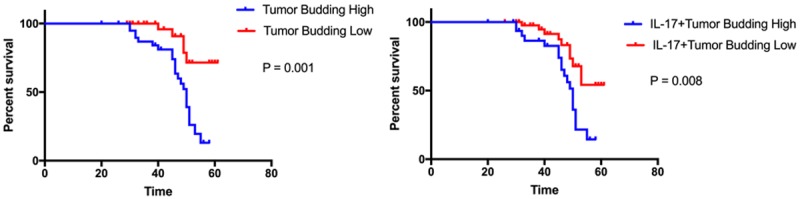
The relationship between tumor budding levels and survival. Left: The overall survival was longer among the patients with low-grade tumor budding than among those with high-grade tumor budding (P = 0.001). Right: The overall survival was slightly longer among the patients without IL-17-positive tumor budding than among those with IL-17-positive tumor budding (P = 0.008).
Table 2.
Cox proportional hazard models for overall survival prediction
| Characteristics | Multivariate analysis | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P | |
| Sex | 0.538 | 0.203-1.427 | 0.213 |
| Age | 1.542 | 0.601-3.955 | 0.368 |
| T classification | 2.751 | 0.967-7.826 | 0.058 |
| Lymph node metastasis | 1.857 | 0.600-5.743 | 0.283 |
| Distant metastasis | 3.222 | 1.009-10.290 | 0.048* |
| Clinical stage | 1.443 | 0.355-5.860 | 0.608 |
| Recurrence | 4.423 | 1.137-17.212 | 0.032* |
| Tumor budding | 4.347 | 1.126-16.776 | 0.033* |
| IL-17 with tumor budding | 4.800 | 1.280-18.002 | 0.020* |
P < 0.05.
Discussion
This retrospective study revealed that tumor budding located in the TIF was positively correlated with IL-17 expression in the adjacent stroma. Excessive expression of IL-17 and high-grade tumor budding were both predictive of poor survival in OSCC. Previous reports have seldom focused on the underlying mechanisms of tumor budding, while some works simply reported the numbers of tumor budding sites in OSCC [7,12,13]. Our results suggest that IL-17 expression interacts with tumor budding in the TIF.
Our findings support previous results showing that IL-17 was overexpressed in the tumor-stroma interface [20]. IL-17 promotes the generation of many other chemokines (IL-8, MCP-1, and GRO-α), cytokines (such as IL-6, IL-1β, TGF-β, G-CSF, GM-CSF, and TNF-α), and prostaglandins (e.g., PGE2) and thus influences the initiation and progression of many autoimmune disorders [24]. Recently, the role of IL-17 was investigated in several cancer types [16-19]. The role of IL-17 in malignancies is controversial, however, because both anti-tumor and pro-tumor effects have been documented. There is accumulating evidence that IL-17 has a positive impact on the carcinogenesis and progression of tongue cancer and OSCC [21]. As one of the most critical proinflammatory cytokines in the tumor microenvironment, IL-17 has been recognized as having a stimulating effect on the epithelial-mesenchymal transition (EMT) of gastric cancer stem cells [25]. Considering that tumor budding was correlated with EMT-like changes in OSCC [26], as well as our current findings, it is reasonable to hypothesize that one of the effects of IL-17 is to promote the formation and progression of tumor budding. Previous results suggest that the tumor stroma is crucial in the formation of tumor budding and that the presence of inflammatory cells is associated with tumor budding [27-31]. Together with those previous results, our data shed new light on the molecular mechanisms underlying tumor budding and the affinity between the inflammatory tumor microenvironment and tumor budding.
The prognostic value of IL-17-positive tumor budding in OSCC is supported by previous studies [12]. High-grade tumor budding in OSCC implies a high risk of poor prognosis. We combined IL-17 and tumor budding together as a single factor in our analysis. Correlation analysis showed that IL-17-positive tumor budding was significantly associated with T classification, lymph node metastasis, distant metastasis, clinical stage, and recurrence. Thus, our results provide additional evidence that IL-17 together with tumor budding can act as an independent prognostic marker in multivariate analysis.
