Abstract
Objective: Omentin-1, an adipokine released from visceral fat tissue, is associated with diabetes and stroke. The purpose of this study was to assess the impact of serum omentin-1 levels on functional prognosis in nondiabetic patients with ischemic stroke. Methods: From March 2016 to December 2017, consecutive patients with first-ever ischemic stroke admitted to our hospital, China, were recorded. Functional impairment was evaluated at 3-month after admission using the modified Rankin scale (mRS). Uni-and multivariate analyses with Cox proportional hazard regression was used for assessing the relationship between serum level of omentin-1 and functional outcome. Results: We recorded 209 stroke patients, 52 of them (24.9%) experienced as poor functional outcome. The obtained omentin-1 level in patients with poor outcome was lower than in those patients with good outcome [100.8 (80.9-131.6) ng/ml vs. 137.6 (IQR, 106.1-171.5) ng/ml; Z=4.692; P<0.001). Multivariate analysis models were used to assess stroke outcome according to omentin-1 quartiles (the highest quartile [Q4] as the reference), the 1st and 2nd quartile of omentin-1 were compared against the Q4, and the risks were increased by 505% (HR=6.05; 95% CI: 2.13-12.15; P=0.007) and 215% (31.5; 1.21-7.98; P=0.03), respectively. The inclusion of omentin-1 in the routine prediction model for the prediction of poor functional outcome, enhanced the NRI (P=0.006) and IDI (P=0.001) values, confirming the effective reclassification and discrimination. Kaplan-Meier analysis suggested that the patients with low serum omentin-1 levels had a higher risk of death than those patients with high levels of omentin-1 (log-rank test P=0.033). Conclusion: In this cohort of nondiabetic patients with ischemic stroke, a reduced baseline level of serum omentin-1 was related with an increased risk for poor functional outcome or death, independent of baseline variables.
Keywords: Nondiabetic, ischemic stroke, adipokine, omentin-1, functional outcome
Introduction
From 2002 to 2013, the incidence of stroke in China increased rapidly (an overall annual increase of 8.3%) [1]. Furthermore, stroke is one of the most common cause of death and disability in China [2]. Approximately 1.6 million people per year died due to stroke, and there are 7.5 million of stroke survivors (15% to 30% of those patients permanently disabled) [3]. Early prediction of prognosis can influence therapeutic strategies and change stroke outcomes.
Adipokines, secreted by adipose tissue, play roles in the adjusting of glucose metabolism, inflammation and cardiovascular homeostasis [4]. Some adipokines, such as leptin [5], adiponectin [6], retinol-binding protein 4 [7] and interleukin-6 [8] have been discovered and suggested as prediction biomarkers for stoke risk and functional outcome. As a novel adipokine, omentin had been suggested play role in anti-apoptosis, anti-oxidation and anti-inflammatory [9]. Two isoforms of omentin: omentin-1 and omentin-2 were observed and omentin-1 was the predominant form of in the human blood was mainly [10].
Omentin-1, an adipocytokine released from visceral fat tissue, is associated with metabolic syndrome [11], diabetes and hypertension [12]. A previous study found that that omentin-1 was down regulated by insulin and glucose [13]. The influence of the omentin-1 on vascular health had been suggested [14]. Previous studies revealed that omentin-1 might have role in atherosclerosis in patients with metabolic syndrome [15], coronary artery disease (CAD) [16], cardiovascular dysfunction in diabetes mellitus [17], and peripheral artery disease (PAD) [18]. However, the association of low serum omentin-1 with carotid atherosclerosis was not found by Kadoglou et al. [19].
As a novel adipokine, the role of omentin-1 in the stroke had been proposed [20]. Yue et al. suggested that the omentin-1 could be promising indicator of ischemic stroke and its severity [9]. Furthermore, higher baseline serum levels of omentin-1 levels were negatively associated with poor functional outcome among patients with ischemic stroke [21]. Diabetes mellitus was a recognized risk factor for stroke [22], and insulin resistance was independently associated with poor functional outcome after acute ischemic stroke [23]. A previous study pointed toward a role of omentin-1 in insulin resistance and type 2 diabetes mellitus [24]. Thus, the role of omentin-1 in stroke risk and progression might be due to a possible association with insulin resistance and abnormal glucose metabolism. However, data on the association between serum levels of omentin-1 and functional prognosis in nondiabetic patients with ischemic stroke are lacking. The purpose of this study was to assess the impact of serum omentin-1 levels on functional prognosis in nondiabetic patients with ischemic stroke.
Patients and methods
Patients
From March 2016 to December 2017, consecutive patients with first-ever acute ischemic stroke admitted to Jiangsu University Affiliated Hospital, China, were recorded. The participants were exclusively Chinese. Ischemic stroke was diagnosed and identified according to World Health Organization recommendations (neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours) [25]. The clinical diagnoses were validated according to magnetic resonance imaging (MRI). The exclusion criteria included: (1) malignant tumor; (2) symptoms onset more than 48 h; (3) liver and kidney function insufficiency; (4) diabetes mellitus and/or metabolic syndrome; (5) lipodystrophy or chronic coexistent inflammatory disease; (6) other neurological diseases (such as Parkinson’s disease and Alzheimer’s disease).
