Abstract
Dental pulp stem cell (DPSC)-based odontogenic regeneration in inflammatory conditions is important in the process of pulpitis. DPSCs have been reported to lose potential for odontogenic regeneration in inflammatory conditions. This study aims to determine the mechanism of impaired odontogenic differentiation of DPSCs in an inflammatory microenvironment. We regulated Wnt4 expression using recombinant lentiviral Wnt4 and Wnt4 siRNA. Alkaline phosphatase (ALP) and Alizarin red S (ARS) staining as well as Real-Time PCR were performed to evaluate the osteogenic differentiation potential of DPSCs with either upregulated or downregulated Wnt4. Furthermore, SP600125 was used to inhibit the potential downstream factor JNK1, and the osteogenic differentiation ability of DPSCs was evaluated. As shown, Wnt4 was downregulated in DPSCs under inflammatory conditions. Inhibition of Wnt4 expression in DPSCs negatively regulated odontogenic differentiation. Overexpression of Wnt4 in LPS-treated DPSCs promoted odontogenic differentiation. In addition, JNK1 was responsible for Wnt4-mediated odontogenic differentiation of DPSCs. Taken together, Wnt4 may function by affecting JNK signaling pathways in the process of impaired odontogenic regeneration by DPSCs under inflammatory conditions.
Keywords: Dental pulp stem cells, Wnt4, inflammatory, microenvironment
Introduction
As one of the most important endodontic diseases, pulpitis threatens people’s oral health and causes great inconvenience to people’s daily lives [1]. To date, the most commonly used treatment is root canal therapy, which removes the infected pulp in the pulp cavity and fills the cavity with dental materials [2]. The limitation of this treatment is the loss of a nutrient supply, which causes increased brittleness and shortens the survival time of a tooth [3,4]. Stem cell-based regeneration of odontogenic tissues has attracted more attention in recent years due to the noninvasive process and the longer survival time of the original tooth [5,6]. Dental pulp stem cells (DPSCs) from dental pulp tissues are the most important contributors to odontogenic regeneration [7,8].
DPSCs from dental pulp tissues repair and regenerate dental tissues [9]. Under physiological conditions, DPSCs differentiate into odontoblasts and express the dentin-specific proteins DSPP and DMP [10,11]. Odontoblasts secrete dentin components and proteoglycans to maintain the normal structure and function of dentin. Factors, such as inflammation, that affect the normal microenvironment of DPSCs and inhibit odontogenic differentiation influence the repair and regeneration of dental tissues [12].
Wnt signaling pathways are considered one of the most important regulated pathways in humans that are involved in crucial aspects of organogenesis, cell migration, and cell fate determination [13,14]. Both canonical and noncanonical pathways are important components of Wnt signaling [15]. The canonical Wnt pathway has been reported to regulate stem cell differentiation in many aspects [16]. Canonical Wnt signaling regulates pluripotent stem cell differentiation. During the embryonic stem cell differentiation process, canonical Wnt plays key roles in hematopoietic stem cells [17]. Inhibition of the canonical Wnt signaling pathway has been shown to prevent neural differentiation of mesenchymal stem cells [18]. However, the role of the noncanonical Wnt pathway in cell differentiation is not well understood.
Wnt4 is reported as a noncanonical Wnt4 pathway component, which plays crucial roles in both physiological and pathological conditions [19]. It can be considered a potential noninvasive biomarker of cisplatin-induced acute kidney injury, as its expression is significantly upregulated in the early stages of injury [20]. It is also an important contributor to the regeneration of limbs [21]. More importantly, Wnt4 has been shown to inhibit bone resorption that inhibited osteoclast formation [22]. Whether Wnt4 also contributes to odontogenic differentiation in dental regeneration attracted our attention.
Functioning in many physiological processes, such as embryonic development, cell proliferation and differentiation, the C-Jun N-terminal kinase (JNK) pathway is considered one of the most critical signaling pathways in humans [23]. Disruptions of JNK signaling leads to many pathological consequences. Abnormal JNK activation is responsible for pancreatic tumors [24]. Loss of JNK1 expression has been shown to cause impaired bone formation, which results in severe osteopenia in mice [25]. In addition, crosstalk between the JNK pathway and the canonical Wnt pathway is important for epithelial morphogenesis [26]. However, whether the noncanonical Wnt pathway also interacts with the JNK pathway to regulate the physiological and pathological processes is still not well understood but merits investigation.
