Abstract
An increasing amount of research is demonstrating the role of long noncoding RNAs (lncRNAs) in human cardiovascular disease, and in particular, atherosclerosis. To date, the mechanism through which lncRNA OIP5-AS1 regulates the oxidative low-density lipoprotein (ox-LDL)-mediated endothelial cell apoptosis is still unclear. Results from this study found that lncRNA OIP5-AS1 was significantly over-expressed in the human umbilical vein endothelial cells (HUVECs) administered with ox-LDL. The silencing of OIP5-AS1 inhibited apoptosis and promoted proliferation via inducing G0/G1 cycle arrest. Chromatin immunoprecipitate (ChIP) revealed that lncRNA OIP5-AS1 reduced GSK-3β expression through recruiting EZH2, a critical element of the Polycomb Repressive Complex 2 (PRC2) complex that directly bind with the GSK-3β promoter region. Rescue experiments validated that GSK-3β could eliminate the effect of OIP5-AS1 on HUVECs. Overall, these findings suggest that lncRNA OIP5-AS1 accelerates ox-LDL mediated vascular endothelial cell apoptosis through targeting GSK-3β via recruiting EZH2, providing potential therapeutic strategies for atherosclerosis.
Keywords: Vascular endothelial cells, OIP5-AS1, EZH2, ox-LDL, GSK-3β
Introduction
Atherosclerosis (AS) is a cardiovascular disease where the arteries harden and narrow due to a build-up of plaque, and is the main cause of coronary heart disease, cerebral infarction and peripheral vascular disease [1-3]. Atherosclerosis is a complicated pathophysiological processe, involving lipid disorders, endothelial dysfunction, inflammatory cell infiltration and vascular smooth muscle cell differentiation [4,5]. The pathological basis of atherosclerosis is characterized by the involvement of arterial lesions in vascular endothelial cells (VECs) found in lipid metabolic disorders [6].
The rapid expansion and sophistication of genome sequencing technology has allowed for a more complete understanding of the role of noncoding RNAs (ncRNAs) [7]. Among the types of ncRNAs, long noncoding RNAs (lncRNAs) are an essential element in epigenetic regulation via multiple mechanisms, including transcriptional and posttranscriptional mechanisms. For example, lncRNA LINC00305 expression is significantly up-regulated in hypoxia induce human umbilical vein endothelial cells (HUVECs), and enhances apoptosis and suppresses proliferation of HUVECs via targeting miR-136 [8]. Further, lncRNA TCONS_00024652 is highly expressed in TNF-α-induced HUVECs and regulates cell proliferation and angiogenesis via microRNA-21 [9].
An increasing number of studies support the finding that lncRNAs have an important regulatory function in vascular endothelial cells [10]. To further investigate this mechanism, we conducted a series of experiments on the role of lncRNA OIP5-AS1 in the ox-LDL induced HUVECs in an atherosclerosis simulation environment. In the current study, lncRNA OIP5-AS1 was found to be highly expressed in the ox-LDL induced HUVECs, and regulates HUVEC proliferation and apoptosis via inhibiting GSK-3β by recruiting the EZH2 at GSK-3β promotor.
Materials and methods
Vascular endothelial cells culture
Vascular endothelial cell (human umbilical vein endothelial cell, HUVECs) were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). HUVECs were cultured in DMEM (Dulbecco’s modified Eagle medium) supplemented with the 10% fetal bovine serum (FBS, Gibco, Gran Island, NY, USA) and 100 µg/ml penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc. USA).
Transfection
For small interfering RNA oligonucleotides (siRNA) transfection experiments, sequences were transfected into HUVECs with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The HUVECs were harvested after 48 hours. All siRNA were designed and synthesized chemically (GenePharma, RiBio). The sequences of siRNAs are presented in Table S1.
Quantitative RT-PCR
The total RNAs from HUVECs were extracted with TRIzol reagent (Invitrogen, Carlsbad, Calif, USA). Complementary DNA (cDNA) was isolated from the total RNA by reverse transcription using SuperScript First-Stand Synthesis system (Invitrogen, Carlsbad, Calif, US). PCR was carried out using the ReverTra Ace RT-PCR Kit (Toyobo, Osaka, Japan) on an ABI7500 real-time PCR instrument (TaKaRa, Dalian, China). GAPDH was used as internal control and the final data was measured using the 2-ΔΔCt method.
