Abstract
Aging is tightly associated with various diseases, such as cardiovascular diseases; however, there is no effective biomarker to detect and evaluate the aging process in vivo. Therefore, it is critical to identify new aging biomarkers for earlier diagnose of aging-related diseases. This study investigated the profile of cytokines in serum samples of young and aged mice with the purpose of exploring new biomarkers that have remarkable alterations in aging. A solid-phase antibody array was used to screen 200 proteins in the mouse serum, among which 32 cytokines differentially expressed between young and aged mice were screened. The major proteins were secreted frizzled-related protein 3 (sFRP3), Fractalkine, IGFBP-5, IGFBP-6, etc. We select secreted frizzled-related protein 3 (sFRP3) in follow-up study. Then, enzyme-linked immunosorbent assay (ELISA) was used to detect the expression levels of sFRP3. Our results revealed that the expression levels of sFRP3 in serum samples from aged mice were significantly higher than those in samples from young mice. ELISA data were identical to those obtained by the antibody array. Our findings indicated that sFRP3 has remarkable significance in senescence. Furthermore, we detected sFRP3 level in culture supernatants of primary endothelial cells, and the variation trend of sFRP3 levels in culture supernatant was consistent with serum data. We also detected serum sFRP3 amounts in healthy young and elderly individuals. Interestingly, serum sFRP3 amounts in the elderly were significantly increased compared with those of young individuals.
Keywords: sFRP3, aging, antibody array, serum biomarker, ELISA
Introduction
Aging is a complex life process that affects several aspects of human biology, and endothelial cell senescence is an important aspect thereof. A decline in aging-related immune function may explain why the elderly are more susceptible to cardiovascular disease, cancer and infectious diseases [1,2]. Aging is associated with different types of heart diseases, and Wnt signaling may be involved in the development of heart disease associated with aging. Recent studies have shown a link between Wnt signaling and premature aging or aging-related phenotypes [3]. Furthermore, premature senescence of endothelial cells is a major cause of various cardiovascular diseases [4]. The incidence rates of cardiovascular diseases and tumors annually increase worldwide [5]. Therefore, in-depth identification and studying aging-related proteins is significant for the diagnosis and treatment of senile diseases.
The Wnt signaling pathway involves Wnt proteins, Wnt receptors and Wnt regulatory proteins. It regulates the proliferation and differentiation of cells, and plays a crucial role in the development and functions of various tissues [6]. Some reports demonstrated that Wnt signaling also regulates aging in a variety of tissues. Wnt proteins typically bind to the Frizzled (Fzd) receptor located in the plasma membrane, and induce a variety of intracellular responses [7,8]. Wnt proteins can activate intracellular Wnt/β-catenin signaling to participate in the senescence of various tissues and organs, Wnt signaling in bone, kidney, intestine and adipose tissue and interorgan interaction in aging [9]. Secreted frizzled-related protein 3 (sFRP3) is a member of the sFRP family that inhibits Wnt signaling by binding directly to Wnt proteins via their regions of homology to the Wnt-binding domain of Fzd receptor [10]. The sFRP3 protein acts as a regulator of the Wnt signaling pathway, and has been observed in various human tissues, especially in the placenta and heart. sFRP3 plays an important role in embryonic development and carcinogenesis [11]. However, the sFRP3 protein has not been reported in available aging-related studies.
In this work, protein microarray technology was used to assess differential proteins in mouse aging. A solid-phase antibody array was used to screen 200 proteins in the mouse serum, among which 32 differentially expressed proteins were identified between young and elderly mice. We investigated the profiles of cytokines in serum of young and aged mice. Then, sFRP3 expression was detected by enzyme-linked immunosorbent assay (ELISA) in young and elderly mice. We also detected the variation of sFRP3 in culture supernatants of primary endothelial cells in mice. In addition, we detected the expression levels of sFRP3 in serum from healthy young and the elderly individual.
sFRP3 may be a specific protein marker of aging, participating in the aging process of endothelial cells. These findings provide new targets for the pathogenesis and treatment of aging-related diseases.
Materials and methods
Mice
Male C57BL/6 mice were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, Jiangsu province, China) and housed in specific pathogen-free conditions at Central Animal Laboratory of Anhui Provincial Hospital (Hefei, Anhui province, China). Young mice were purchased at the age of 8 weeks and used for experiments within 1 week after arrival. Elderly mice were purchased at the age of 10 months, and housed for another 8 months before the experiments. During the feeding period, body weights were recorded, closely monitoring the animals to ensure healthy growth (Table 1). The animal protocols were approved by the Biomedical Ethics Committee of Anhui Provincial Hospital.
