Abstract
Mesenchymal stem cells (MSCs) can be recruited to damaged tissues directly for regeneration. Exosomes, acting as an important ingredient of MSCs-involved intercellular communication through paracrine actions, also play significant roles in tissue damage repair and have a prospect of potential clinical application. It is generally recognized that MSC-derived exosomes (MSC-exosomes) enhance tissue regeneration and repair through reducing inflammatory responses, promoting proliferation, inhibiting apoptosis and facilitating angiogenesis. This review summarizes the positive effects of human umbilical cord mesenchymal stem cells (hucMSCs) and hucMSC-derived exosomes (hucMSC-exosomes) on tissue damage and the specific mechanisms of repair action.
Keywords: HucMSC, exosomes, tissue injury repair
Introduction
Recently, mesenchymal stem cells (MSCs) and MSCs-exosomes have aroused widespread concern in the field of tissue repair and regeneration. As pluripotent stem cells, MSCs possess self-renewal and multi-directional differentiation potential [1]. MSCs can be easily obtained from a variety of tissue organs, such as bone marrow, adipose tissue and umbilical cord [2]. Compared with other MSCs, human umbilical cord mesenchymal stem cells (hucMSCs) are of interest for tissue injury repair because of low cost, minimal invasiveness, convenient isolation, large cell content, high gene transfection efficiency and low immunogenicity [3]. On account of their bioactive advantages, hucMSCs are likely to become a promising new approach for tissue repair and regeneration. Nevertheless, increasing evidence shows that hucMSCs exert their therapeutic effects mainly through the extracellular vesicles (EVs) produced by paracrine actions [4]. EVs have emerged as important mediators of intercellular communication to regulate a diverse range of biological processes [5].
Studies have shown that the EVs secreted by cells are generally referred to as microvesicles, apoptotic bodies and exosomes [4]. Exosomes are naturally present in body fluids including blood, saliva, urine and cerebrospinal fluid [6,7]. Exosomes, acting as an integral component of the interaction between cells, are being increasingly valued in the cellular microenvironment. Exosomes can regulate the biological activities of the recipient cell through shuttling bioactive molecules including proteins, nucleic acids and lipids [8]. HucMSC-exosomes, acquired by extensively expanding hucMSCs in vitro, are convenient to extract, store and transport, lower in immunogenicity and better in biocompatibility [9,10]. Simultaneously, hucMSC-exosomes have been shown to be therapy targets for tissue injury repair.
In this paper, the recent status of studies on hucMSCs and hucMSC-exosomes in animal models such as renal, hepatic and heart failure is reviewed. The problems in the clinical application of exosomes and their application prospects are evaluated.
Biological characteristics of hucMSCs and hucMSC-exosomes
When culturing the hucMSCs in suitable conditions, it can be found that the cells appear to be long spindle-shaped and adherently fibroblastic under a microscope. In 2008, our laboratory has succeeded in isolating MSCs from human umbilical cord tissue, demonstrating that their general biological characteristics are similar to those of bone marrow MSCs [11]. HucMSCs have the ability to differentiate into bone, fat, cartilage, liver, epithelium, muscle and various other types of cells [12-15]. Thanks to the availability and easy separation of and the fewer ethical restrictions on hucMSCs, more and more attention has been paid to hucMSCs worldwide. With the exhaustive investigation on hucMSCs, a great many studies are focused on their paracrine products, especially on exosomes.
The membrane of the late endosome sprouts inward to form a lumen structure and then gradually separates from the basement membrane to form vesicular structure called multivesicular bodies (MVBs). MVBs fuse with cell membranes and then release exosomes extracellularly so that exosomes exert positive effects on cell-to-cell communication [16]. Exosomes are a lipid membrane vesicle with a diameter of 30-150 nm and a density of about 1.13-1.19 g/ml [17,18]. The structure under the transmission electron microscope is like “cup” or “disk”. Exosomal surface, which carries specific markers such as CD9, CD63, CD81, Alix and TSG101, contains a variety of biologically active substances such as proteins, nucleic acids (DNA, mRNA, non-coding RNA) and lipids [16,19]. More importantly, exosomes from different sources contain specific biological substances relating to the original cells, which can not only reflect the cell types of the source, but also closely mirror the physiological function or pathological changes of the original cells [20].