In this study, we aim to determine the mechanism of impaired odontogenic differentiation of DPSCs. Wnt4 was found to be a key contributor to the process, and the JNK pathway was further influenced.
Materials and methods
Cell culture
Normal and inflamed dental pulp were collected from the Department of Oral and Maxillofacial Surgery and Department of Endodontics at the Xi’an Jiaotong University Stomatological Hospital. Normal pulp was derived from the mandibular third molar that was voluntarily removed from the patients. Inflamed pulps were derived from pulps extracted before root canal treatment of patients with irreversible pulpitis. Dental pulps were washed with phosphate buffered saline (PBS) containing 1% penicillin/streptomycin three times. 3 mg/ml type IV collagenase with 4 mg/ml dispase II were used to digest cells for 1 h at 37°C. After digestion, cells were suspended in Dulbecco’s-modified Eagle’s media (DMEM) containing 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Stem cell characterization
DPSCs were collected for characterization at passage 3. The mesenchymal stem cell markers Strol-1, CD90, and CD105 were considered positive markers, and CD34 and CD45 were considered negative markers. All antibodies were obtained from Abcam (Cambridge, England). For immunofluorescence staining, cells were fixed with 4% paraformaldehyde (PFA) for 15 minutes and then washed with PBS three times. Then, cells were blocked with 5% bovine serum albumin (BSA) for 1 h and incubated with primary antibodies at 4°C overnight. Secondary antibodies with labeled fluorescence probes were added and incubated the following day.
LPS treatment
Then, 1 µg/ml lipopolysaccharide (Sigma-Aldrich, St Louis, MO) was added to the culture media of normal pulp-derived DPSCs to simulate inflammation of DPSCs.
Osteogenic differentiation
DPSCs at passage 3 were cultured with osteogenic differentiation media for 7 and 21 days for alkaline phosphatase (ALP) and Alizarin red S (ARS) staining. Osteogenic differentiation media contained 10 mmol/L beta sodium glycerophosphate, 0.05 mmol/L ascorbic acid, and 100 mmol/L dexamethasone. Osteogenic differentiation media was changed every 2 days. For ALP and ARS staining, cells were fixed with 70% ethanol for 10 minutes, and ALP or ARS staining buffer was then added for 15 minutes of incubation.
Cell transfection
DPSCs from normal dental pulp were transfected with Wnt4 siRNA to knockdown the expression of Wnt4. DPSCs from inflamed dental pulps were transfected with recombinant lentiviral Wnt4 to upregulate the expression of Wnt4.
The concentration of siRNA and the multiplicity of infection (MOI) of the Wnt4 recombinant lentivirus were 50 nM and 10 nM.
Real-time PCR
First, RNA was extracted from cells. The extraction procedure was performed according to the manufacturer’s instructions (Takara, Japan). The extracted RNA was then quantified and reverse transcribed into cDNA with a PrimeScript RT regent kit (Takara, Japan). SYBR Premix EX Taq (Perfect Real Time) was used to quantitatively determine gene expression. Reverse transcription parameters were set as follows: 37°C for 60 min, 85°C for 5 s. For qPCR assay, each reaction was performed as follows: 10 μl Premix Ex Taq (2X), 0.4 μl PCR forward primer (10 μM), 0.4 μl PCR reverse primer (10 μM), 0.4 μl ROX Reference Dye II (50X), 2 μl cDNA template, and 6 μl sterile purified water. Number of cycles: 40, 95°C for 5 s, 60°C for 34 s.
Western blotting
Cells were lysed in cell lysis buffer, and the protein concentration was then quantified with a protein quantification kit. After electroporation, proteins were transferred to PVDF membranes. Then, the membranes were blocked in 5% nonfat milk for 1 h. Primary antibodies Wnt4 (1:1000), JNK1 (1:1000), p-JNK1 (1:500), Stat3 (1:2000) were used for incubation in a cold environment overnight. Secondary antibodies were incubated for 2 h at room temperature and washed 3 times in TBS-T for 10 minutes. Finally, membranes were exposed to ECL Plus for protein detection. Details of the antibodies are listed as follows: Wnt4, JNK1, p-JNK1, Stat3 are all rabbit monoclonal antibodies and secondary antibody was goat polyclonal secondary antibody to rabbit all purchased from Abcam, Cambridge.
