Abstract
This study examined the relationship between the expression of Ras guanyl nucleotide-releasing protein 3 (RasGRP3) and disease activity in systemic lupus erythematosus (SLE) and explored the possible mechanisms in MRL/lpr mice. We detected the expression of RasGRP3 in peripheral blood mononuclear cells (PBMCs) of SLE patients (n=26) and healthy controls (n=20) by employing RT-PCR and studied the association between the mRNA expression of RasGRP3 in PBMCs and the clinical findings. We also measured the protein level of RasGRP3 in PBMCs by Western blotting (n=10). In addition, we isolated the B cells from PBMCs with magnetic bead separation and determined the RasGRP3 expression by RT-PCR (n=10). Furthermore, we extracted spleen B cells from MRL/lpr mice and knocked down RasGRP3 by siRNA transfection to study the role of RasGRP3 in the pathway of B cell receptor (BCR) activation and the production of pro-inflammatory cytokines. Compared with healthy volunteers, the expression of RasGRP3 was significantly elevated in PBMCs and purified B cells from SLE patients. The mRNA expression of RasGRP3 in PBMCs was positively correlated with SLE disease activity index (SLEDAI). Moreover, silencing RasGRP3 could inhibit Akt and Erk1/2 activation in marginal zone (MZ) and follicular (FO) B cells of MRL/lpr mice. Additionally, the production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), was decreased whereas activation of caspase-3 cleavage was induced in vitro. In conclusion, over-expression of RasGRP3 is associated with disease activity and might be involved in the pathogenesis of SLE.
Keywords: Systemic lupus erythematosus, B cell, RasGRP3, MRL/lpr mice, SLE disease activity index
Introduction
Systemic lupus erythematosus (SLE) represents a systemic autoimmune disease with multi-organ damage, and is characterized by production of pathogenic autoantibodies directed against nucleic acids and their binding proteins [1]. B- and T-lymphocytes play central roles in the development of SLE [2-5]. The extensive infiltration of T cells in skin, kidney and other involved organs activates immune cells and elevates the level of pro-inflammatory cytokines. B cells not only serve as effectors of immune response by secreting antibodies and forming autoimmune complex, but also are important initiators of the event by presenting antigens and producing pro-inflammatory cytokines [6]. Dysfunction of antigen receptor signaling pathways in B and T cells can lead, in general, to a wide array of pathological events, and in particular, to the development of autoimmunity [3,7,8].
Ras guanyl nucleotide-releasing protein (RasGRP), expressed primarily in hematopoietic cell lineages, is a member of the CDC25 family of Ras guanine nucleotide exchange factors (GEFs) that contain an N-terminal GEF domain and C-terminal calcium- and diacylglycerol (DAG)-binding domains [9]. In mice, RasGRP3 is principally expressed in B cells whereas RasGRP1 is highly expressed in T cells and, to a lesser extent, in B cells [10-12]. These proteins are involved in the signaling of T and B cell receptors, and are believed to act as a link between T/BCR signaling and Ras activation [13-15]. Ras can regulate cell proliferation and survival, especially the fate of T or B cells [11,16]. Indeed, RasGRP1-/- mice tend to be autoimmune-prone and develop a lupus-like phenotype. These mice were found to have an increased number of autoreactive CD4+ T cells, which can facilitate the activation of B cells and the production of auto-antibodies (Ab) [12]. On the other hand, in RasGRP3-/- mice the Ab production was suppressed and double-mutant mice did not develop signs of autoimmunity [11,12]. Therefore, inhibiting RasGRP1 is believed to promote autoimmunity by activating B cells via autoreactive CD4+ T cells, while RasGRP3 inhibition renders B cells less sensitive to T cell signals [11].
In SLE patients, incidence of defective isoforms of RasGRP1 was found to be increased, which led researchers to postulate that the isoforms could cause the dysfunction of the GEF domain of RasGRP1 and defective signaling in lymphocytes [17,18]. Although there exists a relationship between single nucleotide polymorphisms (SNPs) of RasGRP3 gene and SLE disease [19,20], so far, no defects in the RasGRP3 transcript have been found in SLE patients [17]. Until now, little is known about the expression of RasGRP3 in immune cells from SLE patient and the role of RasGRP3 in the activation of B cells by BCR cross-linking.
Therefore, in this study, we studied the expression of RasGRP3 in PBMCs and B cells in SLE patients and healthy controls, and examined the relationship between RasGRP3 mRNA expression in PBMC and disease activity of SLE and other clinical and laboratory features of SLE. Moreover, we extracted spleen B cells from MRL/lpr mice and knocked down RasGRP3 by siRNA transfection, with an attempt to understand the role of RasGRP3 in BCR activation pathway.