Proliferative viability assay
HUVECs were seeded into 96-well plates at a density of 5×103 cells per well. After the transfection with siRNAs and the culture for 48 hours, proliferative viability of HUVECs was detected using Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s specifications.
Western blotting
Total cell lysates were extracted from the HUVECs using the RIPA lysis buffer (Cell Signal Technology, Danvers, MA, USA) supplemented with protease inhibitors (Roche, Basel, Switzland). The lysates were transferred to the SDS loading buffer and separated by SDS-PAGE and transferred to PVDF member and blocked with 5% skim milk in TBST. The primary antibodies were incubated overnight at 4°C, and horseradish peroxidase-conjugated secondary antibody at room temperature. The final vision blot was visualized using chemiluminescence reagent (ECL) kit (Beyotime Biotechnology).
Flow cytometric apoptosis analysis
Apoptosis was measured on the harvested cells (200 ml) using flow cytometric apoptosis analysis using the apoptosis Kit. For the apoptosis, after being trypsinized and resuspended, HUVECs (1×105) were stained with Annexin V-FITC (5 ml) and propidium iodide (PI, 5 ml) for 15 min at room temperature in the dark using the Apoptosis Detection kit (KeyGEN). For cycle analysis, the HUVECs were stained with propidium iodide using Cell Cycle Detection kit (KeyGen) and analyzed with a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA).
Subcellular fractionation
The cellular localization of OIP5-AS1 was measured using the NUCLEI EZ PREP NUCLEI ISOLATION KIT (Sigma-Aldrich, St. Louis, MO, USA). The cytoplasm and nuclear fraction were isolated according to the manufacturer’s instructions. After extraction, the precipitate was washed with PBS twice and resuspended with Nuclei EZ storage buffer.
Chromatin immunoprecipitation (ChIP)-PCR
The potential binding within the PRC2 complex (EZH2, H3K27me3 and LSD1) was detected using the ChIP assay using the EZ-Magna ChIPTM A/G Chromatin Immunoprecipitation Kit (Millipore) as previously described [11]. In the first step, the cross-linked chromatin DNA was unlocked to be short fragments using the sonication method. The antibodies (anti-EZH2, anti-H3K27me3) against each segment were then administrated to immunoprecipitate. Antibody against immunoglobulin (IgG) acted as negative control. The value was analyzed by real-time PCR.
Statistical analyses
Statistical analysis was carried out using SPSS (Chicago, IL, USA, vision 19.0) and GraphPad Prism (La Jolla, CA, USA). The statistical approaches were Student’s t-test and ANOVA analysis. P-value less than 0.05 was considered statistically significance.
Results
LncRNA OIP5-AS1 expression is up-regulated in ox-LDL treated HUVECs
Results found that lncRNA OIP5-AS1 is a transcript of noncoding RNA located in the chromosome 15 (chr15: 41,576,201-41,591,795) (Figure 1A). In the HUVECs treated with ox-LDL (0-150 mg/L), lncRNA OIP5-AS1 was significantly over-expressed following an increasing concentration gradient (Figure 1B). When the HUVECs were treated with ox-LDL (100 mg/L), lncRNA OIP5-AS1 was significantly over-expressed following an increasing time gradient (Figure 1C). These results suggest that lncRNA OIP5-AS1 expression is up-regulated in ox-LDL treated HUVECs.
Figure 1.
