Abstract
Nonalcoholic steatohepatitis represents a significant and rapidly growing unmet medical need. The development of novel therapies has been hindered in part, by the limitations of existing preclinical models. There is a strong need for physiologically relevant in vivo and in vitro liver fibrosis models that are characterized by better translational predictability. In this study, we used the InSphero 3D InSightTM three-dimensional (3D) human liver microtissue (3D-hLMT) system prepared by co-culturing primary human hepatocytes with hepatic stellate cells, Kupffer cells and endothelial cells to develop a model of NASH with a severe fibrotic phenotype. In our model, palmitic acid (PA) induced a robust proinflammatory and profibrogenic phenotype in the 3D-hLMT. PA significantly increased several markers of the inflammatory and profibrotic process including gene expression of collagens, α-sma, tissue inhibitor of matrix metalloprotease 1 (timp1) and the stellate cell activation marker pdgfrβ as well as secreted CXCL8 (IL8) levels. We also observed TGFβ pathway activation, increase in active collagen synthesis and significant overall increase in tissue damage in the 3D-hLMTs. Immunohistochemistry analysis demonstrated the upregulation of collagen, cleaved caspase 3 as well as of the PDGFRβ protein. We further validated the model using a phase 3 clinical compound, GS-4997, an apoptosis signal-regulating kinase 1 (ASK-1) inhibitor and showed that GS-4997 significantly decreased PA induced profibrotic and proinflammatory response in the 3D-hLMTs with decreases in apoptosis and stellate cell activation in the microtissues. Taken together we have established and validated an in vitro 3D-hLMT NASH model with severe fibrotic phenotype that can be a powerful tool to investigate experimental compounds for the treatment of NASH.
Keywords: NASH, fibrosis, liver micro tissue, ASK-1
Introduction
Nonalcoholic steatohepatitis (NASH) is a severe form of nonalcholic fatty liver disease (NAFLD) and is characterized by the presence of hepatic steatosis (accumulation of lipids), hepatocyte injury and inflammation [1]. NASH can be progressive and may ultimately result in advanced fibrosis and cirrhosis characterized by disrupted hepatic architecture, vascular structure and aberrant regeneration that can eventually lead to mortality. Currently NASH is the most common liver disorder in Western countries and there are no approved drugs for this debilitating disease.
Due to the significant unmet medical need, there has been a lot of interest in recent years in the development of new therapies for NASH with fibrosis. In spite of significant progress in our understanding of the pathogenic mechanisms of liver fibrosis, effective antifibrotic therapies are still lacking. This is, in part, due to the lack of validated translational models and the poor predictability of animal models that have considerably hindered progress [2].
The most widely used 2D human cellular models fall short due to diminished longevity and absence of critical hepatocyte-non parenchymal cell interactions [3]. Therefore physiologically more relevant human NASH models with a robust fibrotic phenotype are needed that are characterized by in vitro longevity and contain the main liver cell types involved in the pathophysiology. Such in vitro models will be important tools to reliably investigate and develop transformational drugs to treat NASH with fibrosis.
Three-Dimensional (3D) human liver microtissue models prepared by co-culturing primary human hepatocytes with non-parenchymal cells such as hepatic stellate cells, Kupffer cells and endothelial cells have shown great promise for in vitro toxicology studies [4]. The 3D-human liver micro-tissues (3D-hLMTs) can be maintained for over 5 weeks and are well characterized with respect to adenosine triphosphate level, albumin secretion, native human liver gene expression, histological assessment and with the expression of the bile salt export pump (BSEP) and the multidrug resistance protein 2 (MRP2) [4]. 3D-hLTMs have also been successfully used recently to develop in vitro models of liver fibrosis using various inflammatory and profibrotic mediators [5].
High levels of unsaturated free fatty acids play a crucial role in the development of NASH. The free fatty acids cause lipid accumulation followed by damage to the hepatocytes by lipo-apoptosis [6], which then cascades into the activation of Kupffer cells triggering a pro-inflammatory response and activation of hepatic stellate cells (HSCs). The stellate cells transdifferentiate into a fibrogenic myofibroblast phenotype that produce smooth muscle actin and collagens, particularly type I, III and VI causing excessive deposition of extracellular matrix, which is conducive for the development of fibrosis during experimental and chronic human liver injury [7,8].