Clinical variables and laboratory testing
The general information including age, gender, body mass index (BMI), blood pressure (diastolic blood pressure [DBP] and systolic blood pressure [SBP]) and vascular risk factors (hypertension, hypercholesterolemia, atrial fibrillation, smoking, drinking, previous myocardial infarction, and a history of transient ischemic attack [TIA]) were collected. Therapy received before stroke (oral anticoagulants, antiplatelet agents and antihypertensive treatment) and acute treatment (Intravenous [IV] thrombolysis and/or mechanical thrombectomy) was also recorded.
National Institutes of Health Stroke Scale (NIHSS) was used to assess clinical severity at admission. Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria were used to determine the stroke etiology, including large-artery arteriosclerosis, small-artery occlusion, cardioembolism, the other causative factor, and undetermined causative factor [26]. Functional outcome at 3-month after admission was defined as the endpoint, which was assessed by the modified Rankin Scale (mRS) [27]. The mRS of 0-2 points was indicated as a good functional outcome, while 3-6 points was defined as poor outcome [28].
The fasting serum samples were collected at 8:00 on the first morning of admission for testing levels of omentin-1 (within 0-6 h [n=52], 6-24 h [n=73], 24-48 h [n=53], and 48-72 h [n=31] from the symptom onset). A commercial enzyme-linked immunosorbent assay (ELISA) kit was used for determining omentin-1 in serum (Immuno-Biological Laboratories CO., Ltd., Gunma, Japan). The variation coefficients of intra- and inter-assay were 4.0%-8.0% and 4.8%-9.5%, respectively. In addition, serum biomarkers of glucose, C-reactive protein (CRP) and interleukin-6 (IL-6) were also tested by ELISA in the laboratory of our hospital.
Statistical analyses
The results of categorical variable and continuous variable were presented as percentage and median value (interquartile ranges, IQRs). Chi-square and Mann-Whitney U test were applied for comparing the proportions and medians values between groups. Correlations between omentin-1 and other parameters were studied by Spearman correlation analysis. Uni- and multivariate analyses with Cox proportional hazard regression was used for assessing the relationship between serum level of omentin-1 and functional outcome (defined as m-RS of 3-6 and 0-2). In multivariate analyses, significant factors which confirmed in the univariate analyses, including age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6 were adjusted. The results were showed as hazard ratios (HR) with 95% confidence intervals (CI). For a more detailed exploration of the omentin-1 and stroke outcome, multivariate analysis models to estimate adjusted HR and 95% CIs of poor outcome for omentin-1 quartiles (the 4th quartile as reference) was applied.
Second, the accuracy of omentin-1 for predicting outcome of stroke was evaluated with Receiver operating characteristic (ROC) curves and results were calculated with Area under the curve (AUC). In addition, the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) were used to measure the quantity of improvement for the correct classification and sensitivity according to the addition of serum omentin-1 levels to the prediction model [29].
Lastly, cumulative overall survival rates were computed using the Kaplan-Meier method and were compared using the log-rank test. We divided the patients into two groups according to the median serum omentin-1 levels (high vs. low). Statistical analysis was performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA) and the ROCR package (version 1.0-2). P<0.05 was considered statistically significant.
Ethics
The design of study was reviewed and approved by investigational review board of the Jiangsu Normal University. Informed consents were obtained from patients or their relatives (patients unable to communicate) prior to their inclusion in this study, under the guidance of Declaration of Helsinki.
Results
During the inclusion period, 305 patients were screened. Two hundred and sixteen patients with ischemic stroke were included (37 with transient ischemic attack, 43 with onset of symptoms >48 hours, 3 without informed consent, 3 with malignant tumor and 3 with surgical procedures within the last 3 months) and 209 completed follow-ups (5 lost to follow-up and 2 withdraw). However, these 209 patients were similar in terms of baseline characteristics [age (P=0.38), gender (P=0.88), NIHSS (P=0.19) and weight (P=0.63)] compared to the overall cohort. Lastly, we recorded 209 stroke patients, and the median omentin-1 serum level in those patients was 129.0 ng/ml (IQR, 97.1-163.7 ng/ml). The median NIHSS scores on admission was 7 points (IQR, 4 to 12). The patients received acute treatment were 12.9% for IV thrombolysis and 8.6% for mechanical thrombectomy. The general information of patients was provided in Table 1.
Table 1.