HucMSCs and hucMSC-exosomes were confirmed to produce measurable benefits in tissue damage repair when administered to different animal models. 15-LOX-1, an enzyme secreted by macrophages, could be inhibited by hucMSCs resulting in repairing the dextran sulfate sodium (DSS)-induced inflammatory bowel disease (IBD) [21]. In ischemia-induced brain injury, hucMSCs contributed to the Th17/Treg differentiation through modulating the production of TGF-β1 on peripheral immune response significantly [22]. A novel finding provided a perspective that hucMSC-exosomes improved the functional recovery in spinal cord injury (SCI) mice in the way of down-regulating the inflammatory cytokines such as TNF-α, IFN-γ, IL-6 [23]. In addition, this review summarizes the functions of hucMSCs and hucMSC-exosomes in the main tissue organs damage, especially the kidney, skin, liver, lung, and heart.
HucMSCs and hucMSC-exosomes in kidney injury repair
In China, the incidence of kidney injury is increasing year by year and the types of injury are various. The number of patients with nephropathy has been growing by 100 million a year [24]. Over the past decades, nephrologists have divided kidney failure into two distinct syndromes--acute renal failure and chronic renal failure according to serum creatinine (Scr) concentration or glomerular filtration rate (GFR) [25]. At present the clinical treatments of kidney injury are confined to drugs, surgery and renal transplantation [26]. With the deepening of stem cell research, the protective effects of hucMSC and hucMSC-exosomes on renal tissue injury have been demonstrated.
Roles of hucMSCs and hucMSC-exosomes in acute kidney injury repair
Acute kidney injury (AKI) refers to the clinical syndrome that occurs due to the rapid decline of renal function caused by multiple factors. The diverse causes of acute kidney injury can be divided into three categories according to its anatomical location: pre-renal, renal and post-renal AKI [27].
Common causes of pre-renal acute kidney injury include decreased blood volume (such as fluid loss and bleeding due to various causes), reduction in effective arterial blood volume and changes in intrarenal hemodynamics. Sepsis that can cause AKI is a new research hotspot. Song et al. established a sepsis model by cecal ligation and puncture (CLP) and then treated it with hucMSCs [28]. The evidence showed that hucMSCs significantly improved the general condition of septic mice, facilitated kidney function and reduced tissue damage. It is reported that when pretreating hucMSCs with IL-1β, the immunomodulatory efficacy of MSCs could be highly improved. MiR-146a, a well-known anti-inflammatory microRNA, was strongly upregulated by IL-1β stimulation and packaged into exosomes selectively. This exosomal miR-146a was transferred to macrophages, resulting in M2 polarization, and finally leading to the amelioration of kidney damage in septic mice [29].
Renal AKI features renal parenchymal damage, including damage caused by renal tubules, renal interstitial, renal blood vessels and glomerular diseases. Ischemia/reperfusion injury (IRI) will lead to the dysfunction of renal cells as a result of extensive apotosis and inflammation [30]. In 2010, on the basis of the successful establishment of an IRI rat model, we made it clear that hucMSCs could ameliorate ischemia/reperfusion-induced acute renal failure in rats via a paracrine action [31]. In the following year, HGF-hucMSCs (HGF transduction to hucMSCs by using adenovirus-HGF) transplantation has been found to efficiently accelerate the recovery of IRI-induced AKI via anti-apoptotic and anti-inflammatory mechanisms [32]. There is a research indicating that hucMSCs can not only reduce the infiltration of macrophages into injured kindeys, but also increase the proportion of M2-like macrophages during repairing process, resulting in the amelioration of mouse renal IRI effectively [33].
