Abstract
Epidural fibrosis causes serious complications in patients who have undergone laminectomy. Pirfenidone is an effective antifibrotic agent but its effect on epidural fibrosis remains unclear. In this study, we aimed to investigate the effect of pirfenidone on epidural fibrosis and to evaluate its mechanism of action on human epidural scar fibroblasts. In a rat model of laminectomy, the degree of epidural fibrosis was quantified via Rydell standard classification, histological analysis, and collagen density analyses. In cultured human epidural scar fibroblasts, cell proliferation was measured using a Cell Counting Kit-8 and EdU assay. Cell apoptosis was detected using Annexin V/propidium iodide staining, and cytotoxicity was evaluated via lactate dehydrogenase assay. Relative mRNA levels of α-smooth muscle actin (α-SMA) and collagen type I were analyzed using quantitative polymerase chain reaction. The protein expression of α-SMA and collagen type I and the phosphorylation status of Smad2, Smad3, protein kinase B (Akt), and p38 were determined via western blotting. Pirfenidone reduced epidural fibrosis by inhibiting fibroblast proliferation and suppressing collagen formation in rats. It also inhibited human epidural scar fibroblast proliferation with no cytotoxic or apoptotic effects. Pirfenidone inhibited fibroblast differentiation by decreasing TGF-β1-induced transcriptional and translational expression of α-SMA. It inhibited TGF-β1-induced phosphorylation of Smad2, Smad3, Akt, and p38. This study suggests that topical application of pirfenidone could reduce epidural scar adhesion after laminectomy, and that its mechanism of action may be the inhibition of TGF-β1-induced epidural scar fibroblast proliferation and differentiation into myofibroblasts through the attenuation of TGF-β1-induced Smad-dependent and -independent pathways.
Keywords: Epidural fibrosis, pirfenidone, fibroblast, mechanism
Introduction
Laminectomy is an effective and widely used surgical option for patients with spinal stenosis. The operation is performed to decompress the spinal cord and nerves by removing the roof of bone and thickened ligaments that lie over the spinal canal [1]. However, this procedure may cause fibrosis formation around the surgical site and lead to intractable back and leg pain, which constitute the main features of failed back surgery syndrome (FBSS) [2-4]. Unfortunately, excessive fibrosis formation may render re-exposure of the same surgical field technically difficult and dangerous, and may cause more intraoperative complications [2].
In response to this challenge, numerous attempts have been made to halt the fibrosis formation process. A series of biological, pharmaceutical, and synthetic materials, such as recombinant tissue plasminogen activator [5], cholesterol [6], mitomycin C [7], and polyester materials [8], have been used to relieve fibrosis formation after laminectomy. In spite of this, there is no effective and widely used method in clinical practice.
Fibrosis formation is a normal physiological process that occurs during wound healing [9], although several key factors contribute to excessive fibrosis formation after laminectomy. Oxidative stress and the release of inflammatory mediators around the surgical site may promote the recruitment of inflammatory cells to local damaged tissues. These cells in turn secrete various wound-healing/profibrotic cytokines, including transforming growth factor-β1 (TGF-β1), platelet-derived growth factor (PDGF), and interleukin (IL)-1, which promote the proliferation of local fibroblasts and the deposition of extracellular matrix (ECM) components such as fibronectin and type I collagen [10,11]. Meanwhile, some fibroblasts may transform into myofibroblasts, which are characterized by the expression of α-smooth muscle actin (α-SMA). Myofibroblasts secrete much more ECM than fibroblasts [12]. Excessive deposition of ECM progressively distorts normal tissue architecture and promotes the formation of excessive scar tissue around the nerve root, finally resulting in FBSS [13].
Pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) has demonstrated its antifibrotic potential both in vitro and in vivo [14-19]. It exerts anti-inflammatory effects by reducing the production of inflammatory mediators such as TGF-β, thus reducing fibroblast proliferation, myofibroblast differentiation, and ECM accumulation [16,20,21]. Pirfenidone reportedly prevents the proliferation, migration, and collagen contraction of human Tenon’s fibroblasts [22]. Furthermore, pirfenidone shows potential as an antifibrotic drug in the treatment of idiopathic pulmonary fibrosis [23], hepatic cirrhosis [24], and diabetic kidney disease [25] in clinical practice. Topically administered pirfenidone could reduce local fibrosis formation in animal models [15,17]. Furthermore, pirfenidone has shown promising antifibrotic effects in patients with skin scarring caused by burns [14] and facial trauma after severe dog bite [26].
In light of these findings, we hypothesized that pirfenidone could suppress epidural fibrosis and provide a potential treatment method to inhibit fibrosis formation after laminectomy. The aim of the study was to investigate the effect of pirfenidone on epidural fibrosis formation in a rat model of laminectomy, and to further explore the mechanism of pirfenidone on human epidural scar fibroblasts in vitro. These findings may provide experimental evidence for pirfenidone’s potential as a new therapeutic agent for the prevention of epidural scar adhesions after laminectomy.
Materials and methods
Ethics statement
Human epidural scar fibroblasts were donated by the Nanjing Medical University. Briefly, a primary fibroblast cell line was established from epidural scar fibroblasts isolated from patients who took part in a clinical study approved by the Ethical Committee of the First Affiliated Hospital of Nanjing Medical University in accordance with the provisions of the Declaration of Helsinki. The clinical trial registration number is ChiCTR-TRC-10001079. We have used this cell line in a previous study focused on the relationship between endoplasmic reticulum stress and the apoptotic effect of mitomycin C on human epidural scar fibroblasts [27].