Materials and methods
Ethics statement
The study was carried out in strict accordance with the guidelines of good clinical practice, laws, state and local, and the Declaration of Helsinki, and was approved by the Ethics Committee of Huazhong University of Science and Technology, Wuhan, China. Informed consent, with signature, was obtained from all subjects. All animal studies were conducted strictly according to protocols approved by the Health Sciences Animal Policy and Welfare Committee of Huazhong University of Science and Technology, Wuhan, China.
Human subjects
Forty-six SLE patients were studied from 2011 to 2015 in the Department of Dermatology at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. The patients were diagnosed against the criteria of the American College of Rheumatology (1997). At the same time, forty healthy gender- and age-matched volunteers were also recruited. The demographic characteristics of the SLE patients and healthy controls are shown in Table 1. Disease activity of the SLE patients was quantitatively rated on the SLEDAI scale. Peripheral blood samples (10 ml from each patient) were taken from healthy controls and SLE patients.
Table 1.
Demographic characteristics of SLE patients and healthy controls
| Demographic characteristics | SLE patients (n=46) | Healthy controls (n=40) |
|---|---|---|
| Age, years | 34.1±12.8 | 30.5±10.6 |
| Female, n (%) | 42 (91.3%) | 34 (85%) |
| Male, n (%) | 4 (8.7%) | 6 (15%) |
Laboratory measurements of SLE patients
In all SLE patients, clinical features were examined and recorded by two professional doctors. Peripheral blood cells were counted by routine laboratory tests. Urinalysis was performed by microscopic analysis of urinary sediments. Proteinuria was defined as urine protein >+++, or total quantity of protein in a 24-hour urine collection test >0.5 g; Hematuria was considered present if urine contained >5 red blood cells per high-power field. Serum levels of complement component 3 (C3), C4 and IgG, and autoantibodies such as ANA, anti-dsDNA and anti-SM antibodies, were detected by ELISA. The laboratory tests were all carried out in the Clinical Laboratory of Union hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Mice
MRL/lpr mice were kindly provided by Prof. Qianjin Lu at the Second Xiangya Hospital, Central South University, Hunan, China. Mice were bred in SPF animal center of Union Hospital, Tongji Medical College, Wuhan, China.
Cell preparation and cell culture
PBMCs were isolated by Ficoll-Paque Plus gradient centrifugation (Hao Yang Biotechnology Company, Tianjin, China). Human B cells were isolated from PBMCs by using human B cell MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany). B cells counts ranged from 4×105 to 8×105. Mouse MZ and FO B cells were separated from spleen cell suspensions by employing mouse MZ and FO B cell MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 15% FCS (Gibco of Life Technologies, Grand Island, NY, USA).
SiRNA nucleofection and cell activation
After isolation of MZ and FO B cells, the cells were incubated for 24 hours in RPMI-1640 medium with LPS (50 µg/ml, Sigma). Nucleofection of MZ and FO B cells was performed using the Amaxa Stimulated Mouse B Cells Nucleofector™ Kit (program Z-001), and the non-target control siRNA and RasGRP3 siRNA (5’-CCGAUGGUAUUUAUCUUCCACUGAA-3’, 5’-UUCAGUGGAAGAUAAAUACCAUCGG-3’) were purchased from Invitrogen, USA. The cells were incubated in humidified incubator (37°C, 5% CO2) for 18 hours and 36 hours. MZ and FO B cells were stimulated with affiniPure F(ab’)2 fragment goat anti-mouse anti-IgM (10 µg/ml, Jackson ImmunoResearch Laboratories, PA, USA) and anti-CD40 (500 ng/ml, Pepro Tech company, NJ, USA) and IL-4 (20 ng/ml, Pepro Tech company, NJ, USA) for 30 minutes and 6 hours.
Reverse transcriptase-PCR (RT-PCR) and real-time PCR
mRNAs of human PBMCs and B cells were extracted by using Trizol reagent (Invitrogen, USA) and RT-PCR was carried out using PrimeScriptTM RT reagent Kit (Takara Bio, Shiga, Japan). RasGRP3 was amplified using specific primers from Invitrogen Biotechnology, and β-actin, serving as a control, was also amplified. The primer sequences were: 5’-GAGTTGGTCTCCTCCAACGG-3’ and 5’-AATTTCTAGGCTCCAGCACCA-3’ for Homo sapiens RasGRP3, and 5’-GTCCACCGCAAATGCTTCTA-3’ and 5’-TGCTGTCACCTTCACCGTTC-3’ for Homo sapiens β-actin. Total mRNAs of mouse MZ FO B cells were extracted using Trizol reagent (Invitrogen, USA) and reverse transcribed into cDNA using PrimeScriptTM RT reagent kit with gDNA Eraser real-time PCR reverse transcriptase kit (Takara Bio, Shiga, Japan). Real-time PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR system using the SYBR Premix Ex Tag (Takara Bio, Shiga, Japan). The data were normalized to the amount of β-actin transcript. The primer sequences were: 5’-GGGAAAGCGGCAACACTAGAT-3’ and 5’-GGGCAAGTAACTGTCGTTCAG-3’ for mouse RasGRP3, 5’-GAGTTGTGCAATGGCAATTCTG-3’ and 5’-GCAAGTGCATCATCGTTGTTCAT-3’ for mouse IL-6, 5’-CCCTCACACTCAGATCATCTTCT-3’ and 5’-GCTACGACGTGGGCTACAG-3’ for mouse TNF-α, and 5’-AGTGTGACGTTGACATCCGT-3’ and 5’-GCAGCTCAGTAACAGTCCGC-3’ for mouse β-actin.