The kidney is an important organ for drug metabolism and excretion. The clinical use of cisplatin is limited by its side effects of nephrotoxicity. Our previous research findings have shown that hucMSCs could accelerate the recovery of renal function by reducing the production of inflammatory cytokines and promoting the proliferation of renal tubular cells. Then we further discovered that hucMSCs transplantation reduced the content of MDA in renal tissues, indicating that hucMSCs could protect renal cells from mitochondrial dysfunction and oxidative damage [34]. Afterwards, we found that cisplatin-induced nephrotoxicity was mainly caused by oxidative stress, which could be inhibited by hucMSC-exosomes through suppressing p38MAPK (p38 mitogen-activated protein kinase) pathway [35]. In other words, our research group pretreated cisplatin-induced AKI with hucMSC-exosomes and renal damage could be alleviated by reducing the number of apoptotic cells, increasing the expression level of PCNA and activating autophagy both in vitro and in vivo [36]. 14-3-3ζ, a protein transported by hucMSC-exosomes may up-regulate the autophagic level in HK-2 (human renal proximal tubular) cells, which could prevent the cisplatin-induced nephrotoxicity [37]. On the basis of previous researches, we further indicated that hucMSC-exosomes-delivered 14-3-3ζ interacted with ATG16L to activate autophagy. Further data confirmed that hucMSC-exosomes increased ATG16L expression and that 14-3-3ζ interacted with ATG16L, promoting the localization of ATG16L at autophagosome precursors [38].
Post-renal acute kidney injury originates from acute urinary tract obstruction that may occur from the renal pelvis to any level of the urethra. Unilateral ureteral obstruction (UUO) is a kidney injury characterized by progressive renal interstitial fibrosis [39]. HucMSCs conditioned medium (hucMSC-CM) significantly reduced the expression of TGF-β1, α-SMA, TNF-α and Collagen-I in UUO kidney, promoted the proliferation of renal tubular epithelial cells (RTEs) and inhibited their apoptosis [40]. In another research, it is suggested that hucMSC-CM had protective effects against UUO-induced renal fibrosis and exhibited its anti-inflammatory effects through inhibiting TLR4/NF-κB signaling pathway [41] (Figure 1).
Figure 1.
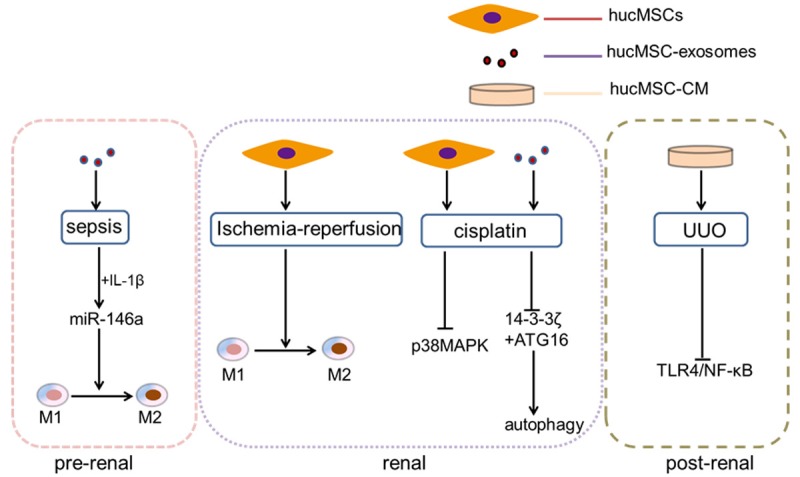
The processes of hucMSCs and hucMSC-exosomes take part in the kidney recovery in three categories of acute kidney injury.