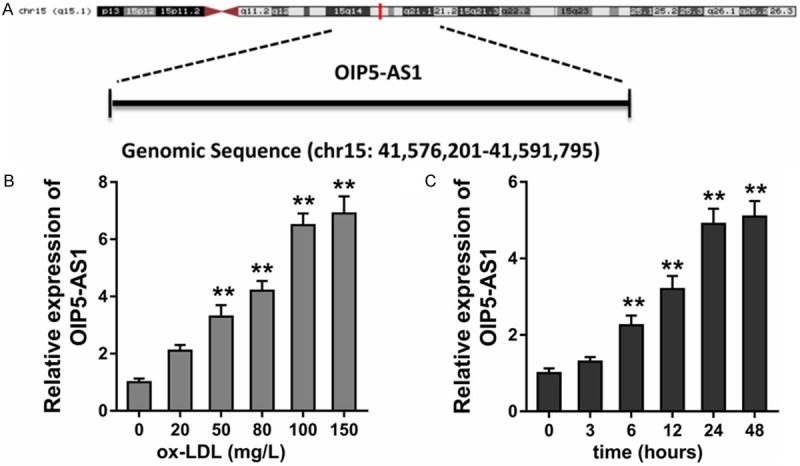
lncRNA OIP5-AS1 expression is up-regulated in the ox-LDL treated HUVECs. A. Schematic diagram for the location of OIP5-AS1 in chromosome 15 (chr15: 41,576,201-41,591,795). B. RT-PCR showed the level of OIP5-AS1 in HUVECs treated with ox-LDL (0-150 mg/L). C. RT-PCR showed the level of OIP5-AS1 in HUVECs treated with ox-LDL (100 mg/L) following an increasing time gradient. **p value less than 0.01.
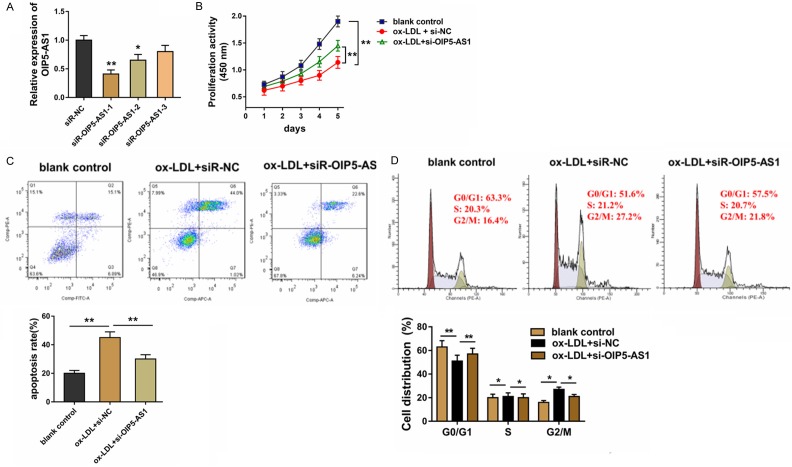
Silencing of lncRNA OIP5-AS1 accelerated the apoptosis of HUVECs
Given that OIP5-AS1 is over-expressed in the ox-LDL treated HUVECs, loss-of-function experiments were performed to investigate its role in HUVECs. The synthesized siRNAs targeting OIP5-AS1 were transfected into HUVECs to knockdown the level of OIP5-AS1 expression (Figure 2A). CCK-8 proliferative analysis found that ox-LDL administration decreased the absorbance of HUVECs, whereas the siRNA-OIP5-AS1 co-transfected recovered compared with other groups (Figure 2B). Flow cytometry apoptosis analysis revealed that ox-LDL administration enhanced the apoptosis of HUVECs and this was restored by siRNA-OIP5-AS1 transfection (Figure 2C). Flow cytometry cycle analysis showed that ox-LDL administration inhibited the cellular processes from the G1/G0 phase to the S phase, inducing the cycle arrest of HUVECS (Figure 2D) and siRNA-OIP5-AS1 transfection rescued it. Therefore, this data show that silencing of lncRNA OIP5-AS1 accelerated HUVEC apoptosis.
Figure 2.
Silencing of lncRNA OIP5-AS1 accelerated the apoptosis of HUVECs. A. Specifically synthesized siRNAs targeting OIP5-AS1 were transfected into HUVECs. B. CCK-8 proliferative analysis stated the absorbance of HUVECs administrated by ox-LDL and siRNA-OIP5-AS1 transfection. C. Flow cytometry apoptosis analysis revealed apoptosis of HUVECs administered with ox-LDL and siRNA-OIP5-AS1 transfection. D. Flow cytometry cycle analysis cycle distribution of HUVECs administered with ox-LDL and siRNA-OIP5-AS1 transfection. **p value less than 0.01. *p value less than 0.05.
