Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple organ systems with diverse presentation, primarily affecting women of reproductive age. Various genetic and environmental risk factors are involved in the pathogenesis of SLE, and many SLE susceptibility genes have been identified recently; however, gene therapy is not a viable clinical option at this time. Thus, environmental risks factors, particularly regional characteristics that can be controlled, need to be further investigated. Here, we systematically explored these risk factors, including ultraviolet radiation, seasonal distribution, geographical distribution, and climate factors, and also summarized the mechanisms related to these risk factors. Probable mechanisms were explicated in at least four aspects including inflammatory mediators, apoptosis and autophagy in keratinocytes, epigenetic factors, and gene-environment interactions. This information is expected to provide practical insights into these risk factors in order to benefit patients with SLE and facilitate the development of potential therapeutic strategies.
Keywords: Risk factors, systemic lupus erythematosus, ultraviolet radiation, season distribution, geographical distribution, climate factors
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple organ systems with diverse presentation, primarily affecting women of reproductive age. SLE can persist throughout the entire life of the patient, exhibiting possible frequent relapses. The etiology of SLE is not well understood, although the disease is known to be caused by genetic and environmental interactions. A study by Deapen et al. showed that the SLE concordance rate in monozygous twins was 24%, which was substantially lower than a prior estimation [1], indicating that environmental risk factors cannot be neglected. Environmental factors can work together to cause epigenetic changes, resulting in immune dysregulation, loss of tolerance, and autoimmunity and leading to onset or recurrence of SLE. Although many studies have evaluated susceptibility-related genes, research on environmental risk factors and the mechanisms through which these risk factors contribute to the development of SLE remains limited. Moreover, compared with the complexity and technical difficulties of genetherapy, changing environmental factors is much more practical.
In this review, we discuss environmental risk factors, including ultraviolet radiation (UVR), climate factors, and geographical distribution, in the pathogenesis of SLE and illustrate the underlying mechanisms with the goal of facilitating the development of new therapeutic strategies for the management of SLE.
Environmental risk factors for SLE
UVR is the most important environmental factor inducing SLE, as demonstrated in various studies of human populations and experimental studies [2-7]. UVR includes UVA, UVB, and UVC.UVA (wavelength range: 320-400 nm) is abundant in terrestrial sunlight, but is not strongly absorbed by proteins and nucleic acids and induces erythema; UVB (wavelength range: 290-320 nm) strongly induces erythema and is present in the terrestrial solar spectrum; and UVC (wavelength range: 200-290 nm) is absorbed by the earth’s ozone layer and is germicidal, although its effects on the development of SLE appear negligible [8]. UVA exposure induces cutaneous lupus skin lesions, but requires nearly 1000 times more energy than UVB to induce erythema [8]. The role of UVA in the development of SLE remains controversial. McGrath showed that in a New Zealand White/New Zealand Black mouse model of lupus, low-dose UVA markedly decreased mortality, prolonged survival, improved immune function, and had significant therapeutic effects [9]. In a follow-up human study, McGrath et al. found that low-dose UVA with long-term therapy significantly decreased clinical disease activity in SLE, such as remission of joint pain and rashes, reversal of brain dysfunction, elimination of anticardiolipin antibodies, and cessation of cognitive decline [10-15].
In contrast, UVB is known to be involved in the pathogenesis of SLE development. UVB exposure is responsible for photosensitivity, skin rashes, and recurrence in patients with pre-existing SLE. Additionally, Cheng et al. found that annual sunshine duration is related to disease activity [16]. Indeed, SLE has been shown to have seasonal variation, with higher incidence in the summer, during which UVR is the strongest [17]. However, a counter-season phenomenon has also been observed with regard to the seasonal distribution of SLE disease activity. For example, some studies have demonstrated that there are more cases of new onset and recurrence of SLE in winter and spring than in summer and autumn [18-25]. Moreover, different organs were shown to exhibit changes in seasonal variation patterns in a prospective longitudinal cohort study of 2102 patients with SLE; significantly more photosensitive rash and arthritis activity were observed in spring and summer, decrease in renal activity was found in the summer, higher serositis activity was found from August to October, and higher anti-double-stranded DNA levels were observed during October and November [26]. Additionally, some geographical environment factors, such as climate factors (temperature, atmospheric pressure, mean humidity, wind speed, and precipitation) and geographical distribution (latitude, longitude and altitudes), are also closely associated with UVR and have been studied in the context of susceptibility to SLE.
Based on hypotheses drawn from epidemiological or experimental animal studies, climate factors and geographical distribution maybe risk factors for the development of SLE [18,22-25,27,28]. Climate factors, as an important part of the geographical environment, have been shown to be correlated with autoimmune diseases and may influence the progression of SLE. Several studies have reported that the activity and incidence of SLE are correlated with temperature, atmospheric pressure, mean humidity, wind speed, and precipitation [16,18,22-25]. In addition, Pan et al. showed that the proportion of lupus nephritis increased significantly with the decreasing geographic latitude from the northern to the southern part of China, although no significant correlation was found with the change in geographic longitude, potentially because most studies were performed within a particular longitudinal band in China [27]. Cheng et al. also showed that living in the southern part of China is a risk for disease activity in SLE [16]. This epidemiology of the geographical distribution of SLE suggests that latitude may be an important environmental factor contributing to the development of SLE. In contrast, Deng et al. found that there was no significant correlation between SLE activity and altitude; Generally speaking, in patients with active or inactive SLE, clinical features and organ activities had different patterns of altitudinal variations. The development of SLE can also be affected by specific environmental factors at high altitudes [28]. These findings are summarized in Table 1. Further studies on the season distribution (temporal distribution) and geographical distribution (spatial distribution) patterns in SLE will improve our understanding of these SLE-related climate factors and geographical distributions in order to establish seasonal treatment programs for vulnerable groups.
Table 1.
Association between natural factors and SLE
| Definite | Probable | ||
|---|---|---|---|
| UVR | Season distribution | Climate factors | Geographical distribution |
| UVB | Winter and spring | Temperature | Latitude |
| UVA | Atmospheric pressure | Longitude | |
| Mean humidity | Altitude | ||
| Precipitation | |||
| Wind speed | |||
Abbreviation: UVR: ultraviolet radiation, UVB: ultraviolet B, UVA: ultraviolet A.
Pathogenic mechanisms
Inflammatory mediators
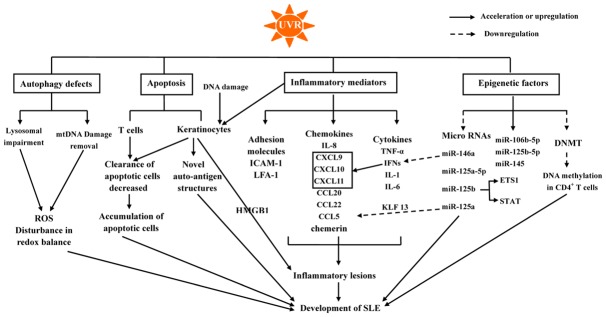
Inflammatory mediators regulated by UVR and climate factors may propagate inflammatory responses, recruit immune cells, suppress immune system tolerance, and promote B- and T-cell activation, giving rise to the development of SLE (Figure 1).
Figure 1.
The role of UVR in the development of SLE. Abbreviation: SLE, systemic lupus erythematosus; UVR, ultraviolet radiation; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; CXCL, chemokine (C-X-C motif) ligand; CCL, chemokine (C-C motif) ligand; ICAM-1, intercellular adhesion molecule 1; HMGB1, high-mobility group protein B1; LFA-1, lymphocyte function-associated antigen; DNA methyl transferase 1, DNMT1.