Characteristics of stroke patients according to functional outcomes
| Total | Good outcomes | Poor outcomes | P | |
|---|---|---|---|---|
| N | 209 | 157 | 52 | - |
| Age, years median (IQR) | 66 (55-75) | 63 (52-71) | 72 (60-81) | 0.012 |
| Female gender, n (%) | 83 (39.7) | 60 (38.2) | 23 (44.2) | 0.44 |
| BMI, kg/m2 median (IQR) | 24.5 (23.1-26.8) | 24.1 (22.5-26.1) | 26.1 (24.2-28.5) | 0.018 |
| Smoking, n (%) | 64 (30.6) | 46 (29.3) | 18 (34.6) | 0.47 |
| Drinking, n (%) | 52 (24.9) | 42 (26.8) | 10 (19.2) | 0.28 |
| SBP, mmHg median (IQR) | 148 (130-169) | 142 (125-163) | 160 (139-181) | 0.007 |
| DBP, mmHg median (IQR) | 80 (72-95) | 77 (70-90) | 88 (77-100) | 0.011 |
| Prior vascular risk factors, n (%) | ||||
| Hypertension | 136 (65.1) | 100 (63.7) | 36 (69.2) | 0.48 |
| Hypercholesterolemia | 71 (34.0) | 51 (32.5) | 20 (38.5) | 0.38 |
| Atrial fibrillation | 25 (12.0) | 19 (12.1) | 6 (11.5) | 0.91 |
| Myocardial infarction | 24 (11.5) | 16 (10.2) | 8 (15.4) | 0.31 |
| Previous TIA | 18 (8.6) | 10 (6.4) | 8 (15.4) | 0.045 |
| Pre-stroke treatment, n (%) | ||||
| Antihypertensive treatment | 142 (67.9) | 105 (66.9) | 37 (71.2) | 0.57 |
| Antiplatelet agents | 63 (30.1) | 49 (31.2) | 14 (26.9) | 0.56 |
| Acute treatment, n (%) | ||||
| IV thrombolysis | 27 (12.9) | 25 (15.9) | 2 (3.8) | 0.024 |
| Mechanical thrombectomy | 18 (8.6) | 16 (10.2) | 2 (3.8) | 0.26 |
| Stroke etiology, n (%) | ||||
| Small-vessel occlusive | 39 (18.7) | 28 (17.8) | 11 (21.1) | 0.59 |
| Large-vessel occlusive | 45 (21.5) | 29 (18.5) | 16 (30.8) | 0.061 |
| Cardioembolic | 78 (37.3) | 60 (38.2) | 18 (34.6) | 0.64 |
| Other | 21 (10.0) | 18 (11.5) | 3 (5.8) | 0.24 |
| Unknown | 26 (12.5) | 22 (14.0) | 4 (7.7) | 0.23 |
| NIHSS at admission, median (IQR) | 7 (4-12) | 6 (4-11) | 9 (5-15) | <0.001 |
| Lesion volumes, ml median (IQR) | 18.6 (10.2-31.3) | 17.3 (9.6-29.4) | 20.5 (12.7-36.9) | 0.002 |
| Time from onset to admission, hours | 14.0 (10.5-18.5) | 13.5 (10.0-18.0) | 15.0 (11.5-20.0) | 0.07 |
| Laboratory findings, medians (IQR) | ||||
| Glucose level, mmol/L | 5.22 (4.93-5.92) | 5.03 (4.88-5.77) | 5.55 (5.10-6.03) | 0.015 |
| CRP, mg/l | 6.33 (4.13-9.76) | 5.95 (3.88-8.94) | 6.99 (4.55-10.39) | 0.009 |
| IL-6, pg/ml | 7.55 (5.14-10.33) | 7.12 (5.00-9.55) | 8.32 (5.94-12.10) | 0.006 |
| Omentin-1, ng/ml | 129.0 (97.1-163.7) | 137.6 (106.1-171.5) | 100.8 (80.9-131.6) | <0.001 |
SBP, Systolic blood pressure; DBP, Diastolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; IL-6, interleukin-6; IQR, interquartile ranges.
Serum omentin-1 levels and stroke severity
At admission, 115 patients (55.0%) had a minor stroke (NIHSS<5). In these patients, the omentin-1 level (median: 139.5 ng/ml; [IQR, 110.4-170.4]) was higher than in patients with moderate-to-high clinical severity (115.6 ng/ml [88.5-142.4]; P<0.001). As a continuous variable, a correlation between NIHSS score and serum levels of omentin-1 was found (r=-0.363; P<0.001). Furthermore, omentin-1 was found to be associated with lesion size. A negative association between omentin-1 and the infarct volume (r=-0.304, P<0.001) had been reported. Similarly, the data suggested negative associations between omentin-1 and glucose (r=-0.281, P=0.001), IL-6 (r=0.295, P<0.001).
Association between serum omentin-1 levels and functional outcome
Among the 209 patients with ischemic stroke, 52 of them (24.9%) experienced as poor functional outcome among the 3-month follow-up period. As shown in the Table 1, stroke patients with poor outcome were more likely older, higher BMI, SBP, DBP and to have high blood levels of glucose, CRP and IL-6 but less likely to receive acute treatment. In addition, they also have a higher NIHSS at larger lesion size at admission. The obtained omentin-1 level in patients with poor outcome was lower than in those patients with good outcome [100.8 (80.9-131.6) ng/ml vs. 137.6 (IQR, 106.1-171.5) ng/ml; Z=4.692; P<0.001; Figure 1).
