Abstract
The PI3K/AKT/mTOR signaling pathway is considered as a promising therapeutic target in the treatment of ovarian cancer (OC); however, inhibition of this pathway only exhibited moderate clinical efficacy when tested clinically. Combination of mTOR inhibitors with other anticancer compounds could improve the anticancer efficiency. Therefore, the concurrent inhibition of Fibroblast Growth Factor Receptor (FGFR) signaling pathway was evaluated in the present study. OC cell lines were treated with FGFR inhibitor BGJ398, mTOR inhibitor Rapamycin, or combined inhibition of both BGJ398 and Rapamycin. The results revealed that the growth and motility, expression of angiogenic markers and phosphorylation of associated proteins were affected in treated OC cells. Additionally, the anticancer effects of aforementioned inhibitors were evaluated using a murine tumor xenograft model. Combined treatment with BGJ398 and Rapamycin exhibited stronger inhibitory effects on the growth and motility of OC cells compared with BGJ398 or Rapamycin alone group. Furthermore, combined inhibition of FGFR and mTOR pathways by BGJ398 and Rapamycin induced remarkable cell cycle arrest and apoptosis in OC cells. Reduced tumor size in the xenograft was also observed following combined treatment but not in BGJ398 or Rapamycin alone group. The results in the present study revealed that combined inhibition of FGFR and mTOR pathways could be a promising therapeutic strategy in the treatment of patients with OC.
Keywords: FGFR, mTOR, epithelial ovarian cancer, BGJ398, rapamycin
Introduction
Ovarian cancer (OC) is the fourth leading tumor in women, and the most common type of gynecological malignancy characterized by high mortality rate. Annually, >200,000 cases of OC patients are diagnosed with >100,000 mortality worldwide [1]. Most OC initiated from epithelial cells and are thus composed of poorly differentiated epithelial cells [2]. Surgical treatment of OC, such as radical hysterectomy together with bilateral salpingo-oophorectomy combined with platinum-based chemotherapy, is able to increase the 5-year survival rate to 45%, and <40% of patients can be cured [3]. Therefore, it is important to identify the novel targets and underlying molecular mechanisms that promote the pathogenesis of OC.
Recent study suggested that the Fibroblast Growth Factor (FGF)/FGF Receptor (FGFR) family, which consist of 19 FGFs and four FGFRs, can interact with PI3K/AKT pathway and subsequently involve in the carcinogenesis of OC [4]. In addition, FGFRs recruit stromal cells, which are essential participants in the growth and motility of OC cells. Furthermore, the copy number of FGFR1 was elevated in both serous tumor and endometrioid carcinoma [5]. Additionally, individual FGFRs including FGFR2 IIIb are not expressed in normal ovarian epithelium, but are overexpressed in OC [6]. Mutations activated by FGFR2/3 could result in OC cancerogenesis [7].
The aforementioned PI3K/AKT pathway can activate the serine/threonine kinase called the mechanistic target of Rapamycin (mTOR), which is also implicated in OC [8]. Thus, the PI3K/AKT/mTOR signaling pathway is considered a promising therapeutic target in the treatment of patients with OC. Numerous inhibitors have been developed against various components of this pathway; however, none of these inhibitors exhibited notable clinical efficacy [9]. Complementary therapeutic strategies combined with the inhibition of the PI3K/AKT/mTOR pathway could be developed. For instance, FGFR inhibitors function as tumor suppressors, such as inhibition of stromal cells. Furthermore, a synergistic activity of combined inhibition of mTOR and FGFR pathways has been reported [10].
Therefore, the present study aimed at investigating whether the combined inhibition of mTOR and FGFR pathways would enhance the anticancer effects in the treatment of OC.