LncRNA OIP5-AS1 reduced the GSK-3β expression through PRC2 complex
To investigate the underlying mechanism by which OIP5-AS1 regulates the pathological process, the subcellular location of OIP5-AS1 in HUVECs was determined. Results found that lncRNA OIP5-AS1 is primarily located in the the nucleus rather than cytoplasm (Figure 3A). The level of GSK-3β protein was under-expressed in the ox-LDL treated HUVECs, which was negatively correlated with that of OIP5-AS1 (Figure 3B). To investigate the relationship between OIP5-AS1 and GSK-3β, siRNA and enhanced plasmid was respectively transfected for OIP5-AS1 into HUVECs, and data showed that OIP5-AS1 was negatively correlated with GSK-3β protein in HUVECs (Figure 3C, 3D). Chromatin immunoprecipitate (ChIP) followed by qRT-PCR showed that EZH2, H3K27me3 and LSD1 were directly bound to the GSK-3β promoter region in HUVECs (Figure 3E). OIP5-AS1 silencing inhibited the EZH2 and LSD1 binding and reduced the H3K27me3 occupation at GSK-3β promoter region (Figure 3F). In conclusion, we found that lncRNA OIP5-AS1 can reduce GSK-3β expression through PRC2 complex binding at its promoter region.
Figure 3.

LncRNA OIP5-AS1 reduced GSK-3β through PRC2 complex. A. The subcellular location of OIP5-AS1 in HUVECs was measured using cell cytoplasm/nucleus fraction analysis. B. Western blot analysis revealed that GSK-3β protein was under-expressed in the ox-LDL treated HUVECs, which was negatively correlated with that of OIP5-AS1. C, D. Western blot analysis showed the GSK-3β protein in HUVECs transfected with siRNA and enhanced plasmid for OIP5-AS1. E, F. Chromatin immunoprecipitate (ChIP) followed by qRT-PCR showed the levels relative to IgG immunoprecipitate in HUVECs of EZH2 and LSD1 binding and H3K27me3 occupany at GSK-3β promoter region. **p value less than 0.01. *p value less than 0.05.
GSK-3β rescues the role of lncRNA OIP5-AS1 in HUVECs
Results found that lncRNA OIP5-AS1 reduces GSK-3β expression through EZH2, and the expression of EZH2 and GSK-3β was negative (Figure 4A). Rescue experiments were performed using CCK-8 assay and flow cytometry analysis. Cycle analysis revealed that GSK-3β silencing induced by si-GSK-3β could accelerate the cycle progression, whereas the co-transfection of si-GSK-3β and siRNA OIP5-AS1 could recover it (Figure 4B). CCK-8 analysis found that GSK-3β silencing decreased HUVECs proliferative ability, and the co-transfection of si-GSK-3β and siRNA OIP5-AS1 rescued it (Figure 4C). GSK-3β silencing promoted apoptosis, while the co-transfection of si-GSK-3β and siRNA OIP5-AS1 reduced apoptosis (Figure 4D). Thus, we concluded that GSK-3β rescues the role of lncRNA OIP5-AS1 in HUVEC apoptosis and proliferation.
Figure 4.