UVR
In genetically predisposed individuals, UVR, as a predisposing factor of SLE, has important roles in the pathogenesis of lupus by inducing a pro-inflammatory environment and leading to abnormal long-lasting photoreactivity via inflammatory mediators, such as pro-inflammatory cytokines, chemokines, and adhesion molecules (Table 2). UVR exposure upregulates pro-inflammatory cytokines expression, such as interferon (IFN)-α, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α [4,5,29-35]. In particular, IFNs, which have important roles in the early activation of the immune system, are involved in the development of UVB-induced inflammatory skin lesions in patients with SLE [36].
Table 2.
Inflammatory mediators in the development of SLE
| Classification | Details of Inflammatory mediators |
|---|---|
| Cytokines | IFN-α, IL-1, IL-6, TNF-α, IL-12. |
| Chemokines | CXCL9, CXCL10, CXCL11, IL-8, CCL 5, CCL20, CCL22, chemerin. |
| Adhesion molecules | ICAM-1, LFA-1, e-selectin, vascular cell adhesion molecule-1. |
| Proteins | HMGB1. |
Abbreviation: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; CXCL: chemokine (C-X-C motif) ligand; CCL, chemokine (C-C motif) ligand; ICAM-1, intercellular adhesion molecule 1; HMGB1, high-mobility group protein B1; LFA-1, lymphocyte function-associated antigen.
UVR and neutrophil extracellular traps induce oxidative modifications in DNA, which can result in resistance to degradation by the intracellular nuclease three prime repair exonuclease 1. Subsequently, oxidized DNA produces various type I IFNs, which are involve in the pathogenesis of SLE [37,38]. Additionally, type I/III IFNs increase the expression of pro-inflammatory chemokines, including chemokine (C-X-C motif) ligand (CXCL) 9, CXCL10, and CXCL11, which recruit chemokine (C-X-C motif) receptor 3 effector cells and induce keratinocyte apoptosis [5,39,40]. However, another study in IFN-α receptor-knockout mouse considered type I IFNs protective against skin inflammation induced by UVB irradiation [41].
UVR also upregulates intracellular adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen 1 [32,36,42-44], and increase the secretion of chemokines, including IL-8, chemokine (C-C motif) ligand (CCL) 5, CCL20, CCL22, and chemerin [3,34,45], which are important for recruiting immune cells to areas of inflammation. Yin et al. reported that chemerin, which was found to be elevated in UVB-irradiated skin, was chemotactic for plasmacytoid dendritic cells (pDCs) via its functional receptor chemR23 and recruited PDCs to areas of inflammation [45]. PDCs contribute to the pathogenesis of SLE by producing type I IFNs. Additionally, Abdulahad et al. revealed that UVB exposure induced high-mobility group protein B1 (HMGB1) release, which is related to the number of apoptotic cells in patients with SLE. HMGB1 released from apoptotic keratinocytes exerts inflammatory effects through binding to its receptors, resulting in the development of inflammatory lesions in the skin of patients with SLE upon UVB exposure [46].
Low temperature
Low temperature also plays an important role in the occurrence, development and recurrence of SLE. Pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12, which are produced by monocytes, can be upregulated by low temperature. The proportions of pro-inflammatory cytokines (IL-12/IL-10 and TNF-α/IL-10) may then increase [47-50]. In parallel, cold stimulation induces the expression of the inflammatory adhesion molecules e-selectin, ICAM-1, and vascular cell adhesion molecule-1 [51], and complement is activated at low temperature [52-54]. Also, cold exposure can induce cell apoptosis [55,56]. These factors may lead to the development of SLE.
Pressure
Extracellular pressure may alter some aspects of macrophage and monocyte functions. Singhal et al. showed that pressure increases monocytes migration in a dose-dependent manner when compared with normal atmospheric pressure [57], and Hironosuke et al. revealed that pressure enhances the expression of scavenger receptors in macrophages [58]. Interestingly, extracellular pressure regulates the production of TNF-α and IL-1β, which are involved in the development of SLE, by regulating monocytes and macrophages [59].
Humidity
Ye et al. demonstrated that humidity may be a risk factor for SLE and may decrease the body’s resistance to bacterial infections [60]. Zhang et al. also showed that a damp environment may reduce cellular immune function and alter some aspects of the ultra structure, resulting in pathological changes in the joints, lungs, and kidneys and causing function damage to multiple systems and organs in rats [61]. In particular, they also demonstrated that dampness and wind may increase the production of TNF-α and IL-6, resulting in organ damage in rats [62].
Apoptosis and autophagy in keratinocytes
Apoptosis
UVR, particularly UVB, is a strong inducer of apoptosis [6] and has dose-dependent effects on the rate of apoptosis in keratinocytes. Low doses induce apoptosis without inflammation, intermediate doses induce apoptosis and IL-1α production, and high doses induced necrosis and dramatic increases in IL-α production [63]. The DNA of keratinocytes absorbs UVR, leading to strand breaks or cyclobutan pyrimidine dimmers [64]. Pro-inflammatory mediators and DNA damage, which are influenced by UVR, jointly results inkeratinocyte death [65]. Moreover, UVR can upregulate the Fas antigen on peripheral T cells in patients with SLE, resulting in apoptosis in T cells [66]. Furthermore, decreased clearance of apoptotic cells has been observed. Some studies have shown that UV exposure can induce accumulation of apoptotic cells due to impaired clearance of apoptotic cells in the skin of patients with cutaneous lupus [6,67-69]. In contrast, Reefman et al. reported that UVB exposure did not induce apoptosis in the skin of patients with SLE compared with that in controls [70], and in vivo, there were no significant differences in clearance rates of apoptotic cells after UVB irradiation between patients with SLE and controls [71]. However, the skin of patients with SLE after UVB irradiation can induce infiltrates and inflammatory lesions due to an altered, inflammatory clearance of apoptotic cells; this may have a crucial role in the development of lupus-related skin lesions [71]. Many studies have also shown that UVB radiation can lead to a redistribution of nuclear antigens, including Ro, La, nuclear RNP, and Sm, which are related to cutaneous forms of lupus, to the cell surface in human keratinocytes [72-74]. Additionally, UVB radiation upregulates Ro52 expression in keratinocytes in inflammatory skin, which may generate auto-antibodies for Ro52 and disrupttolerance [75,76]. Also, UVB irradiation can generate novel auto-antigen structures in apoptotic keratinocytes after UVB irradiation, e.g., covalent RNA-protein complexes involved in antigen capture and processing [77]. Overall, these effects, which promote an autoimmune state, are thought to be involved in SLE pathogenesis.
Autophagy
Studies of receiver biases have suggested that autophagy is involved in UVR-induced damage. Moreover, exposure to UVA, UVB, and UVC induces autophagy, which may be a protective response to UVR [78-84]. Exposure to UVA and UVA-oxidized phospholipids, which leads to oxidative stress, such as accumulation of protein aggregates and elevated levels of reactive oxidized phospholipids, induce autophagy to promote the removal of oxidized phospholipids and protein aggregates in epidermal keratinocytes [82]. Additionally, autophagy reduces reactive oxygen species and maintains the redox balance upon UVA-induced oxidative damage in limbal stem cells [84]. Chronic UVA has also been shown to inhibit the enzymatic activities of cathepsin B (CB) and cathepsin L (CL) and to impair autophagic flux; downstream CB and CL inactivation results in UVA-induced lysosomal impairment in human skin fibroblasts, consequently causing skin damage in patients with SLE [85,86]. Notably, however, UVB exposure activates autophagy, which may be a protective response to UVB-induced damage, such as DNA damage and apoptosis, in epidermal cells. Studies have also shown that UVB-induced autophagy is mediated by inhibition of glycogen synthase kinase 3β and activation of AMP-activated protein kinase (AMPK) [79]. UVC exposure induces irreparable mitochondrial DNA (mtDNA) damage, and mitochondrial autophagy, which is increased after UVC exposure, can remove mtDNA damage in primary human fibroblasts [78,81]. Overall, autophagy may play a protective role in UVR-induced damage, and autophagy defects may promote the development of SLE.