Animals
This study included 48 adult, healthy, Sprague-Dawley rats, with weights ranging from 260 to 310 g, that were purchased from the Xuzhou Laboratory Animal Center, Xuzhou, China. All animals received care in accordance with the international principles of Laboratory Animal Care. The 48 healthy rats were divided into the following four groups (12 rats in each group): 80 mg/ml pirfenidone group (Sigma-Aldrich, St. Louis, MO, USA), 40 mg/ml pirfenidone group, 10 mg/ml pirfenidone group, and control group (saline).
Establishment of a rat model of laminectomy and in vivo drug intervention
Rats were anesthetized using 1% pentobarbital sodium via intraperitoneal injection and fixed in a prone position. After depilation and disinfection of the surgical area around the first and second lumbar vertebrae (L1 and L2), a longitudinal incision was made along the spinous processes to expose the entire lamina. The spinous processes and laminas of L1 or L2 were removed using a rongeur. Next, intact 5 mm × 2 mm areas of the dura mater were exposed followed by careful hemostasis. Pirfenidone was dissolved in saline to prepare solutions with varying concentrations (10 mg/ml, 40 mg/ml, and 80 mg/ml). Cotton slices with size of 5 mm × 2 mm were immersed in the pirfenidone solution, and those used for the control group were soaked in saline. Next, the cotton slices were removed, and the laminectomy areas were immediately covered by paraspinal muscles. Finally, the wounds were closed in layers. Rats were postoperatively injected with 1,600,000 U/kg penicillin to prevent infection.
Macroscopic assessment of epidural fibrosis
After 4 weeks, six rats were randomly selected from each group for macroscopic evaluation. The surgical sites were reopened through the previous operative incision, and the extent of epidural scar fibrosis was evaluated under double-blind trials according to the Rydell standard [28]: grade 0, little epidural scar tissue without adherence to the dura mater; grade 1, moderate epidural scar tissue with slight adherence to the dura mater; grade 2, moderate epidural scar tissue with tight adherence to the dura mater and dissected with difficulty without disrupting the dura matter; and grade 3, epidural scar tissue was firmly adherent to the dura mater and could not be dissected.
Histological analysis
The other six rats in each group were also assessed 4 weeks after operation. Rats were anesthetized using pentobarbital sodium and perfused with 4% paraformaldehyde solution intracardially for histological analysis. The entire L1 spine columns and surrounding tissues were removed. Specimens were soaked in 10% buffered formalin for 4 days, decalcified in ethylenediaminetetraacetic acid (EDTA) and glycerol solution for 30 days, and embedded in paraffin. Vertebrae were divided into 12 continuous transversal sections of 4 μm thickness, and of these, six odd sections were stained with hematoxylin and eosin (H&E). The epidural scar adhesion was observed through a light microscope with 40 × magnification. Fibroblast density was calculated from three fields in each section at 400 × magnification. The other six even sections were treated with Masson’s trichrome stain, and the process of collagen tissue proliferation was observed through a light microscope with 200 × magnification. Image-Pro Plus 6.0 image analysis software was used to determine the optical density of positively stained collagen.
Cell culture and drug intervention in vitro
Fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco BRL) and penicillin (100 IU/ml)/streptomycin (100 μg/ml, PS; Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in 5% CO2. A fibroblast monolayer seeded in 96-well plates or 10-cm dishes was allowed to grow overnight and the cells were treated with pirfenidone (0, 0.01, 0.1, 0.5, 1.0, and 1.5 mg/ml) dissolved in DMEM. Cells were used in in vitro experiments after four to seven passages.
Cell viability assay
Cell viability was determined using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Cells were plated in replicates of six in 96-well plates (2 × 103 cells/well) in 100 μL DMEM and subjected to various treatments. Next, 10 μL CCK-8 solution was added to each well and incubated for 3 h at 37°C. The absorbance was measured at 570 nm using an absorbance microplate reader (ELx800 Absorbance Microplate Reader, Bio-Tek, Winooski, VT, USA). Cell viability was expressed as a percentage relative to that of control cells and reported as mean ± standard deviation (SD).
5-ethynyl-2-deoxyuridine (EdU) incorporation assay
Incorporated EdU was detected using Click-iT® EdU Imaging Kits (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cell photographs were captured using a fluorescent microscope (Olympus BX51, Olympus, Tokyo, Japan) and Image-Pro Plus 5.0 image analysis software (Media Cybernetics, Shanghai, China). We randomly selected at least 50 cells from a single captured field and calculated the average nuclear fluorescence intensity. Data points presented in the study were obtained from five different fields.
Annexin V/propidium iodide (PI) double staining
Annexin V/PI double staining was used to detect apoptosis. Human epidural scar fibroblasts were plated in 60-mm dishes (3 ml, 1 × 106 cells/well) and incubated for 48 h at 37°C. After treatment with pirfenidone, the detached and adherent cells were collected and washed twice with ice-cold phosphate-buffered saline (PBS). The cells were then resuspended in binding buffer at a concentration of 1 × 106 cells/ml and incubated with annexin V-fluorescein isothiocyanate (FITC) and PI (BD Biosciences, Franklin Lakes, NJ, USA) to achieve double staining, according to the manufacturer’s instructions. The mixture was incubated in the dark for 15 min at 25°C prior to analysis on a Beckman Coulter FC500 flow cytometry system and CXP software (Beckman Coulter, Brea, CA, USA).