Statistical analysis
Unpaired Student’s t-tests were performed between two groups. One-way ANOVA was used for multiple group comparisons. A significant difference was defined as a P-value less than 0.05.
Results
Wnt4 was downregulated in DPSCs under inflammatory conditions
The expression of Wnt4 was first investigated in both normal and inflamed dental pulp tissues. As shown in Figure 1A, Wnt4 was downregulated in inflamed dental pulp tissues at both the mRNA and protein levels. Next, we investigated the expression level of Wnt4 of DPSCs in normal dental pulp tissues and DPSCs treated with 1 µg/ml LPS. LPS was used to imitate an inflammatory microenvironment. The results showed that when treated with 1 µg/ml LPS for 72 h, the expression level of Wnt4 was downregulated compared with DPSCs in normal dental pulp tissues (Figure 1B).
Figure 1.
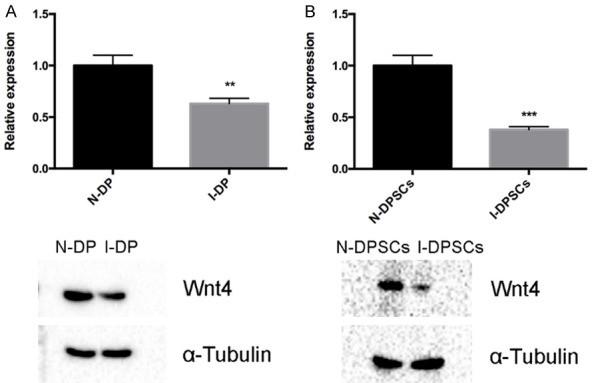
Wnt4 was downregulated in DPSCs under inflammatory conditions. A. Wnt4 expression in normal dental pulps (N-DP) and inflamed dental pulps (I-DP) at both the mRNA (upper) and protein (lower) levels. B. Relative Wnt4 expression in DPSCs from normal dental pulp (N-DPSCs) and DPSCs treated with 1 µg/ml LPS (I-DPSCs).
Inhibition of Wnt4 expression in DPSCs negatively regulated odontogenic differentiation
We knocked down the expression of Wnt4 with siRNA in DPSCs. The knockdown efficiency was evaluated in Figure 2A. After 7 days of osteogenic/odontogenic induction, a reduction of ALP-stained cells was observed with the inhibition of Wnt4 expression (Figure 2B). In addition, after 14 days of osteogenic/odontogenic induction, the Wnt4 knockdown group showed decreased ARS-stained mineralized modules (Figure 2C). The expression of Runx2, DSPP, IBSP and COL1A1 was negatively regulated by Wnt4 inhibition (Figure 2D).
Figure 2.
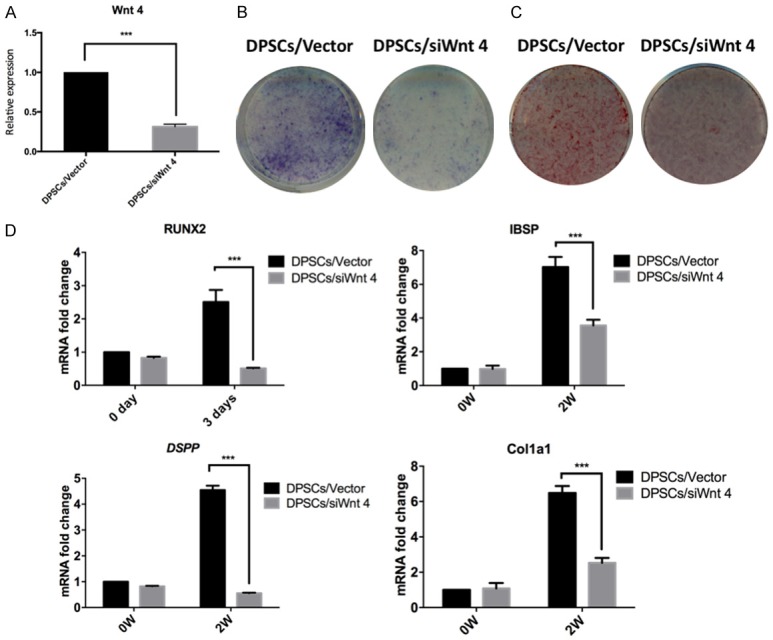
Inhibition of Wnt4 expression in DPSCs negatively regulated odontogenic differentiation. A. The transfection efficiency of Wnt4 siRNA in DPSCs. B. ALP staining of DPSCs with inhibited Wnt4 expression. C. ARS staining of DPSCs with inhibited Wnt4 expression. D. Relative expression of Runx2, IBSP, DSPP and COL1A1 in DPSCs with Wnt4 inhibition. ***P<0.001.