Materials and methods
Cell lines and reagents
OC cell lines (CAOV-3, SKOV-3 and OVCAR-5) and vascular smooth muscle cells (as stromal control) were obtained from the Cell Bank of the Chinese Academy of Science (Beijing, China). Inhibitors of FGFR (BGJ398) and mTOR (Rapamycin), paraformaldehyde and dimethyl sulphoxide (DMSO) were purchased from Selleck Chemicals (Houston, Texas, USA). For the treatment of mice, BGJ398 and Rapamycin were dissolved in water. For the treatment of cells, stock solutions of BGJ398 and Rapamycin were prepared in DMSO and further diluted in cell culture medium. Monoclonal antibodies against phosphorylated AKT and total AKT, phosphorylated ERK1/2 and total ERK1/2, phosphorylated PI3K and total PI3K, phosphorylated S6 and total S6 and horseradish peroxidase conjugated goat anti-mouse antibody were purchased from Cell Signaling (Beverly, Massachusetts, USA). Other antibodies (anti-b-actin antibody, horseradish peroxidase conjugated donkey anti-mouse/rabbit antibody) were from obtained from Santa Cruz Biotechnology (Shanghai, China).
Patient specimens
Tumor specimens were obtained from five patients who underwent tumor resection at the Department of Surgery, Jinzhou Medical University (Liaoning, China). None of the patients had received radiotherapy, chemotherapy or other anticancer treatments prior to surgery. RNA was isolated using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands). The study design was approved by the Human Ethics Committee of Jinzhou Medical University, and informed written consents were obtained from patients for the use of their tissues.
Cell proliferation assay
CyQuant Cell Proliferation Assay Kit (Invitrogen, Carlsbad, CA, USA) was used in the evaluation of cell growth. Exponentially growing cells were seeded into 96 well plates. The values of effective concentration at 50% maximum inhibition (EC50) were determined for both inhibitors on each OC line and defined as 1×. Cells were then incubated for 24 h, and the treatments of rapamycin and BGJ398 were performed with a fixed ratio of 1:1 for each compound. Two-fold serial dilutions above and below the EC50 were tested. The values ranging from 0.125 to 8 were added to the cells for 72 h.
Transwell assay
The assay was performed using a dual chamber system. A total of 2.5×105 cells were resuspended in serum free DMEM onto a BD FalconTM Cell Culture and seeded into an insert with the pore size of 8 mm (BD, Heidelberg, Germany). The cells were treated with BGJ398, rapamycin or both inhibitors and added to the upper chamber. To evaluate the invasive ability, membranes were coated with BD MatrigelTM (BD Biosciences, Franklin Lakes, NJ, USA). Then cells were incubated at 37°C for 48 hours, and non-invasive cells were removed using a cotton swab. The cells attached to the lower membranes of the Transwell chambers were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The invasive capacities were examined by counting the number of invasive cells in three randomly selected fields/membranes by using an inverted microscope (magnification, ×40, IX83; Olympus Corporation, Tokyo, Japan) non-invasive cells were removed from the upper chamber.
Western blot analysis
Cells were harvested using radioimmunoprecipitation buffer (50 mM Tris-HCl [pH 7.4], 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM PMSF and protease inhibitor cocktail). Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, California, USA). Equal amounts of protein samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes and incubated with primary antibodies overnight at 4°C. Following three washes, the membranes were incubated with the secondary peroxidase-conjugated antibody (1:1000) for one hour at room temperature. Bands were visualised using an enhanced chemiluminescence protein detection kit (Pierce Biotechnology; Thermo Fisher Scientific, Inc).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol® reagent (Thermo Fisher, Beijing, China) according to the manufacturer’s protocols. Reverse transcription was performed using the TOYOBO ReverTra Ace reverse transcription kit (Toyobo, Shanghai, China). Then qPCR was conducted and primer sequences were presented in Table 1. The program used was as follows: 95°C for 15 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec). Gene expression levels were analyzed in triplicate and normalized to housekeeping genes using 2-ΔΔCt method.
Table 1.