Epigenetic factors
DNA methylation
Previously evidence has shown that DNA hypomethylation is implicated in the pathogenesis of SLE. Normal CD4+ T cells develop auto-reactivity when inhibiting DNA methylation, and these auto-reactive cells promote autoantibody production [43,87-90]. Recent studies have shown that UVB exacerbates the development of SLE by decreasing the levels of DNA methylation in CD4+ T cells in a dose-dependent manner [91-94]. Additionally, methylation-related molecules, such as DNA methyl transferase 1 (DNMT1) and methyl CpG binding domain protein 2 (MBD2), which maintain methylation and demethylation, respectively, may be involved in UVB-induced DNA hypomethylation in CD4+ T cells [95]. Zhu et al. demonstrated that UVB exposure decreases the levels of DNMT1 mRNA at higher dosages in patients with active SLE and but not affect MBD2 mRNA expression [92]. Wu et al. also found that UVB can inhibit DNMT1 activity in CD4+ T cells from patients with SLE [96]. However, Wang et al. and Wu et al. found that UVB exposure did not affect mRNA and protein expression of DNMT1 in CD4+ T cells from patients with SLE [91,93]. Moreover, Wu et al. suggested that UVB enhances global DNA hypomethylation in CD4+ T cells by inhibiting DNMT1 catalytic activity in patients with SLE [93]. Another study concluded that loss of DNMT1 catalytic activity resulted in aberrant DNA methylation [97]. However, the exact roles of DNMT1 in the pathogenesis of SLE are still unclear.
Overall, these findings demonstrated that the process through which DNA hypomethylation occurs in patients with SLE is complicated and that further studies are needed to evaluate the multiple factors involved in DNA methylation and demethylation.
MicroRNAs
UVB exposure induces microRNA-mediated gene regulation earlier than most transcriptional responses [98] and can cause variations in the expression of microRNAs (Table 3) [99,100], which modulate the UVR-induced DNA-damage response [101]. These deregulated microRNAs may be potentially involved in the pathogenesis of SLE. Xu et al. found that miR-146a and miR-125a-5p were downregulated after UVB exposure in mouse skin [102]. When miR-146a, which negatively regulates the IFN pathway, is expressed at low levels, the expression of type I IFNs is increased by targeting key signaling proteins in patients with lupus [103]. Indeed, miR-146a expression is negatively correlated with SLE activity [104]. Moreover, overexpression of miR-125a markedly reduces the levels of its target gene kruppel-like factor 13 (KLF 13) [105] and may induce CCL5 expression in late-activated T cells [106]. The level of CCL5 [105] modulates the recruitment of T cells to inflammatory sites, leading to tissue and organ inflammation [107-109]. In contrast, UVB exposure decreases the level of miR-125a, which can result in elevated levels of inflammatory chemokines, such as CCL5, and promote the development of SLE [105]. Dong et al. showed that miR-145 is overexpressed and contributes to IL-6-induced increases insensitivity to UVB irradiation by decreasing the levels of MyD88 [110].
Table 3.
Expression levels of MicroRNAs in SLE patients
| Expression levels | Details of Micro RNA |
|---|---|
| Upregulation | miR-145, miR-106b-5p, miR-125b-5p. |
| Downregulation | miR-146a, miR-125a-5p, miR-125a, miR-125b. |
In a study of UVB-mediated microRNA expression in peripheral blood T cells from patients with SLE, UVB was found to induce significant upregulation of miR-106b-5p and miR-125b-5p [111]. However, few studies have evaluated the associations of miR-106b-5p and miR-125b-5p with SLE. Luo et al. reported that miR-125b levels were reduced, showing a negative association with lupus nephritis, in T cells from patients with active SLE. Additionally, downregulation of miR-125b regulates the expression of ETS1 and STAT3 genes, triggering the development of SLE [112]. Gao et al. also demonstrated that the level of miR-125b-5p is decreased in peripheral blood mononuclear cells from patients with SLE and that miR-125b inhibits autophagy in Jurkat cells by targeting UVR resistance-associated gene protein, indicating that miR-125b maybe a therapeutic target for SLE [113]. Further studies are needed to determine the complex processes through which microRNAs are deregulated in patients with SLE.
Gene-environmental interactions
As external factors, climate factors, which have been shown to affect various polymorphic loci related to the immune response, can influence the roles of these polymorphic loci in disease processes by altering the allele frequency distribution. Many studies have shown that multiple polymorphic loci are strongly correlated with climate factors, such as UVR, humidity, temperature, and latitude [114,115]. For example, two human-specific polymorphisms, p53 codon 72 (rs1042522) and MDM2 single nucleotide polymorphism (SNP) 309 (rs2279744), which influence the activities of p53, have strong correlations with minimum winter temperature, latitude, and summer downward solar radiation [114]. Some findings of the gene-environment interaction hypothesis have shown that climate factors may alter the allele frequency distributions of multiple polymorphic loci involved the development of SLE [114,115]. Interestingly, a study in a Korean population showed an association of the p53 codon 72 polymorphism with SLE susceptibility, and individuals with the Pro allele were found to be more susceptible to SLE than those carrying the Arg allele [116]. Furthermore, two case-control studies from Anhui province in China and Shiraz in Iran also revealed that p53 codon 72 (rs1042522) may be associated with susceptibility of SLE in Chinese and Iranian populations [117,118].
Recent findings have shown that p53 may be a crucial factor in the pathogenesis of SLE. The tumor suppressor p53 has been shown to play central roles in apoptosis, cell proliferation, and DNA repair [119-121]. In addition, p53 suppresses autoimmunity. Indeed, overexpression of p53 and the presence of autoantibodies to the C-terminal domain of p53 inhibit the functions of p53 in patients with SLE and murine lupus [122-127]. Moreover, mutations in the TP53 tumor-suppressor gene are prognostic factors for the development of lymph proliferative disorders in patients with autoimmune diseases, including rheumatoid arthritis, SLE, dermatomyositis, progressive systemic sclerosis, and autoimmune hemolytic anemia [128]. p53 reduces regulatory T cells, consequently suppressing the development of autoimmunity [129,130]. However, the roles of genetic polymorphisms in p53 in SLE remain unclear. The p53 codon 72 polymorphism was not associated with SLE in Spanish and Polish populations [131,132]. Moreover, a study in Caucasian, African American, and Asian children and adults also demonstrated a lack of association of the TP53 Arg72Pro SNP and the MDM2 SNP309 with SLE [133]. However, a meta-analysis of associations between p53 codon 72 polymorphisms and SLE demonstrated that p53 codon 72 may explain why Asians but not Europeans are susceptible to SLE [134]. In contrast, MDM2 SNP309 may promote the expression of the MDM2 gene by increasing the affinity of transcriptional activator of nuclear hormone receptors (Sp1), leading to the higher levels of MDM2 RNA and protein and attenuating the p53 pathway [135,136]. The SNP309 may also affect the roles of hormones, such as estrogen, in tumorigenesis because the G-allele of SNP309 increases the affinity of the protein for Sp1 [137]. Activation of MDM2 may also reduce the numbers of plasma cells and CD3+CD4-CD8- T cells, leading to the production of autoantibodies and immune complexes and aggravating the development of SLE and lupus nephritis in a mouse model of lupus [138].
Taken together, these findings demonstrate that polymorphisms in both p53 codon 72 (rs1042522) and MDM2 SNP309 (rs2279744) are involved in the pathogenesis of SLE and that climate factors, such as minimum winter temperature, latitude, and summer downward solar radiation, may affect SLE by modulating the allele frequency distributions of p53 codon 72 and MDM2 SNP309.