Lactate dehydrogenase (LDH) assay
Cell viability was evaluated via measurement of LDH released from the cytosol of damaged cells. Cells were treated with pirfenidone at concentrations of 0, 0.1, 0.5, or 1.5 mg/ml for 48 h. The culture medium was collected and LDH release activity from damaged cells was measured using a Cytotoxicity Detection Kit (Roche, Basel, Switzerland). Cell death was determined as LDH release and expressed as a percentage of the mean absorbance measured in untreated control cultures.
Western blot analysis
The protein concentration was determined using a Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of proteins were resolved via electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA). The primary antibodies (diluted 1:500 to 1:1000) including anti-α-SMA, anti-type collagen type-I, anti-Smad2, anti-Smad3, anti-P38, anti-Akt, anti-phospho-Smad2, anti-phospho-Smad3, anti-phospho-p38, and anti-phospho-Akt, were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse anti-β-actin antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Membranes were washed three times in Tris-buffered saline with Tween® 20 (TBST) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (IgG, diluted 1:5000) for 1 h. The immune complexes were visualized via fluorography using enhanced chemiluminescence (ECL) western blotting detection reagents (Merck Millipore).
Quantitative polymerase chain reaction (qPCR)
qPCR was used to investigate gene expression in human epidural scar fibroblasts treated with TGF-β1 and pirfenidone. After treatment, total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized using random primers (Invitrogen), and qPCR was performed using a ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) and SYBR Green PCR Master Mix (Applied Biosystems). The primers used were as follows: human collagen type 1 α1 (COL1A1) mRNA, 5’-ACGAAGACATCCCACCAATC-3’ (sense) and 5’-AGATCACGTCATCGCACAAC-3’ (anti-sense); human α-SMA mRNA, 5’-CTGCTGAGCGTGAGATTGTC-3’ (sense) and 5’-CTCAAGGGAGGATGAGGATG-3’ (anti-sense); and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, 5’-GGGCTCTCCAGAACATCATCC-3’ (sense) and 5’-GTCCACCACTGACACGTTGG-3’ (anti-sense). The PCR reaction was performed as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 30 s and 60°C for 30 s. Gene expression was quantified using the 2-ΔΔCt method.
Statistical analysis
Data are presented as means ± SD of three independent experiments. Statistical differences between treatment groups were analyzed via one-way analysis of variance (ANOVA) followed by Dunnett test or Student’s t-test using SPSS 16.0 software. Statistical significance was defined as P<0.05.
Results
Macroscopic assessment of epidural adhesion
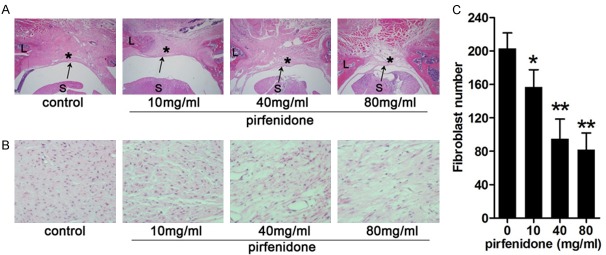
Results showed that all scar adhesions in the control group were classified as grade 3. In the 10-mg/ml and 40-mg/ml pirfenidone-treated group, most rats showed grade 2 or grade 1 adhesions. However, in the 80-mg/ml pirfenidone-treated group, three rats displayed grade 0 adhesions and no rats showed grade 3 adhesions (Table 1). This suggests that pirfenidone inhibits epidural adhesion in a rat model of laminectomy.
Table 1.
Epidural scar formation according to the Rydell standard
| Group | Grade | |||
|---|---|---|---|---|
|
| ||||
| 0 | 1 | 2 | 3 | |
| pirfenidone (0 mg/ml) | 0 | 0 | 0 | 6 |
| pirfenidone (10 mg/ml) | 0 | 2 | 3 | 1 |
| pirfenidone (40 mg/ml) | 1 | 4 | 1 | 0 |
| pirfenidone (80 mg/ml) | 3 | 2 | 1 | 0 |
Six rats were randomly selected from each group. The values within the table represent the number of rats.
Histological analysis of pirfenidone on epidural adhesions
Histopathological evaluation and epidural cell density analysis were performed to detect the effect of pirfenidone on reducing epidural fibrosis. In the control group, severe epidural fibrosis adhesions to the dura mater and a particularly high number of fibroblasts were observed in the laminectomy sites. However, moderate epidural fibrosis and a reduction in the number of fibroblasts around the laminectomy sites were observed in the 10-mg/ml and 40-mg/ml pirfenidone-treated group. Furthermore, little epidural fibrosis and fewer fibroblasts were observed in the 80-mg/ml pirfenidone-treated group (Figure 1). These results indicate that pirfenidone reduced scar formation by reducing epidural fibrosis and inhibiting fibroblast proliferation.
Figure 1.
Histological images of epidural scar adhesion and fibroblast proliferation in control and pirfenidone-treated rats after laminectomy. After surgery, rats were treated with saline or pirfenidone (10 mg/ml, 40 mg/ml, and 80 mg/ml) at the laminectomy site. (A) Representative histological images of epidural scar adhesions. Dense scar tissue (asterisk) with tight adherence to the dura mater (arrow) was observed in the control group (grade 3). Moderate scar tissue was observed in 10-mg/ml pirfenidone-treated group (grade 2) and 40-mg/ml pirfenidone-treated group (grade 1). Loose scar tissue without adherence to the dura mater was observed in 80-mg/ml pirfenidone-treated group (grade 0). (B) Representative histological images of fibroblast proliferation. The number of cells decreased as the concentration of pirfenidone increased. (C) Number of cells in the epidural scar tissue in each group, expressed as the number per counting area. *P<0.05, **P<0.01 versus the control group (saline). The magnification used was 40 × in (A) and 400 × in (B). “S” represents the spinal cord and “L” represents the laminectomy defect.