Primer sequences
| FGFR1 | 5’-CAGTGACACCACCTACTTC | 3’-GCACCACAGAGTCATTAT |
| FGFR2IIIb | 5’-TGCTGGCTCTGTTCAATGTG | 3’-GGCGATTAAGAAGACCCCTA |
| FGFR2IIIc | 5’-ACACCACGGACAAAGAGATT | 3’-GGCGATTAAGAAGACCCCTA |
| FGFR3 | 5’-ATGGGCGCCCCTGCCTGC | 3’-GTGGTGTGTTGGAGCTCATG |
| FGFR4 | 5’-CGGCCTCTCCTACCAGTCT | 3’-TGCCGGAAGAGCCTGAC |
| VEGF-A | 5’-GCAGCTTGAGTTAACGAACG | 3’-GGTTCCCGAAACCCTGAG |
| PDGF-B | 5’-TCGAGTTGGAAAGCTCATCTC | 3’-GTCTTGCACTCGGCGATTA |
| bFGF | 5’-AGCGGCTGTACTGCAAAAAC | 3’-TTCTGCTTGAAGTTGTAGCTTGAT |
Flow cytometry
The apoptosis and cell cycle were evaluated using flow cytometry. Cells were washed with phosphate-buffered saline and 70% pre-cooled ethanol at 4°C overnight. Fixed cells were then resuspended using 1 mL of propidium iodide (20 mg/L, Sigma-Aldrich) containing RNAase. Cells were filtrated by using nylon mesh after keeping in dark for 30 min. The red fluorescence of excitation wavelength at 488 nm was recorded by flow cytometry (FACS Calibur, BD Biosciences, San Jose, California, USA).
Animal studies
The present study was approved by the Animal Ethics Committee of Jinzhou Medical University. Female, 5 weeks old, nude mice (nu/nu strain) weighing 17-20 g were used. A total of 5×104 SKOV-3 cells were injected into the right flank of each mouse as previously described [10]. Tumor size was calculated using the following formula: volume = (length × width2)/2. When the average volume of the tumor reached 200 mm3, mice (n=5 mice/group) were administered BGJ398 (10 or 30 mg/kg, 1 per day by oral gavage) or rapamycin (0.4 or 1 mg/kg per day, intra-peritoneally) or a combination of both compounds. The treatment lasted for 21 days. The nude mice were sacrificed by cervical dislocation, and the tumors were removed and weighed.
Statistical analysis
All experiments were repeated at least three times. The data were analyzed using GraphPad Prism 6 (San Diego, California, USA) and presented as mean ± standard deviation (SD). Student t-test or one-way analysis of variance (ANOVA) was used for comparison between different groups. P<0.05 was considered to indicate a statistically significant difference.
Results
The expression level of FGFR in OC, vascular smooth muscle cells and patient samples
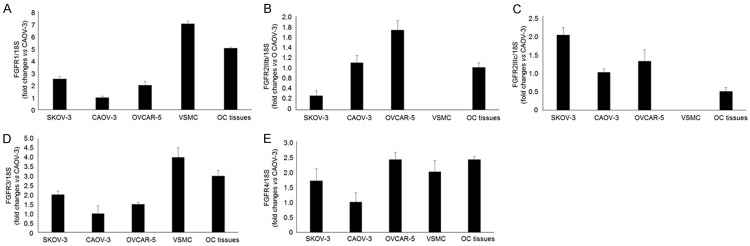
The expression of FGFR in OC cell lines (CAOV-3, SKOV-3 and OVCAR-5), vascular smooth muscle cells and OC tissues was quantified using qPCR The expression of FGFR1, 3, 4 and FGFR2IIIc were detected in all three tested OC cell lines (Figure 1A-E). In addition, FGFR2IIIb was abundantly expressed in CAOV-3 and OVCAR-5 cells, but not in SKOV-3 (Figure 1B). The expression levels of FGFR2IIIb and c were extremely low in vascular smooth muscle cells, where FGFR1, 3 and 4 were strongly expressed (Figure 1A-E). In OC tissues, mRNAs of all five tested FGFRs were detected, and the expression levels of FGFG1 and 4 were abundant (Figure 1A-E). These results revealed that potential targets of BGJ398 are expressed in OC cells and tissues.
Figure 1.
The expression level of FGFRs in OC cells and tissues. A. The mRNA level of FGFR1 in OC cells, vascular smooth muscle cells and OC tissues. B. The expression of FGFR2IIIb in OC cells and tissues. C. The mRNA level of FGFR2IIIc in OC cells and tissues. D. The expression of FGFR3 in OC cells, vascular smooth muscle cells and OC tissues. E. The mRNA level of FGFR4 in OC cells, vascular smooth muscle cells and OC tissues. VSMC: vascular smooth muscle cells, OC: ovarian cancer.