Hancock et al. showed that the SNP rs2313132, located in the upstream promoter region of PCDH18, was strongly correlated with summer UVR from a worldwide analysis. Additionally, the SNP rs2187668, located in the region of the first intron of HLA-DQA1, was strongly correlated with relative humidity in Africa and Western Eurasia. Both polymorphic loci were confirmed to be related to SLE genetic susceptibility [115]. However, a case-control study from Anhui province in China found a lack of association of PCDH18 (SNP rs2313132), HLA-C (SNP rs10484554), and TLR6 (SNP rs5743810) with susceptibility to SLE in Asians, although these polymorphic loci were strongly correlated with climate factors [117]. Despite these findings, these SNPs were found to be correlated with the clinical symptoms of patients with SLE. For example, PCDH18 (SNPs rs2313132), which was strongly correlated with summer UVR, was correlated with leucopenia; TP53 (rs1042522), which was strongly correlated with minimum winter temperature, latitude, and summer shortwave radiation, was correlated with discoid erythema; HLA-C (rs10484554), which was strongly correlated with summer precipitation rate, was correlated with leucopenia, alopecia, and fever; and TLR6 (rs5743810), which was strongly correlated with winter UVR, was correlated with pericarditis, oral ulcers, and photosensitivity. These SNPs may be associated with the geographical distribution of patients with SLE in China [117].
Sun et al. suggested that the SNP rs11868112 in the RPTOR gene was strongly correlated with latitude and winter temperature and hypothesized that the frequency of the derived T allele may increase with decreasing temperature and increasing latitude. These changes may promote regulation of the immune response through mammalian target of rapamycincomplex 1, consequently reducing the expression of RPTOR to maintain the balance between pathogen pressure and immune response. Conversely, low latitudes and high temperatures, under which conditions pathogen diversity is increased [139], induce the production of RPTOR to enhance the immune response; this can result in increased risk of susceptibility to autoimmune diseases, such as SLE [140]. Further studies of the association of RPTOR (SNP rs11868112) with SLE are required.
Overall, differentiation between polymorphic loci and ethnic groups may explain why different populations exhibit differences in racial compositions when exposed to distinct environmental factors, such as UVR, temperature, and latitude (Table 4). These factors can affect the roles of these polymorphic loci in the development of SLE by changing the allele frequency distribution.
Table 4.
SLE susceptibility genes
| SLE susceptibility genes | Induction factors | Symptoms |
|---|---|---|
| p53 codon 72 (rs1042522) | Minimum winter, temperature, latitude, summer downward solar radiation. | Rheumatoidarthritis, SLE. |
| MDM2 SNP 309 (rs2279744) | Minimum winter, temperature, latitude, summer downward solar radiation. | Aggravating the development of SLE and lupus nephritis in a mouse model of lupus. |
| PCDH18 (SNP rs2313132) | Summer UVR. | SLE genetic susceptibility. |
| HLA-DQA1 SNP rs2187668 | Humidity. | SLE genetic susceptibility. |
| TP53 (rs1042522) | Latitude, minimum winter temperature, summer shortwave radiation. | Discoid erythema. |
| HLA-C (rs10484554) | Summer precipitation rate. | Leucopenia, alopecia, fever. |
| TLR6 (rs5743810) | Winter UVR. | Pericarditis, oralulcers photosensitivity. |
| RPTOR (SNP rs11868112) | Latitude, winter Temperature. | Susceptibility to Autoimmune diseases. |
Conclusion
In this review, we summarized environmental risk factors, including UVR, season distribution, climate factors, and geographical distributions, affecting the development of SLE. The probable mechanism was assessed based on inflammatory mediators, apoptosis, autophagy in keratinocytes, epigenetic factors, and gene-environment interactions. This information is expected to facilitate the development of new strategies for preventing the occurrence and progression of SLE. Susceptible individuals should avoid environmental risk factors if possible. However, the effects of some environmental factors, particularly seasonal distribution, climate factors, and geographical distributions, on SLE are still controversial, and the information is limited. Accordingly, further studies are required to clarify the environmental determinants of SLE.
Acknowledgements
Supported by grants from the National Natural Sciences Foundation of China (81471530, 81202346), Department of Education of Guangdong Province (2017 Key Platform and Scientific Research Project, No. 2017KTSCX077).
Disclosure of conflict of interest
None.
References
- 1.Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35:311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann P, Hölzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22:181–187. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 3.Meller S, Winterberg F, Gilliet M, Müller A, Lauceviciute I, Rieker J, Neumann NJ, Kubitza R, Gombert M, Bünemann E. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: an amplification cycle triggering cutaneous lupus erythematosus. Arthritis Rheum. 2005;52:1504–1516. doi: 10.1002/art.21034. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof MG, Dutz JP. The immunopathology of cutaneous lupus erythematosus. Rheum Dis Clin North Am. 2014;40:455–474. doi: 10.1016/j.rdc.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn A, Wenzel J, Weyd H. Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol. 2014;47:148–162. doi: 10.1007/s12016-013-8403-x. [DOI] [PubMed] [Google Scholar]
- 6.Bijl M, Kallenberg CG. Ultraviolet light and cutaneous lupus. Lupus. 2006;15:724–727. doi: 10.1177/0961203306071705. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn A, Beissert S. Photosensitivity in lupus erythematosus. Autoimmunity. 2005;38:519–529. doi: 10.1080/08916930500285626. [DOI] [PubMed] [Google Scholar]
- 8.Kochevar IE. Action spectrum and mechanisms of UV radiation-induced injury in lupus erythematosus. J Invest Dermatol. 1985;85:140s–143s. doi: 10.1111/1523-1747.ep12275658. [DOI] [PubMed] [Google Scholar]
- 9.Bak E, Michalski JP. Ultraviolet-a light prolongs survival and improves immune function in (new zealand black× new zealand white) F1 hybrid mice. Arthritis Rheum. 1987;30:557–561. doi: 10.1002/art.1780300510. [DOI] [PubMed] [Google Scholar]
- 10.McGrath H Jr. Ultraviolet-A1 irradiation decreases clinical disease activity and autoantibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:129–135. [PubMed] [Google Scholar]
- 11.McGrath H, Martinez-Osuna P, Lee F. Review: Ultraviolet-A1 (340-400 nm) irradiation therapy in systemic lupus erythematosus. Lupus. 1996;5:269–274. doi: 10.1177/096120339600500405. [DOI] [PubMed] [Google Scholar]
- 12.Molina J, McGrath H Jr. Longterm ultraviolet-A1 irradiation therapy in systemic lupus erythematosus. J Rheumatol. 1997;24:1072–1074. [PubMed] [Google Scholar]
- 13.McGrath H Jr. Ultraviolet A1 (340-400 nm) irradiation and systemic lupus erythematosus. J Investig Dermatol Symp Proc. 1999;4:79–84. doi: 10.1038/sj.jidsp.5640187. [DOI] [PubMed] [Google Scholar]
- 14.Menon Y, McCarthy K, McGrath H Jr. Reversal of brain dysfunction with UV-A1 irradiation in a patient with systemic lupus. Lupus. 2003;12:479–482. doi: 10.1191/0961203303lu374oa. [DOI] [PubMed] [Google Scholar]