Table 1.
Male mice growth data
| Group | N | Age (month) | Weight (g) |
|---|---|---|---|
| Young Mice | 19 | 2 | 22.1±0.93 |
| Aged Mice | 19 | 18 | 35.7±1.05 |
Human subjects and blood samples
Healthy young and elderly subjects were recruited for this study. Clinical data regarding all subjects were in Table 2. We collected the subject’s peripheral blood (PB) (all subjects, in the fasted state, in the morning for a physical exam). All serum was obtained after centrifugation and stored at -80°C until further use. This study conformed to protocols approved by the institutional review boards of Anhui Provincial Hospital.
Table 2.
Healthy young and elderly group characteristics
| Group | Young | Elderly |
|---|---|---|
| Age (year) | 28.17±3.89 | 70.87±7.22 |
| Weight (Kg) | 60.83±8.81 | 62.93±6.73 |
| BFS (mmol/L) | 4.60±0.33 | 4.91±0.45 |
| TC (mmol/L) | 4.09±0.57 | 4.43±0.48 |
| TG (mmol/L) | 0.87±0.30 | 1.01±0.26 |
| LDL-C (mmol/L) | 2.14±0.48 | 2.40±0.53 |
| Gender (male/female) | 15/15 | 17/13 |
Values are expressed as mean ± SD or number. BFS: Blood-fasting sugar; TC: Total cholesterol; TG: Total triglyceride; LDL-C: Low density lipoprotein cholesterol.
Antibody array processing
A combination of 5 non-overlapping arrays for quantitative measurement of 200 Mouse cytokines (QAM-CAA-4000, Raybiotech, Norcross GA, USA) was used to detect protein in serum samples from eight groups of mice. The array consisted of eight glass slides imprinted with 200 antibodies. Briefly, serum was incubated in these assay pools for 2 h. After washing, the array glass slides were incubated with Biotinylated Antibody Cocktail for another 1 h. The samples were washed again and incubated with Cy3 Equivalent Dye-Streptavidin for 1 h at room temperature. The slides were scanned with InnoScan 300 Microarray Scanner (Innopsys Parc d’Activités Activestre; 31 390 Carbonne-France), and the signal extraction was performed with the InnoScan 300 Microarray software. The extracted data were analyzed with the GSM-CAA-4000 data analysis software.
Detection of cytokine levels in serum and primary endothelial cells by ELISA
Taking the aorta of mice, fully exposed endothelial cells were exfoliated in sterile phosphate buffered saline (PBS) under a phase-contrast microscope. The obtained primary endothelial cells were cultured, after they grew to confluency, primary culture supernatants was subject to were assessed by detection with ELISA. Based on the results of antibody arrays, the most relevant cytokines were selected, sample size was expanded, and cytokine levels in young and aged mice were assessed by ELISA (Raybiotech, Norcross GA, USA), according to the manufacturer’s instructions. Serum dilution factors were specific to the individual serum biomarkers. After dilution, samples were incubated in plates coated with capture antibodies for 2.5 h at room temperature. The plates were then washed and successively incubated with a biotinylated antibody for 1 h and streptavidin-horseradish peroxidase (HRP) solution for 45 min at room temperature. After adding 3, 3’, 5, 5’-tetramethylbenzidine (TMB) substrate, the reaction was stopped by addition of 50 μL sulfuric acid, and optical density (OD=450) was recorded ona Multiskan MK3 microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Serum samples from healthy young people and the elderly are also tested in the same way.
Statistical analysis
Protein microarray data were statistically analyzed with the “R” programming language. After raw data were normalized by the software, differential proteins were screened by fold-change and P-value. Data are mean ± standard deviation (SD). Values for normal mouse serum and endothelial cells were validated for use in ELISA, and statistically analyzed by the Mann-Whitney U test. All statistical analyses were performed with the SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Differences between groups were determined by the Mann-Whitney U test, and were considered statistically significant at P<0.05.