In this study, we used 3D-hLMTs as a tool to develop a model of NASH with a severe fibrotic phenotype. To recapitulate liver fibrosis as observed during NASH we explored if treatment of the 3D-hLMT with high concentrations of palmitic acid would induce a phenotype similar to that observed in NASH patients with fibrosis. Our data show that 0.5 mM palmitic acid (PA) induced a robust pro-inflammatory and fibrogenic phenotype in the 3D-hLMT. PA significantly induced the expression of several collagen genes, α-sma (alpha smooth muscle actin), tissue inhibitor of matrix metalloprotease 1 (timp1) and the stellate cell activation marker pdgfrβ (platelet derived growth factor receptor beta) as shown both by gene expression analysis and imaging as well as secreted CXCL8 (IL8) levels. We also observed TGFβ (transforming growth factor beta) pathway activation, increase in active collagen synthesis and significant overall increase in tissue damage in the 3D-hLMTs. We then further validated this translational in vitro model with GS-4997, an apoptosis signal-regulating kinase 1 (ASK-1) inhibitor and showed that GS-4997 decreased the PA induced profibrotic and proinflammatory response in the 3D-hLMTs with significant decrease in apoptosis in the microtissues. Thus, we have established and validated an in vitro model that can be used to test experimental compounds for the treatment of NASH.
Materials and methods
Spheroid culturing
3D InSightTM Human Liver Microtissues (MT-02-302-05) and Human Liver Maintenance Medium (hLiMM) TOX (CS-07-001a) were purchased from InSphero, Switzerland. Microtissues were incubated with 70 μL of fresh hLiMM TOX medium at 37°C in 5% CO2 incubator for 4 days. The medium was changed one more time 24 hr later-and treated with 0.5 M Palmitic acid (day 0, Figure 1). The same treatment of the hLiMTs was repeated after 2 days (day 2). The dilutions of GS-4997 stocks in DMSO and the vehicle controls were aliquoted and frozen for each dosing. On the day of treatment, aliquots were diluted to working concentration with hLiMM TOX. The final volume of hLiMM TOX medium for the treatments was 50 ul. The experiment was terminated on day 9 and the supernatants were collected for ELISA and luminex assays. The liver microtissues were lysed in the RNA buffer from the ReliaPrepTM RNA Miniprep Systems (Promega) and stored at -80°C for RNA extraction.
Figure 1.
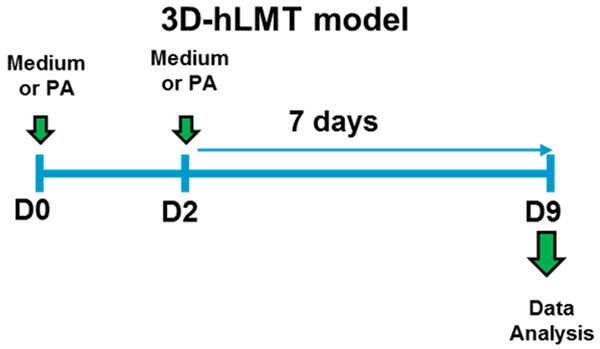
3D human liver microtissue NASH model with severe fibrotic phenotype. The microtissues (MTs) were treated with 0.5 mM palmitic acid (PA) in the presence or absence of 1 uM of Selonsertib or with Medium+DMSO on day 0 and day 2. On day 9 all the MTs and supernatants were collected and analyzed for various endpoints.
Real-time PCR
Total RNA was extracted from liver microtissues with ReliaPrepTM RNA Miniprep Systems (Promega). The RNA was reverse transcribed to cDNA with reverse transcription kit (QuantaBio qScript cDNA kit 95048-500). The Applied Biosystems TaqMan Gene Expression Assays and the best coverage primers for each gene were purchased from ThermoFisher Scientific. mRNA expression of target genes was calculated using a comparative Ct (ΔCt) method and normalized by geomean of GAPDH, B2M and RPL30.
ELISA and luminex assays
The levels of human ProCol1 (R&D Systems), PIIINP (Cloud-Clone Corp) and PAI-1 (ThermoFisher Scientific) was measured in the microtissue supernatants collected on day 9. Briefly, the sups from eight microtissues were combined and appropriate amount of material was used for the ELISA according to the respective manufacturer’s instructions. For the ProCol1 ELISA the samples were diluted 1:10, undiluted for PIIINP and for the PAI-1 ELISA the samples were diluted 1:2. The ELISA plates were read by measuring absorption as specified in the respective manufacturer’s protocol. All experiments were performed in triplicates and repeated at least twice to assess reproducibility. IL-8 levels in the liver microtissue supernatants were measured by Bio-Rad bioplex assays using the Luminex platform.