Western blotting
Cells were washed twice with ice-cold PBS and lysed in RIPA buffer (Biyuntian Company, Shanghai, China) which contained 1% PMSF (Guge Company, Wuhan, China). The cell lysates were resolved in 12% SDS-PAGE gels and transferred to a PVDF membrane (Millipore, MA, USA). The membranes were incubated with antibodies against phosphor-T133 RasGRP3, RasGRP3, ERK, phospho-ERK, phospho-AKT, Caspase 3 (Cell Signaling Technology, MA, USA), AKT (Santa Cruz, TX, USA), with β-actin as the internal control. Antibody binding was shown by incubating the sample with anti-rabbit and anti-mouse IgG peroxidase conjugate secondary antibody. Chemiluminescence was detected using Western Bright Sirius Chemiluminescent HRP Substrate. The amount of target protein was normalized to the control and analyzed by utilizing Quantity One software package (Bio-Rad Laboratories, California, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Prism Software Inc., California, USA). Means between two groups were compared by using two-tailed unpaired t-test. The Pearson’s correlation test was used to assess the correlation between the RasGRP3 mRNA levels in PBMCs and SLEDAI scores. P<0.05 was considered to be statistically significant.
Results
Expression of RasGRP3 mRNA and protein in PBMCs and B cells from SLE patients and healthy controls
Overall, 46 SLE patients were randomly divided into three groups: group 1 included 26 blood samples, and group 2 and 3 each had 10 samples. With group 1, PBMCs were isolated and detected for the mRNA level of RasGRP3. In group 2, the expression of RasGRP3 protein was determined in PBMCs. For group 3, blood samples were taken for the determination of RasGRP3 mRNA expression in B cells. RT-PCR showed that the RasGRP3 mRNA expression in PBMCs was up-regulated in SLE patients as compared with the healthy controls (Figure 1A). Moreover, the mRNA level of RasGRP3 in PBMCs was significantly higher in SLE patients than in the controls (P<0.05) (Figure 1B). What is more, Western blotting showed that the protein expression of RasGRP3 in PBMCs was higher in SLE patients (n=10) than in the controls (n=10) (P<0.05) (Figure 1C, 1D). In addition, RT-PCR exhibited that the RasGRP3 mRNA expression in B cells was significantly higher in the SLE patients (n=10) than in the controls (n=10) (P<0.05) (Figure 1E, 1F).
Figure 1.
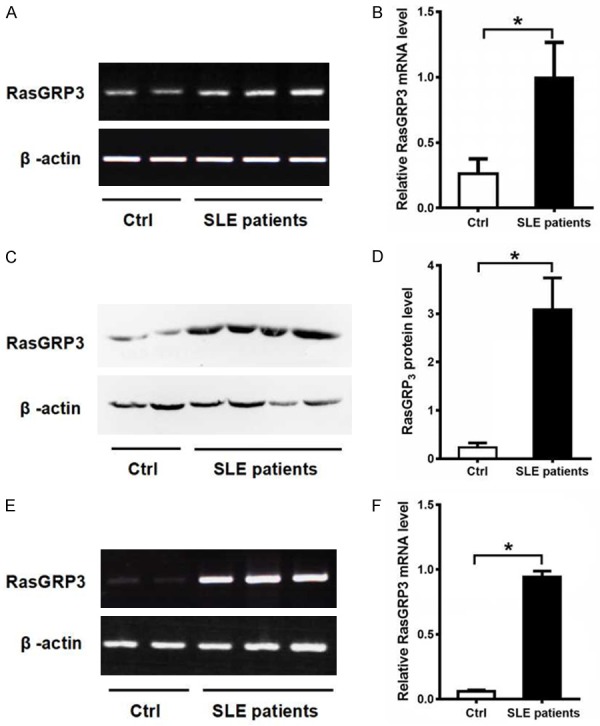
Expression levels of RasGRP3 mRNA and protein in PBMC and B cells of patients with SLE and healthy controls. A. Expression levels of RasGRP3 mRNA in PBMC from patients with SLE and healthy controls by RT-PCR. B. Statistical analysis of RasGRP3 mRNA levels of PBMC by RT-PCR in SLE patients (n=26) and healthy controls (n=20). C. Expression levels of RasGRP3 protein in PBMC from SLE patients and healthy controls by western blot. D. Comparison of RasGRP3 protein expression in SLE patients (n=10) with healthy controls (n=10). E. RT-PCR was performed to show the RasGRP3 mRNA expression level in B cells from patients with SLE and healthy controls. F. Expression levels of RasGRP3 mRNA levels of B cells by RT-PCR in SLE patients (n=10) and healthy controls (n=10). Horizontal bars indicate medians; P<0.05 means a significant difference.