Collagen density analysis of pirfenidone on epidural adhesions
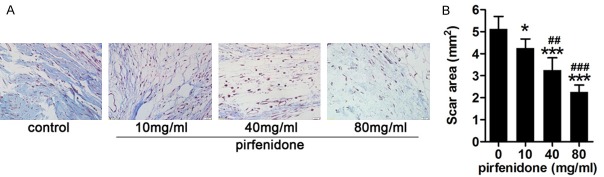
Masson’s trichrome staining was used to detect the effect of pirfenidone on reducing collagen secretion. In the control group, high-density collagen was observed. However, in the pirfenidone treatment groups, the collagen density was relatively low. We observed a dramatic decrease in collagen density in the 40-mg/ml and 80-mg/ml pirfenidone treatment groups compared to that in the 10-mg/ml pirfenidone treatment group and control group. Representative images of Masson’s trichrome staining in each group are shown in Figure 2A and statistical analysis of the optical density of the collagen tissues in each group is shown in Figure 2B. These results suggest that pirfenidone may inhibit the secretion of ECM.
Figure 2.
Pirfenidone inhibited collagen secretion. A. Histological images of collagen tissue hyperplasia in the laminectomy sites of rats treated with saline or pirfenidone (10 mg/ml, 40 mg/ml, and 80 mg/ml). The collagen density in scar tissue decreased with the increasing of pirfenidone concentration. B. The area of scar tissue for each rat was defined as the mean scar area of six sections and the data are expressed as means ± standard deviation (SD) in mm2. *P<0.05, ***P<0.001 versus the area of scar tissue in the control group. ##P<0.01, ###P<0.001 versus the 10-mg/ml pirfenidone-treated group. The magnification used was 200 ×.
Pirfenidone inhibited human epidural scar fibroblast proliferation
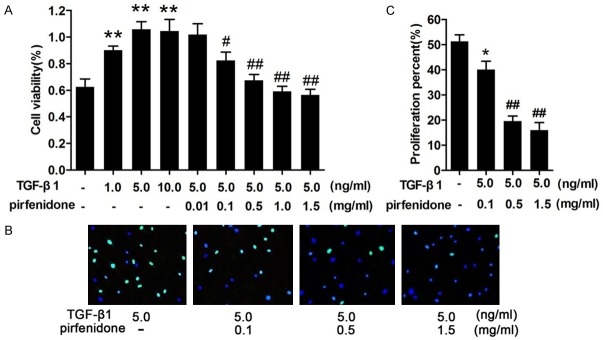
To gain further insight into the mechanism of action of pirfenidone on stimulated human epidural scar fibroblasts, a CCK-8 was used to measure cell viability after stimulation with different concentrations of TGF-β1 (0, 1.0, 5.0, and 10.0 ng/ml). Cell proliferation increased in a dose-dependent manner, and the median effective concentration of TGF-β1 was 5 ng/ml (Figure 3A). Next, cells were treated with 5 ng/ml TGF-β1 and different concentrations of pirfenidone (0, 0.01, 0.1, 0.5, 1.0, and 1.5 mg/ml) for 48 h. The results showed that pirfenidone inhibited TGF-β1-induced fibroblast proliferation in a dose-dependent manner. The optimum concentration of pirfenidone was 0.5 mg/ml (Figure 3A). Consistent with the findings of the CCK-8 assay, the EdU assay also demonstrated the significantly anti-proliferative effects of pirfenidone on TGF-β1-treated fibroblasts (Figure 3B and 3C). These data suggest that pirfenidone inhibits TGF-β1-induced fibroblast proliferation.
Figure 3.
Pirfenidone inhibited the proliferation of human epidural scar fibroblasts. A. Cells were treated with 0, 1, 5, or 10 ng/ml transforming growth factor-β1 (TGF-β1) for 48 h or pretreated with 5 ng/ml TGF-β1 and then incubated with different concentrations (0.01, 0.1, 0.5, 1.0, and 1.5 mg/ml) of pirfenidone for 48 h. Cell viability after different treatments was detected using a Cell-Counting Kit-8 (CCK-8). B. Cells pretreated with 5 ng/ml TGF-β1 were stained with 5-ethynyl-2-deoxyuridine (EdU) 48 h after pirfenidone treatment and then visualized using a fluorescence microscope. Nuclei are shown in blue, and EdU staining is shown in green. C. The histograms represent the means ± SD of three independent experiments. *P<0.05 versus the control group, ##P<0.01 versus the TGF-β1 only treatment group.