Figure 1.
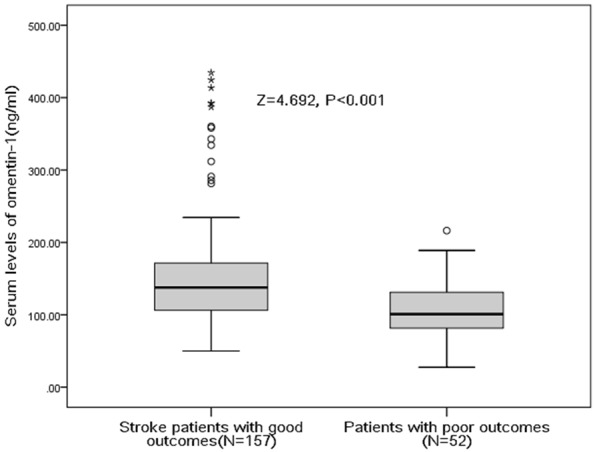
Serum levels of omentin-1 in patients with poor functional outcomes and good outcomes. A good outcome was defined as a mRS score of 0 to 2 points, while poor outcome was defined as 3-6 points. All data are medians and in-terquartile ranges (IQR). P values refer to Mann-Whitney U tests for differences between groups.
As a continuous variable, omentin-1 was associated with decreased risk of stroke poor outcome (OR 0.981, 95% CI: 0.973-0.990; P<0.001) in the univariate model. In multivariate regression analysis model, omentin-1 was still associated with decreased risk of poor outcome (HR 0.990, 95% CI: 0.982-0.996; P=0.002) after adjusting for other significant factors which confirmed in the Table 1, including age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6. In addition, multivariate analysis models were used to assess stroke outcome according to omentin-1 quartiles (the highest quartile [Q4] as the reference), with the adjusted HR with 95% CIs were recorded. As shown in the Table 2 and Figure 2, the 1st and 2nd quartile of omentin-1 were compared against the Q4, and the risks were increased by 505% (HR=6.05; 95% CI: 2.13-12.15; P=0.007) and 215% (31.5; 1.21-7.98; P=0.03), respectively. Furthermore, classified according to cut-off value, the low level of serum omentin-1 was a predictor of poor outcomes, with an adjusted HR of 2.43 (95% CI, 1.29-4.82; P=0.09).
Table 2.
Univariate and multivariate analyses for poor functional outcomes according to omentin-1 quartiles※
| MIF† | PFO/N, (%) | Crude HR (95% CI), P # | Multivariable-adjusted‡, P # |
|---|---|---|---|
| Quartile 1 | 23/52, (44.2) | 9.52 (2.99-30.29), <0.001 | 6.05 (2.13-12.15), 0.007 |
| Quartile 2 | 15/53, (28.3) | 4.74 (1.45-15.45), 0.006 | 3.15 (1.21-7.98), 0.03 |
| Quartile 3 | 10/52, (19.2) | 2.86 (0.83-9.79), 0.09 | 1.75 (0.59-4.11), 0.21 |
| Quartile 4 | 4/52, (7.7) | Reference | Reference |
| Low levels vs. high | 38/105 vs. 14/104 | 3.65 (1.83-7.27), <0.001 | 2.43 (1.29-4.82), 0.009 |
Poor functional outcome was defined as an mRS >2.
Omentine-1 in Quartile 1 (<97.1 ng/ml), Quartile 2 (97.1-129.0 ng/ml), Quartile 3 (129.1-163.7 ng/ml), and Quartile 4 (>163.7 ng/ml). High omentin-1 serum level was defined as ≥129.0 ng/ml (median).
Adjusted for significant factors which confirmed in the Table 1, including age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6.
P value for the trend <0.001.
PFO, poor functional outcomes; HR, hazard ratio; CI, confidence interval; mRS, modified Rankin Scale; BMI, body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; IL-6, interleukin-6; IQR, interquartile ranges.
Figure 2.
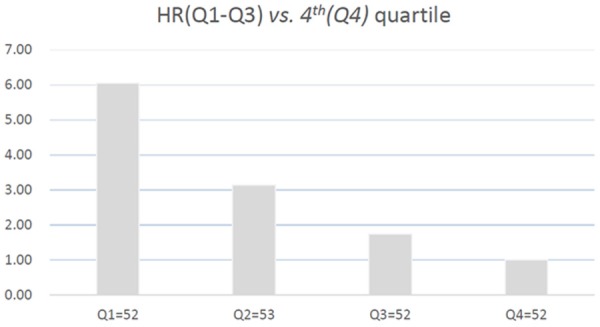
Hazard ratio of the quartiles of omentin-1 levels for poor functional outcomes after adjustment of age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6.