Clinical manifestations and laboratory measurements of SLE patients
The clinical manifestations and laboratory findings of SLE patients are presented in Table 2. The disease duration ranged from two weeks to 25 years (mean: 3.27 years). Clinical features included malar rash, discoid rash, photosensitivity, nasal or oral ulcer and arthritis. 13 of the 26 SLE patients had lupus nephritis (LN). Arthritis, serositis and central nervous system (CNS) disease were found in eight, four and one patient(s) respectively. The mean score on SLEDAI scale was 12.19 in the patients, ranging from 4 to 22. The ANA, anti-dsDNA, anti-Sm autoantibodies were detected in 100%, 38.5% and 61.5% of patients, respectively. Serum C3 and C4 was low in 473.1% and 50% of SLE patients and IgG was high in 26.9% of the patients. 13, 8 and 7 patients suffered from leukocytopenia, anemia and thrombocytopenia respectively. Urinalysis revealed that 10 and 13 patients had hematuria and proteinuria respectively.
Table 2.
Clinical manifestations and laboratory measurements of twenty-six SLE patients
| Clinical manifestation | Laboratory measurements | ||
|---|---|---|---|
| Disease duration, years | 3.27±2.37 | ANA positive, n (%) | 26 (100%) |
| Malar rash, n (%) | 23 (88.5%) | Anti-dsDNA positive, n (%) | 10 (38.5%) |
| Discoid rash, n (%) | 15 (57.7%) | Anti-Sm positive, n (%) | 16 (61.5%) |
| Photosensitivity, n (%) | 14 (53.8%) | C3 hypocomplementemia, n (%) | 19 (73.1%) |
| Nose or oral ulcers, n (%) | 6 (23.1%) | C4 hypocomplementemia, n (%) | 13 (50%) |
| Arthritis, n (%) | 8 (30.8%) | High IgG, n (%) | 7 (26.9%) |
| CNS disease, n (%) | 1 (3.8%) | Leukocytopenia, n (%) | 13 (50%) |
| Lupus nephritis, n (%) | 13 (50%) | Anemia, n (%) | 8 (30.8%) |
| Serositis, n (%) | 4 (15.4%) | Thrombocytopenia, n (%) | 7 (26.9%) |
| SLEDAI | 12.19±3.76 | Hematuria, n (%) | 10 (38.5%) |
| Proteinuria, n (%) | 13 (50%) | ||
SLE: Systemic lupus erythematosus; SLEDAI: SLE disease activity index; CNS: Central nervous system; ANA: Anti-nuclear antibody; Anti-dsDNA: Anti-double stranded DNA antibody; Anti-Sm: Anti Smith antibody; IgG: immunoglobulin G.
Relationships between RasGRP3 mRNA expression and clinical features or laboratory findings in SLE patients
In order to understand the association between RasGRP3 expression and clinical features or laboratory findings in SLE patients, we compared the RasGRP3 mRNA level in PBMCs and clinical characteristics of SLE patients (Table 2). The results showed that the expression level of RasGRP3 mRNA was positively correlated with SLEDAI scores (r=0.4238, P<0.05) (Figure 2A). Furthermore, RasGRP3 mRNA expression was significantly higher in SLE patients than in their counterparts without such conditions (P<0.05) (Figure 2B, 2C). No significant correlation was found between RasGRP3 mRNA expression levels and other clinical manifestations in SLE patients, such as malar rash, discoid rash, photosensitivity, nasal or oral ulcer and arthritis. We did not make comparison in terms of CNS disease and serositis due to limited case numbers. There was no statistically significant correlation between RasGRP3 mRNA expression levels and other laboratory findings, such as, positive anti-dsDNA or anti-Sm antibodies, hypocomplementemia, high IgG and aberrant blood cell counts.
Figure 2.