The effects of combined treatment using BGJ398 and rapamycin on cancer cell growth
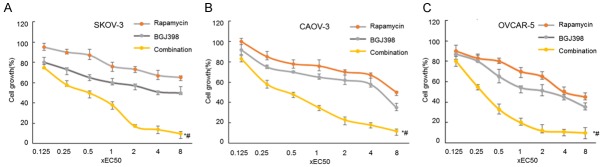
Potential synergistic effects of BGJ398 and rapamycin on cancer cell growth were investigated using previously established method [11]. BGJ398 and rapamycin inhibit FGFR and mTOR signaling pathway respectively. The inhibitors were tested both individually and in combination. IC50 of BGJ398 were 40, 100 and 200 nM in SKOV-3, CAOV-3 and OVCAR-5 cells, where the IC50 of rapamycin IC50 were 0.5, 1.0 and 1.5 nM (data not shown). Furthermore, serial concentrations from 0.125 to 8× IC50 of the inhibitors were tested alone or in combination. Combined treatment using BGJ398 and rapamycin exhibited enhanced inhibition of cell proliferation in a dose-dependent manner (Figure 2).
Figure 2.
The effects of combined treatment using BGJ398 and rapamycin on cancer growth. A-C. SKOV-3, CAOV-3 and OVACR-5 cells were treated with serial concentrations of BGJ398, Rapamycin, or both inhibitors at a ratio of 1:1. The dose range was 0.125-8× IC50 of each compound.
The influences of combined treatment using BGJ398 and Rapamycin on SKOV-3 cells
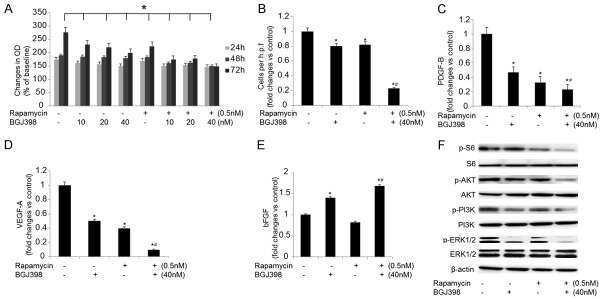
To investigate whether the growth of SKOV-3 cells was suppressed by combined treatment of BGJ398 and rapamycin, further experiments were conducted. Cells were treated with either individual inhibitor or combined compounds for up to 72 hours. The cell growth was inhibited by BGJ398 significantly, and addition of rapamycin enhanced the effects (Figure 3A).
Figure 3.
The influences of combined treatment on SKOV-3 cells. A. The growth of SKOV-3 cells treated with 40 nM BGJ398 (with or without 0.5 nM rapamycin) for 72 hours. B. The motility of SKOV-3 cells following the treatments. C-E. The expression levels of PDGF-B, VEGF-A and bFGF in SKOV-3 cells treated with 40 nM BGJ398 (with or without 0.5 nM rapamycin) for 72 hours. F. The expression of p-S6, p-Akt, p-PI3K and p-ERK1/2 following the treatments. *P<0.05 vs. the control group; #P<0.05 vs. the treatment with BGJ398 or rapamycin alone.
In addition, the mechanisms of rapamycin- and BGJ398-modulated motility of SKOV-3 cells were investigated. The cell growth was significantly suppressed by rapamycin and BGJ398 especially with combined treatment (Figure 3B). The effects on angiogenic factors in treated cells were evaluated using qPCR, and the results suggested that the expression levels of PDGF-B and VEGF-A were significantly downregulated in SKOV-3 cells, where the level of bFGF was remarkably upregulated by combined treatment of BGJ398 and rapamycin (Figure 3C-E). To further explore the pathways involved in the combined treatment, the expression level and phosphorylation status of the proteins associated with mTOR and FGFR signaling pathways were evaluated (Figure 3F). Treatment with BGJ398 alone led to partially inhibited phosphorylation of Akt, PI3K and ERK1/2, whereas the phosphorylation of S6 remained unchanged; however, the phosphorylation of S6 was significantly inhibitied by rapamycin. Furthermore, combined treatment resulted in completely inhibited phosphorylation of S6, Akt, PI3K and ERK1/2. In summary, SKOV-3 cells could be susceptible to co-inhibition of BGJ398 and rapamycin.