Roles of hucMSCs and hucMSC-exosomes in chronic kidney injury repair
Diabetic nephropathy has become the main cause of chronic kidney diseases in worldwide. The incidence rate of diabetic nephropathy is second to that of primary glomerulonephritis, but it has also increased significantly in recent years [42]. The changes of podocytes in morphology and function can play important roles in the occurrence and development of diabetic nephropathy. Hepatocyte growth factor (HGF), which is secreted by hucMSCs through paracrine pathway, may ameliorate podocytic apoptosis and injury [43]. Renal fibrosis is a common pathway leading to end-stage renal failure in chronic kidney disease (CKD). In the issue of kidney failure, Huang et al. provided insight into a phenomenon, that is, infused mesenchymal stem cells could reach damaged kidney tissues with obstructive chronic progressive renal interstitial fibrosis (RIF) after a vein graft [44].
HucMSCs and hucMSC-exosomes in cutaneous wound healing
Wound healing refers to the healing process after the external force, and thus the skin appears to be broken or defective. There are three basic stages of wound healing: acute inflammation phase, cell proliferative phase and epidermal and other tissue regeneration phase [45]. In order to summarize the important roles of hucMSCs and hucMSC-exosomes in each phase of the wound healing process briefly, novel researches and their underlying mechanisms will be reviewed below. It is reported that hucMSCs enhance wound healing and establish a foundation for the role of a potential therapeutic modality for skin wound healing [46].
Functioning of hucMSCs and hucMSC-exosomes in acute inflammation phase
Early changes in wounds: there are different degrees of tissue necrosis and vascular rupture in the wound and inflammatory reactions occur within a few hours [47]. The acute inflammation phase is characterized by the accumulation of white blood cells mainly composed of neutrophils and then after 3 days, it is converted into macrophage-based enrichment [48]. MiR-21 induced cell proliferation in macrophages, which subsequently promoted efferocytosis progression. Studies have shown that miR-21 controlled the inflammatory response and promoted wound healing through silencing PTEN and GSK3β, dampening NF-κB activation and promoting c-Jun/AP1 activities [49]. In addition, when enhancing the transcription of miR-146a in macrophages, the intracorporal pro-inflammatory macrophage responses could be inhibited in order to suppress NF-κB-mediated inflammation reactions [50]. Besides, miR-181c is an essential noncoding RNA derived from hucMSC-exosomes, which could take part in anti-inflammation treatment. It is highlighted that miR-181c regulated the burn-induced inflammation through decreasing TLR4 expression and reducing NF-κB/p65 activation [51].
Functioning of hucMSCs and hucMSC-exosomes in cell proliferative phase
Proliferation of endothelial cells and fibroblasts promotes angiogenesis and new extracellular matrix (ECM) synthesis during cell proliferative phase. In the model of diabetic-induced wound closure, with the injection of hucMSCs and their conditioned media (CM), amounts of KGF (keratinocyte growth factor) and PDGF (platelet-derived growth factor) increased in the wounds. What’s more, VEGF (vasculoendothelial growth factor), the representation of angiogenesis, was more expressed in both groups. In a word, injection of hucMSCs and CM increased the angiogenesis of wounded tissue [52].
As far as we know, Wnt4 is the key factor in activating β-catenin signaling pathway. In our laboratory, Zhang et al. have found that hucMSC-exosomes had the ability to deliver Wnt4 in order to enhance wound healing and to inhibit heat stress-induced skin cell apoptosis via the activation of AKT pathway [53]. Later in 2016, our novel research supported the perspective on both the key regulators of the Hippo pathway: YAP (YES-associated protein) and p-LATS (Large Tumor Suppressor) were involved in cutaneous repair. HucMSC-exosomal 14-3-3ζ protein recruited p-LATS to induce Ser127 phosphorylation of YAP by forming a complex, which contributed to the regulation of skin cell proliferation through coordinating the self-control of Wnt4 activity effectively [54]. Analogously, it is noteworthy that in the second-degree burn injury rat model, 3,3’-diindolylmethane (DIM) enhanced the stemness of hucMSCs and their proliferation through increased exosomal Wnt11 autocrine signaling pathway, which was related to the activation of Wnt/β-catenin signaling [55].