Cell cytotoxicity
Cell death and cell lysis were quantified based on the measurement of lactate dehydrogenase (LDH) activity released from the cytosol of damaged cells into the supernatant. LDH activity (Cytotoxicity Detection Kit-Sigma-Aldrich) was measured in undiluted supernatants and according to the manufacturer’s protocol. All experiments were performed in triplicates and repeated at least twice to assess reproducibility.
Quantification of P1NP
The concentration of P1NP fragments in the supernatants was measured by competitive ELISA assay for human N-terminal propeptide of collagen type 1 (Nordic Bioscience) as described previously [9].
GS-4997 and palmitic acid
GS4997 was purchased from Selleckchem and a stock solution of 10 mM was prepared in DMSO. A 10 mM stock of Palmitic Acid was prepared following the protocol as describe previously [10].
Immunofluorescence
Microtissues were collected and processed for histology following the protocol (TP006) from InSphero Histology Protocols -(https://insphero.com/support/protocols-manuals). PDGF Receptor β (28E1) Rabbit mAb (Cell Signaling Technology), cleaved caspase 3 antibody (Cell Signaling) and Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody Alexa Fluor Plus 555 (Thermo Fisher Scientific) were used for staining of the 5 uM FFPE sections.
Histology
Microtissues were collected and processed for histology following the protocol (TP006) -(https://insphero.com/support/protocols-manuals) from InSphero Histology Protocols. Masson’s trichrome (Poly Scientific R&D) staining was done using standard protocols.
Statistical analysis
The experimental data are expressed as mean ± SD. One-way ANOVA with Dunnett’s post-test for multiple comparisons was used to determine whether sample mean values between groups were significantly different. P<0.05 was considered statistically significant.
Results
PA induced tissue damage and a robust fibrotic phenotype in the 3D-hLMTs
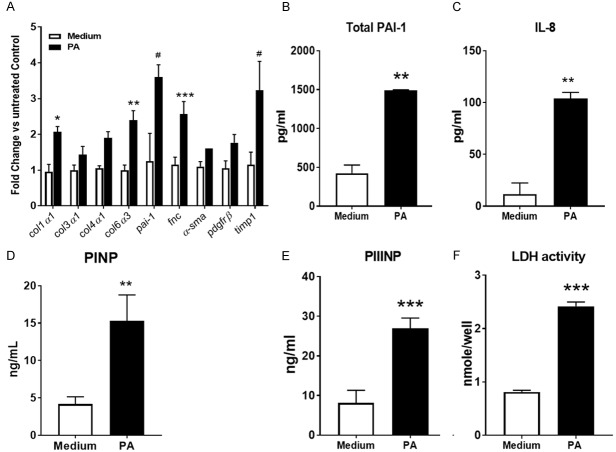
Unsaturated free fatty acids play an important role in the development of NAFLD and NASH. Oleic acid and palmitic acid are the 2 most abundant fatty acids in Western diets and both are likely to be involved in the development of NASH. However, they have differential effects on hepatocytes in vitro in that palmitic acid more robustly induces injury/apoptosis and therefore it is likely to be a more effective inducer of a severe fibrotic phenotype in the context of a relatively short term model. High concentrations of palmitic acid are hepatotoxic and induce lipoapoptosis in the parenchymal cells of the liver, which in turn can cause inflammation followed by the induction of pro-fibrotic processes. To recapitulate liver fibrosis as observed during NASH, we explored if treatment of the 3D human liver microtissues with high concentrations of palmitic acid would induce a phenotype similar to that observed in human NASH patients. During the development of the model, we optimized the treatment regime with respect to time course, treatment frequency and concentration (not shown) and established an optimized protocol as shown in Figure 1. According to this protocol, we treated the 3D human liver microtissues with 0.5 mM of palmitic acid twice on day 0 and day 2 and then the MTs and supernatants were collected for further analysis after 7 days on day 9. Palmitic acid significantly increased the expression of collagen genes, α-sma, fibronectin and timp1 as well as of pdgfrβ, a marker of hepatic stellate cell activation in the 3D-hLMT (Figure 2A). The expression of the pai-1 gene, a downstream target of TGFβ as well as PAI-1 protein levels were increased with palmitic acid treatment indicating the engagement and activation of the TGFβ pathway during the fibrotic response in the microtissues (Figure 2B). IL-8 levels increased in the supernatant of the microtissues treated with PA, suggesting a proinflammatory microenvironment in the 3D system (Figure 2C). To assess active collagen synthesis in the 3D model, the levels of P1NP and PIIINP, products of procollagen 1 and procollagen 3 synthesis, respectively were measured in the supernatants. Palmitic acid significantly increased the levels of both of these markers of collagen synthesis in the microtissues (Figure 2D and 2E). To assess the viability and functionality of the microtissues upon PA treatment we measured LDH, a clinical biochemical marker of tissue damage. We observed significant increase in the levels of LDH (Figure 2F) suggesting ensuing microtissue damage concomitant with the development of the fibrotic phenotype in response to palmitic acid treatment in the 3D microtissues. Taken together, palmitic acid induced tissue damage, a proinflammatory and a severe fibrotic phenotype in the 3D human liver microtissues, a phenotype closely resembling that of human NASH with fibrosis. Thus, our model of NASH with severe fibrotic phenotype in 3D-hLMTs provides us a translational tool to investigate experimental compounds for the treatment of NASH.