Apoptotic effect and toxicity of pirfenidone
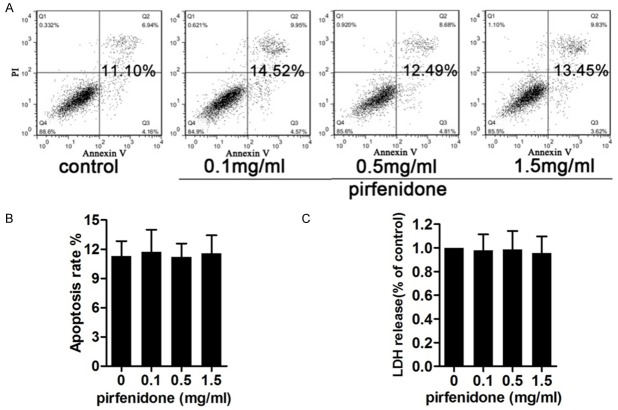
To investigate whether the anti-proliferative effects of pirfenidone were mediated by apoptosis or cellular toxicity, annexin V/PI double staining (Figure 4A and 4B) and an LDH (Figure 4C) release assay were carried out. Human epidural scar fibroblasts were exposed to various concentrations of pirfenidone (0, 0.1, 0.5, and 1.5 mg/ml) for 48 h. Results indicated that pirfenidone showed no significant apoptotic effects and cytotoxicity on cultured human epidural scar fibroblasts at the concentrations and time periods tested. These results suggest that pirfenidone exert its anti-proliferative effects in a non-apoptotic and -cytotoxic manner.
Figure 4.
Toxicology and apoptotic effects of pirfenidone. Cells were treated with 0, 0.1, 0.5, or 1.5 mg/ml pirfenidone for 48 h and then used for subsequent experiments. A. Apoptotic rates were assessed using annexin V/propidium iodide (PI) double staining and flow cytometry. The cells shown in the bottom right quadrant are annexin V-fluorescein isothiocyanate (FITC)-positive and PI-negative, indicating an early stage of apoptosis. The cells in the top right quadrant stained positive for annexin V-FITC and PI, indicating late apoptotic/necrotic cells. The total percentage of cell apoptosis is shown in bold. B. Statistical analysis of the total recorded apoptotic cells was performed. The results are shown in the bar graphs. C. A lactate dehydrogenase (LDH) assay was performed to detect the cytotoxicity of pirfenidone. Data represent means ± standard error of the mean (SEM) of the percentage cytotoxicity calculated in cells from three independent experiments. No significant cytotoxic effects were observed for any treatment.
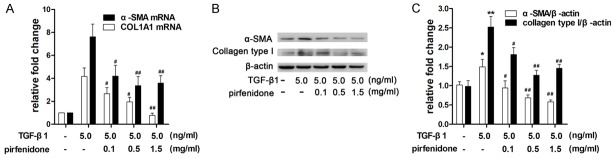
Effects of pirfenidone on TGF-β1-induced α-SMA and collagen type I protein expression
Fibroblast differentiation is considered to be an important process in fibrosis formation. Because the expression of α-SMA is characteristic of myofibroblasts, the effects of pirfenidone on TGF-β1-induced α-SMA mRNA and protein levels were measured via qPCR and western blotting, respectively. Pirfenidone treatment reduced TGF-β1-induced α-SMA mRNA expression in a dose-dependent manner (Figure 5A). Consistent with this result, treatment with 0.5 mg/ml pirfenidone reduced α-SMA protein expression in the presence of TGF-β1 stimulation (Figure 5B and 5C). Collagen type I protein is a major ECM component that leads to the formation of fibrosis. Next, we performed qPCR and western blotting to investigate the effect of pirfenidone on the TGF-β1-induced synthesis of COL1A1 mRNA (encoding pro-collagen type-I) as well as collagen type I protein. The results indicate that pirfenidone inhibited TGF-β1-induced expression levels of COL1A1 mRNA and collagen type I protein in a dose-dependent manner (Figure 5B and 5C).
Figure 5.
Pirfenidone inhibited TGF-β1-induced α-SMA and type I collagen (COL1A1) expression. Cells were treated with 5 ng/ml TGF-β1 and then incubated with different concentrations (0, 0.1, 0.5, and 1.5 mg/ml) of pirfenidone for 48 h. A. Relative α-SMA and COL1A1 mRNA levels were detected via quantitative real-time polymerase chain reaction (PCR) and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. These values were compared with those of the TGF-β1 only treatment group. The values represent means ± SD of three separate experiments. B. Equal amounts of whole cell lysates were analyzed via western blotting with antibodies specific for α-SMA, type I collagen, and β-actin (loading control). C. The band intensities for α-SMA and type I collagen are shown as a histogram. Those of the control group were normalized to a value of 1. Gel data are derived from experiments performed in triplicate with similar results. The bar graphs represent the average results from three different experiments. *P<0.05, **P<0.01 versus the control group. #P<0.05, ##P<0.01 versus the TGF-β1 only treatment group.
Effects of pirfenidone on TGF-β1-induced Smad2, Smad3, Akt, and p38 activity
TGF-β1 induces tissue fibrosis via activation of both Smad-dependent and -independent signaling pathways, which results in the activation of fibroblasts and the excessive production of ECM. To further elucidate the molecular mechanism of pirfenidone on TGF-β1-treated fibroblasts, the expression of related proteins was detected via western blotting. The results indicate that TGF-β1 activated the phosphorylation of Smad2, Smad3, p38, and Akt, and that pirfenidone significantly impaired the phosphorylation of Smad2, Smad3, p38, and Akt in a dose-dependent manner (Figure 6A and 6B).
Figure 6.