Overexpression of Wnt4 in LPS-treated DPSCs promoted odontogenic differentiation
Wnt4 was overexpressed in LPS-treated DPSCs with a lentiviral vector for 48 h. The MOI is shown in Figure 3A. Increased amounts of ALP-stained cells were observed after 7 days of osteogenic/odontogenic induction compared to cells transfected with the negative control (Figure 3B). Furthermore, increased amounts of ARS-stained mineralized modules were observed in the Wnt4-upregulated DPSCs compared with the negative control group after 14 days of osteogenic/odontogenic induction (Figure 3C). The expression of Runx2, DSPP, IBSP and COL1A1 was upregulated by Wnt4 overexpression (Figure 3D).
Figure 3.
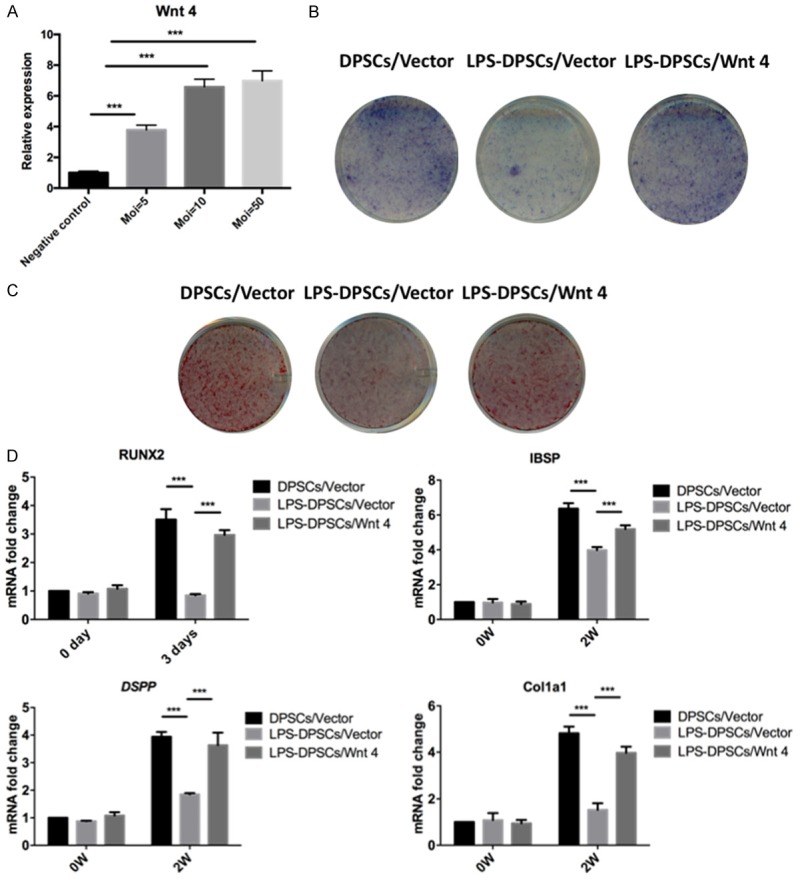
Overexpression of Wnt4 in LPS-treated DPSCs promoted odontogenic differentiation. A. The MOI and transfection efficiency of the lentiviral vector used for Wnt4 overexpression in LPS-induced DPSCs (LPS-DPSCs). B. ALP staining of LPS-DPSCs with upregulated Wnt4 expression. C. ARS staining of LPS-DPSCs with upregulated Wnt4 expression. D. Relative expression of Runx2, IBSP, DSPP and COL1A1 in LPS-DPSCs overexpressing Wnt4. ***P<0.001.