Tumor budding is defined as single cell or small clusters of tumor cells separated from the main tumor mass [32-34]. Tumor budding was characterized first in colon cancers [8,34] and subsequently in other tumor types such as breast, pancreatic, esophagus, and larynx cancers [29,32,33,35-39]. Tumor budding in the initial metastatic process has EMT-like features [27,34], which confers a strong invasive ability on the budding cells, leading to unfavorable effects on patient survival [37,40,41]. In addition, tumor budding is associated with aberrations of E-cadherin and loss of the expression of epithelial cell adhesion molecules, which contributes to the loss of intercellular adhesions [42]. Moreover, tumor budding is closely related to local metastasis. All of those features of tumor budding may contribute to the poor prognosis associated with high-grade tumor budding. In several types of cancer [21,22,25], IL-17 has been shown to induce pro-invasive factors and to promote the invasion of cancer cells. Furthermore, STAT3 phosphorylation was up-regulated in patients with elevated IL-17 expression [43], suggesting that STAT3 signaling might be activated by IL-17. The correlation between tumor budding and aggressive phenotypes and the close relation between tumor budding and IL-17 suggest that the IL-17-STAT3 pathway may play a role in the biological process of tumor budding, which may explain how IL-17 with tumor budding acts as a prognostic marker in OSCC.
There were some limitations in our study. Our sample of OSCC patients was not large enough, which might have affected the results of the Cox proportional hazard regression model. Therefore, more prospective studies are needed. In subsequent studies, efforts should be focused on elucidating the role of IL-17 in the development of tumor budding as well as on the genetic background of tumor budding.
In conclusion, we found enhanced expression of IL-17 in the tumor and tumor margins, which was positively associated with tumor budding in the TIF. In addition, IL-17 combined with tumor budding was an independent predictor of prognosis for patients with OSCC.
Acknowledgements
This work was supported by Science and Technology Commission Foundation of Shanghai (No. 10DZ1951300, to C.P.Z), the National Natural Science Foundation of China (No. 81771046 and 81270015, to L.W), programs of Shanghai Talent Development (No. 2018042, to L.W), Clinical Research Program of 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYLJ201817, to L.W) and Shanghai Summit & Plateau Disciplines (to C.P.Z and L.W). This manuscript has been proofread and edited by a professional English editing company, BioScience Writers (BSW), located in Houston, Texas.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884–11894. [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera C, Oliveira A, Costa R, De Rossi T, Paes Leme A. Prognostic biomarkers in oral squamous cell carcinoma: a systematic review. Oral Oncol. 2017;72:38–47. doi: 10.1016/j.oraloncology.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2015;33:3305–3313. doi: 10.1200/JCO.2015.62.0963. [DOI] [PubMed] [Google Scholar]
- 5.Irani S. Distant metastasis from oral cancer: a review and molecular biologic aspects. J Int Soc Prev Community Dent. 2016;6:265–271. doi: 10.4103/2231-0762.186805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola S, Nieminen O, Soini Y, Kosma VM, Koivunen P, Grénman R, Leivo I, Salo T. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–818. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson H, Koelzer VH, Karamitopoulou E, Economou M, Hammer C, Muller DE, Lugli A, Zlobec I. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology. 2014;64:577–584. doi: 10.1111/his.12294. [DOI] [PubMed] [Google Scholar]
- 9.Luo WR, Gao F, Li SY, Yao KT. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology. 2012;61:1072–1081. doi: 10.1111/j.1365-2559.2012.04350.x. [DOI] [PubMed] [Google Scholar]
- 10.Almangush A, Karhunen M, Hautaniemi S, Salo T, Leivo I. Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology. 2016;68:173–182. doi: 10.1111/his.12781. [DOI] [PubMed] [Google Scholar]