Functioning of hucMSCs and hucMSC-exosomes in epidermal and other tissue regeneration phase
During this process, the matrix is continuously reconstituted under the action of myofibroblasts. The proportion of type I collagen increases and the proportion of type III collagen decreases. Fibroblasts give up space to further strengthen the ECM [56]. As mentioned above, it is undeniably clear that exosomal protein 14-3-3ζ controlled YAP activities and phosphorylation, so that it combined p-LATS with YAP at high cell density and the complex thus formed restricted excessive fibroblast expansion and collagen deposition during cutaneous remodeling. In brief, hucMSC-exosomes functioned as a “brake” of the signal by modulating YAP to orchestrate controlled cutaneous regeneration [54]. It is demonstrated that hucMSC-exosomes derived specific microRNAs such as miR-21, miR-23a, miR-125b and miR-145 were essential for suppressing myofibroblast formation by inhibiting the TGF-β/Smad2 pathway [57]. In a word, hucMSC-exosomes might provide a strategy to prevent scar formation during wound healing. More importantly, hucMSCs were comfirmed to be a tool that exhibited significantly better wound-healing capabilities and promoted the deposition of collagen, even reducing scar formation and avoiding the occurrence of scar [58] (Figure 2).
Figure 2.
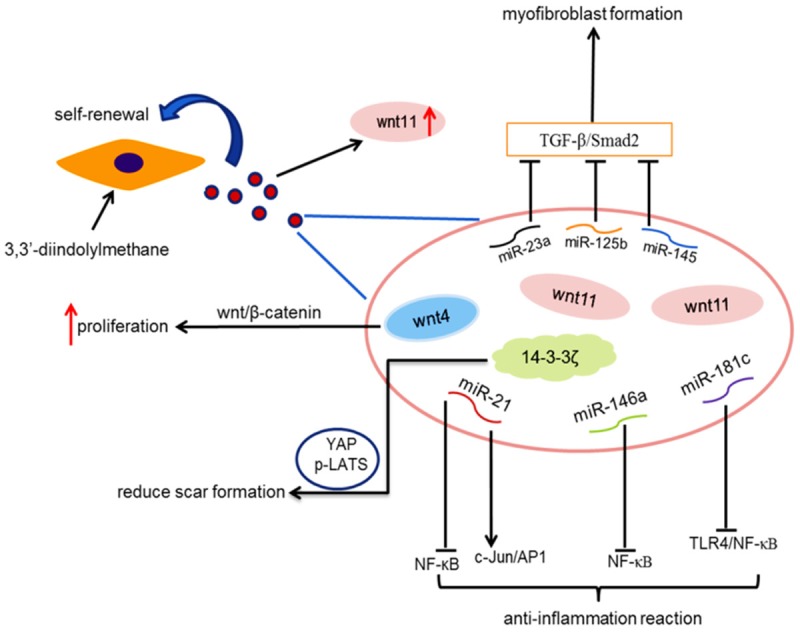
This graphic indicates that hucMSCs and hucMSC-exosomes play important roles in cutaneous wound healing through various mechanisms.
HucMSCs and hucMSC-exosomes in liver injury repair
Due to the lack of liver tissue and hepatocyte source, in recent years, researches on stem cell therapy have begun [59]. HucMSCs may be an ideal source of cells that can be transplanted for the treatment of liver diseases. In our research team, Yan et al. have succeeded in founding a model of CCl4-injured mouse liver failure [60]. HucMSCs could not only differentiate into hepatocyte-like cells through activating ERK1/2 signaling pathway [61], but also weaken mouse hepatic injury in vivo by decreasing inflammation, apoptosis and denaturation and by enhancing proliferation and recovery [62]. On the basis of previous studies on hucMSCs and liver injury, hucMSC-exosomes attenuated CCl4-induced acute liver injury/fibrosis through decreasing oxidative stress and apoptosis [63]. HucMSCs could obviously inhibit liver fibrosis, which might be related to the downregulation of TGF-β1 expression and the induction of HGF and IL-10 productions.