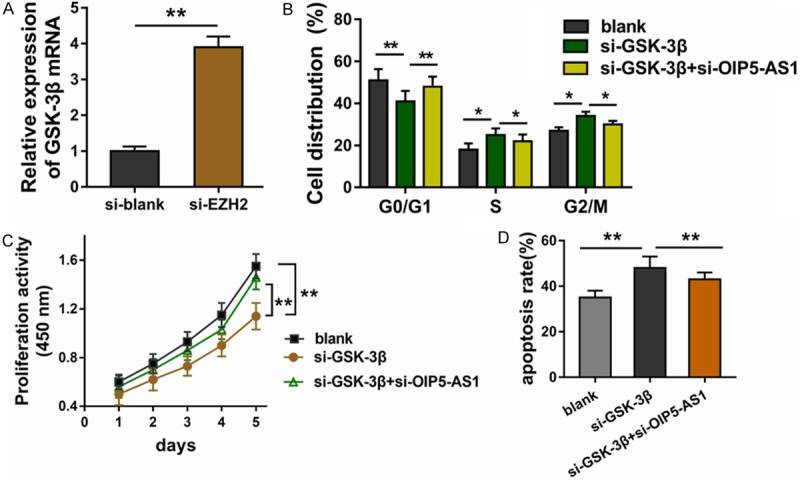
GSK-3β rescues the role of lncRNA OIP5-AS1 on HUVECs. A. RT-PCR showed the negative expression of EZH2 and GSK-3β. B. Cycle analysis revealed the cycle progression of HUVECs induced by si-GSK-3β transfection and siRNA OIP5-AS1 transfection. C. CCK-8 assay measured the proliferation of HUVECs after transfection. D. Apoptosis of HUVECs transfected with si-GSK-3β and siRNA OIP5-AS1. **p value less than 0.01. *p value less than 0.05.
Discussion
Increasing evidence shows that lncRNAs are involved in numerous pathophysiological processes in humans including cardiovascular disease [12-14]. This study aimed to investigate the potential role of lncRNAs in vascular endothelial cells in atherosclerosis and characterize the underlying mechanism.
Research shows that lncRNA OIP5-AS1 is an oncogene involved in human cancers, including osteosarcoma, colorectal cancer, and hepatoblastoma [15,16]. For example, OIP5-AS1 is increased in osteosarcoma tissue and cells and silencing OIP5-AS1 expression significantly reduced proliferation and accelerated apoptosis. It also triggered G0/G1 phase cycle arrest via inhibiting miR-223 by targeting CDK14 Mrna [17]. In lung adenocarcinoma, OIP5-AS1 directly sponges miR-448 and Bcl-2 to regulate cell proliferation, migration and invasion [18]. Taken together, this research suggests that OIP5-AS1 plays a role in human disease.
In this study, OIP5-AS1 level was significantly increased in ox-LDL administrated HUVECs in a dose- and time-dependent manner. Functional experiments showed that OIP5-AS1 silencing inhibits proliferation, accelerates apoptosis and induces the cycle arrest of HUVECs. Therefore, OIP5-AS1 might acts as a risk factor for atherosclerosis. The above results suggest that high levels of lncRNA OIP5-AS1 in the ox-LDL treated HUVECs can aggravate VEC injury and stimulate atherosclerosis.
Current research suggests that primary regulative mechanism for lncRNA is transcriptional control and post-transcriptional control [19,20]. For example, competing endogenous RNA (ceRNA), a vital category by which lncRNAs regulate downstream factors, interprets the most common epigenetics findings. In this work, OIP5-AS1 is primarily located in the nucleus rather than the cytoplasm, suggesting its potential role in transcriptional regulation. For the underlying mechanism, we found that OIP5-AS1 could decrease GSK-3β expression via recruiting PRC2 complex, including EZH2, H3K27me3 and LSD1. Through the complex, OIP5-AS1 directly inhibited the transcription of GSK-3β, resulting in a negative correlation between OIP5-AS1 and GSK-3β.
The mechanism by which lncRNA directly or indirectly regulates the functional protein has been widely reported [21-24]. In osteosarcoma cells, HOXD-AS1 is over-expressed and epigenetically represses p57 by recruiting enhancer of zeste homolog 2 (EZH2) to the promoter region of p57, indicating its oncogenic role [25]. In diabetic nephropathy, lncRNA LINC00968 accelerates proliferation, extracellular matrix (ECM) protein (fibronectin, collagen IV) expression and fibrosis of mesangial cells by epigenetically repressing p21 via recruiting EZH2 [26].