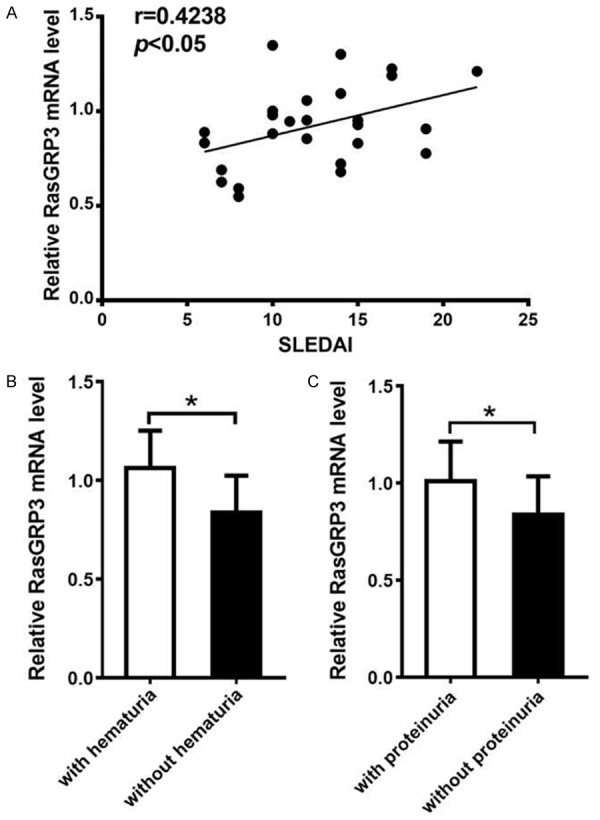
The relationship between RasGRP3 mRNA expression and clinical characteristics or laboratory parameters in the patients with SLE. RT-PCR was performed to semi-quantify the expression of RasGRP3 gene in patients with SLE (n=26). A. Positive correlation between RasGRP3 mRNA expression and SLEDAI in the patients with SLE. B. RasGRP3 mRNA expression in PBMC from SLE patients with hematuria was significantly higher than those without hematuria. C. RasGRP3 expression in SLE patients with proteinuria was significantly higher compared with those without proteinuria. Horizontal lines indicate medians. P<0.05 means a significant difference.
RasGRP3 silencing inhibits Akt and Erk1/2 activation in MZ and FO B cells of MRL/lpr mice
Some studies reported that RasGRP3 was required for optimal activation of B cell receptor cross-linking. Our above results demonstrated that expression of RasGRP3 was elevated or up-regulated in PBMCs and B cells of SLE patients. In order to further understand the role of high RasGRP3 expression in the pathogenesis of SLE, we separated MZ and FO B cells from spleen cells of MRL/lpr mice and knocked down RasGRP3 by siRNA transfection.
Compared to controls, we found that, the expression of total RasGRP3 was decreased 40 hours after RasGRP3 siRNA nucleotransfection, and the phosphorylation of RasGRP3 was decreased in B cells that had been stimulated for 30 minutes with anti-IgM, anti-CD40 and IL-4 (Figure 3A, 3B). Then we detected the phosphorylation of Akt and Erk1/2 after RasGRP3 silencing. The results revealed that silencing RasGRP3 in B cells decreased the phosphorylation levels of both Akt and Erk1/2 stimulated for 30 minutes with goat anti-mouse anti-IgM, anti-mouse CD40 and IL-4 (Figure 3D, 3F).
Figure 3.
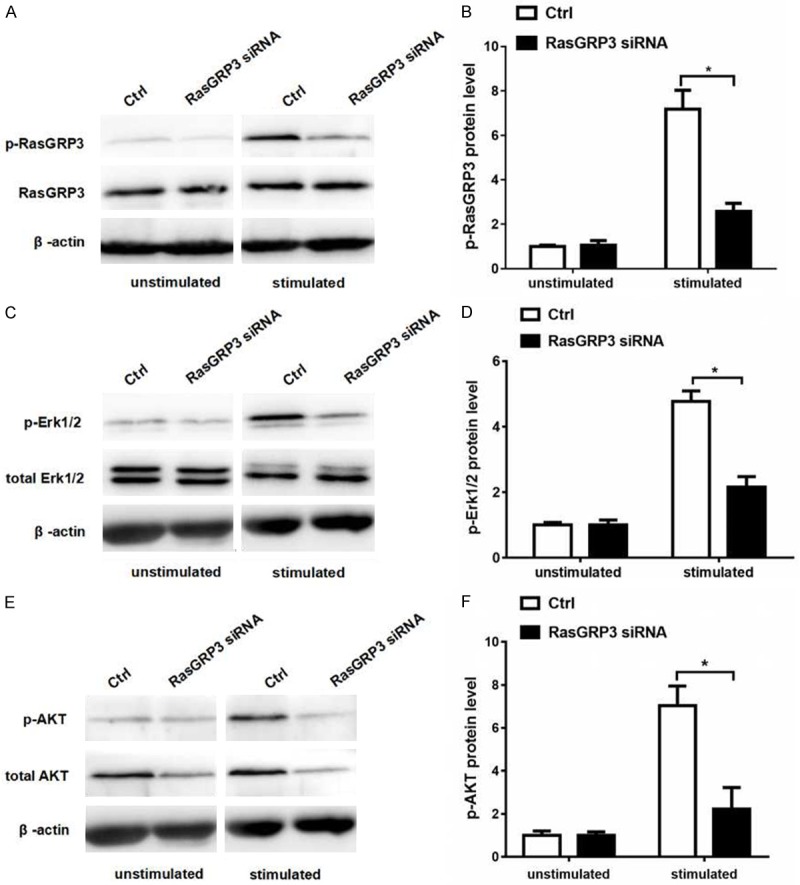
RasGRP3 silence inhibits Akt and Erk1/2 activation in MZ and FO B cells of MRL/lpr mice. After stimulated with anti-mouse anti-IgM, anti-mouse CD40 and IL-4 for 30 minutes, MZ and FO B cells transfected with scramble siRNA or RasGRP3 siRNA of MRL/lpr mice (n=3) were examined for Akt and Erk1/2 expression by western blotting. A. Phosphorylation of Thr133 in RasGRP3 was examined by using phospho-RasGRP3 antibody. B. A significant decrease in