On the one hand, hucMSC-exosomes could ameliorate liver fibrosis by inhibiting EMT, which was activated through TGF-β1/Smad pathway in vivo; on the other hand, hucMSC-exosomes were able to reduce the surface fibrous capsules to soften liver textures and to alleviate hepatic inflammation [64]. It is demonstrated that glutathione peroxidase1 (GPX1), derived from hucMSC-exosomes, was related to the reduction of hepatic oxidative stress and apoptosis caused by CCl4 and H2O2 [65]. Any liver injury has a process of liver fibrosis during repairing. If the damage factor remained for a long time, the process of fibrosis will be developed into cirrhosis. Other researchers drew a conclusion that SB-431542, TGFβ-1 receptor inhibitor, improved the potential of hucMSCs in antifibrosis process through TGF-β1/Smad pathway [66].
HucMSCs and hucMSC-exosomes in cardiomyopathies
Cardiomyopathies, one of the most dangerous diseases all over the world, has a very high fatality rate. The traditional view is that cardiomyocytes cannot be regenerated, but with the development of medical research, it is confirmed that in some pathological conditions, cardiomyocytes can be regenerated [67]. However, there is still a long way to go before cardiomyocyte regeneration is popularized in clinical trials. The differentiative potential of hucMSCs was extensively explored in cell therapy. It is widely known that 5-Azacytidine (5-Aza) induced the differentiation of hucMSCs into cardiomyocytes, which led to changing their morphology and expressing cardiac specific proteins irrespective of the presence of bFGF [68]. However, we also have found that 5-Aza could induce hucMSCs to differentiate into cardiomyocytes in vitro through sustained ERK phosphorylation in 2012 [69]. A recent finding has demonstrated that injected hucMSCs improved cardiac function in a way of attenuating myocardial fibrosis and dysfunction via the downregulation of TGF-β1 and TNF-α expression in dilated cardiomyopathy (DCM) rats [70]. In the animal models of acute myocardial infarction (AMI), our data showed that hucMSC-exosomes could improve cardiac systolic function by protecting myocardial cells from apoptosis and by promoting angiogenesis, which was associated with the expression of Bcl-2 family [71]. What’s more, the exosomes might exhibit protective effects on acute myocardial infarction and promote cells repair through regulating Smad7 in cardiomyocyte [72].
HucMSCs and hucMSC-exosomes in lung injury repair
A novel finding indicated that when pretreated hucMSC with a low concentration of TGF-β1, the upregulation of fibronectin and other extracellular matrix components could improve the survival of a rat model of lipopolysaccharide-induced acute lung injury (ALI) [73]. Huang hypothesized that treatment with hucMSC carrying the Angiopoietin-1 (Ang1) gene could improve both systemic inflammation and alveolar permeability in ALI [74]. Similarly, in the model of endotoxin-induced ALI rats, hucMSC-exosomes could inhibit mitogen-activated protein kinase phosphorylation [75]. In recent studies, it is shown that canine radiation-induced lung injury could be reduced in the forms of reduction in oxidative stress and inflammatory reactions and the activation of TGF-β/Smad2/3 pathway after transplanting hucMSC in the models [76]. As far as we know, angiotensin-converting enzyme 2 gene (ACE2) is regarded as a homologue of ACE which may prevent lung injury resulting from acid inhalation, endotoxin shock and septicemia [77]. The combination hucMSCs with ACE2 will produce the best therapeutic effect on acute lung ischemia-reperfusion injury in rats [78]. So will it work on the bleomycin-induced lung fibrosis injury animal models [79].