Results from this study suggest that lncRNA OIP5-AS1 regulates proliferation and apoptosis in the HUVECs via recruiting the EZH2 to the promotor region of GSK-3β to reduce its expression. These finding might provide a novel target for atherosclerosis treatment.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Cui Y, Qi X, Huang A, Li J, Hou W, Liu K. Differential and predictive value of galectin-3 and soluble suppression of tumorigenicity-2 (sST2) in heart failure with preserved ejection fraction. Med Sci Monit. 2018;24:5139–5146. doi: 10.12659/MSM.908840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Cui Y, Huang A, Li Q, Jia W, Liu K, Qi X. Additional diagnostic value of growth differentiation factor-15 (GDF-15) to N-terminal B-type natriuretic peptide (NT-proBNP) in patients with different stages of heart failure. Med Sci Monit. 2018;24:4992–4999. doi: 10.12659/MSM.910671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang JH, Kim EA, Park HJ, Sung EG, Song IH, Kim JY, Woo CH, Doh KO, Kim KH, Lee TJ. Methylglyoxal-induced apoptosis is dependent on the suppression of c-FLIPL expression via down-regulation of p65 in endothelial cells. J Cell Mol Med. 2017;21:2720–2731. doi: 10.1111/jcmm.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Y, Li C, Zeng C, Li J, Zhu Z, Chen W, Huang A, Qi X. Tongmai Yangxin pills anti-oxidative stress alleviates cisplatin-induced cardiotoxicity: network pharmacology analysis and experimental evidence. Biomed Pharmacother. 2018;108:1081–1089. doi: 10.1016/j.biopha.2018.09.095. [DOI] [PubMed] [Google Scholar]
- 5.Wang TM, Chen KC, Hsu PY, Lin HF, Wang YS, Chen CY, Liao YC, Juo SH. microRNA let-7g suppresses PDGF-induced conversion of vascular smooth muscle cell into the synthetic phenotype. J Cell Mol Med. 2017;21:3592–3601. doi: 10.1111/jcmm.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J Cell Mol Med. 2017;21:1457–1462. doi: 10.1111/jcmm.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Zhao W, Wang Z, Xiang X, Zhang S, Liu L. Long non-coding RNA SNHG20 promotes the tumorigenesis of oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J Cell Mol Med. 2019;23:680–688. doi: 10.1111/jcmm.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang BY, Jin Z, Zhao Z. Long intergenic noncoding RNA 00305 sponges miR-136 to regulate the hypoxia induced apoptosis of vascular endothelial cells. Biomed Pharmacother. 2017;94:238–243. doi: 10.1016/j.biopha.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 9.Halimulati M, Duman B, Nijiati J, Aizezi A. Long noncoding RNA TCONS_00024652 regulates vascular endothelial cell proliferation and angiogenesis via microRNA-21. Exp Ther Med. 2018;16:3309–3316. doi: 10.3892/etm.2018.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z, Vajen T, Karshovska E, Dickhout A, Schmitt MM, Megens RTA, von Hundelshausen P, Koeppel TA, Hackeng TM, Weber C, Koenen RR. Deletion of junctional adhesion molecule a from platelets increases early-stage neointima formation after wire injury in hyperlipidemic mice. J Cell Mol Med. 2017;21:1523–1531. doi: 10.1111/jcmm.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Huo X, Yang XR, He J, Cheng L, Wang N, Deng X, Jin H, Wang N, Wang C, Zhao F, Fang J, Yao M, Fan J, Qin W. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16:136. doi: 10.1186/s12943-017-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Tang C, Li Y, Liu Y, Lv P, Wang W, Mu Y. The interplay of LncRNA ANRIL and miR-181b on the inflammation-relevant coronary artery disease through mediating NF-kappaB signalling pathway. J Cell Mol Med. 2018;22:5062–5075. doi: 10.1111/jcmm.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Sun Y, Zhong L, Xiao Z, Yang M, Chen M, Wang C, Xie X, Chen X. The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs by long non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr Metab Cardiovasc Dis. 2018;5:666–673. doi: 10.1016/j.numecd.