Results
Cytokine data analysis
After analysis with the Raybiotech analysis software, the values of mean flourscence indensity (MFI) were statistically analyzed. Thirty-two cytokines were differentially expressed between young and aged mice. The levels of Fractalkine, IGFBP5, IL-17E, I-TAC, MDC, IL-15, IL-21, Leptin, MIG, MIP-1a, GITR L, Lymphotactin, Osteoactivin, OX40 Ligand, PIGF-2, ANG-3, CCL28, Epigen, Galectin-7, Gremlin, IFNg R1, MIP-16, Persephin, sFRP3, Shh-N, SLAM, TECK, TGFb1 and TWEAK were increased, while IGFBP-6, CD6 and DLL4 amounts were decreased in serum samples from aged mice compared with values for young animals. The mean values of these thirty-two biomarkers and fold-changes in serum samples from young and aged mice showed clear differences (Table 3). Among these differentially expressed proteins, we found that the difference in fluorescence signal intensity of sFRP3 in aged mice and young mice reached 3.5 fold (young mice =2983.914089, aged mice =10410.41235), and the Fold-change reached 1.8 fold. The findings revealed that the expression of the screened proteins were significantly different between young and aged mice (Figure 1).
Table 3.
Protein microarray data of differential cytokines in serum samples of young and aged mice
| Cytokines | Young (n=4) | Aged (n=4) | Fold-change | P-value |
|---|---|---|---|---|
| Fractalkine | 374.22±280.02 | 927.14±303.20 | 1.30891259 | 0.036789943 |
| IGFBP-5 | 3770.46±933.67 | 7525.70±1712.77 | 0.997084988 | 0.013845086 |
| IGFBP-6 | 51424.19±13712.88 | 23607.81±14511.04 | -1.123182752 | 0.031831847 |
| IL-17E | 196.77±142.51 | 524.08±43.83 | 1.413255694 | 0.015197574 |
| I-TAC | 252.24±187.10 | 911.67±419.45 | 1.853697356 | 0.043482921 |
| MDC | 54266.88±11681.25 | 92906.56±15581.87 | 0.775708524 | 0.008600027 |
| IL-15 | 882.86±226.03 | 1725.10±301.56 | 0.966422561 | 0.005095772 |
| IL-21 | 918.25±297.93 | 1432.93±133.05 | 0.642019988 | 0.032639773 |
| Leptin | 713.21±155.99 | 1491.44±347.22 | 1.06431491 | 0.013813349 |
| MIG | 818.21±173.53 | 1229.16±145.51 | 0.58711845 | 0.011561618 |
| MIP-1a | 725.58±49.16 | 1439.61±253.84 | 0.988474281 | 0.009637616 |
| GITR L | 405.28±77.57 | 965.72±340.77 | 1.252692934 | 0.042730809 |
| Lymphotactin | 293.35±105.07 | 740.02±229.24 | 1.334946457 | 0.022030626 |
| CD6 | 4055.37±955.71 | 1599.53±788.84 | -1.342186224 | 0.007969166 |
| DLL4 | 9408.85±2784.62 | 4209.44±1345.84 | -1.160388175 | 0.025022401 |
| Osteoactivin | 614.20±242.38 | 1947.85±694.65 | 1.665102441 | 0.02518712 |
| OX40 Ligand | 929.52±642.38 | 2273.15±631.55 | 1.29014186 | 0.024547193 |
| PlGF-2 | 390.71±402.33 | 1754.32±483.98 | 2.166756368 | 0.005300427 |
| ANG-3 | 1938.68±348.27 | 3833.80±1175.91 | 0.983699131 | 0.043347546 |
| CCL28 | 2287.08±569.18 | 4046.59±1117.35 | 0.823200158 | 0.042875092 |
| Epigen | 4499.02±818.32 | 7284.07±1485.46 | 0.695134832 | 0.024184841 |
| Galectin-7 | 3781.47±271.12 | 5723.31±851.63 | 0.597901321 | 0.015325596 |
| Gremlin | 996.30±172.44 | 3158.25±1096.32 | 1.664468628 | 0.027487912 |
| IFNg R1 | 5846.41±766.84 | 9667.36±2284.79 | 0.725570281 | 0.038186943 |
| MIP-1b | 1650.89±262.54 | 3722.14±748.91 | 1.172882197 | 0.007793881 |
| Persephin | 3406.18±477.69 | 6336.31±1699.62 | 0.895487101 | 0.036339497 |
| sFRP-3 | 2983.91±661.45 | 10410.41±2760.77 | 1.802749335 | 0.010307478 |
| Shh-N | 4212.82±683.68 | 6998.47±1447.66 | 0.732254669 | 0.022772598 |
| SLAM | 3678.37±529.28 | 6831.67±1439.58 | 0.893172087 | 0.016360262 |
| TECK | 740.79±118.78 | 1753.84±397.18 | 1.243386449 | 0.011079997 |
| TGFb1 | 17821.92±2221.25 | 29742.05±6181.41 | 0.738851146 | 0.024621678 |
| TWEAK | 5547.25±796.23 | 10870.80±2261.85 | 0.970614612 | 0.013238362 |
The vaules of mean flourscence indensity (MFI) are represented by mean ± SD.