- 15.McGrath H Jr. Elimination of anticardiolipin antibodies and cessation of cognitive decline in a UV-A1-irradiated systemic lupus erythematosus patient. Lupus. 2005;14:859–861. doi: 10.1191/0961203305lu2164cr. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Li M, Zhao J, Ye Z, Li C, Li X, Zhu P, Wang Z, Zheng Y, Li X, Zhang M, Huang C, Zeng X. Chinese SLE Treatment and Research Group (CSTAR) registry:VIII. Influence of socioeconomic and geographical variables on disease phenotype and activity in Chinese patients with SLE. Int J Rheum Dis. 2018;21:716–724. doi: 10.1111/1756-185X.13057. [DOI] [PubMed] [Google Scholar]
- 17.Amit M, Molad Y, Kiss S, Wysenbeek A. Seasonal variations in manifestations and activity of systemic lupus erythematosus. Rheumatology (Oxford) 1997;36:449–452. doi: 10.1093/rheumatology/36.4.449. [DOI] [PubMed] [Google Scholar]
- 18.Léone J, Pennaforte J, Delhinger V, Detour J, Lefondre K, Eschard J, Etienne J. Influence of seasons on risk of flare-up of systemic lupus: retrospective study of 66 patients. Rev Med Interne. 1997;18:286–91. doi: 10.1016/s0248-8663(97)84013-1. [DOI] [PubMed] [Google Scholar]
- 19.Krause I, Shraga I, Molad Y, Guedj D, Weinberger A. Seasons of the year and activity of SLE and Behcet’s disease. Scand J Rheumatol. 1997;26:435–439. doi: 10.3109/03009749709065715. [DOI] [PubMed] [Google Scholar]
- 20.Hasan T, Pertovaara M, Yli-Kerttula U, Luukkaala T, Korpela M. Seasonal variation of disease activity of systemic lupus erythematosus in Finland: a 1 year follow up study. Ann Rheum Dis. 2004;63:1498–1500. doi: 10.1136/ard.2003.012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlesinger N, Schlesinger M, Seshan SV. Seasonal variation of lupus nephritis: high prevalence of class V lupus nephritis during the winter and spring. J Rheumatol. 2005;32:1053–1057. [PubMed] [Google Scholar]
- 22.Szeto CC, Mok HY, Chow KM, Lee TC, Leung JY, Li EK, Tsui TK, Yu S, Tam LS. Climatic influence on the prevalence of noncutaneous disease flare in systemic lupus erythematosus in Hong Kong. J Rheumatol. 2008;35:1031–1037. [PubMed] [Google Scholar]
- 23.Hua-Li Z, Shi-Chao X, De-Shen T, Dong L, Hua-Feng L. Seasonal distribution of active systemic lupus erythematosus and its correlation with meteorological factors. Clinics. 2011;66:1009–1013. doi: 10.1590/S1807-59322011000600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiche L, Jourde N, Ulmann C, Mancini J, Darque A, Bardin N, Dicostanzo MP, Thomas G, Harlé JR, Vienne J. Seasonal variations of systemic lupus erythematosus flares in southern France. Eur J Intern Med. 2012;23:250–254. doi: 10.1016/j.ejim.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Lu YW, Pan HF, Tao JH, Zou YF, Bao W, Ye DQ. Seasonal distribution of systemic lupus erythematosus activity and its correlation with climate factors. Rheumatol Int. 2012;32:2393–2399. doi: 10.1007/s00296-011-1971-2. [DOI] [PubMed] [Google Scholar]
- 26.Duarte-García A, Fang H, To CH, Magder LS, Petri M. Seasonal variation in the activity of systemic lupus erythematosus. J Rheumatol. 2012;39:1392–1398. doi: 10.3899/jrheum.111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Q, Li Y, Ye L, Deng Z, Li L, Feng Y, Liu W, Liu H. Geographical distribution, a risk factor for the incidence of lupus nephritis in China. BMC Nephrol. 2014;15:67. doi: 10.1186/1471-2369-15-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian G, Ran X, Zhou C, Deng D, Zhang P, Guo Y, Luo J, Zhou X, Xie H, Cai M. Systemic lupus erythematosus patients in the low-latitude plateau of China: altitudinal influences. Lupus. 2014;23:1537–1545. doi: 10.1177/0961203314544186. [DOI] [PubMed] [Google Scholar]
- 29.Skov L, Hansen H, Allen M, Villadsen L, Norval M, Barker J, Simon J, Baadsgaard O. Contrasting effects of ultraviolet A1 and ultraviolet B exposure on the induction of tumour necrosis factor- in human skin. Br J Dermatol. 1998;138:216–220. doi: 10.1046/j.1365-2133.1998.02063.x. [DOI] [PubMed] [Google Scholar]
- 30.Avalos-Diaz E, Alvarado-Flores E, Herrera-Esparza R. UV-A irradiation induces transcription of IL-6 and TNF alpha genes in human keratinocytes and dermal fibroblasts. Rev Rhum Engl Ed. 1999;66:13–19. [PubMed] [Google Scholar]
- 31.Brink N, Szamel M, Young A, Wittern K, Bergemann J. Comparative quantification of IL-1β, IL-10, IL-10r, TNFα and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res. 2000;49:290–296. doi: 10.1007/PL00000209. [DOI] [PubMed] [Google Scholar]
- 32.Foltyn V, Golan T. In vitro ultraviolet irradiation induces pro-inflammatory responses in cells from premorbid SLE mice. Lupus. 2001;10:272–283. doi: 10.1191/096120301680416968. [DOI] [PubMed] [Google Scholar]
- 33.Narbutt J, Lesiak A, Sysa-Jedrzejowska A, Wozniacka A, Cierniewska-Cieslak A, Boncela J, Jochymski C, Kozlowski W, Zalewska A, Skibinska M. Repeated low-dose ultraviolet (UV) B exposures of humans induce limited photoprotection against the immune effects of erythemal UVB radiation. Br J Dermatol. 2007;156:539–547. doi: 10.1111/j.1365-2133.2006.07670.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, Kimura H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32:1405–1411. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-α production in keratinocytes through enhanced gene transcription. J Invest Dermatol. 2009;129:994–1001. doi: 10.1038/jid.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reefman E, Kuiper H, Limburg PC, Kallenberg CG, Bijl M. Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythaematosus patients. Ann Rheum Dis. 2008;67:11–18. doi: 10.1136/ard.2007.070359. [DOI] [PubMed] [Google Scholar]
- 37.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tüting T, Hartmann G, Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, Gudjonsson JE, Kahlenberg JM. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type i interferon loop. J Invest Dermatol. 2017;137:115–122. doi: 10.1016/j.jid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenzel J, Tüting T. Identification of type I interferon-associated inflammation in the pathogenesis of cutaneous lupus erythematosus opens up options for novel therapeutic approaches. Exp Dermatol. 2007;16:454–463. doi: 10.1111/j.1600-0625.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 40.Zahn S, Rehkämper C, Kümmerer BM, Ferring-Schmidt S, Bieber T, Tüting T, Wenzel J. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNλ) in cutaneous lupus erythematosus. J Invest Dermatol. 2011;131:133–140. doi: 10.1038/jid.2010.244. [DOI] [PubMed] [Google Scholar]
- 41.Sontheimer C, Liggitt D, Elkon KB. Ultraviolet B irradiation causes stimulator of interferon genes-dependent production of protective type I interferon in mouse skin by recruited inflammatory monocytes. Arthritis Rheumatol. 2017;69:826–836. doi: 10.1002/art.39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heckmann M, Eberlein-König B, Wollenberg A, Przybilla B, Plewig G. Ultraviolet-A radiation induces adhesion molecule expression on human dermal microvascular endothelial cells. Br J Dermatol. 1994;131:311–318. doi: 10.1111/j.1365-2133.1994.tb08516.x. [DOI] [PubMed] [Google Scholar]