ROC curve, NRI and IDI analysis
With the AUC of 0.73 (95% CI, 0.65-0.81), the omentin-1 showed an improved discriminatory ability for poor outcome than age (0.65, 0.59-0.72; P=0.006), CRP (0.62, 0.57-0.69; P<0.001), glucose (0.57; 0.50-0.62; P<0.001) and IL-6 (0.68, 0.60-0.75; P=0.013), as well as in the range of NIHSS score (0.75, 0.68-0.83; P=0.27). Interestingly, the combined model (omentin-1 and NIHSS) improved the NIHSS score to predict poor outcomes (AUC of the combined model: 0.80; 95% CI, 0.84-0.87; P=0.041). The NRI and the IDI were used to test the quantity of improvement for the correct reclassification and sensitivity according to the addition of omentin-1 serum levels to the prediction model I. As shown in the Table 3, the inclusion of omentin-1 in the prediction model I (includes age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6) for the prediction of poor functional outcome, enhanced the NRI (P=0.006) and IDI (P=0.001) values, confirming the effective reclassification and discrimination.
Table 3.
Statistics for model fit and improvement with addition of serum omentin-1 level predicted on the prediction of poor functional outcome
| Prediction Model I† | Prediction Model II (Model I + omentin-1) | P | |
|---|---|---|---|
| NRI (95% CI) | Reference | 0.248 (0.125-0.404) | 0.006 |
| IDI (95% CI) | Reference | 0.139 (0.083-0.206) | 0.001 |
Prediction Model I included age, BMI, SDP, BDP, previous TIA, IV thrombolysis, NIHSS at admission, lesion volumes, serum levels of glucose, CRP and IL-6.
BMI, body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; IL-6, interleukin-6; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Sub-group analysis
Among the 209 patients, 21 of them (10.0%) died. The median serum level of omentin-1 was lower in the cases of death than that observed in cases of survival (98.1 [80.3-131.9] vs. 131.8 [99.4-165.3] ng/ml; Z=2.829, P<0.001). As shown in the Figure 3, the patients were divided into two groups according to the median omentin-1 serum level (high vs. low). Kaplan-Meier analysis suggested that the patients with low serum omentin-1 levels had a higher risk of death than those patients with high levels of omentin-1 (log-rank test P=0.033).
Figure 3.
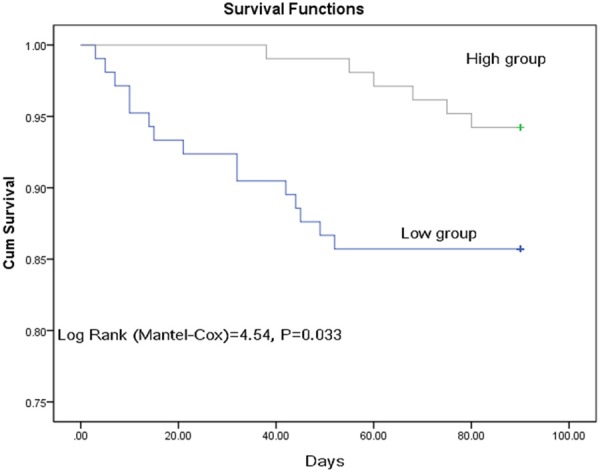
Kaplan-Meier analysis. The patients with low omentin-1 serum levels had a higher risk of death compared to those with high serum omentin-1 levels (log-rank test P=0.033). High omentin-1 serum level was defined as ≥129.0 ng/ml (median).
Discussion
Omentin-1 is a 38 kDa novel adipokine identified in 2004 from visceral adipose tissue [30]. A previous study suggested an elevated omentin-1-related diabetes risk among participants with high adiponectin concentrations [31]. Here we found for the first time, to our knowledge, data on the association between omentin-1 and functional prognosis in nondiabetic patients with ischemic stroke. This study reported that low serum level of omentin-1 might be an independent predictor for the poor stroke outcome. Thus, the prognostic value of omentin-1 in stroke patients was not just caused by the insulin resistance and abnormal glucose metabolism. In addition, the prognosis prediction accuracy of NIHSS score could be significantly improved by the omentin-1. Consistent with our finding, low serum omentin-1 levels were linked with a poor cardiac outcome in heart failure patients [32]. Similarly, another study showed that omentin-1 might represent a biomarker for predicting poor stroke functional outcome [21]. It should be noted that patients with diabetes were not excluded in above two studies.
Omentin-1 had been suggested to play a beneficial role in preventing atherosclerosis [14,32]. Some data indicated that low levels of omentin were closely linked with the presence of CAD and its severity [33,34]. Similarly, in this study, we also confirmed that low levels of omentin-1 were related to stroke severity (defined by NIHSS and infarct size). Another study reported that the association between the adipokine omentin-1 and risk of heart failure may differ according to pre-existing coronary heart disease (CHD) [35]. Xu et al. [36] showed that Omentin-1 might represent a biomarker for predicting carotid plaque instability of acute ischemic stroke patients. Similarly, one study suggested that omentin-1 may be an alternative marker for adequate coronary collateral circulation in patients with ≥90% coronary occlusion [37]. A previous study found that irisin and omentin-1 were inversely associated with each other [38]. Tu et al., illuminated that irisin was an independent prognostic marker improving currently used risk stratification of stroke patients [39].