Figure 1.
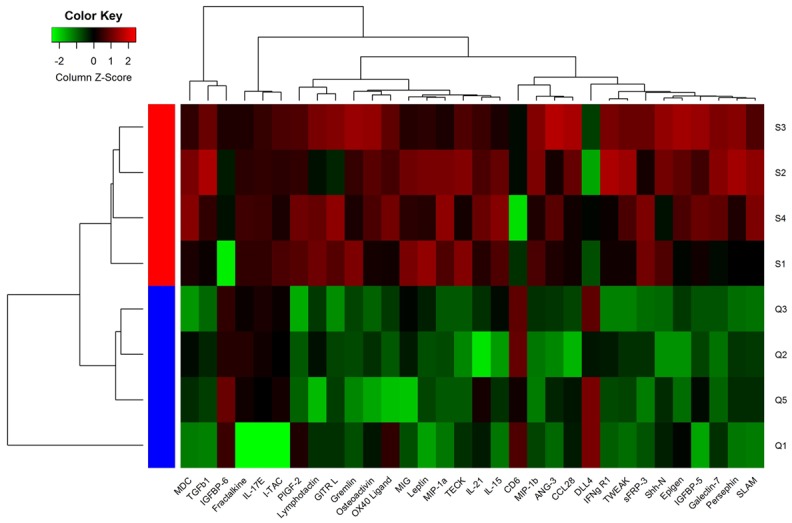
Unsupervised-hierarchical cluster analysis of differentially expressed cytokines. Array data of the 32 differentially expressed biomarkers were used for unsupervised-hierarchical cluster analysis by the Cluster 3.0 software. The results showed that young and aged mice were discriminated accurately. Green, black and red represent low, median and high serum protein levels, respectively.
sFRP-3 expression
The fluorescent signals of sFRP3 were captured, and their intensities are proportional to protein amounts. Each antibody was assessed in duplicate. The locations of sFRP3 were noted in colored boxes (Figure 2A). The levels of sFRP3 were significantly higher in serum samples from aged mice than in those from young animals (P=0.0103) (Table 2; Figure 2B).
Figure 2.
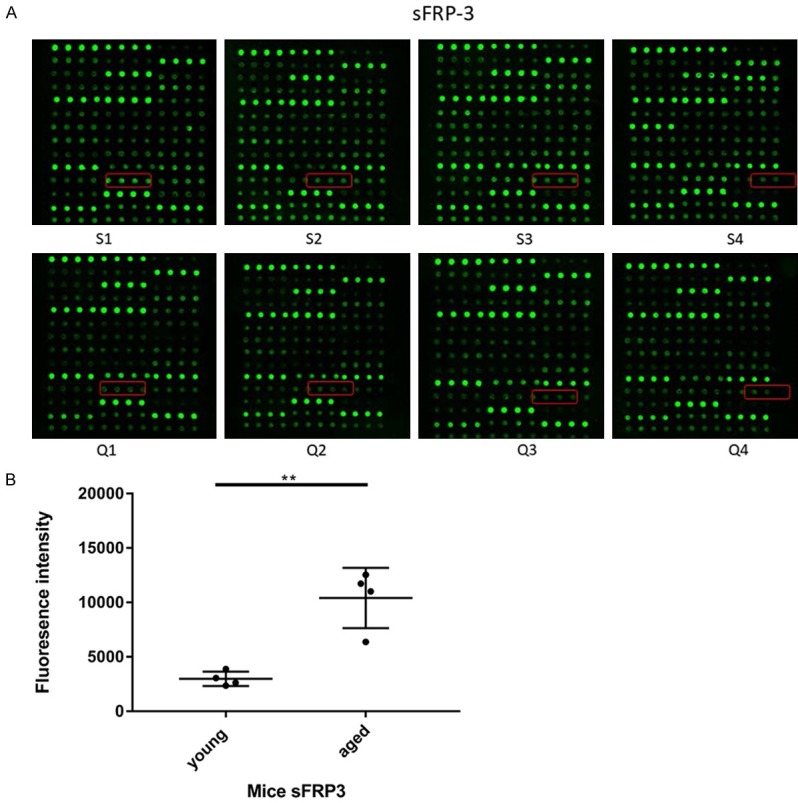
(A) Assay of sFRP3 by cytokine array. The fluorescent signals of the sFRP3 were acquired and their signal intensities were proportional to protein amounts. Each antibody was assessed in duplicate. The locations of sFRP3 were noted in colored boxes. Q1-Q4, young mice; S1-S4, aged mice. (B) Scatter diagram of the sFRP3 from antibody array data. After performing statistical analysis by using Mann-Whitney U test, the data of serum sFRP3 expressed differentially between the young and aged samples are shown by Scatter diagram (P<0.05). The centerline in the Scatter diagram indicates the median in each group.