The effects of combined treatment of BGJ398 and Rapamycin on CAOV-3 cells
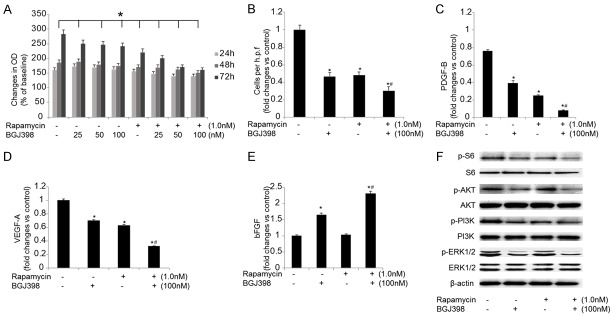
The growth of CAOV-3 cells were significantly inhibited by combined treatment using BGJ398 and rapamycin (Figure 4B). In addition, the potential effects of the inhibitors on the migration of CAOV-3 cells were investigated. Combined inhibition of mTOR and FGFR pathways resulted in significant suppression on cell migration (Figure 4B). Furthermore, inhibition of FGFR notably reduced the mRNA levels of PDGF-B and VEGF-A and increased the expression of bFGF (Figure 4C-E). The effects on phosphorylation status of S6, Akt, PI3K and ERK1/2 were also investigated (Figure 4F), and the phosphorylation of these proteins was inhibited by the combined treatment. Therefore, combination of BGJ398 and rapamycin exhibited similar effects on CAOV-3 cells as seen in SKOV-3 cells.
Figure 4.
The effects of combined treatment of BGJ398 and rapamycin on CAOV-3 cells. A. The growth of CAOV-3 cells growth treated with 100 nM BGJ398 (with or without 1.0 nM rapamycin) for 72 hours. B. The motility of CAOV-3 cells following the treatments. C-E. The expression levels of PDGF-B, VEGF-A and bFGF in CAOV-3 cells treated with 100 nM BGJ398 (with or without 1.0 nM rapamycin) for 72 hours. F. The expression of p-S6, p-Akt, p-PI3K and p-ERK1/2 following the treatments. *P<0.05 vs. the control group; #P<0.05 vs. the treatment with BGJ398 or rapamycin alone.
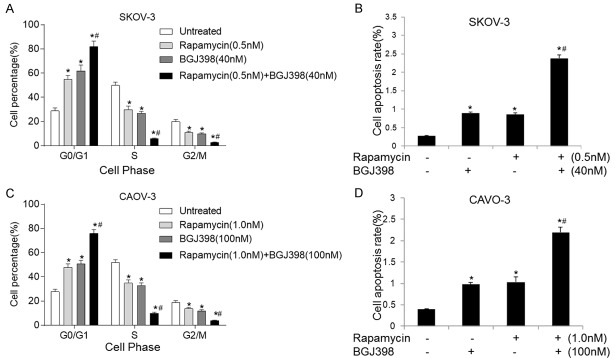
Combination of BGJ398 and rapamycin promote cell cycle arrest and apoptosis in SKOV-3 and CAOV-3 cells
The effects of combined treatment on cell cycle and apoptosis on SKOV-3 and CAOV-3 cells were evaluated. As presented in Figure 5A and 5C, treatment with individual compound led to decreased proportion of cells in S and G2/M phase, with a concomitant accumulation of cells in the G0/G1 phase. The changes were statistically significant in SKOV-3 and CAOV-3 cells. In comparison, effects of combined treatment were more pronounced and induced G0/G1 arrest. Furthermore, treatment with BGJ398 and rapamycin significantly promoted the apoptosis of SKOV-3 (Figure 5B) and CAOV-3 (Figure 5D) cells. These influences could be caused by cell cycle arrest, and combination of both inhibitors may enhance the effects on cell cycle arrest and apoptosis in OC.
Figure 5.