Figure 2.
Model characterization-PA induced fibrotic and proinflammatory phenotype with microtissue tissue damage in the 3D-hLMTs. A. Profibrotic gene expression in the MTs quantified by real time PCR. B, C. Total PAI-1 and IL-8 measured by ELISA and Luminex respectively. D, E. The levels of P1NP and PIIINP were quantified by ELISA based methods. F. LDH activity was measured in the supernatants using a substrate-based assay. Data is expressed as mean ± SD and *P<0.05; **P<0.01; ***P<0.001; #P=0.0001.
Anti-fibrotic and anti-inflammatory effects of GS-4997 in 3D-hLMT
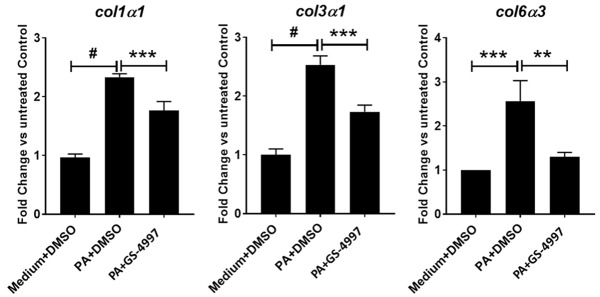
To validate the 3D-hLMT NASH model to support the investigation of compounds for the treatment of NASH, we used a Phase 3 clinical compound, GS-4997 (Selonsertib), an ASK-1 inhibitor that has shown promise for the treatment of NASH in clinical trials. The 3D-microtissues were treated with palmitic acid (0.5 mM) in the presence or absence of GS-4997 (1 uM) or left untreated as medium only control. GS-4997 significantly decreased palmitic acid induced gene expression of col1α1, col3α1 and col6α3 in the microtissues (Figure 3). Hepatic stellate cell activation is a key feature during the development of fibrosis in NASH. In our model, palmitic acid induced stellate cell activation was reduced with GS-4997 as indicated by the reduction of pdgfrβ expression. Furthermore, GS-4997 also decreased the expression of the proinflammatory cytokine IL-12 in the 3D-microtissues (Figure 4A and 4B).
Figure 3.
GS-4997 decreased collagen gene expression. Gene expression of col1α1, col3α1 and col6α3 in the microtissues was quantified by real time PCR. Data is expressed as mean ± SD and **P<0.01; ***P<0.001; #P=0.0001.
Figure 4.
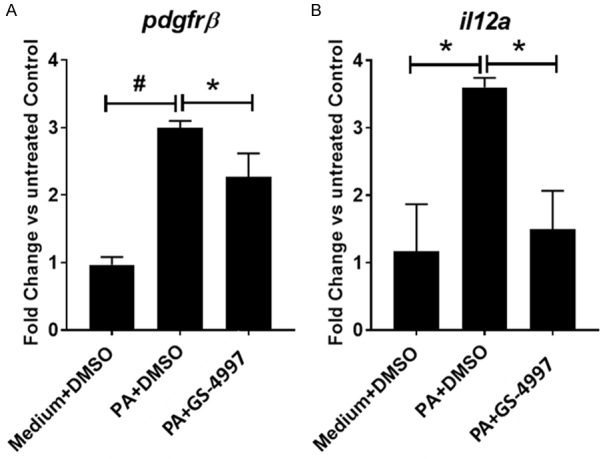
GS-4997 decreased expression of stellate cell activation and inflammation marker. (A) Stellate cell activation marker-pdgfrβ and (B) proinflammatory il12a gene expression in the microtissues was measured by real time PCR. Data is expressed as mean ± SD and *P<0.05; #P=0.0001.