Verification of ELISA data
After cytokine microarray analysis, ELISA was conducted to validate microarray date. Validation of sFRP3 identified by antibody array was performed with additional samples by ELISA. The expression levels of the sFRP3 was significantly higher in serum samples from aged mice compared with those from young mice (P<0.0001, Figure 3A), confirming the reliability of protein microarray results. Besides, ELISA detected sFRP3 levels in culture supernatants of primary mouse endothelial cells, and sFRP3 levels in primary endothelial cells from aged mice were also remarkably higher than those of cells from young mice (P<0.05, Figure 3B). Besides, the level of sFRP3 expression in the serum of the elderly is significantly different from that of young people (P<0.0001, Figure 4).
Figure 3.
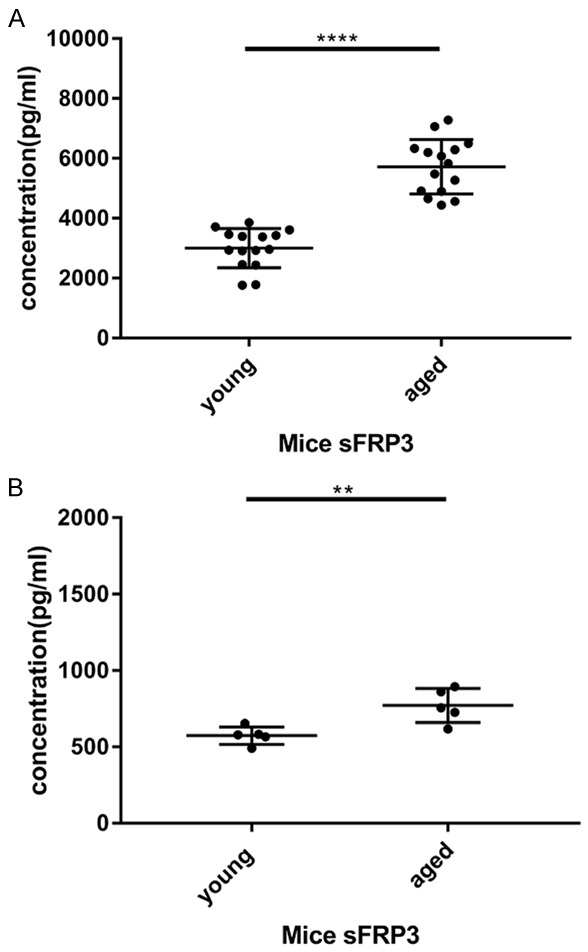
A. The sFRP3 level in serum samples of young and aged mice was detected by ELISA assay. The data are shown in scatter diagram with mean ± standard deviation (SD). ****P<0.0001 vs. aged mice. B. The sFRP3 level in the primary endothelial cells of young and aged mice was detected by ELISA assay. The data are illustrated in a scatter diagram with mean ± standard deviation (SD). **P<0.01 vs. aged mice.
Figure 4.