phospho-RasGRP3 protein expression in MZ and FO B cells transfected with RasGRP3 siRNA of MRL/lpr mice after stimulation. C. The expression of phosphorylated Erk1/2 was examined by western blotting. D. Statistical analysis of the level of phospho-Erk1/2 protein in MZ and FO B cells of MRL/lpr mice by western blotting. E. The expression of phosphorylated Akt was examined by western blotting. F. Statistical analysis of the level of phospho-Akt protein in MZ and FO B cells of MRL/lpr mice by western blotting. P<0.05 means a significant difference.
RasGRP3 silencing reduces pro-inflammatory cytokine production and induces activation of caspase-3 cleavage in vitro
Engagement of the BCR in B cells activates multiple signaling pathways, such as AKT, Erk pathways, to release several pro-inflammatory cytokines. IL-6 and TNF-α, produced following BCR activation, are important pro-inflammatory cytokines for SLE. We then examined the effects of RasGRP3 silencing on the production of IL-6 and TNF-α in B cells. We found that, after treatment or stimulation with anti-IgM, anti-CD40 and IL-4, the levels of TNF-α and IL-6 mRNA were significantly lowered in B cells transfected with RasGRP3 siRNA as compared with those transfected with scramble siRNA (Figure 4A, 4B). These data indicated that inhibiting RasGRP3 could decrease the production of B cell pro-inflammatory cytokines in the B cells from lupus mice.
Figure 4.
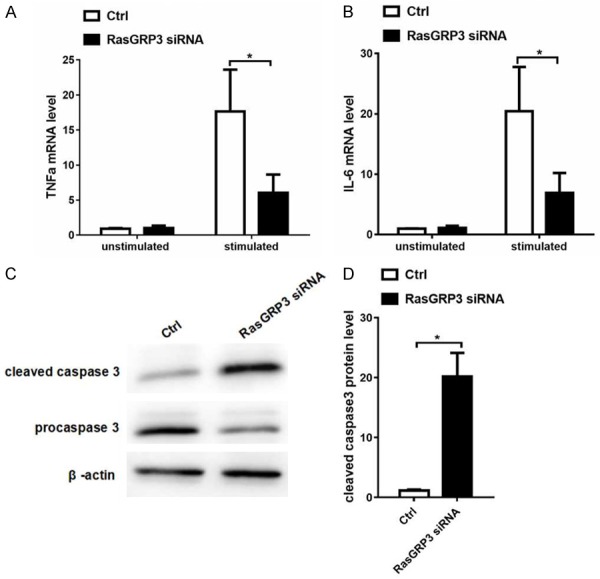
RasGRP3 silencing reduces pro-inflammatory cytokine production and induces activation of cleaved caspase-3 in vitro. MZ and FO B cells of MRL/lpr mice (n=3) transfected with scramble siRNA or RasGRP3 siRNA were stimulated with anti-mouse anti-IgM, anti-mouse CD40 and IL-4 for 6 hours, and the level of TNF-α and IL-6 mRNA was examined by Realtime-PCR. A. Expression levels of TNF-α mRNA in MZ and FO B cells transfected with scramble siRNA or RasGRP3 siRNA. B. Expression levels of IL-6 mRNA in MZ and FO B cells transfected with scramble siRNA or RasGRP3 siRNA. C. Expressions of caspase-3 and cleaved caspase-3 was examined by western blotting. D. Statistical analysis of cleaved caspase-3 of MZ and FO B cells transfected with scramble siRNA or RasGRP3 siRNA by western blotting. P<0.05 means a significant difference.