Effect of GS-4997 on markers of collagen synthesis
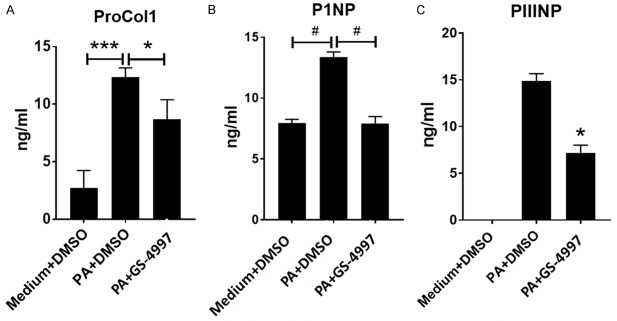
Excessive collagen synthesis is an integral part of the development of fibrosis. The synthesis of collagen can be measured by quantifying the amount of pro-collagen released into the supernatant following a fibrotic insult to the microtissues. During the synthesis of collagens these proteins undergo proteolytic maturation during which the precursor proteins, the pro-collagens, are cleaved by different proteases releasing N- and C-terminal pro-peptides thus creating the mature collagen proteins that organize into fibrils and form the mature collagen. These cleaved fragments of the processed procollagens are indicators of active collagen synthesis during fibrosis. To investigate the effect of GS-4997 on collagen 1 and collagen 3 synthesis in our 3D-hLMT model of NASH, the microtissues were treated with palimitic acid in the presence or absence of GS-4997. GS-4997 significantly decreased palmitic acid mediated increase in proCol1 (procollagen 1) (Figure 5A), P1NP (N-terminal pro-peptide of pro-collagen 1) (Figure 5B) and PIIINP (N-terminal pro-peptide of pro-collagen 3) (Figure 5C). The data suggest that GS-4997 reduced the active synthesis of collagen 1 and 3 in the 3D-hLMT NASH model. Collagen levels were also measured by trichrome staining of the 3D-microtissues. As shown in Figure 6, palmitic acid significantly increased the collagen levels (orange arrows on the blue stain) compared to the medium only group. Based on the image, the collagen deposition appears to resemble bridging fibrosis, a phenomenon seen in patients with NASH and severe fibrosis. In addition, palmitic acid induced a characteristic morphological change in the microtissues, suggestive of diminished tissue integrity, causing an overall reduction in the size of the microtissue. GS-4997 treatment decreased the collagen levels and reversed the morphological changes in the liver microtissues indicating an overall recovery of palmitic acid induced injury of the liver microtissues.
Figure 5.
ECM markers of collagen 1 and collagen 3 synthesis decreased with GS-4997. (A) Soluble proCol1, (B) N-terminal peptide of proCol1 (P1NP) and (C) N-terminal peptide of proCol3 were measured by ELISA in the supernatants of the MTs. Data is expressed as mean ± SD and *P<0.05; ***P<0.001; #P=0.0001.
Figure 6.
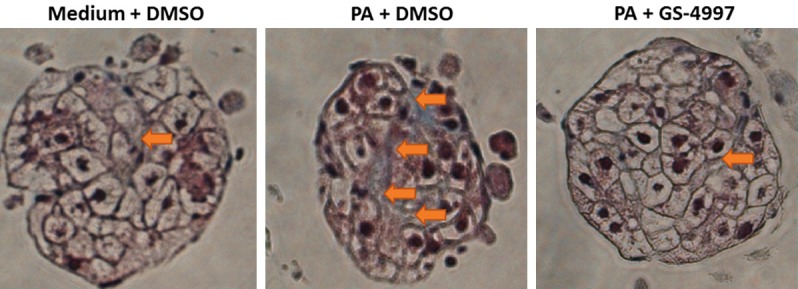
Reduction in collagen levels with GS-4997. Collagen levels in the microtissues were determined by trichrome staining. The orange arrows indicate collagen deposition (blue) in the microtissues.