Combination of BGJ398 and rapamycin promotes cell cycle arrest and apoptosis in SKOV-3 and CAOV-3 cells. A. Cell cycle in SKOV-3 cells treated with 40 nM BGJ398 (with or without 0.5 nM rapamycin) for 72 hours. B. The apoptosis rate of SKOV-3 cells following the treatments. C. Cell cycle in CAOV-3 cells treated with 100 nM BGJ398 (with or without 1.0 nM rapamycin) for 72 hours. D. The apoptosis of SKOV-3 cells following the treatments. *P<0.05 vs. the control group; #P<0.05 vs. the treatment with BGJ398 or rapamycin alone.
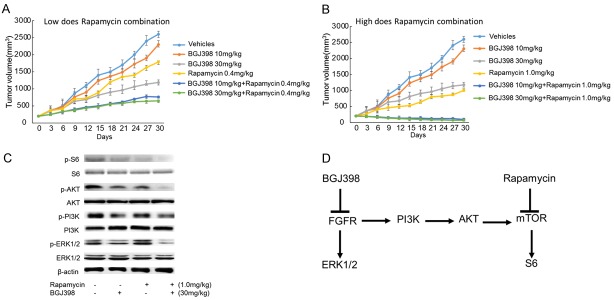
Effects of combined treatment of BGJ398 and rapamycin on the growth of tumor in vivo
To investigate whether the aforementioned signaling pathways could be inhibited in vivo, a mouse tumor xenograft model was established. No regression of the tumor was observed when the mice were treated with each compound alone, albeit a dose-dependent inhibition of tumor growth was detected (Figure 6A and 6B). Furthermore, BGJ398 inhibited tumor growth by 21 and 76% respectively, when used at 10 and 30 mg/kg (Figure 6A and 6B). In comparison, rapamycin exhibited inhibition by 50 and 74% at the doses of 0.4 and 1 mg/kg (Figure 6A and 6B). A combination of low-dose BGJ398 (10 mg/kg) and rapamycin (0.4 mg/kg) resulted in significant reduction of tumor size (Figure 6A). In addition, combined treatment using high-dose BGJ398 and rapamycin induced more substantial inhibition on tumor growth (Figure 6B).
Figure 6.
The effects of combined treatment on the growth of tumor in vivo. Mice with SKOV3 endometrial xenografts (200 mm3) were randomized into treatment with vehicle, BGJ398 (10 or 30 mg/kg), Rapamycin (0.4 or 1.0 mg/kg), or a combination of both inhibitors. A. Tumor growth in low-dose BGJ398 group (with or without Rapamycin). B. The growth of tumor in high-dose BGJ398 group (with or without Rapamycin). C. The expression levels of p-S6, p-Akt, p-PI3K and p-ERK1/2 in mice treated with 30 mg/kg BGJ398 (with or without 1.0 mg/kg rapamycin). D. The network of FGFR signaling pathway. Inhibition of FGFR results in the phosphorylation of FRS2-α and activation of downstream molecules such as ERK1/2 and PI3K, and the PI3K/AKT pathway activates the mTOR/S6 signalling, which is negatively regulated by rapamycin.
Following the harvest of tumors, the phosphorylation status of key regulators in the two signaling pathways (S6, Akt, PI3K and ERK1/2) were evaluated by western blotting. The results revealed that rapamycin diminished the phosphorylation of S6, whereas the phosphorylation of S6, Akt, PI3K and ERK1/2 were all inhibited by BGJ398 (Figure 6C). Furthermore, combination of rapamycin and BGJ398 inhibited the phosphorylation of S6, Akt, PI3K and ERK1/2. The proposed intracellular effects of the associated molecules were presented in Figure 6D.
Discussion
OC is one of the most common types of gynecological malignancies with poor prognosis, and the median survival time is ~18 months [3,12]. Compared with current treatments, potential targeted therapies could be more effective. The mTOR pathway is involved in OC tumorigenesis [9,13]. In previous phase I study, patients treated with mTOR inhibitor exhibited longer survival time [14]. However, in recent phase II studies, moderate effects was achieved by inhibition of the mTOR pathway [15,16]. Furthermore, in other types of cancer, previous study revealed that combined inhibition of mTOR and FGFR signalings could be a promising anticancer approach [10]. Therefore, whether inhibition of both aforementioned pathways is more effective in the treatment of OC was investigated.