Adipokines can contribute to the crosstalk between adipose tissue and brain. Adipokines markers have been proposed as potential prognostic biomarkers of cardiovascular mortality/morbidity in patients with cardiometabolic diseases [40]. Wolk et al. found that adipokines were independent predictors of composite outcome events in patients with type 2 diabetes and coronary artery disease [41]. Determining blood levels of leptin and adiponectin, mediating proatherogenic and antiatherogenic responses, may act as a novel prognostic biomarker for inflammation and atherosclerosis in stroke [42]. A study reported that visfatin level was a predictor of cardiovascular mortality and morbidity in stroke [43], while another study suggested that the serum levels of chemerin might be related with atherosclerotic process and/or oxidative stress associated with brain damage occurring after stroke [44]. Furthermore, an elevated baseline level of monocyte chemotactic protein-1 (MCP-1) had been showed associated with an increased risk for death or myocardial infarction [45].
The mechanism of omentin-1’s roles on stroke outcome need further demonstration. First, omentin-1 improved vasodilation by through endothelium-derived nitric oxide [46]. In addition, omentin-1 could increase the phosphorylation of adenosine 5-monophosphate (AMP)-activated protein kinase to promote function and enhance differentiation of endothelial cells [47]. Second, omentin has been shown to increase insulin sensitivity and enhance insulin-mediated Akt-phosphorylation and glucose uptake in adipocytes [30]. Third, some data suggested that decreased omentin expression was implicated in a variety of chronic inflammatory diseases [16]. In this study, we also found a negative relationship between omentin-1 and IL-6. Similarly, Pan et al. found that expression and production of omentin-1 were decreased with elevated inflammatory adipokines (including tumor necrosis factor-alpha and IL-6), in patients with newly diagnosed type 2 diabetes mellitus [48]. Omentin-1 play role in anti-inflammatory action by its ability to reduce the induction of migration, angiogenesis, and activation of AMPK signaling pathway [49], nuclear factor kappa B (NF-kB) and p38 by proinflammatory factors in endothelial cells and smooth muscle cells [50]. Lastly, circulating omentin concentration could be a useful marker of endothelial function [51]. Omentin induces endothelium-dependent relaxation via endothelium-derived NO through phosphorylation of eNOS in rat isolated aorta [18]. Dysfunction of the endothelial lining of lesion-prone areas of the arterial vasculature can lead to the pathobiology of atherosclerotic cardiovascular disease [52]. As a whole, based on above reports, low omentin-1 might be involved in the development of poor functional outcomes through vasodilation, dysfunction of endothelial, insulin resistance and abnormal glucose metabolism and inflammatory action.
In this study, in order to eliminate the influence of diabetes mellitus on results, patients with diabetes were excluded. Furthermore, a variety of statistical methods had been used to assess the prognostic value of omentin-1. In addition to those advantages, some shortcomings also need acknowledge. First, the sample size was relatively small (N=209) and it was a single center study. Second, there were no data for other adipocytokines, such as vaspin, apelin, visfatin and ghrelin, RBP4 and irisin. The association between serum omentin-1 and other adipocytokines should be clarified. Hence, the possibility that these adipokines confounded the true association between omentin-1 and ischemic stroke functional outcome cannot be completely eliminated. Third, omentin-1 levels in our study were determined with a single measurement at baseline. However, a previous work demonstrated that serum omentin-1 levels are stable over time [2]. In addition, only the blood samples had been collected. Interestingly, a previous study found that circulating and epicardial adipose tissue (EAT)-derived omentin-1 levels were reduced in patients with CAD [20]. Lastly, any causal relationship could not be proved due to the cross-sectional study design.
Conclusions
To put it simple, in this cohort of nondiabetic patients with ischemic stroke, a reduced baseline level of serum omentin-1 was related both with stroke severity as well as an increased risk for poor functional outcome or death, independent of baseline variables. Because it plays an important role at multiple stages of stroke progression, omentin-1 is magnetic as a prognostic biomarker and be worthy of further research as a possible therapeutic target.
Acknowledgements
We are grateful to the staff in the Emergency Department of Jiangsu University Affiliated Hospital for their support with patient recruitment. We also grateful to the patients who were included this study. This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); the 2016 “333 Project” Award of Jiangsu Province, the 2013 “Qinglan Project” of the Young and Middle-aged Academic Leader of Jiangsu College and University, the National Natural Science Foundation of China (81571055, 81400902, 81271225, 31201039, 81171012, and 30950031), the Major Fundamental Research Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (13KJA180001), and grants from the Cultivate National Science Fund for Distinguished Young Scholars of Jiangsu Normal University. The funding plays no role in the design and concept of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Written informed consents were obtained from all patients.