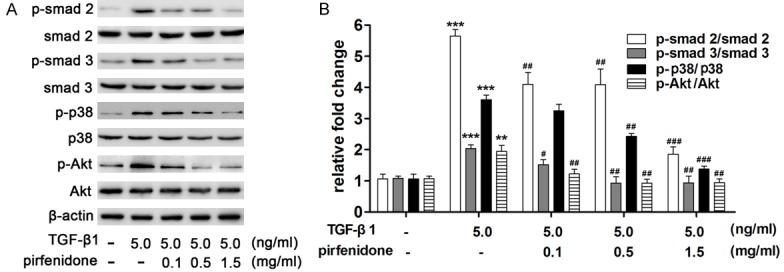
Effect of pirfenidone on TGF-β1-induced Smad 2, Smad 3, p38, and Akt expression. Cells were treated with 5 ng/ml TGF-β1 and then incubated with different concentrations (0, 0.1, 0.5, and 1.5 mg/ml) of pirfenidone for 48 h. A. Equal amounts of whole cell lysates were analyzed via western blotting with antibodies specific for Smad2, Smad3, p38, Akt, and their phosphorylation statuses were determined. Expression of β-actin was used to normalize sample loading. Pirfenidone significantly impaired TGF-β1 activated the phosphorylation of Smad2, Smad3, p38, and Akt in a dose-dependent manner. B. The band intensities for each protein are shown as a histogram. Those of the control group were normalized to a value of 1. Gel data are derived from experiments performed in triplicate with similar results. The bar graphs represent the average results from three different experiments. *P<0.05, **P<0.01, ***P<0.001 versus the control group. #P<0.05, ##P<0.01, ###P<0.001 versus the TGF-β1 only treatment group.
Discussion
The formation of fibrosis in the epidural space is a natural healing process after laminectomy [29]. It occurs via the same mechanism as wound scarring and proceeds through three overlapping dynamic phases. First is the inflammatory phase, in which multiple cytokines such as TGF-β and basic fibroblast growth factors are released from damaged tissue. Next is the cell proliferation phase in which cytokines promote the proliferation and differentiation of fibroblasts. The increasing number of myofibroblasts leads to increased production of ECM. The third is the scar formation phase, involving the imbalanced deposition and degradation of ECM that lead to fibrosis formation [30]. In all three phrases, fibroblasts perform the most important function. Therefore, various drugs including non-steroidal anti-inflammatory agents are used in the treatment of epidural scar formation as they inhibit the proliferation and differentiation of epidural fibroblasts. To date, there is no satisfactory treatment for this pathological process.
Pirfenidone is the only drug approved for the treatment of idiopathic pulmonary fibrosis in Europe and Japan [23]. Scholars are increasingly investigating its antifibrotic properties in a variety of in vitro and in vivo models of lung, liver, renal, and ocular tissue fibrosis [15,19-22,31,32]. In this study, multiple parameters, including the histological analysis, area of scar tissue, and number of fibroblasts, were used to evaluate the effect of pirfenidone on epidural fibroblast proliferation and scar formation in a rat model of laminectomy. Our results indicate that pirfenidone inhibited epidural fibroblast proliferation and reduced fibrosis formation at laminectomy sites in a rat model.
Next, we studied the underlying mechanism of action of pirfenidone on the inhibition of fibrosis formation. Numerous studies have established that TGF-β is part of a superfamily of related growth factors. Three separate TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3) have been identified in mammals. These control proliferation, differentiation, and other functions in many cell types [10]. Among the three isoforms, TGF-β1 plays a dominant role in the initiation and progression of fibrosis formation [10,11,33]. During the inflammatory phase of tissue repair, TGF-β1, which is secreted by damaged tissue, recruits and promotes the differentiation of fibroblasts, and regulates the secretion of ECM [11]. In this experiment, we stimulated fibroblasts with TGF-β1 and observed the effect of pirfenidone on these. The CCK-8 and EDU assays showed that pirfenidone inhibited the TGF-β1-induced proliferation of cultured human epidural scar fibroblasts in a dose-dependent manner.
To further confirm the apoptotic and toxic effects of pirfenidone, annexin V/PI double staining and LDH assays were performed. Pirfenidone exerted no significant apoptotic and cytotoxic effects on cultured human epidural scar fibroblasts. Thus, these results suggest that pirfenidone inhibited fibroblast activity by inhibiting cell proliferation rather than by inducing cell apoptosis or cytotoxicity. In agreement with our study, similar results were observed in cardiac fibroblasts [34], human lung fibroblasts [21], and human Tenon’s fibroblasts [22]. Pirfenidone showed no cytotoxic effects on various cell lines, suggesting that it may be used as a promising scar-suppressing drug in clinical practice. However, its potential side effects on epidural fat cells and nerve cells require further study.
TGF-β1 induces the transition of fibroblasts to myofibroblasts [10,12,13,30,33,35,36]. Myofibroblasts, which are characterized by the expression of α-SMA, play a crucial role in wound healing, fibrosis formation, contraction, and connective tissue remodeling. Furthermore, myofibroblasts can promote more endogenous TGF-β1 secretion, thereby promoting the formation of pathological scarring [36]. In this study, we demonstrated that pirfenidone inhibited exogenous TGF-β1-induced expression of α-SMA. This finding is in agreement with previous studies on human Tenon’s fibroblasts [22], keloid fibroblasts [19], and corneal fibroblasts [15]. This result is noteworthy considering that prevention of fibroblast differentiation may represent a crucial target for therapies aimed at limiting fibrosis formation in the epidural space after laminectomy. Furthermore, using qPCR and western blot analysis, we clearly demonstrated that pirfenidone inhibited TGF-β1-induced synthesis of collagen type I protein at both mRNA and protein levels.