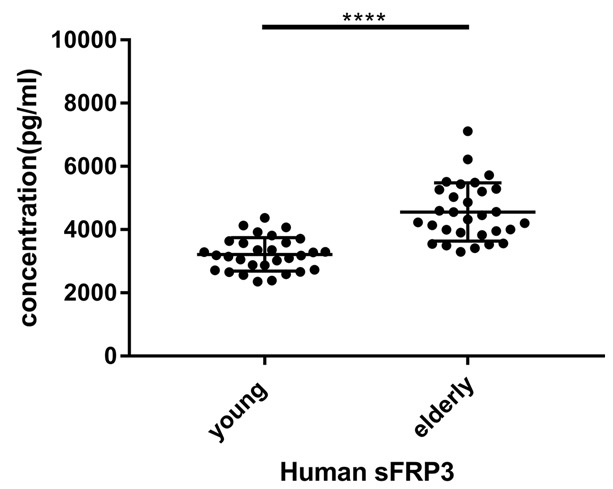
The sFRP3 level in serum samples of healthy young and elderly people was detected by ELISA assay. The data are shown in a scatter diagram with mean ± standard deviation (SD). ****P<0.0001 vs. elderly people.
Discussion
Although there are a number of studies assessing changes of selected cytokines during aging through body fluid [12], in the present study, we firstly presented a high-throughput solid protein array approach with sufficient clinical specificity and sensitivity to identify novel serum biomarkers of aging. The protein chip technology has been rapidly developed in recent years as an advanced new tool [13-15]. Its basic principle is to make various proteins orderly fixed on all kinds of medium carrier. Tagged antibodies that are specified will be matched by chromatin immunoprecipitation (ChIP). Then, the fluorescent-antibody can be matched with the relevant protein. Meanwhile, the corresponding signal indicates the expression levels of the protein. All the other antibodies, which are not complementary, would be washed out, and samples are assessed with a fluorescent scanner or by laser scanning technology. The fluorescence intensity of each point on the ChIP and interaction among proteins was analyzed, yielding the expression levels of related proteins.
In this study, the primary screen deployed a novel cytokine antibody array that can simultaneously detect 200 cytokines. Furthermore, 32 cytokines differentially expressed between the young and aged mice were screened. The unsupervised-hierarchical cluster analysis accurately differentiated young and aged mouse samples using the microarray data.
Most of the 32 selected cytokines belong to the chemokine family, including Fractalkine, I-TAC, MDC, MIG, MIP-1a, Lymphotactin, CCL28, MIP-1b, TECK. Growth factors include IGFBP-5, IGFBP-6, PlGF-2, ANG-3, DLL4, Epigen. Tumor necrosis factor family include GITR L, OX40 Ligand, TGFb1. Some other cytokines such as IL17E is a pro-inflammatory factor. Leptin is a protein hormone secreted by adipose tissue. Osteoactivin is a highly glycosylated secreted glycoprotein. Galectin-7 is a member of the animal lectin family and plays an important role in tumor transformation and apoptosis. Gremlin is a member of the cysteine knot superfamily and plays an important role in embryonic growth and development. IFNg R1 is an immunoregulatory factor. Persephin and Shh-N belong to Neurotrophic factor. TWEAK is a multifunctional cytokine involved in cell proliferation, migration, differentiation, apoptosis, angiogenesis and inflammation. These genes may participate in some biological processes or cellular components to play an important role in the natural aging of mice through their own unique molecular functions.
We selected sFRP3 with significant differences in protein chip results and conducted follow-up studies. Although the difference in PIGF-2 is greater, its fluorescence signal expression intensity is much lower than that of sFRP3 in protein chip results. In addition, PIGF-2 has been reported in many studies on aging-related diseases, However, sFRP3 has not been reported in aging-related study.
To confirm the results derived from the microarray analysis, ELISA were carried out to detect the expression level of sFRP3. ELISA assay showed that the sFRP3 level in serum sample of aged mice was significantly higher than that of young mice, which was identical compared to those obtained from the antibody array. We also detected the levels of sFRP3 from culture supernatant of the primary endothelial cells of mice. Similarly, the levels of sFRP3 in the serum of healthy young people are higher than that in elderly. These results suggested that protein array is a potent tool in biomedical discovery. sFRP3 may play important roles in specific patho-physiological processes of aging, particularly, in endothelial cell senescence, which may become potential biomarkers in serum.