- 43.Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, Yang J, Chang S, Hemati N, Richardson B. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyberg F, Hasan T, Skoglund C, Stephansson E. Early events in ultraviolet light-induced skin lesions in lupus erythematosus: expression patterns of adhesion molecules ICAM-1, VCAM-1 and E-selectin. Acta Derm Venereol. 1999;79:431–6. doi: 10.1080/000155599750009852. [DOI] [PubMed] [Google Scholar]
- 45.Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity. 2014;47:185–192. doi: 10.3109/08916934.2013.866105. [DOI] [PubMed] [Google Scholar]
- 46.Abdulahad DA, Westra J, Reefman E, Zuidersma E, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) Lupus. 2013;22:597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]
- 47.Monroy FP, Banerjee SK, Duong T, Aviles H. Cold stress-induced modulation of inflammatory responses and intracerebral cytokine mRNA expression in acute murine toxoplasmosis. J Parasitol. 1999;85:878–886. [PubMed] [Google Scholar]
- 48.Knight RJ, Liu H, Fishman E, Reis ED. Cold ischemic injury, aortic allograft vasculopathy, and pro-inflammatory cytokine expression. J Surg Res. 2003;113:201–207. doi: 10.1016/s0022-4804(03)00199-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Yu B, Liu J, Sun Y, Li K, Su Y. Effect of sub-hypothermiain exposure on the normal monocytes of cytokines in vitro [article in Chinese. Shan Dong Yi Yao. 2007;47:44–45. [Google Scholar]
- 50.Aibiki M, Maekawa S, Nishiyama T, Seki K, Yokono S. Activated cytokine production in patients with accidental hypothermia. Resuscitation. 1999;41:263–268. doi: 10.1016/s0300-9572(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 51.Awad E, Khan S, Sokolikova B, Brunner P, Olcaydu D, Wojta J, Breuss J, Uhrin P. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 2013;11:1716–1726. doi: 10.1111/jth.12357. [DOI] [PubMed] [Google Scholar]
- 52.Atkinson JP, Gorman JC, Curd J, Hyla JF, Deegan MJ, Keren DF, Abdou NI, Walker SE. Cold dependent activation of complement in systemic lupus erythematosus. Arthritis Rheum. 1981;24:592–601. doi: 10.1002/art.1780240405. [DOI] [PubMed] [Google Scholar]
- 53.Yukiyama Y, Yoshida K, Hirose S. Complement activation at low temperature. I. The profile of complement component of patients’ sera[Article in Japanese] . Allergy. 1984;33:275–281. [PubMed] [Google Scholar]
- 54.Mathews KP, Mentyka RA, Chambers SL, Hugli TE, Herschbach JH, Zuraw BL. Cold-dependent activation of complement: recognition, assessment, and mechanism. J Clin Immunol. 1992;12:362–370. doi: 10.1007/BF00920794. [DOI] [PubMed] [Google Scholar]
- 55.Crenesse D, Gugenheim J, Hornoy J, Tornieri K, Laurens M, Cambien B, Lenegrate G, Cursio R, De Souza G, Auberger P. Protein kinase activation by warm and cold hypoxia-reoxygenation in primary-cultured rat hepatocytes-JNK1/SAPK1 involvement in apoptosis. Hepatology. 2000;32:1029–1036. doi: 10.1053/jhep.2000.19065. [DOI] [PubMed] [Google Scholar]
- 56.Fransen J, Dieker J, Hilbrands L, Berden J, van der Vlag J. Synchronized turbo apoptosis induced by cold-shock. Apoptosis. 2011;16:86–93. doi: 10.1007/s10495-010-0546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singhal P, Sagar P, Gupta S, Arya M, Gupta M, Prasad A, Loona R, Sharma P, Mattana J. Pressure modulates monocyte migration. Am J Hypertens. 1997;10:1297–1301. doi: 10.1016/s0895-7061(97)00271-9. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto H, Aikawa M, Hill CC, Weiss D, Taylor WR, Libby P, Lee RT. Biomechanical strain induces class a scavenger receptor expression in human monocyte/macrophages and THP-1 cells a potential mechanism of increased atherosclerosis in hypertension. Circulation. 2001;104:109–114. doi: 10.1161/hc2701.091070. [DOI] [PubMed] [Google Scholar]
- 59.Shiratsuch H, Basson MD. Differential regulation of monocyte/macrophage cytokine production by pressure. Am J Surg. 2005;190:757–762. doi: 10.1016/j.amjsurg.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Ye D, Li X, Zheng H, Wu X, Wang Y. The risk factors of systemic lupus erythematosus in Hefei City [article in Chinese] . Chin J Public Health. 1997;13:338–339. [Google Scholar]
- 61.Zhang L, Mei J, Huang Z, Qiu G, Zhou A, Cheng Q. Research on pathogenesis mechanisms of exogenous dampness pathogen [article in Chinese] . J Tradit Chin Med. 1999;40:496–498. [Google Scholar]
- 62.Zhang W, Cao Y, Liu H. Effects of pathogenic wind-dampness on lung tissue cytokines in rats with syndrome due to pathogenic cold invading lung. [article in Chinese] . J Chin Integr Med. 2008;6:748–751. doi: 10.3736/jcim20080717. [DOI] [PubMed] [Google Scholar]
- 63.Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–5786. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- 64.Casciola-Rosen L, Rosen A. Ultraviolet light-induced keratinocyte apoptosis: a potential mechanism for the induction of skin lesions and autoantibody production in LE. Lupus. 1997;6:175–180. doi: 10.1177/096120339700600213. [DOI] [PubMed] [Google Scholar]
- 65.Batista LF, Kaina B, Meneghini R, Menck CF. How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutat Res Rev Mutat Res. 2009;681:197–208. doi: 10.1016/j.mrrev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Zhao L, Cui N, Yang P, Zhao X, Lu J, Xiao W. The effect of ultraviolet ray on Fas antigen of T-lymphocytes in patients with systemic lupus erythematosus [article in Chinese] . Chin J Phys Med Rehabil. 2005;27:92–94. [Google Scholar]
- 67.Bijl M, Reefman E, Limburg PC, Kallenberg CG. Inflammatory clearance of apoptotic cells after UVB challenge. Autoimmunity. 2007;40:244–248. doi: 10.1080/08916930701357125. [DOI] [PubMed] [Google Scholar]
- 68.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Kuhn A, Herrmann M, Kleber S, Beckmann-Welle M, Fehsel K, Martin-Villalba A, Lehmann P, Ruzicka T, Krammer PH, Kolb-Bachofen V. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939–950. doi: 10.1002/art.21658. [DOI] [PubMed] [Google Scholar]
- 70.Reefman E, Kuiper H, Jonkman MF, Limburg PC, Kallenberg CG, Bijl M. Skin sensitivity to UVB irradiation in systemic lupus erythematosus is not related to the level of apoptosis induction in keratinocytes. Rheumatology (Oxford) 2006;45:538–544. doi: 10.1093/rheumatology/kei249. [DOI] [PubMed] [Google Scholar]
- 71.Reefman E, De Jong M, Kuiper H, Jonkman MF, Limburg PC, Kallenberg CG, Bijl M. Is disturbed clearance of apoptotic keratinocytes responsible for UVB-induced inflammatory skin lesions in systemic lupus erythematosus? Arthritis Res Ther. 2006;8:R156. doi: 10.1186/ar2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeFeber W, Norris D, Ryan S, Huff J, Lee L, Kubo M, Boyce S, Kotzin B, Weston W. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol. 1990;94:77–85. doi: 10.1111/1523-1747.ep12873930. [DOI] [PubMed] [Google Scholar]
- 74.Golan TD, Elkon KB, Gharavi AE, Krueger JG. Enhanced membrane binding of autoantibodies to cultured keratinocytes of systemic lupus erythematosus patients after ultraviolet B/ultraviolet A irradiation. J Clin Invest. 1992;90:1067. doi: 10.1172/JCI115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oke V, Vassilaki I, Espinosa A, Strandberg L, Kuchroo VK, Nyberg F, Wahren-Herlenius M. High Ro52 expression in spontaneous and UV-induced cutaneous inflammation. J Invest Dermatol. 2009;129:2000–2010. doi: 10.1038/jid.2008.453. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Xu M, Min X, Wu K, Zhang T, Li K, Xiao S, Xia Y. TWEAK/Fn14 activation participates in Ro52-mediated photosensitization in cutaneous lupus erythematosus. Front Immunol. 2017;8:651. doi: 10.3389/fimmu.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrade F, Casciola-Rosen LA, Rosen A. Generation of novel covalent RNA-protein complexes in cells by ultraviolet B irradiation: implications for autoimmunity. Arthritis Rheum. 2005;52:1160–1170. doi: 10.1002/art.20992. [DOI] [PubMed] [Google Scholar]