Disclosure of conflict of interest
None.
References
- 1.Guan T, Ma J, Li M, Xue T, Lan Z, Guo J, Shen Y, Chao B, Tian G, Zhang Q, Wang L, Liu Y. Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology. 2017;89:53–61. doi: 10.1212/WNL.0000000000004056. [DOI] [PubMed] [Google Scholar]
- 2.Tu WJ, Zhao SJ, Xu DJ, Chen H. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin Sci. 2014;126:339–346. doi: 10.1042/CS20130284. [DOI] [PubMed] [Google Scholar]
- 3.Tu WJ, Ma GZ, Ni Y, Hu XS, Luo DZ, Zeng XW, Liu Q, Xu T, Yu L, Wu B. Copeptin and NT-proBNP for prediction of all-cause and cardiovascular death in ischemic stroke. Neurology. 2017;88:1899–1905. doi: 10.1212/WNL.0000000000003937. [DOI] [PubMed] [Google Scholar]
- 4.Bouziana S, Tziomalos K, Goulas A, Hatzitolios AI. The role of adipokines in ischemic stroke risk stratification. Int J Stroke. 2016;11:389–398. doi: 10.1177/1747493016632249. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Guo W, Li J, Cao S, Zhang J, Pan J, Wang Z, Wen P, Shi X, Zhang S. Leptin concentration and risk of coronary heart disease and stroke: a systematic review and meta-analysis. PLoS One. 2017;12:e0166360. doi: 10.1371/journal.pone.0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, Daskalopoulou SS. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: a systematic review and meta-analyses. Metabolism. 2017;69:51–66. doi: 10.1016/j.metabol.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Che Y. Retinol-binding protein 4 predicts lesion volume (determined by MRI) and severity of acute ischemic stroke. Neurotox Res. 2019;35:92–99. doi: 10.1007/s12640-018-9933-z. [DOI] [PubMed] [Google Scholar]
- 8.Bustamante A, Sobrino T, Giralt D, García-Berrocoso T, Llombart V, Ugarriza I, Espadaler M, Rodríguez N, Sudlow C, Castellanos M, Smith CJ, Rodríguez-Yánez M, Waje-Andreassen U, Tanne D, Oto J, Barber M, Worthmann H, Wartenberg KE, Becker KJ, Chakraborty B, Oh SH, Whiteley WN, Castillo J, Montaner J. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. J Neuroimmunol. 2014;274:215–224. doi: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Yue J, Chen J, Wu Q, Liu X, Li M, Li Z, Gao Y. Serum levels of omentin-1 association with early diagnosis, lesion volume and severity of acute ischemic stroke. Cytokine. 2018;111:518–522. doi: 10.1016/j.cyto.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 10.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 11.Shibata R, Ouchi N, Takahashi R, Terakura Y, Ohashi K, Ikeda N, Higuchi A, Terasaki H, Kihara S, Murohara T. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012;4:37. doi: 10.1186/1758-5996-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Randeva HS. Omentin-1, a novel adipokine, is decreased in overweight insulin resistant women with the polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–8. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 14.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol. 2011;10:103. doi: 10.1186/1475-2840-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21–25. doi: 10.1016/j.diabres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhong X, Zhang H, Tan H, Zhou Y, Liu FL, Chen FQ, Shang DY. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–8. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greulich S, Chen WJ, Maxhera B, Rijzewijk LJ, van der Meer RW, Jonker JT, Mueller H, de Wiza DH, Floerke RR, Smiris K, Lamb HJ, de Roos A, Bax JJ, Romijn JA, Smit JW, Akhyari P, Lichtenberg A, Eckel J, Diamant M, Ouwens DM. Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS One. 2013;8:e59697. doi: 10.1371/journal.pone.0059697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Kadoglou NP, Lambadiari V, Gastounioti A, Gkekas C, Giannakopoulos TG, Koulia K, Maratou E, Alepaki M, Kakisis J, Karakitsos P, Nikita KS, Dimitriadis G, Liapis CD. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis. 2014;235:606–612. doi: 10.1016/j.atherosclerosis.2014.05.957. [DOI] [PubMed] [Google Scholar]
- 20.Menzel J, di Giuseppe R, Biemann R, Wittenbecher C, Aleksandrova K, Pischon T, Fritsche A, Schulze MB, Boeing H, Isermann B, Weikert C. Omentin-1 and risk of myocardial infarction and stroke: results from the EPIC-Potsdam cohort study. Atherosclerosis. 2016;251:415–421. doi: 10.1016/j.atherosclerosis.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Xu T, Zuo P, Wang Y, Gao Z, Ke K. Serum omentin-1 is a novel biomarker for predicting the functional outcome of acute ischemic stroke patients. Clin Chem Lab Med. 2018;56:350–355. doi: 10.1515/cclm-2017-0282. [DOI] [PubMed] [Google Scholar]
- 22.Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D’Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: the Framingham offspring study. Arch Intern Med. 2006;166:106–111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- 23.Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, Kamouchi M Fukuoka Stroke Registry Investigators. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. 2018;90:e1470–e1477. doi: 10.1212/WNL.0000000000005358. [DOI] [PubMed] [Google Scholar]