GS-4997 decreased apoptosis and stellate cell activation in 3D-hLMT
To investigate the mechanism of the recovery from palmitic acid induced tissue injury of the liver microtissues that resulted in overall reduction in the size of the tissue and reduction in the fibrotic response, we stained for cleaved caspase 3, which is a marker of apoptosis and PDGFRβ, the marker for hepatic stellate cell activation, respectively. We hypothesized that the inhibition of ASK-1 with GS-4997 will decrease cellular apoptosis and stellate cell activation and thereby reverse the palmitic acid induced tissue injury and fibrotic response. We observed significant increase in apoptosis with palmitic acid indicated by yellow punctate staining of cleaved caspase 3 distributed across the microtissue parenchyma (Figure 7A) and there was complete inhibition of the cleaved caspase 3 staining with GS-4997. These data suggest that GS-4997 inhibits hepatocyte apoptosis in the 3D-hLMT. Furthermore, GS-4997 decreased PA induced hepatic stellate cell activation as shown in Figure 7B, thereby decreasing the fibrotic response in the microtissues. Reduced cleaved caspase 3 and PDGFRβ levels with GS-4997 indicate an impact on both hepatocytes and hepatic stellate cells and may explain the reversal of the palmitic acid induced tissue injury.
Figure 7.
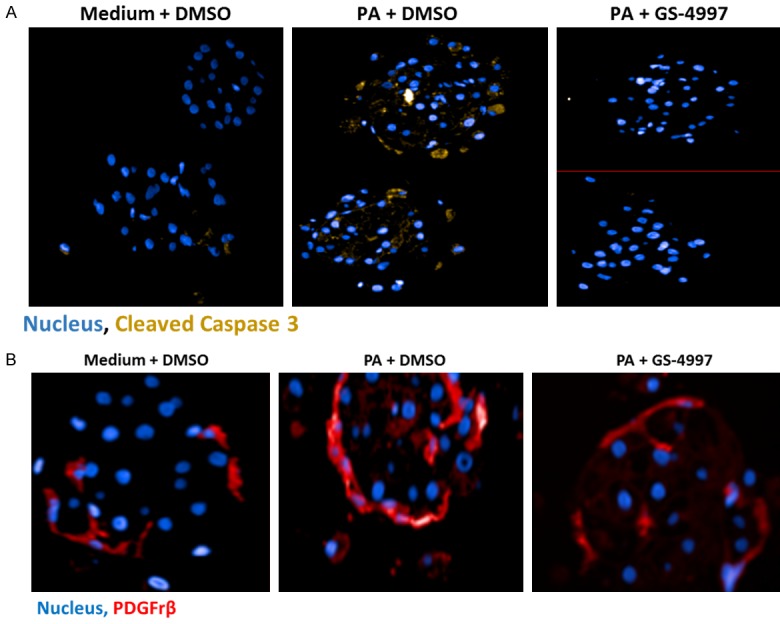
GS-4997 decreased PA induced apoptosis and stellate cell activation in microtissues were observed by immune-fluorescent confocal microscopy. A. Cleaved caspase 3 (yellow stain), a marker of apoptosis; B. PDGFRβ (red stain), a marker of stellate cell activation.
Discussion
There is a growing interest in the use of organ specific and physiologically relevant 3D multicellular spheroids in academic research and in the pharmaceutical industry. The 3D spheroids overcome many of the limitations of the monocultures or 2D culture systems in vitro by providing longevity to the cellular system, significant parenchymal-non parenchymal cell-to-cell interactions and enhanced normal tissue phenotype. Currently the most frequently used mono cultures for liver specific models or responses, are i) the hepatocyte culture system in which primary hepatocytes are plated on 2D collagen coated dishes [11-13]; ii) hepatic stellate cell cultures in various matrices with varying tensile strength and iii) Kupffer cell cultures for the measurement of inflammatory responses. The currently used 2D co-culture systems include i) co-cultures of hepatocytes with stromal fibroblasts [14] and ii) Kupffer cells co-cultured with stellate cells. These cellular models, however, lose liver-specific functionality and responses within days [11,15] hampering long-term and repeated dosing studies. On the other hand, the 3D spheroid culture systems in the recent past have emerged as promising tools to assess the mechanisms of hepatotoxicity due to their enhanced resemblance of the liver structure and phenotype, metabolic activity and stability in culture. Due to their proven functionality and close resemblance of many aspects of liver physiology, the 3D human multi-cellular spheroids seemed and ideal system for the development of organ specific disease models of human NASH.