Upregulation of Wnt4 in inflamed dental pulp-derived DPSCs restored odontogenic differentiation potential
DPSCs were isolated from inflamed dental pulps of patients. Characterization of stem cell properties was performed as previously described. Wnt4 overexpression was induced by a lentiviral vector that was transfected into DPSCs from inflamed dental pulps for 48 h. The MOI is shown in Supplementary Figure 1A. Decreased amounts of ALP-stained cells were observed in DPSCs from inflamed dental pulps, while overexpression of Wnt4 restored this effect (Supplementary Figure 1B). A similar trend was observed with ARS staining (Supplementary Figure 1C). In addition, the expression of Runx2, DSPP, IBSP and COL1A1 was restored by Wnt4 overexpression (Supplementary Figure 1D). Taken together, the upregulation of Wnt4 restored the impaired odontogenic differentiation potential of DPSCs derived from inflamed dental pulps.
JNK activity is closely related in Wnt4-mediated odontogenic differentiation of DPSCs
DPSCs were transfected with a lentiviral vector to overexpress and Wnt4 siRNA to knockdown Wnt4 expression for 48 h, and the kinetics of JNK phosphorylation were evaluated by western blotting. As shown in Figure 4A, JNK1 increased in the Wnt4 overexpressed group and decreased in the Wnt4 knockdown group. However, p-JNK1 was not affected in both groups. Next, we inhibited JNK with SP600125 and evaluated the odontogenic differentiation ability of DPSCs. After 7 days of osteogenic/odontogenic induction, a reduction in the amount of ALP stained cells was observed with the inhibition of JNK. In addition, after 14 days of osteogenic/odontogenic induction, DPSCs showed reduced amounts of ARS-stained mineralized modules with JNK inhibition (Figure 4B). Nuclear proteins were extracted to evaluate the expression level of STAT3, as shown in Figure 4C. STAT3 was downregulated with the inhibition of JNK. Finally, we recovered JNK activity in Wnt4 downregulated DPSCs. As shown in Figure 4D, the odontogenic differentiation potential was restored, as indicated by ALP and ARS staining.
Figure 4.
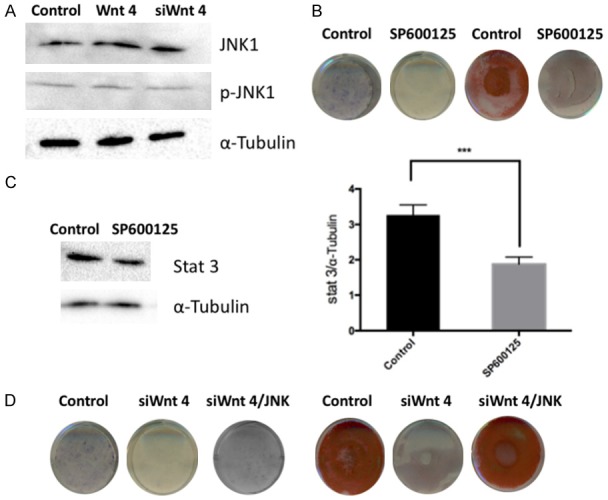
JNK activity is closely related in Wnt4-mediated odontogenic differentiation of DPSCs. A. Evaluation of JNK1 and JNK phosphorylation in DPSCs with Wnt4 upregulation and downregulation by western blotting. B. ALP and ARS staining of DPSCs with JNK inhibition by SP600125. C. Expression of STAT3 with JNK inhibition by SP600125. D. ALP and ARS staining of DPSCs with Wnt4 inhibition and subsequent JNK rescue.
Discussion
This study revealed that Wnt4 is crucial for DPSC odontogenic differentiation in both normal and inflammatory conditions. Loss of Wnt4 in DPSCs in the inflammatory microenvironment prevented normal odontogenic differentiation potential. Restoration of Wnt4 promoted odontogenic differentiation of LPS-treated DPSCs and DPSCs from inflamed tissues. In addition, the downstream JNK-mediated signaling pathway was responsible for the reduced odontogenic differentiation of DPSCs.