- 24.Gürsoy G, Kırnap NG, Eşbah O, et al. The relationship between plasma omentin-1 levels and insulin resistance in newly diagnosed type 2 diabetıc women. Clinical Reviews and Opinions. 2010;2:49–54. [Google Scholar]
- 25.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Bonita R, Beaglehole R. Modification of rankin scale: recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 28.Tu WJ, Zeng XW, Deng A, Zhao SJ, Luo DZ, Ma GZ, Wang H, Liu Q. Circulating FABP4 (fatty acid-binding protein 4) is a novel prognostic biomarker in patients with acute ischemic stroke. Stroke. 2017;48:1531–1538. doi: 10.1161/STROKEAHA.117.017128. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wittenbecher C, Menzel J, Carstensen-Kirberg M, Biemann R, di Giuseppe R, Fritsche A, Isermann B, Herder C, Aleksandrova K, Boeing H, Weikert C, Schulze MB. Omentin-1, adiponectin, and the risk of developing type 2 diabetes. Diabetes Care. 2016;39:e79–e80. doi: 10.2337/dc15-2702. [DOI] [PubMed] [Google Scholar]
- 32.Narumi T, Watanabe T, Kadowaki S, Kinoshita D, Yokoyama M, Honda Y, Otaki Y, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc Diabetol. 2014;13:84. doi: 10.1186/1475-2840-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata R, Ouchi N, Kikuchi R, Takahashi R, Takeshita K, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–814. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism. 2014;63:1265–1271. doi: 10.1016/j.metabol.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menzel J, Di Giuseppe R, Biemann R, Wittenbecher C, Aleksandrova K, Eichelmann F, Fritsche A, Schulze MB, Boeing H, Isermann B, Weikert C. Association between chemerin, omentin-1 and risk of heart failure in the population-based EPIC-Potsdam study. Sci Rep. 2017;7:14171. doi: 10.1038/s41598-017-14518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T, Zuo P, Cao L, Gao Z, Ke K. Omentin-1 is associated with carotid plaque instability among ischemic stroke patients. J Atheroscler Thromb. 2018;25:505–511. doi: 10.5551/jat.42135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou JP, Tong XY, Zhu LP, Luo JM, Luo Y, Bai YP, Li CC, Zhang GG. Plasma omentin-1 level as a predictor of good coronary collateral circulation. J Atheroscler Thromb. 2017;24:940–948. doi: 10.5551/jat.37440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang FJ, Wang JP, Liu XT, Zheng QS, Xue YS, Wang B, Zhao LY. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers. 2011;16:657–662. doi: 10.3109/1354750X.2011.622789. [DOI] [PubMed] [Google Scholar]
- 39.Tu WJ, Qiu HC, Cao JL, Liu Q, Zeng XW, Zhao JZ. Decreased concentration of irisin is associated with poor functional outcome in ischemic stroke. Neurotherapeutics. 2018;15:1158–1167. doi: 10.1007/s13311-018-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opatrilova R, Caprnda M, Kubatka P, Valentova V, Uramova S, Nosal V, Gaspar L, Zachar L, Mozos I, Petrovic D, Dragasek J, Filipova S, Büsselberg D, Zulli A, Rodrigo L, Kruzliak P, Krasnik V. Adipokines in neurovascular diseases. Biomed Pharmacother. 2018;98:424–432. doi: 10.1016/j.biopha.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 41.Wolk R, Bertolet M, Singh P, Brooks MM, Pratley RE, Frye RL, Mooradian AD, Rutter MK, Calvin AD, Chaitman BR, Somers VK BARI 2D Study Group. Prognostic value of adipokines in predicting cardiovascular outcome: explaining the obesity paradox. Mayo Clin Proc. 2016;91:858–866. doi: 10.1016/j.mayocp.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gairolla J, Kler R, Modi M, Khurana D. Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke. Rev Neurosci. 2017;28:295–306. doi: 10.1515/revneuro-2016-0055. [DOI] [PubMed] [Google Scholar]
- 43.Kadoglou NP, Fotiadis G, Lambadiari V, Maratou E, Dimitriadis G, Liapis CD. Serum levels of novel adipokines in patients with acute ischemic stroke: potential contribution to diagnosis and prognosis. Peptides. 2014;57:12–16. doi: 10.1016/j.peptides.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Hasırcı B, Koçer A, Toprak A, et al. The relationship between prognosis and serum chemerin levels in acute ischemic stroke patients. J Neurol Sci. 2015;357:e387. [Google Scholar]
- 45.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 46.Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 47.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-alpha-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686:116–123. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity. 2011;19:1552–1559. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]
- 52.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]