In order to recapitulate human liver disease in vitro, it is necessary to culture all the main liver specific cells in proportions similar to that of the native liver to enable complex autocrine and paracrine cell-cell and cell-matrix interactions. The 3D-hLMTs provides such a multicellular system and seemed ideal to develop a NASH model with severe fibrotic phenotype. There are several physiologically relevant hepatic in vitro models in development including 3D bioprinted liver tissues comprising several hepatic cell types [16] and 3D spheroid cultures with hepatic non-parenchymal cells (NPC) [17,18]. However, we believe that the extensive characterization of the 3D-hLMTs used in this study with respect to their long term stability without detectable necrosis or loss of viability [4,17] together with a complex liver specific microenvironment offers distinct advantages for the development of in vitro human NASH models.
Indeed, Prestigiacomo et al. have recently described [5] the characterization of a 3D multicellular model of liver fibrosis utilizing the same InSphero technology to create the microtissues as we have in our current study. However, they have used various profibrotic and proinflammatory mediators, including TGF-β1, LPS and TNF-α as well as other non-specific profibrotic chemicals such as methotrexate (MTX) and thioacetamide (TAA) and showed strong fibrotic phenotypes in their studies. While their model is undoubtedly robust and is well suited for studying anti-fibrotic compounds and mechanisms, our aim was to establish a model that more specifically relates to the etiology of NASH. Furthermore, we set out to validate our model using a clinical phase NASH antagonist compound.
We used palmitic acid to challenge the 3D-hLMTs as a surrogate for high fat diet induced liver damage, and were able to demonstrate a robust fibrogenic and proinflammatory response in the microtissues. Palmitic acid induced significant apoptosis in the parenchymal cells and activated the stellate cells. Further activation of the TGFβ pathway is the cornerstone of a classical fibrotic response and PAI-1, a downstream target of the TGFβ pathway was increased significantly with palmitic acid in the microtissues. This led us to hypothesize that the fibrotic responses in the 3D-hLMTs with palmitic acid is due to the activation of the hepatic stellate cells and activation of the TGFβ pathway that led to increased synthesis of collagen 1 and collagen 3. In addition to increased expression of the collagen genes, there was a significant increase in the levels of P1NP and PIIINP indicating ongoing active synthesis of the collagens in the microtissues. We also observed that the collagen deposition in the tissue was reminiscent to that of bridging fibrosis, which is a much-advanced stage in the fibrotic process in patients with NASH. Moreover in the 3D-hLMT model we were able to capture multiple and robust cell type specific responses of liver damage and fibrosis, a response more akin to a damaged human liver that has many different types of cells and that are not possible to generate in mono or 2D culture systems. The responses were a combination of the effects on multiple cell types that are in contact to each other and were able to cascade the responses to injury caused by palmitic acid treatment.
Next, we further validated this fibrotic NASH model with GS-4997, an ASK-1 inhibitor compound in Phase 3 trials for the treatment of NASH. Using GS-4997 we were able to show that palmitic acid induced proinflammatory and fibrotic responses in the 3D-hLMTs were significantly decreased. GS-4997 decreased profibrotic gene expression and active synthesis of collagen 1 and 3. The reduction in collagen synthesis is likely to be due to the combined effect of GS-4997 on parenchymal hepatocytes and hepatic stellate cells. In hepatocytes, it decreases PA induced apoptosis thereby limiting further injury that would otherwise trigger inflammatory and profibrotic responses in chronic liver disease. In hepatic stellate cells GS-4997 decreased HSC activation implicating the involvement of the stress kinase pathways in that process. The inhibition of the TGFβ pathway, as demonstrated by the reduction of PAI-1 expression is also directly contributing to the reduction of collagen synthesis. If this effect is a direct effect on hepatic stellate cells or if TGFβ synthesis is inhibited in hepatocytes or Kupffer cells in this complex model remains to be investigated.