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Huang GQ, Ke ZP. Silence of long intergenic noncoding RNA HOTAIR ameliorates oxidative stress and inflammation response in ox-LDL-treated human macrophages by upregulating miR-330-5p. J Cell Physiol. 2019;234:5134–5142. doi: 10.1002/jcp.27317. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Liu F, Yang F, Liu Y. Kockdown of OIP5-AS1 expression inhibits proliferation, metastasis and EMT progress in hepatoblastoma cells through up-regulating miR-186a-5p and down-regulating ZEB1. Biomed Pharmacother. 2018;101:14–23. doi: 10.1016/j.biopha.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y, Yao S, Chen X, Liu D, Wang J, Yuan X, Rao J, Xiong H, Yu S, Yuan X, Zhu F, Hu G, Wang Y, Xiong H. LncRNA OIP5-AS1 regulates radioresistance by targeting DYRK1A through miR-369-3p in colorectal cancer cells. Eur J Cell Biol. 2018;97:369–378. doi: 10.1016/j.ejcb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Dai J, Xu L, Hu X, Han G, Jiang H, Sun H, Zhu G, Tang X. Long noncoding RNA OIP5-AS1 accelerates CDK14 expression to promote osteosarcoma tumorigenesis via targeting miR-223. Biomed Pharmacother. 2018;106:1441–1447. doi: 10.1016/j.biopha.2018.07.109. [DOI] [PubMed] [Google Scholar]
- 18.Deng J, Deng H, Liu C, Liang Y, Wang S. Long non-coding RNA OIP5-AS1 functions as an oncogene in lung adenocarcinoma through targeting miR-448/Bcl-2. Biomed Pharmacother. 2018;98:102–110. doi: 10.1016/j.biopha.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Sun J. LncRNA SRA deregulation contributes to the development of atherosclerosis by causing dysfunction of endothelial cells through repressing the expression of adipose triglyceride lipase. Mol Med Rep. 2018;18:5207–5214. doi: 10.3892/mmr.2018.9497. [DOI] [PubMed] [Google Scholar]
- 20.Yari M, Bitarafan S, Broumand MA, Fazeli Z, Rahimi M, Ghaderian SMH, Mirfakhraie R, Omrani MD. Association between long noncoding RNA ANRIL expression variants and susceptibility to coronary artery disease. Int J Mol Cell Med. 2018;7:1–7. doi: 10.22088/IJMCM.BUMS.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Gu M, Liu C, Wan X, Shi Q, Chen Q, Wang Z. Long noncoding RNA FOXC2-AS1 facilitates the proliferation and progression of prostate cancer via targeting miR-1253/EZH2. Gene. 2018;686:37–42. doi: 10.1016/j.gene.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 22.Jin L, Cai Q, Wang S, Wang S, Mondal T, Wang J, Quan Z. Long noncoding RNA MEG3 regulates LATS2 by promoting the ubiquitination of EZH2 and inhibits proliferation and invasion in gallbladder cancer. Cell Death Dis. 2018;9:1017. doi: 10.1038/s41419-018-1064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Yang X, Xue X, Sun D, Cai P, Song Q, Zhang B, Qin L. HANR promotes hepatocellular carcinoma progression via miR-214/EZH2/TGF-beta axis. Biochem Biophys Res Commun. 2018;506:189–193. doi: 10.1016/j.bbrc.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Chen X, Lin K, Zeng K, Liu X, Pan B, Xu X, Xu T, Hu X, Sun L, He B, Pan Y, Sun H, Wang S. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer. 2018;17:141. doi: 10.1186/s12943-018-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu W, Zhang E, Song L, Tu L, Wang Z, Tian F, Aikenmu K, Chu G, Zhao J. Long noncoding RNA HOXD-AS1 aggravates osteosarcoma carcinogenesis through epigenetically inhibiting p57 via EZH2. Biomed Pharmacother. 2018;106:890–895. doi: 10.1016/j.biopha.2018.06.173. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Yu Z, Meng X, Yu P. LncRNA LINC00968 accelerates the proliferation and fibrosis of diabetic nephropathy by epigenetically repressing p21 via recruiting EZH2. Biochem Biophys Res Commun. 2018;504:499–504. doi: 10.1016/j.bbrc.2018.08.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.