In this study, we demonstrated that Wnt4 was downregulated in inflamed dental pulps compared with normal dental pulps. According to previous reports, undifferentiated mesenchymal stem cells are one of the most important components in dental pulp tissues [27,28]. Therefore, we speculated whether the expression of Wnt4 also changed in DPSCs. The results showed that Wnt4 expression was decreased in DPSCs from inflamed dental pulps, which suggested that the loss of Wnt4 expression may affect the function of DPSCs, mainly in odontogenic differentiation. To determine the effects of inflammatory conditions on DPSCs, we stimulated DPSCs from normal dental pulp tissues with LPS, which mimicked the inflammatory microenvironment of DPSCs. When treated with LPS, the expression of Wnt4 decreased, consistent with the observations of DPSCs from inflamed dental pulps.
When the expression of Wnt4 was inhibited in DPSCs, the odontogenic potential decreased. ALP activity was evaluated, which is a functional indicator of odontoblasts and is important in the calcification process of odontoblasts and enamel [29,30]. The early odontogenic marker Runx2 was downregulated in the Wnt4 inhibition group, which suggested that the loss of Wnt4 expression affected DPSCs from an early stage of injury. As one of the most important markers of odontoblast differentiation [31], the expression of DSPP was inhibited by the downregulation of Wnt4. These results may explain why there are no functional DPSCs in inflamed dental cavities.
We also investigated the effects of inflammatory conditions on DPSCs. LPS was used to simulate the microenvironment DPSCs in which the expression of Wnt4 was downregulated, and the odontogenic differentiation of DPSCs was inhibited. However, when we restored the expression of Wnt4 in LPS-treated DPSCs, we also restored the odontogenic differentiation potential, which suggested that Wnt4 inhibition in inflammatory conditions was a contributor to nonfunctional DPSCs.
In our study, a decrease in Wnt4 expression affected the expression of JNK instead of JNK phosphorylation. Activated or inhibited downstream molecules that may be involved include c-Jun, SMAD4, p53, and STAT3 [32-34]. The expression of STAT3 in the nucleus was downregulated by JNK inhibition, which suggested that STAT3 may be a downstream effector of Wnt4-regulated odontogenic differentiation. Therefore, JNK was shown to regulate odontogenic differentiation of DPSCs in normal and inflammatory conditions.
In conclusion, inflammation in dental pulps decreased the expression of Wnt4 in DPSCs. Loss of Wnt4 expression impaired the odontogenic differentiation potential of DPSCs. However, restoration of Wnt4 was able to rehabilitate the impaired odontogenic differentiation potential. In addition, we determined that Wnt4 may function through its effect on JNK signaling pathways. These results may provide a potential method of reconstructing odontogenic tissues through DPSCs in inflammatory conditions. Therefore, this study provides a therapeutic approach for treating irreversible pulpitis and may extend the survival of afflicted teeth.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81371348).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102:411–418. doi: 10.2105/AJPH.2011.300362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonzar F, Fonzar A, Buttolo P, Worthington HV, Esposito M. The prognosis of root canal therapy: a 10-year retrospective cohort study on 411 patients with 1175 endodontically treated teeth. Eur J Oral Implantol. 2009;2:201–208. [PubMed] [Google Scholar]
- 3.Kröncke A. Possiblities and limitations of treating the root canal in pulp necrosis and chronic apical periodontitis. SSO Schweiz Monatsschr Zahnheilkd. 1965;75:1114–1125. [PubMed] [Google Scholar]
- 4.Mounce R. Endodontic retreatment possibilities: evaluation, limitations, and considerations. Compend Contin Educ Dent. 2004;25:364–6. 368. [PubMed] [Google Scholar]
- 5.Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715–722. doi: 10.1016/j.tcb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudry A, Uzunoglu E, Schneider B, Kellermann O, Goldberg M. From pulpal stem cells to tooth repair: an emerging field for dental tissue engineering. Evidence-Based Endodontics. 2016;1:2. [Google Scholar]