sFRPs are a family of soluble proteins known for their ability to inhibit signaling pathway by binding to Wnt ligands and/or Fz receptors [16-18]. sFRPs were the first Wnt antagonists identified and are structurally associated with Fz proteins. The N-terminal of these proteins (about 300 amino acids in length) possess an Fz-like cysteine-rich domain (CRD), displaying similar sequence homology to the CRD on the extracellular portion of the Fz receptors. However, unlike Fz receptors, sFRPs do not possess transmembrane or cytosolic domains. Upstream the CRD, there is a signal peptide. In addition to the CRD region, sFRPs have a hydrophilic region on the C-terminal that appears to confer heparin-binding properties [17-19]. Previous studies demonstrated aberrant expression of sFRPs in different types of cancer [20-22]. Although sFRPs were initially considered tumor suppressors that exert an inhibitory role in Wnt signaling (as they have been found to be downregulated in the majority of tumors), recent findings indicate that sFRPs could also stimulate and activate the Wnt signaling pathway [17,23]. Furthermore, there are numerous reports concerning the overexpression of sFRPs in cancer, therefore, this behavior can no longer be considered as inconsistent, and it should be noted that sFRPs exert a dual-role in Wnt signaling, which has been shown to be deleterious [21-23]. A previous study reported SFRP downregulation in various types of cancer, indicating loss of function [21-24]. Besides, a sFRP family member was shown to strongly promote the growth of intracranial glioma xenografts in nude mice as well as glioma cell growth in vitro [25]. Other studies revealed that sFRP3 is an important morphogen of mouse neurogenesis [26-28]. The sFRP3 protein has also been shown to play a role in the development of plasma cell myeloma, medulloblastoma, malignant melanoma, and gastric carcinoma [29]. The present study revealed that sFRP3 amounts in serum samples from aged mice were higher than those obtained from young subjects, suggesting that sFRP3 may be a novel biomarker of aging.
In summary, this study assessed young and aged mice for serum biomarkers of aging by protein arrays, whose results were confirmed by ELISA. Importantly, sFRP3 levels were firstly found to be higher in serum samples from aged mice compared with those from young subjects. These results suggest that sFRP3 may be a specific biomarker of aging, participating in the aging process of endothelial cells, and provide new targets for the pathogenesis and treatment of aging-related diseases.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81472018).
Disclosure of conflict of interest
None.
References
- 1.Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, Larbi A, Weinberger B, Cossarizza A. Aging of the immune system-focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castaneda-Delgado JE, Frausto-Lujan I, Gonzalez-Curiel I, Montoya-Rosales A, Serrano CJ, Torres-Juarez F, Enciso-Moreno JA, Rivas-Santiago B. Differences in cytokine production during aging and its relationship with antimicrobial peptides production. Immunol Invest. 2017;46:48–58. doi: 10.1080/08820139.2016.1212873. [DOI] [PubMed] [Google Scholar]
- 3.Naito AT, Shiojima I, Komuro I. Wnt signaling and aging-related heart disorders. Circ Res. 2010;107:1295–1303. doi: 10.1161/CIRCRESAHA.110.223776. [DOI] [PubMed] [Google Scholar]
- 4.Luo WW, Wang Y, Yang HW, Dai CM, Hong HL, Li JY, Liu ZP, Guo Z, Chen XY, He P, Li ZQ, Li F, Jiang JM, Liu PQ, Li ZM. Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling. Aging (Albany NY) 2018;10:1722–1744. doi: 10.18632/aging.101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf TU, Cubas RA, Ghneim K, Cartwright MJ, Grevenynghe JV, Richner JM, Olagnier DP, Wilkinson PA, Cameron MJ, Park BS, Hiscott JB, Diamond MS, Wertheimer AM, Nikolich-Zugich J, Haddad EK. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 2015;14:421–432. doi: 10.1111/acel.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimaki S, Wakabayashi T, Takemasa T, Asashima M, Kuwabara T. The regulation of stem cell aging by Wnt signaling. Histol Histopathol. 2015;30:1411–1430. doi: 10.14670/HH-11-657. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Sethi JK, Vidalpuig A. Wnt signalling and the control of cellular metabolism. Biochem J. 2010;427:1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D, Xie R, Shu B, Landay AL, Wei C, Reiser J, Spagnoli A, Torquati A, Forsyth CB, Keshavarzian A, Sumner D. Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann N Y Acad Sci. 2018 doi: 10.1111/nyas.13945. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao XF, Huang H, Chen YD, Liu Y, Zhang ZY, Ma QF, Qiu MS. Dynamic expression of secreted frizzled-related protein 3 (sFRP3) in the developing mouse spinal cord and dorsal root ganglia. Neuroscience. 2013;248:594–601. doi: 10.1016/j.neuroscience.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partl JZ, Fabijanovic D, Skrtic A, Vranic S, Martic TN, Serman L. Immunohistochemical expression of SFRP1 and SFRP3 proteins in normal and malignant reproductive tissues of rats and humans. Appl Immunohistochem Mol Morphol. 2014;22:681–687. doi: 10.1097/PAI.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 12.Mathelin C, Cromer A, Wendling C, Tomasetto C, Rio MC. Serum biomarkers for detection of breast cancers: a prospective study. Breast Cancer Res Treat. 2006;96:83–90. doi: 10.1007/s10549-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 13.Banks R. Protein chip technology. Curr Opin Chem Biol. 2005;7:55–63. doi: 10.1016/s1367-5931(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger SR, Dalmasso EA, Fung ET. Current achievements using ProteinChip® array technology. Curr Opin Chem Biol. 2002;6:86–91. doi: 10.1016/s1367-5931(01)00282-4. [DOI] [PubMed] [Google Scholar]
- 15.Weinrich D, Jonkheijm P, Niemeyer CM, Waldmann H. Applications of protein biochips in biomedical and biotechnological research. Angew Chem Int Ed Engl. 2009;48:7744–7751. doi: 10.1002/anie.200901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang B, Moos M Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- 17.Jablons DM. Roles of secreted frizzled-related proteins in cancer. Acta Pharmacol Sin. 2007;28:1499–1504. doi: 10.1111/j.1745-7254.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopezrios J. Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 19.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 20.Hirata HA, Hinoda YB, Ueno KA, Majid SA, Saini SA, Dahiya R. Role of secreted frizzled-related protein 3 in human renal cell carcinoma. Cancer Res. 2010;70:1896–1905. doi: 10.1158/0008-5472.CAN-09-3549. [DOI] [PubMed] [Google Scholar]
- 21.Surana R, Sikka S, Cai W, Shin EM, Warrier SR, Tan HJ, Arfuso F, Fox SA, Dharmarajan AM, Kumar AP. Secreted frizzled related proteins: implications in cancers. Biochim Biophy Acta. 2014;1845:53–65. doi: 10.1016/j.bbcan.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Schlensog M, Magnus L, Heide T, Eschenbruch J, Steib F, Tator M, Kloten V, Rose M, Noetzel E, Gaisa NT, Knüchel R, Dahl E. Epigenetic loss of putative tumor suppressor SFRP3 correlates with poor prognosis of lung adenocarcinoma patients. Epigenetics. 2018;13:214–227. doi: 10.1080/15592294.2016.1229730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mii Y, Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted frizzled-related proteins. Dev Growth Differ. 2011;53:911–923. doi: 10.1111/j.1440-169X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 24.Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene. 2010;29:3017–3024. doi: 10.1038/onc.2010.32. [DOI] [PubMed] [Google Scholar]
- 25.Lin YW, Shih YL, Lien GS, Suk FM, Hsieh CB, Yan MD. Promoter methylation of SFRP3 is frequent in hepatocellular carcinoma. Dis Markers. 2014;2014:351863. doi: 10.1155/2014/351863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikuševa-Martić T, Šerman L, Zeljko M, Vidas Ž, Gašparov S, Zeljko HM, Kosović M, Pećina-Šlaus N. Expression of secreted frizzled-related protein 1 and 3, T-cell factor 1 and lymphoid enhancer factor 1 in clear cell renal cell carcinoma. Pathol Oncol Res. 2013;19:545–551. doi: 10.1007/s12253-013-9615-3. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen IB, Haaber J, Lyng MB, Knudsen LM, Rasmussen T, Ditzel HJ, Abildgaard N. Myeloma plasma cell expression of osteoblast regulatory genes: overexpression of SFRP3 correlates with clinical bone involvement at diagnosis. Leuk Lymphoma. 2013;54:425–427. doi: 10.3109/10428194.2012.708027. [DOI] [PubMed] [Google Scholar]
- 28.Moskalev EA, Katrin L, Vorobjev IA, Mastitsky SE, Gladkikh AA, Stephan A, Schrenk M, Kaplanov KD, Kalashnikova OB, Pötz O, Joos TO, Hoheisel JD. Concurrent epigenetic silencing of wnt/β-catenin pathway inhibitor genes in B cell chronic lymphocytic leukaemia. BMC Cancer. 2012;12:213. doi: 10.1186/1471-2407-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekström EJ, Sherwood V, Andersson T. Methylation and loss of secreted frizzled-related protein 3 enhances melanoma cell migration and invasion. PLoS One. 2011;6:e18674. doi: 10.1371/journal.pone.0018674. [DOI] [PMC free article] [PubMed] [Google Scholar]