To confirm that FGFR signaling pathway is associated with OC pathogenesis, we the expression levels of FGFRs were evaluated in OC cells and tissues, but not in normal stromal cells. Furthermore, combined inhibition against mTOR and FGFR pathways exhibited prominent anticancer effects in OC cells. In additions, the influences of combination of BGJ398 and rapamycin were further confirmed in a murine tumor xenograft model.
Angiogenesis is crucial for the initiation and development of OC, and anti-angiogenic therapy was effective in OC [17,18]. In the present study, decreased levels of angiogenesis-associated regulators, such as VEGF, PDGF and TGF-β was detected in OC cells, following the combined treatment. Interestingly, the expression of VEGF-A was also reduced in OC cells treated with BGJ398 and rapamycin. VEGF-A is a regulator of tumor angiogenesis, and its expression is associated with cancer progression and overall survival [19]. In addition, the results suggested that combined treatment with BGJ398 and rapamycin led to diminished expression of PDGF-B, which is consistent with previously published studies [20]. PDGF-B regulates the survival and apoptosis of OC cells [21,22]. Therefore, the synergistic effects of inhibiting both FGFR and mTOR pathways could disrupt tumor angiogenesis.
Tumor migration, as a major contributor to the pathogenesis and progression of OC, is increasingly perceived [23]. In consistent with previous reseach [20,24], the results of the present study indicated that combined FGFR/mTOR inhibition exhibited enhanced effects on the migration of OC cells. Interestingly, inhibition of FGFR also exerts some influences on OC cells. Compared with previous study [20], this could be a result of tested cancer cell lines originated from various types of tumors. Furthermore, OC cell cycle was significantly shifted from S to G0/G1 phase and cells apoptosis was promoted in SKOV-3 and CAOV-3 cells following the treatment with BGJ398 and rapamycin. The results revealed that combination of both inhibitors induced cell cycle arrest and apoptosis in OC cells.
FGFR signaling network consists of multiple pathways including Akt and ERK1/2, and the regulators of PI3K/AKT/mTOR pathway are differentially activated following the treatment with BGJ398 and rapamycin depending on the cellular context. Interestingly, the results revealed that both ERK and AKT are constitutively activated in OC cells. Furthermore, sole inhibition of mTOR signaling does not affect the phosphorylation of Akt significantly. Akt phosphorylation was previously demonstrated to be associated with the IGF-IR/IRS-1 signaling and the mTORC2 complex [25,26]. These data suggested that exposure of the cells to FGFR inhibitor could be essential to mTOR-promoted Akt phosphorylation in cancer cells. In addition, the PI3K/AKT/mTOR pathway could be the alternative signaling in the presence of FGFR inhibitior. These results suggested that the combined treatment could reduce the phosphorylation of S6, Akt, PI3K and ERK1/2, and blockade of parallel signaling circuits may result in the synergistic activity.
The anticancer effects induced by combined inhibition of mTOR and FGFR pathways were also confirmed in vivo. Furthermore, the potency of anticancer effects was sufficient even with combinated inhibitors at low-dose. In addition, tumor regression was initiated by a combination of BGJ398 and rapamycin, but not by either compound alone.
Conclusion
In summary, the results suggested that simultaneous inhibition of FGFR and mTOR activity could contribute to anti-proliferative effects and tumor regression. Furthermore, the findings revealed that combined treatment of BGJ398 and rapamycin may be a promising therapeutic strategy in the treatment of patients with OC.