- 7.Sloan A, Waddington R. Dental pulp stem cells: what, where, how? Int J Paediatr Dent. 2010;19:61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 8.Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng. 2015;9:1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- 9.Bakopoulou A, About I. Stem cells of dental origin: current research trends and key milestones towards clinical application. Stem Cells Int. 2016;2016:4209891. doi: 10.1155/2016/4209891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Lu Y, Maciejewska I, Galler KM, Cavender A, D’Souza RN. TWIST1 promotes the odontoblast-like differentiation of dental stem cells. Adv Dent Res. 2011;23:280–284. doi: 10.1177/0022034511405387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi M, Yamada Y, Ozawa R, Ohya M, Ito K, Ueda M. Expression of odontoblastic-related genes in human dental follicle cells, dental pulp stem cells, and oral mucosal cells. Int J Oral-Med Sci. 2011;3:41–48. [Google Scholar]
- 12.Nuti N, Corallo C, Chan BM, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. 2016;12:1–13. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 13.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 14.Yuko Komiya RH. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–116. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Tarafdar A, Dobbin E, Corrigan P, Freeburn R, Wheadon H. Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation. PLoS One. 2013;8:e81030. doi: 10.1371/journal.pone.0081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda K, Takahashi N, Kobayashi Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med. 2013;91:15–23. doi: 10.1007/s00109-012-0974-0. [DOI] [PubMed] [Google Scholar]
- 20.He YX, Diao TT, Song SM, Wang CC, Wang Y, Zhou CL, Bai YB, Yu SS, Mi X, Yang XY. Wnt4 is significantly upregulated during the early phases of cisplatin-induced acute kidney injury. Sci Rep. 2018;8:10555. doi: 10.1038/s41598-018-28595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Fu Y, Zhu F, Mu C, Li R, Song W, Shi C, Ye Y, Wang C. Transcriptomic analysis of Portunus trituberculatus reveals a critical role for WNT4 and WNT signalling in limb regeneration. Gene. 2018;658:113. doi: 10.1016/j.gene.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Bo Y, Jia C, Liu Y, Li J, Kevork K, Alhezaimi K, Graves DT, Park NH, Wang CY. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat Med. 2014;20:1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Singh UK, Kini SG, Garg V, Agrawal S, Tomar PK, Pathak P, Chaudhary A, Gupta P, Malik A. JNK pathway signaling: a novel and smarter therapeutic targets for various biological diseases. Future Med Chem. 2015;7:2065–2086. doi: 10.4155/fmc.15.132. [DOI] [PubMed] [Google Scholar]
- 24.Bose C, Udupa KB. Erythropoietin enhancement of rat pancreatic tumor cell proliferation requires the activation of ERK and JNK signals. Am J Physiol Cell Physiol. 2008;295:394–405. doi: 10.1152/ajpcell.00423.2007. [DOI] [PubMed] [Google Scholar]
- 25.Xu R, Zhang C, Shin DY, Kim JM, Lalani S, Li N, Yang YS, Liu Y, Eiseman M, Davis RJ. c-Jun N-terminal kinases (JNKs) are critical mediators of osteoblast activity in vivo. J Bone Miner Res. 2017;32:1811. doi: 10.1002/jbmr.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, Denbesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2003;81:531. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 28.Casagrande L, Cordeiro MM, Nör SA, Nör JE. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99:1–7. doi: 10.1007/s10266-010-0154-z. [DOI] [PubMed] [Google Scholar]
- 29.Couve E, Schmachtenberg O. Autophagic activity and aging in human odontoblasts. J Dent Res. 2011;90:523–528. doi: 10.1177/0022034510393347. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Gluhakheinrich J, Wang YH, Wu YM, Chuang HH, Chen L, Yuan GH, Dong J, Gay I, Macdougall M. Runx2, Osx, and Dspp in tooth development. J Dent Res. 2009;88:904. doi: 10.1177/0022034509342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia J, Bian Z, Song Y. Dspp mutations disrupt mineralization homeostasis during odontoblast differentiation. Am J Transl Res. 2015;7:2379. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Cao J, Pei Y, Zhang J, Wang Q. Smad4 inhibits cell migration via suppression of JNK activity in human pancreatic carcinoma PANC-1 cells. Oncol Lett. 2016;11:3465–3470. doi: 10.3892/ol.2016.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller S, Berger M, Lehembre F, Seeler JS, Haupt Y, Dejean A. c-Jun and p53 activity is modulated by SUMO-1 modification. J Biol Chem. 2000;275:13321. doi: 10.1074/jbc.275.18.13321. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Chen B, Lu Y, Guan Y, Chen F. JNK-dependent stat3 phosphorylation contributes to akt activation in response to arsenic exposure. Toxicol Sci. 2012;129:363–371. doi: 10.1093/toxsci/kfs199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