Although we were able to develop and validate a novel in vitro 3D model for NASH with a severe fibrotic phenotype, the system has several limitations. Importantly, while the model comprises the key liver cell types it lacks recruited circulating cells which can also play critical roles in inflammation and fibrogenesis. Therefore this model is not suitable to study mechanisms where infiltrating cells play a prominent role. From a practical perspective, another limitation is that the 3D-hLMT system is currently not a high throughput screening platform. However, we believe that appropriate utilization of the model to evaluate the mechanism of action and efficacy of experimental drugs to complement in vivo efficacy studies will result in higher quality compounds with greater translatability to the clinic. In addition, the model holds great promise to investigate target engagement, develop pharmacodynamic biomarkers, differentiate compound specific responses and can also be a valuable tool for the validation of clinical biomarkers.
Acknowledgements
The authors would like to thank Judi Wardwell (InSphero) for her technical insights and guidance in the preparation of the microtissues for immunofluorescence and trichrome staining assays. We would also like to thank Nordic Biosciences for providing their ELISA reagents.
Disclosure of conflict of interest
None.
References
- 1.Cassidy S, Syed BA. Nonalcoholic steatohepatitis (NASH) drugs market. Nat Rev Drug Discov. 2016;15:745–746. doi: 10.1038/nrd.2016.188. [DOI] [PubMed] [Google Scholar]
- 2.Feaver RE, Cole BK, Lawson MJ, Hoang SA, Marukian S, Blackman BR, Figler RA, Sanyal AJ, Wamhoff BR, Dash A. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight. 2016;1:e90954. doi: 10.1172/jci.insight.90954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Kang K, Yoon S, Kim JS, Park SA, Kim WD, Lee SB, Ryu KY, Jeong J, Choi D. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis. 2018;14:1–12. doi: 10.1080/15476278.2018.1423931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messner S, Fredriksson L, Lauschke VM, Roessger K, Escher C, Bober M, Kelm JM, Ingelman-Sundberg M, Moritz W. Transcriptomic, proteomic, and functional long-term charecterization of multicellular thre-dimensional human liver microtissues. Appl In Vitro Toxicol. 2018;4:1–12. doi: 10.1089/aivt.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prestigiacomo V, Weston A, Messner S, Lampart F, Suter-Dick L. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and NRF2 activation in a human 3D-multicellular model of liver fibrosis. PLoS One. 2017;12:e0179995. doi: 10.1371/journal.pone.0179995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;28:12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 7.Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39–45. [PubMed] [Google Scholar]
- 8.Dooley S, Streckert M, Delvoux B, Gressner AM. Expression of Smads during in vitro transdifferentiation of hepatic stellate cells to myofibroblasts. Biochem Biophys Res Commun. 2001;283:554–62. doi: 10.1006/bbrc.2001.4811. [DOI] [PubMed] [Google Scholar]
- 9.Leeming DJ, Koizumi M, Qvist P, Barkholt V, Zhang C, Henriksen K, Byrjalsen I, Karsdal MA. Serum N-terminal propeptide of collagen type I is associated with the number of bone metastases in breast and prostate cancer and correlates to other bone related markers. Biomark Cancer. 2011;3:15–23. doi: 10.4137/BIC.S6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha S, Perdomo G, Brown NF, O’Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 11.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto N, Wu J, Zhang Y, Catana AM, Cai H, Strom S, Novikoff PM, Zern MA. An optimal culture condition maintains human hepatocyte phenotype after long-term culture. Hepatol Res. 2006;35:169–177. doi: 10.1016/j.hepres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Tuschl G, Hrach J, Walter Y, Hewitt PG, Mueller SO. Serum-free collagen sandwich cultures of adult rat hepatocytes maintain liver-like properties long term: a valuable model for in vitro toxicity and drug-drug interaction studies. Chem Biol Interact. 2009;181:124–137. doi: 10.1016/j.cbi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 15.Vinken M, Papeleu P, Snykers S, De Rop E, Henkens T, Chipman JK, Rogiers V, Vanhaecke T. Involvement of cell junctions in hepatocyte culture functionality. Crit Rev Toxicol. 2006;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One. 2016;11:e0158674. doi: 10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messner S, Agarkova I, Moritz W, Kelm JM. Multi-cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol. 2013;87:209–213. doi: 10.1007/s00204-012-0968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell CC, Hendriks DF, Moro SM, Ellis E, Walsh J, Renblom A, Fredriksson Puigvert L, Dankers AC, Jacobs F, Snoeys J, Sison-Young RL, Jenkins RE, Nordling A, Mkrtchian S, Park BK, Kitteringham NR, Goldring CE, Lauschke VM, Ingelman-Sundberg M. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:25187. doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]