TGF-β1 induces scar formation by activating both canonical (Smad-based) and non-canonical (non-Smad-based) signaling pathways [11,21,37]. In the canonical pathway, TGF-β1 binds to TGF-β receptor 2 (TGFR2), which then recruits and activates TGFR1. The active TGFR1 then phosphorylates Smad2 and Smad3, which complex with Smad4 and translocate to the nucleus, and then induce transcription of profibrotic molecules, including α-SMA and collagen type I protein [21]. Therefore, we investigated whether pirfenidone was able to inhibit Smad2 and Smad3 activation. In this study, we demonstrated that pirfenidone treatment significantly reduced TGF-β1-induced Smad2 and Smad3 phosphorylation in human epidural scar fibroblasts. These results bear similarities with those of a previous study showing that pirfenidone inhibited TGF-β-induced phosphorylation of Smad2 or Smad3 in many cell lines such as human lung fibroblasts [21], lens epithelial cells [38], and retinal pigment epithelial cells [20].
In non-canonical signaling pathways, TGF-β1 activates Ras and mitogen-activated protein (MAP) kinases, the phosphoinositide 3-kinase (PI3K)/Akt pathway, and Rho GTPases, and regulates cell growth, survival, migration, and cytoskeleton organization [37,39,40]. In this study, we investigated the putative effects of pirfenidone on Akt, p38. We showed that pirfenidone significantly impaired Akt and p38 phosphorylation in TGF-β1-induced human epidural scar fibroblasts. Our data reinforce the work of others who have shown that pirfenidone inhibited Akt and p38 activation in primary human lung fibroblasts [21]. As a complement to the canonical pathway, the role of the non-canonical pathway in the inhibition of fibroblast proliferation and differentiation by pirfenidone and the interaction with canonical pathways requires further study. The role of other signaling pathways, such as the RhoA-Rho kinase signaling pathway, the Ras-Erk MAP kinase pathway, and the JNK MAP kinase pathway, also needs more investigation.
In summary, our data indicate that pirfenidone reduced epidural scar tissue formation by inhibiting fibroblast proliferation and suppressing collagen formation in a rat model of laminectomy. Moreover, we found that pirfenidone modulated TGF-β1-induced Smad2, Smad3, Akt, and p38 phosphorylation in human epidural scar fibroblasts. This study provides preliminary evidence for the future clinical use of pirfenidone.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 81371968, 81871773 and 81672152), the Jiangsu Province Science Foundation for Youths (grant BK20171089), the National Science Foundation for Young Scientists of China (grant 81803473), the Beijing Medicine and Health Foundation (grant YWJKJJHKYJJ-A720), and the Xuzhou Science and Technology Bureau Applied Basic Research Program (grant KC18041), and High-tech Research Program of Jiangsu Science and Technology Department (grant BE2018132).
Disclosure of conflict of interest
None.
References
- 1.Williams MG, Wafai AM, Podmore MD. Functional outcomes of laminectomy and laminotomy for the surgical management lumbar spine stenosis. J Spine Surg. 2017;3:580–586. doi: 10.21037/jss.2017.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH, Lee JH, Song KS, Hong JY. Neuropathic pain after spinal surgery. Asian Spine J. 2017;11:642–652. doi: 10.4184/asj.2017.11.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabb CH. Failed back syndrome and epidural fibrosis. Spine J. 2010;10:454–455. doi: 10.1016/j.spinee.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira MJ, Yeng LT, Garcia OG, Fonoff ET, Paiva WS, Araujo JO. Failed back surgery pain syndrome: therapeutic approach descriptive study in 56 patients. Rev Assoc Med Bras. 2011;57:282–287. [PubMed] [Google Scholar]
- 5.Cekinmez M, Erdogan B, Tufan K, Sarica FB, Ozen O, Caner H. Is topical tissue plasminogen activator application effective on prevention of post-laminectomy epidural fibrosis? An experimental study. Neurol Res. 2009;31:322–326. doi: 10.1179/174313208X332940. [DOI] [PubMed] [Google Scholar]
- 6.Dobran M, Brancorsini D, Costanza MD, Liverotti V, Mancini F, Nasi D, Iacoangeli M, Scerrati M. Epidural scarring after lumbar disc surgery: equivalent scarring with/without free autologous fat grafts. Surg Neurol Int. 2017;8:169. doi: 10.4103/sni.sni_142_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Sui T, Hong X, Wu X, Cao X. Inhibition of epidural fibrosis after microendoscopic discectomy with topical application of mitomycin C: a randomized, controlled, double-blind trial. J Neurosurg Spine. 2013;18:421–427. doi: 10.3171/2013.1.SPINE12564. [DOI] [PubMed] [Google Scholar]
- 8.Wu CY, Huang YH, Lee JS, Tai TW, Wu PT, Jou IM. Efficacy of topical cross-linked hyaluronic acid hydrogel in preventing post laminectomy/laminotomy fibrosis in a rat model. J Orthop Res. 2016;34:299–306. doi: 10.1002/jor.23001. [DOI] [PubMed] [Google Scholar]