- 11.Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115:831–840. doi: 10.1038/bjc.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almangush A, Pirinen M, Heikkinen I, Makitie AA, Salo T, Leivo I. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer. 2018;118:577–586. doi: 10.1038/bjc.2017.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, Vikesaa J, Nielsen FC, Von BC. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505–516. doi: 10.1002/path.4550. [DOI] [PubMed] [Google Scholar]
- 14.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49:887–892. doi: 10.1016/j.oraloncology.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 16.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–10. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–62. e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Avadhani AV, Parachuru VP, Milne T, Seymour GJ, Rich AM. Multiple cells express interleukin 17 in oral squamous cell carcinoma. J Oral Pathol Med. 2017;46:39–45. doi: 10.1111/jop.12465. [DOI] [PubMed] [Google Scholar]
- 21.Wei T, Cong X, Wang XT, Xu XJ, Min SN, Ye P, Peng X, Wu LL, Yu GY. Interleukin-17A promotes tongue squamous cell carcinoma metastasis through activating miR-23b/versican pathway. Oncotarget. 2016;8:6663–6680. doi: 10.18632/oncotarget.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang B, Kang H, Fung A, Zhao H, Wang T, Ma D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediat Inflamm. 2014;2014:623759. doi: 10.1155/2014/623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollaers K, Hinton-Bayre A, Friedland P, Farah C. AJCC 8th edition oral cavity squamous cell carcinoma staging - is it an improvement on the AJCC 7th edition? Oral Oncol. 2018;82:23–28. doi: 10.1016/j.oraloncology.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Yang S, Li P, Luo X, Li Z, Hao Y, Yu P. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene. 2017;36:1256–1264. doi: 10.1038/onc.2016.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 2015;35:6111–6120. [PubMed] [Google Scholar]
- 27.Gujam F, McMillan D, Mohammed Z, Edwards J, Going J. The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br J Cancer. 2015;113:1066–1074. doi: 10.1038/bjc.2015.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing Y, Han Z, Zhang S, Yan L, Wei L. Epithelial-mesenchymal transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadota K, Yeh Y, Villena-Vargas J, Cherkassky L, Drill E, Sima C, Jones D, Travis W, Adusumilli P. Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest. 2015;148:711–721. doi: 10.1378/chest.14-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53:581–586. doi: 10.1136/gut.2003.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2010;212:260–268. doi: 10.1002/path.2164. [DOI] [PubMed] [Google Scholar]
- 32.Salhia B, Trippel M, Pfaltz K, Cihoric N, Grogg A, Lädrach C, Zlobec I, Tapia C. High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat. 2015;150:363–371. doi: 10.1007/s10549-015-3333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thies S, Guldener L, Slotta-Huspenina J, Zlobec I, Koelzer V, Lugli A, Kröll D, Seiler C, Feith M, Langer R. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum Pathol. 2016;52:1–8. doi: 10.1016/j.humpath.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou J, Hansen T, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the international tumor budding consensus conference (ITBCC) 2016. Mod Pathol. 2017;30:1299–1311. doi: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 35.Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer. 2013;49:1032–1039. doi: 10.1016/j.ejca.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z. The prognostic value of tumor budding in invasive breast cancer. Pathol Res Pract. 2013;209:269–275. doi: 10.1016/j.prp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Santamaria P, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11:718–738. doi: 10.1002/1878-0261.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarioglu S, Acara C, Akman F, Dag N, Ecevit C, Ikiz A, Cetinayak O, Ada E. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract. 2010;206:88–92. doi: 10.1016/j.prp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–1590. doi: 10.1002/hed.24282. [DOI] [PubMed] [Google Scholar]
- 40.Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577–1587. doi: 10.1016/j.febslet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221–232. doi: 10.1038/modpathol.3800733. [DOI] [PubMed] [Google Scholar]
- 43.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]