Caspase-3 belongs to the cysteine-aspartic acid protease family and its activation plays a crucial role in the execution-phase of cell apoptosis. Caspase 3 is virtually inactive until it is cleaved by an initiator caspase after apoptotic signaling events occur. In this study, 40 hours after RasGRP3 was knocked down, cleaved caspase-3 was increased (Figure 4C, 4D) and pre-caspase 3 decreased. This result demonstrated that RasGRP3 silencing could induce the activation of cleaved caspase-3 and trigger B cells apoptosis.
Discussion
Co-expression of RasGRP3 and RasGRP1 in most B cells renders it difficult to clarify the role of RasGRP3 in B cells [12]. Although RasGRP1 is the only RasGRP family member implicated in TCR-induced Ras activation, both of RasGRP1 and RasGRP3 contribute to Ras activation downstream of the BCR [12-15]. B cells express less RasGRP1 than do T cells, suggesting that RasGRP3 plays a major role in B cells [12].
This study demonstrated that RasGRP3 was up-regulated in PBMCs and B cells of SLE patients and the mRNA level of RasGRP3 in PBMCs was positively correlated with disease activity (SLEDAI), suggesting that over-expression of RasGRP3 may contribute to the deterioration or progress of the disease. We also compared the mRNA levels of RasGRP3 in different groups in terms of presence or absence of certain clinical features and laboratory findings and found that the mRNA levels of RasGRP3 were significantly higher in patients with hematuria or proteinuria than in those without such conditions. These results revealed that the level of RasGRP3 in SLE patients could reflect the degree of disease activity, and RasGRP3 overexpression might be involved in the pathogenic process of lupus nephritis. The power of the analysis is limited since the number of patients with CNS disease or serositis was small. Further studies involving more SLE patients and more parameters are warranted to confirm the association.
Furthermore, we examined the role of RasGRP3 in BCR activation pathway in the splenic B cells from MRL/lpr mice. Our study suggested that one possible mechanism was that RasGRP3 might play its part via Ras-Erk and AKT signaling pathways after the BCR ligation in SLE patients. Like RasGRP1 working as a downstream target or effector of TCR in T cells, RasGRP3 can be phosphorylated by protein kinase C (PKC) to contribute to Ras activation after BCR ligation [14,15]. Ras regulates growth, proliferation and survival of cells by activating downstream signaling pathways, especially Raf/Mek/Erk and PI3K/Akt/mTOR pathways [21]. RasGRP3-/- mice showed modestly lower levels of some immunoglobulin types and isolated RasGRP3-/- B cells exhibited undetectable basal Ras-GTP levels and defective BCR-induced Ras-Erk signaling activation in vitro [12]. Taking together, the phosphorylated RasGRP3 acts as downstream effector of BCR activation to serve such functions such as Ab production, and pErk is involved in the process. Our results demonstrated that the level of RasGRP3 phosphorylation was markedly elevated in isolated splenic B cells of lupus mice after the stimulation with anti-IgM, anti-CD40 and IL-4, and the Erk and Akt phosphorylation level was also substantially increased. On the other hand, phosphorylation of Erk and Akt could be inhibited significantly after RasGRP3 was specifically silenced. Thus, we are led to infer that high expression of RasGRP3 may, via BCR-mediated pathway, induce the activation of the Erk- and Akt-mediated pathways thereby eliciting autoimmunity in B cells from lupus mice. Furthermore, after RasGRP3-specific silencing, the cleaved caspase-3 was increased in MZ and FO B cells from MRL/lpr mice compared with scramble group. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis [22]. These findings suggested that the inhibition of RasGRP3 might take part in the apoptosis of B cells.
Another possible mechanism is that increased expression of RasGRP3 may enhance the production of multiple cytokines, following antigen engagement with BCRs, resulting in the inducible transcription of genes and cytokines. In this study, decreased expression of RasGRP3 led to a reduced secretion of both IL-6 and TNF-α by B cells from MRL/lpr mice after BCR signaling activation. This might be explained by the fact that the downstream signaling molecules of Ras, such as Ras-Erk signals, acting as key regulators, modulated the expression of IL-6 and TNF-α mRNA [23]. IL-6 was originally identified as an antigen-nonspecific B-cell differentiation factor in the culture supernatants of mitogen- or antigen-stimulated peripheral blood mononuclear cells that induced B cells to produce immunoglobulin. In this study, serum IL-6 level was significantly elevated in active SLE patients and was correlated with SLEDAI scores, erythrocyte sedimentation rate (ESR), and level of C-reactive protein (CRP) [24]. Similar to IL-6, TNF-α is also an inflammatory cytokine implicated in the pathogenesis of SLE. Serum TNF-α level was significantly increased in patients with SLE, and was correlated with higher disease activity and renal involvement [25,26]. We are led to hypothesize that decreased RasGRP3 expression of B cells may reduce disease activity and ameliorate kidney damage via inhibiting the secretion of cytokines such as IL-6 and TNF-α. We failed to detect autoantibody production due to limited amount of cells and antibodies collected, even though it was proved previously that RasGRP3-/- mice had lower level of immunoglobulins, especially IgG2a [27].