HucMSCs and hucMSC-exosomes in blood glucose level regulation
The regulation of blood glucose balance is a part of life activity regulation and an important condition for maintaining the homeostasis of internal environment [80]. In 2018, our own research team investigated the relationship between hucMSC-exosomes and type 2 diabetes mellitus (T2DM) induced hyperglycemia. It should be made clear that hucMSC-exosomes characteristically decreased blood glucose levels in high-fat diet (HFD)/streptozotocin (STZ)-induced T2DM rats. HucMSC-exosomes enhanced the uptake of fluorescent glucose analogue 2-NBDG in myotubes and hepatocytes, which provided an evidence for hucMSC-exosomes-related glucose uptake. Similarly, hucMSC-exosomes promoted the muscle uptake of glucose, increased the glucose-sensitive transporter (GLUT4) expression in T2DM rats and restored the glucose homeostasis in liver through activating insulin signaling. Nothing is more important than the fact that insulin exerts an enormous effect on blood glucose regulation. We also confirmed that hucMSC-exosomes could increase insulin sensitivity both in vivo and in vitro by activating the insulin/AKT-signaling pathway. Moreover, it has been proved that hucMSC-exosomes promoted insulin secretion and islet regeneration by inhibiting STZ-induced cell apoptosis in the form of caspase-3 changes [81].
Conclusion and future directions
In summary, hucMSCs and hucMSC-exosomes participate in many biological processes and may function as novel targets for medical treatment. HucMSC-based therapeutics hold great promise for the development of therapy aimed at repairing damaged human organ tissues, though impeded by several lingering concerns [82]. Considering the disadvantages of hucMSCs, exosomes as their paracrine products are valued in damage repair. Over the past decades, it has been highlighted that hucMSC-exosomes mainly participate in promoting tissue repair and regeneration by transferring proteins, lipids and nucleotides, which boost the development of “cell-free therapy” [83].
However, given all previous researches on hucMSCs and hucMSCs-exosomes, the therapeutic mechanisms mediated by hucMSCs and hucMSCs-exosomes still need to be further explored, especially when the molecules which play effective roles in diseases have not been identified [84]. The exact molecular mechanism involved in the effect of hucMSCs and hucMSC-exosomes on tissue repair and regeneration, requires further investigations before it is actually be available. HucMSCs and hucMSC-exosomes promise to be efficiently applied to the clinic in future [85].
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 81871496), Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (Grant SS2018003), the Major Research Plan of Jiangsu Higher Education (Grant 15KJA320001), Jiangsu Province’s Major Project in Research and Development (Grant BE2016717), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986–8001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann A, Floerkemeier T, Melzer C, Hass R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J Tissue Eng Regen Med. 2017;11:2565–81. doi: 10.1002/term.2153. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Benito-Martin A, Mighty J, Chang L, Ghoroghi S, Wu H, Wong M, Guariglia S, Baranov P, Young M, Gharbaran R, Emerson M, Mark MT, Molina H, Canto-Soler MV, Selgas HP, Redenti S. Retinal progenitor cells release extracellular vesicles containing developmental transcription factors, microRNA and membrane proteins. Sci Rep. 2018;8:2823. doi: 10.1038/s41598-018-20421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, EI Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–34. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 6.Rezaie J, Ajezi S, Avci ÇB, Karimipour M, Geranmayeh MH, Nourazarian A, Sokullu E, Rezabakhsh A, Rahbarghazi R. Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol. 2018;55:3372–93. doi: 10.1007/s12035-017-0582-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka Y, Ochiya T. Investigation into the identities of circulating exosomes. Rinsho Ketsueki. 2016;57:1874–80. doi: 10.11406/rinketsu.57.1874. [DOI] [PubMed] [Google Scholar]