- 9.Keane TJ, Horejs CM, Stevens MM. Scarring vs. functional healing: matrix-based strategies to regulate tissue repair. Adv Drug Deliv Rev. 2018;129:407–419. doi: 10.1016/j.addr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-beta. Expert Rev Mol Med. 2003;5:1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 11.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 13.Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res. 2016;5:752. doi: 10.12688/f1000research.8190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armendariz-Borunda J, Lyra-Gonzalez I, Medina-Preciado D, Gonzalez-Garcia I, Martinez-Fong D, Miranda RA, Magana-Castro R, Pena-Santoyo P, Garcia-Rocha S, Bautista CA, Godoy J, Flores-Montana J, Floresvillar-Mosqueda J, Armendariz-Vazquez O, Lucano-Landeros MS, Vazquez-Del Mercado M, Sanchez-Parada MG. A controlled clinical trial with pirfenidone in the treatment of pathological skin scarring caused by burns in pediatric patients. Ann Plast Surg. 2012;68:22–28. doi: 10.1097/SAP.0b013e31821b6d08. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury S, Guha R, Trivedi R, Kompella UB, Konar A, Hazra S. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS One. 2013;8:e70528. doi: 10.1371/journal.pone.0070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, Alexander KA, Meng J, Roy S, Panoskaltsis-Mortari A, Loschi M, Hill GR, Serody JS, Maillard I, Miklos D, Koreth J, Cutler CS, Antin JH, Ritz J, MacDonald KP, Schacker TW, Luznik L, Blazar BR. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood. 2017;129:2570–2580. doi: 10.1182/blood-2017-01-758854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasdemir PS, Ozkut M, Guvenal T, Uner MA, Calik E, Koltan SO, Koyuncu FM, Ozbilgin K. Effect of pirfenidone on vascular proliferation, inflammation and fibrosis in an abdominal adhesion rat model. J Invest Surg. 2017;30:26–32. doi: 10.1080/08941939.2016.1215578. [DOI] [PubMed] [Google Scholar]
- 18.Lee BS, Margolin SB, Nowak RA. Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab. 1998;83:219–23. doi: 10.1210/jcem.83.1.4503. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, Yamazaki M, Maeda T, Matsumura H, Setoguchi Y, Tsuboi R. Pirfenidone suppresses keloid fibroblast-embedded collagen gel contraction. Arch Dermatol Res. 2012;304:217–222. doi: 10.1007/s00403-011-1184-2. [DOI] [PubMed] [Google Scholar]
- 20.Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-beta1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–1020. [PMC free article] [PubMed] [Google Scholar]
- 21.Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Yu M, Wu K, Yuan H, Zhong H. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon’s fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2009;50:3763–3770. doi: 10.1167/iovs.08-2815. [DOI] [PubMed] [Google Scholar]
- 23.Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, Collard HR. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavez-Tapia NC, Mendez-Sanchez N. Clinical decisions in hepatology: the pirfenidone case analysis. Ann Hepatol. 2014;13:163–165. [PubMed] [Google Scholar]
- 25.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantu-Cantu MZ, Lyra-Gonzalez I, Armendariz-Borunda J. Coadjuvant treatment with surgery and pirfenidone in severe facial trauma due to dog bite. J Craniofac Surg. 2013;24:675–678. doi: 10.1097/SCS.0b013e31828609cb. [DOI] [PubMed] [Google Scholar]
- 27.Shi K, Wang D, Cao X, Ge Y. Endoplasmic reticulum stress signaling is involved in mitomycin C (MMC)-induced apoptosis in human fibroblasts via PERK pathway. PLoS One. 2013;8:e59330. doi: 10.1371/journal.pone.0059330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rydell N. Decreased granulation tissue reaction after installment of hyaluronic acid. Acta Orthop Scand. 1970;41:307–311. doi: 10.3109/17453677008991516. [DOI] [PubMed] [Google Scholar]
- 29.Fransen P. Prevention of scar tissue formation in spinal surgery: state of the art and review of the literature. J Neurosurg Sci. 2011;55:277–81. [PubMed] [Google Scholar]
- 30.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 31.Seniutkin O, Furuya S, Luo YS, Cichocki JA, Fukushima H, Kato Y, Sugimoto H, Matsumoto T, Uehara T, Rusyn I. Effects of pirfenidone in acute and sub-chronic liver fibrosis, and an initiation-promotion cancer model in the mouse. Toxicol Appl Pharmacol. 2018;339:1–9. doi: 10.1016/j.taap.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yang Y, Xu J, Lin X, Wu K, Yu M. Pirfenidone inhibits migration, differentiation, and proliferation of human retinal pigment epithelial cells in vitro. Mol Vis. 2013;19:2626–2635. [PMC free article] [PubMed] [Google Scholar]
- 33.Naim R, Naumann A, Barnes J, Sauter A, Hormann K, Bloching M. TGF-beta1 modulates HGF/SF in keloid fibroblast cell culture. Otolaryngol Head Neck Surg. 2008;139:P32–P33. doi: 10.1007/s00266-007-9078-6. [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, Hu S, Wei Y. In vitro effects of pirfenidone on cardiac fibroblasts: proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS One. 2011;6:e28134. doi: 10.1371/journal.pone.0028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis SC, Perez R. Cosmeceuticals and natural products: wound healing. Clin Dermatol. 2009;27:502–506. doi: 10.1016/j.clindermatol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495–506. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ye Y, Lin X, Wu K, Yu M. Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PLoS One. 2013;8:e56837. doi: 10.1371/journal.pone.0056837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Ko M, Joo CK. Rho plays a key role in TGF-beta1-induced cytoskeletal rearrangement in human retinal pigment epithelium. J Cell Physiol. 2008;216:520–526. doi: 10.1002/jcp.21424. [DOI] [PubMed] [Google Scholar]