- 78.Meyer JN, Bess AS. Involvement of autophagy and mitochondrial dynamics in determining the fate and effects of irreparable mitochondrial DNA damage. Autophagy. 2012;8:1822–1823. doi: 10.4161/auto.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Wang H, Wang S, Xu M, Liu M, Liao M, Frank JA, Adhikari S, Bower KA, Shi X. GSK3β signaling is involved in ultraviolet B-induced activation of autophagy in epidermal cells. Int J Oncol. 2012;41:1782–1788. doi: 10.3892/ijo.2012.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen LH, Chu PM, Lee YJ, Tu PH, Chi CW, Lee HC, Chiou SH. Targeting protective autophagy exacerbates UV-triggered apoptotic cell death. Int J Mol Sci. 2012;13:1209–1224. doi: 10.3390/ijms13011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bess AS, Ryde IT, Hinton DE, Meyer JN. UVC-Induced mitochondrial degradation via autophagy correlates with mtDNA damage removal in primary human fibroblasts. J Biochem Mol Toxicol. 2013;27:28–41. doi: 10.1002/jbt.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y, Zhang CF, Rossiter H, Eckhart L, König U, Karner S, Mildner M, Bochkov VN, Tschachler E, Gruber F. Autophagy is induced by UVA and promotes removal of oxidized phospholipids and protein aggregates in epidermal keratinocytes. J Invest Dermatol. 2013;133:1629–1637. doi: 10.1038/jid.2013.26. [DOI] [PubMed] [Google Scholar]
- 83.Misovic M, Milenkovic D, Martinovic T, Ciric D, Bumbasirevic V, Kravic-Stevovic T. Short-term exposure to UV-A, UV-B, and UV-C irradiation induces alteration in cytoskeleton and autophagy in human keratinocytes. Ultrastruct Pathol. 2013;37:241–248. doi: 10.3109/01913123.2012.756568. [DOI] [PubMed] [Google Scholar]
- 84.Chen YT, Laggner M, Eckhart L, Gruber F, Schmidt-Erfurth U, Pollreisz A. Autophagy regulates redox balance and maintains stemness of limbal stem cells under UVA-induced oxidative stress. Invest Ophthalmol Vis Sci. 2015;56:3455–3455. [Google Scholar]
- 85.Lamore SD, Wondrak GT. Autophagic-lysosomal dysregulation downstream of cathepsin B inactivation in human skin fibroblasts exposed to UVA. Photochem Photobiol Sci. 2012;11:163–172. doi: 10.1039/c1pp05131h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamore SD, Wondrak GT. UVA causes dual inactivation of cathepsin B and L underlying lysosomal dysfunction in human dermal fibroblasts. J Photochem Photobiol B. 2013;123:1–12. doi: 10.1016/j.jphotobiol.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson BC, Liebling MR, Hudson JL. CD4+ cells treated with DNA methylation inhibitors induce autologous B cell differentiation. Clin Immunol Immunopathol. 1990;55:368–381. doi: 10.1016/0090-1229(90)90125-a. [DOI] [PubMed] [Google Scholar]
- 88.Richardson BC, Strahler JR, Pivirotto TS, Quddus J, Bayliss GE, Gross LA, O’Rourke KS, Powers D, Hanash SM, Johnson MA. Phenotypic and functional similarities between 5-azacytidine-treated t cells and at cell subset in patients with active systemic lupus erythematosus. Arthritis Rheum. 1992;35:647–662. doi: 10.1002/art.1780350608. [DOI] [PubMed] [Google Scholar]
- 89.Quddus J, Johnson K, Gavalchin J, Amento E, Chrisp C, Yung R, Richardson B. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest. 1993;92:38. doi: 10.1172/JCI116576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, Richardson B. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–1860. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 91.Wang G, Zhang M, Li X, Zhang H, Chen W, Kan M, Wang Y. Ultraviolet B exposure of peripheral blood mononuclear cells of patients with systemic lupus erythematosus inhibits DNA methylation. Lupus. 2009;18:1037–1044. doi: 10.1177/0961203309106181. [DOI] [PubMed] [Google Scholar]
- 92.Zhu X, Li F, Yang B, Liang J, Qin H, Xu J. Effects of ultraviolet B exposure on DNA methylation in patients with systemic lupus erythematosus. Exp Ther Med. 2013;5:1219–1225. doi: 10.3892/etm.2013.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Z, Li X, Qin H, Zhu X, Xu J, Shi W. Ultraviolet B enhances DNA hypomethylation of CD4+ T cells in systemic lupus erythematosus via inhibiting DNMT1 catalytic activity. J Dermatol Sci. 2013;71:167–173. doi: 10.1016/j.jdermsci.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 94.Zhang M, Fang X, Wang GS, Ma Y, Jin L, Li XM, Li XP. Ultraviolet B decreases DNA methylation level of CD4+ T cells in patients with systemic lupus erythematosus. Inflammopharmacology. 2017;25:203–210. doi: 10.1007/s10787-017-0321-8. [DOI] [PubMed] [Google Scholar]
- 95.Detich N, Theberge J, Szyf M. Promoter-specific activation and demethylation by MBD2/demethylase. J Biol Chem. 2002;277:35791–35794. doi: 10.1074/jbc.C200408200. [DOI] [PubMed] [Google Scholar]
- 96.Wu Z, Mei X, Ying Z, Sun Y, Song J, Shi W. Ultraviolet B inhibition of DNMT1 activity via AhR activation dependent SIRT1 suppression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci. 2017;86:230–237. doi: 10.1016/j.jdermsci.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 97.Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PDS, Shin D, Chi NC, Shin CH, Schlegel A, Halpern M. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol. 2009;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pothof J, Verkaik NS, Hoeijmakers JH, van Gent DC. MicroRNA responses and stress granule formation modulate the DNA damage response. Cell Cycle. 2009;8:3462–3468. doi: 10.4161/cc.8.21.9835. [DOI] [PubMed] [Google Scholar]
- 99.Guo L, Huang ZX, Chen XW, Deng QK, Yan W, Zhou MJ, Ou CS, Ding ZH. Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol. 2009;85:765–773. doi: 10.1111/j.1751-1097.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 100.Li W, Di W, Hua L, Zhou B, Guo Z, Luo D. UVB suppresses PTEN expression by upregulating miR-141 in HaCaT cells. J Biomed Res. 2011;25:135–140. doi: 10.1016/S1674-8301(11)60017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pothof J, Verkaik NS, van IJcken W, Wiemer EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers JH, Persengiev SP. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009;28:2090–2099. doi: 10.1038/emboj.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu Y, Zhou B, Wu D, Yin Z, Luo D. Baicalin modulates microRNA expression in UVB irradiated mouse skin. J Biomed Res. 2012;26:125–134. doi: 10.1016/S1674-8301(12)60022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP. MicroRNA-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 104.Hashad D, Abdelmagid M, Elsherif S. microRNA146a expression in lupus patients with and without renal complications. J Clin Lab Anal. 2012;26:35–40. doi: 10.1002/jcla.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang L, Luo X, Huang X, Li J, Chen S. MicroRNA-125a contributes to elevated inflammatory chemokine RANTES levels via targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum. 2010;62:3425–3435. doi: 10.1002/art.27632. [DOI] [PubMed] [Google Scholar]
- 106.Song A, Chen YF, Thamatrakoln K, Storm TA, Krensky AM. RFLAT-1: a new zinc finger transcription factor that activates RANTES gene expression in T lymphocytes. Immunity. 1999;10:93–103. doi: 10.1016/s1074-7613(00)80010-2. [DOI] [PubMed] [Google Scholar]