In conclusion, this study revealed that RasGRP3 expression was up-regulated and was correlated with disease activity in SLE patients. Moreover, animal study showed that RasGRP3 could positively regulate the production of pro-inflammatory cytokines (IL-6 and TNF-α) in B cells, possibly through the Erk and Akt pathways in response to BCR stimulation. The findings provide further evidence that overexpression of RasGRP3 might be responsible for the B cells autoimmunity after BCR activation in SLE patients.
Acknowledgements
This project was supported by grants from the National Natural Science Foundation of China. Grants: No. 81573047; No. 81602760.
Disclosure of conflict of interest
None.
References
- 1.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Nashi E, Wang Y, Diamond B. The role of B cells in lupus pathogenesis. Int J Biochem Cell Biol. 2010;42:543–550. doi: 10.1016/j.biocel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsuyama T, Tsokos GC, Moulton VR. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol. 2018;9:1088. doi: 10.3389/fimmu.2018.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sang A, Zheng YY, Morel L. Contributions of B cells to lupus pathogenesis. Mol Immunol. 2014;62:329–338. doi: 10.1016/j.molimm.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morawski PA, Bolland S. Expanding the B cell-centric view of systemic lupus erythematosus. Trends Immunol. 2017;38:373–382. doi: 10.1016/j.it.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renaudineau Y, Pers JO, Bendaoud B, Jamin C, Youinou P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun Rev. 2004;3:516–523. doi: 10.1016/j.autrev.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW. Altered B cell signalling in autoimmunity. Nat Rev Immunol. 2017;17:421–436. doi: 10.1038/nri.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng SL. Altered T and B lymphocyte signaling pathways in lupus. Autoimmun Rev. 2009;8:179–183. doi: 10.1016/j.autrev.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 10.Stone JC. Regulation of ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34:858–861. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin JJ, Stang SL, Dower NA, Stone JC. The role of RasGRPs in regulation of lymphocyte proliferation. Immunol Lett. 2006;105:77–82. doi: 10.1016/j.imlet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 13.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood. 2005;105:3648–3654. doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 16.Stone JC. Regulation and function of the RasGRP family of ras activators in blood cells. Genes Cancer. 2011;2:320–334. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda S, Stevens RL, Terada T, Takeda M, Hashimoto T, Fukae J, Horita T, Kataoka H, Atsumi T, Koike T. Defective expression of Ras guanyl nucleotide-releasing protein 1 in a subset of patients with systemic lupus erythematosus. J Immunol. 2007;179:4890–4900. doi: 10.4049/jimmunol.179.7.4890. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport MJ, Bloch O, Amit-Vasina M, Yona E, Molad Y. Constitutive abnormal expression of RasGRP-1 isoforms and low expression of PARP-1 in patients with systemic lupus erythematosus. Lupus. 2011;20:1501–1509. doi: 10.1177/0961203311418790. [DOI] [PubMed] [Google Scholar]
- 19.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, Zhang XJ. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 20.He CF, Liu YS, Cheng YL, Gao JP, Pan TM, Han JW, Quan C, Sun LD, Zheng HF, Zuo XB, Xu SX, Sheng YJ, Yao S, Hu WL, Li Y, Yu ZY, Yin XY, Zhang XJ, Cui Y, Yang S. TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus. 2010;19:1181–1186. doi: 10.1177/0961203310367918. [DOI] [PubMed] [Google Scholar]
- 21.Kurosaki T. Molecular dissection of B cell antigen receptor signaling (review) Int J Mol Med. 1998;1:515–527. doi: 10.3892/ijmm.1.3.515. [DOI] [PubMed] [Google Scholar]
- 22.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 23.Wozniacka A, Lesiak A, Boncela J, Smolarczyk K, McCauliffe DP, Sysa-Jedrzejowska A. The influence of antimalarial treatment on IL-1beta, IL-6 and TNF-alpha mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br J Dermatol. 2008;159:1124–1130. doi: 10.1111/j.1365-2133.2008.08804.x. [DOI] [PubMed] [Google Scholar]
- 24.Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, Kim SH, Park HS, Suh CH. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–466. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 25.Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067–1074. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- 26.Gabay C, Cakir N, Moral F, Roux-Lombard P, Meyer O, Dayer JM, Vischer T, Yazici H, Guerne PA. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol. 1997;24:303–308. [PubMed] [Google Scholar]
- 27.Liu K, Liang C, Liang Z, Tus K, Wakeland EK. Sle1ab mediates the aberrant activation of STAT3 and Ras-ERK signaling pathways in B lymphocytes. J Immunol. 2005;174:1630–1637. doi: 10.4049/jimmunol.174.3.1630. [DOI] [PubMed] [Google Scholar]