Acknowledgements
The present study was funded by Liaoning Province Natural Science Fund Program project (grant no. 2013010134) and Nature united fund project of Liaoning provincial department of science and technology (grant no. 20170540373).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Romero I, Bast RC Jr. Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3:264–79. doi: 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- 5.Gorringe KL, Jacobs S, Thompson ER, Sridhar A, Qiu W, Choong DY, Campbell IG. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin Cancer Res. 2007;13:4731–9. doi: 10.1158/1078-0432.CCR-07-0502. [DOI] [PubMed] [Google Scholar]
- 6.Steele IA, Edmondson RJ, Bulmer JN, Bolger BS, Leung HY, Davies BR. Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene. 2001;20:5878–87. doi: 10.1038/sj.onc.1204755. [DOI] [PubMed] [Google Scholar]
- 7.Cole C, Lau S, Backen A, Clamp A, Rushton G, Dive C, Hodgkinson C, McVey R, Kitchener H, Jayson GC. Inhibition of FGFR2 and FGFR1 increases cisplatin sensitivity in ovarian cancer. Cancer Biol Ther. 2010;10:495–504. doi: 10.4161/cbt.10.5.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobbin ZC, Landen CN. The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int J Mol Sci. 2013;14:8213–27. doi: 10.3390/ijms14048213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–9. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Gozgit JM, Squillace RM, Wongchenko MJ, Miller D, Wardwell S, Mohemmad Q, Narasimhan NI, Wang F, Clackson T, Rivera VM. Combined targeting of FGFR2 and mTOR by ponatinib and ridaforolimus results in synergistic antitumor activity in FGFR2 mutant endometrial cancer models. Cancer Chemother Pharmacol. 2013;71:1315–23. doi: 10.1007/s00280-013-2131-z. [DOI] [PubMed] [Google Scholar]
- 11.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Fearon AE, Gould CR, Grose RP. FGFR signalling in women’s cancers. Int J Biochem Cell Biol. 2013;45:2832–42. doi: 10.1016/j.biocel.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Cheaib B, Auguste A, Leary A. The PI3K/Akt/mTOR pathway in ovarian cancer: therapeutic opportunities and challenges. Chin J Cancer. 2015;34:4–16. doi: 10.5732/cjc.014.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroney J, Fu S, Moulder S, Falchook G, Helgason T, Levenback C, Hong D, Naing A, Wheler J, Kurzrock R. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin Cancer Res. 2012;18:5796–805. doi: 10.1158/1078-0432.CCR-12-1158. [DOI] [PubMed] [Google Scholar]
- 15.Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, Schilder RJ, Cai KQ, Godwin AK, Alpaugh RK. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123:19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsoref D, Welch S, Lau S, Biagi J, Tonkin K, Martin LA, Ellard S, Ghatage P, Elit L, Mackay HJ, Allo G, Tsao MS, Kamel-Reid S, Eisenhauer EA, Oza AM. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. 2014;135:184–9. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti C, Gasparri ML, Ruscito I, Palaia I, Perniola G, Carrone A, Farooqi AA, Pecorini F, Muzii L, Panici PB. Advances in anti-angiogenic agents for ovarian cancer treatment: the role of trebananib (AMG 386) Crit Rev Oncol Hematol. 2015;94:302–10. doi: 10.1016/j.critrevonc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Fu Z, Chen D, Cheng H, Wang F. Hypoxia-inducible factor-1alpha protects cervical carcinoma cells from apoptosis induced by radiation via modulation of vascular endothelial growth factor and p53 under hypoxia. Med Sci Monit. 2015;21:318–25. doi: 10.12659/MSM.893265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheller T, Hellerbrand C, Moser C, Schmidt K, Kroemer A, Brunner SM, Schlitt HJ, Geissler EK, Lang SA. mTOR inhibition improves fibroblast growth factor receptor targeting in hepatocellular carcinoma. Br J Cancer. 2015;112:841–50. doi: 10.1038/bjc.2014.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin V, Liu D, Fueyo J, Gomez-Manzano C. Tie2: a journey from normal angiogenesis to cancer and beyond. Histol Histopathol. 2008;23:773–80. doi: 10.14670/HH-23.773. [DOI] [PubMed] [Google Scholar]
- 22.Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73:108–18. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 23.Davidson B, Trope CG, Reich R. The role of the tumor stroma in ovarian cancer. Front Oncol. 2014;4:104. doi: 10.3389/fonc.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang SA, Moser C, Fichnter-Feigl S, Schachtschneider P, Hellerbrand C, Schmitz V, Schlitt HJ, Geissler EK, Stoeltzing O. Targeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in mice. Hepatology. 2009;49:523–32. doi: 10.1002/hep.22685. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Dong YY, Wang WM, Xie XY, Wang ZM, Chen RX, Chen J, Gao DM, Cui JF, Ren ZG. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-kappaB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32:51. doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]