- 107.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 108.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 109.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 110.Dong H, Jiang W, Chen H, Jiang S, Zang Y, Yu B. MicroRNA-145 attenuates IL-6-induced enhancements of sensitivity to UVB irradiation by suppressing MyD88 in HaCaT cells. Int J Immunopathol Pharmacol. 2018;32:2058738418795940. doi: 10.1177/2058738418795940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Deng D, Hu G, Qian G, Han L. In J. Dermatol.; WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030-5774, NJ USA. 2014;41:65–65. [Google Scholar]
- 112.Luo X, Zhang L, Li M, Zhang W, Leng X, Zhang F, Zhao Y, Zeng X. The role of miR-125b in T lymphocytes in the pathogenesis of systemic lupus erythematosus. Clin Exp Rheumatol. 2012;31:263–271. [PubMed] [Google Scholar]
- 113.Cao W, Qian G, Luo W, Liu X, Pu Y, Hu G, Han L, Yuan L, A X, Deng D. miR-125b is downregulated in systemic lupus erythematosus patients and inhibits autophagy by targeting UVRAG. Biomed Pharmacother. 2018;99:791–797. doi: 10.1016/j.biopha.2018.01.119. [DOI] [PubMed] [Google Scholar]
- 114.Shi H, Tan SJ, Zhong H, Hu W, Levine A, Xiao CJ, Peng Y, Qi XB, Shou WH, Ma RL, Li Y, Su B, Lu X. Winter temperature and UV are tightly linked to genetic changes in the p53 tumor suppressor pathway in Eastern Asia. Am J Hum Genet. 2009;84:534–541. doi: 10.1016/j.ajhg.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hancock AM, Witonsky DB, Alkorta-Aranburu G, Beall CM, Gebremedhin A, Sukernik R, Utermann G, Pritchard JK, Coop G, Di Rienzo A. Adaptations to climate-mediated selective pressures in humans. PLoS Genet. 2011;7:e1001375. doi: 10.1371/journal.pgen.1001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee Y, Rho Y, Choi S, Ji J, Song G. The functional p53 codon 72 polymorphism is associated with systemic lupus erythematosus. Lupus. 2005;14:842–845. doi: 10.1191/0961203305lu2224oa. [DOI] [PubMed] [Google Scholar]
- 117.Yang J. Hospital-based study on temperal-spatial distribution of systemic lupus erythematosus cases and related climate factor. (doctor thesis) [article in Chinese] Hefei, Anhui, China: Anhui Med Univ; 2014. [Google Scholar]
- 118.Nabavi M, Ghaderi A, Fattahi MJ, Danaie N, Zangooie R, Faranoush M. Original paper Association between p53 codon 72 polymorphism and systemic lupus erythematosus. Reumatologia. 2014;52:94–98. [Google Scholar]
- 119.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 120.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herkel J, Erez-Alon N, Mimran A, Wolkowicz R, Harmelin A, Ruiz P, Rotter V, Cohen IR. Systemic lupus erythematosus in mice, spontaneous and induced, is associated with autoimmunity to the C-terminal domain of p53 that recognizes damaged DNA. Eur J Immunol. 2000;30:977–984. doi: 10.1002/(SICI)1521-4141(200004)30:4<977::AID-IMMU977>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 123.Herkel J, Mimran A, Erez N, Kam N, Lohse AW, Märker-Hermann E, Rotter V, Cohen IR. Autoimmunity to the p53 protein is a feature of systemic lupus erythematosus (SLE) related to anti-DNA antibodies. J Autoimmun. 2001;17:63–69. doi: 10.1006/jaut.2001.0518. [DOI] [PubMed] [Google Scholar]
- 124.Chauhan R, Handa R, Das T, Pati U. Over-expression of TATA binding protein (TBP) and p53 and autoantibodies to these antigens are features of systemic sclerosis, systemic lupus erythematosus and overlap syndromes. Clin Exp Immunol. 2004;136:574–584. doi: 10.1111/j.1365-2249.2004.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herkel J, Kam Na, Erez N, Mimran A, Heifetz A, Eisenstein M, Rotter V, Cohen IR. Monoclonal antibody to a DNA-binding domain of p53 mimics charge structure of DNA: anti-idiotypes to the anti-p53 antibody are anti-DNA. Eur J Immunol. 2004;34:3623–3632. doi: 10.1002/eji.200425371. [DOI] [PubMed] [Google Scholar]
- 126.Kovacs B, Patel A, Hershey JN, Dennis GJ, Kirschfink M, Tsokos GC. Antibodies against p53 in sera from patients with systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 1997;40:980–982. doi: 10.1002/art.1780400531. [DOI] [PubMed] [Google Scholar]
- 127.Kuhn HM, Kromminga A, Flammann HT, Frey M, Layer P, Arndt R. p53 autoantibodies in patients with autoimmune diseases: a quantitative approach. Autoimmunity. 1999;31:229–235. doi: 10.3109/08916939908994068. [DOI] [PubMed] [Google Scholar]
- 128.Hoshida Y, Hongyo T, Xu JX, Sasaki T, Tomita Y, Nomura T, Aozasa K. TP53 gene mutation, an unfavorable prognostic factor for malignant lymphomas in autoimmune diseases. Oncology. 2005;69:175–183. doi: 10.1159/000087980. [DOI] [PubMed] [Google Scholar]
- 129.Kawashima H, Takatori H, Suzuki K, Iwata A, Yokota M, Suto A, Minamino T, Hirose K, Nakajima H. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J Immunol. 2013;191:3614–3623. doi: 10.4049/jimmunol.1300509. [DOI] [PubMed] [Google Scholar]
- 130.Takatori H, Kawashima H, Suzuki K, Nakajima H. Role of p53 in systemic autoimmune diseases. Crit Rev Immunol. 2014;34:509–516. doi: 10.1615/critrevimmunol.2014012193. [DOI] [PubMed] [Google Scholar]
- 131.Sanchez E, Sabio J, Callejas J, de Ramón E, de Haro M, Jiménez-Alonso J, Ortego-Centeno N, Sánchez-Román J, González-Gay M, López-Nevot M. Study of a functional polymorphism in thep53 gene in systemic lupus erythematosus: lack of replication in a Spanish population. Lupus. 2006;15:658–661. doi: 10.1177/0961203306070986. [DOI] [PubMed] [Google Scholar]
- 132.Piotrowski P, Lianeri M, Mostowska M, Wudarski M, Chwalińska-Sadowska H, Jagodziński P. Contribution of polymorphism in codon 72 of p53 gene to systemic lupus erythematosus in Poland. Lupus. 2008;17:148–151. doi: 10.1177/0961203307084722. [DOI] [PubMed] [Google Scholar]
- 133.Onel K, Huo D, Hastings D, Fryer-Biggs J, Crow M, Onel K. Lack of association of the TP53 Arg72Pro SNP and the MDM2 SNP309 with systemic lupus erythematosus in Caucasian, African American, and Asian children and adults. Lupus. 2009;18:61–66. doi: 10.1177/0961203308094558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee Y, Bae S, Choi S, Ji J, Song G. Associations between the p53 codon 72 polymorphisms and susceptibility to systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Lupus. 2012;21:430–437. doi: 10.1177/0961203311434941. [DOI] [PubMed] [Google Scholar]
- 135.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 136.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 137.Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, Robins H, Bartel F, Taubert H, Wuerl P, Hait W. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–5110. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 138.Allam R, Sayyed SG, Kulkarni OP, Lichtnekert J, Anders HJ. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22:2016–2027. doi: 10.1681/ASN.2011010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guernier V, Hochberg ME, Guégan JF. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2:740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun C, Southard C, Witonsky DB, Kittler R, Di Rienzo A. Allele-specific down-regulation of RPTOR expression induced by retinoids contributes to climate adaptations. PLoS Genet. 2010;6:e1001178. doi: 10.1371/journal.pgen.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]