- 8.Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105:1384–92. doi: 10.1111/cas.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Shen L, Shi H, Pan Z, Wu L, Yan Y, Zhang X, Mao F, Qian H, Xu W. Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem Cells Int. 2016;2016:1929536. doi: 10.1155/2016/1929536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H, Wang X, Chen Y. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Xue G, Han X, Ma X, Wu H, Qin Y, Liu J, Hu Y, Hong Y, Hou Y. Effect of microenvironment on differentiation of human umbilical cord mesenchymal stem cells into hepatocytes in vitro and in vivo. Biomed Res Int. 2016;2016:8916534. doi: 10.1155/2016/8916534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H, Seo YK, Jeon S, Yoon HH, Choi YK, Park JK. Neural differentiation of umbilical cord mesenchymal stem cells by sub-sonic vibration. Life Sci. 2012;90:591–9. doi: 10.1016/j.lfs.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629–40. doi: 10.1016/j.exphem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Ott L, Seshareddy K, Weiss ML, Detamore MS. Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen Med. 2011;6:95–109. doi: 10.2217/rme.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Bio. 2012;44:2060–4. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319–23. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olver C, Vidal M. Proteomic analysis of secreted exosomes. Subcell Biochem. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 20.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 21.Mao F, Wu Y, Tang X, Wang J, Pan Z, Zhang P, Zhang B, Yan Y, Zhang X, Qian H, Xu W. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease through the regulation of 15-LOX-1 in macrophages. Biotechnol Lett. 2017;39:929–38. doi: 10.1007/s10529-017-2315-4. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Q, Zhang Z, Zhang S, Yang H, Zhang X, Pan J, Weng L, Sha D, Zhu M, Hu X, Xu Y. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res. 2015;1594:2. doi: 10.1016/j.brainres.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 23.Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. HucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–71. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 25.Chao CT, Lin YF, Tsai HB, Wu VC, Ko WJ. Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg. 2013;217:240–50. doi: 10.1016/j.jamcollsurg.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125–35. doi: 10.2174/157016111201140327163930. [DOI] [PubMed] [Google Scholar]
- 27.Caronni GM, Komaromi H, Guillermin A, Schneider M, Fumeaux Z. Acute kidney injury in 2017-management in a secondary care hospital: an example of interdisciplinary collaboration. Rev Med Suisse. 2017;13:1502–8. [PubMed] [Google Scholar]
- 28.Song Y, Zhu M, He X. Therapeutic efficacy of human umbilical cord-derived mesenchymal stem cells in septic mice. Chinese Journal of Cell Biology. 2017;39:1000–7. [Google Scholar]
- 29.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–21. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 30.Ozturk H, Cetinkaya A, Duzcu SE, Tekce BK, Ozturk H. Carvacrol attenuates histopathogic and functional impairments induced by bilateral renal ischemia/reperfusion in rats. Biomed Pharmacother. 2018;98:656–61. doi: 10.1016/j.biopha.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 31.Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–32. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, Peng X, Li W, Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–13. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Zhang Q, Wang M, Wu H, Mao F, Zhang B, Ji R, Gao S, Sun Z, Zhu W, Qian H, Chen Y, Xu W. Macrophages are involved in the protective role of human umbilical cord-derived stromal cells in renal ischemia-reperfusion injury. Stem Cell Res. 2013;10:405–16. doi: 10.1016/j.scr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Peng X, Xu H, Zhou Y, Wang B, Yan Y, Zhang X, Wang M, Gao S, Zhu W, Xu W, Qian H. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med. 2013;238:960–70. doi: 10.1177/1477153513497176. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:1–13. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8:75–88. doi: 10.1186/s13287-016-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Jia H, Zhang B, Yin L, Mao F, Yu J, Ji C, Xu X, Yan Y, Xu W, Qian H. HucMSC exosome-transported 14-3-3ζ prevents the injury of cisplatin to HK-2 cells by inducing autophagy in vitro. Cytotherapy. 2018;20:29–44. doi: 10.1016/j.jcyt.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Jia H, Liu W, Zhang B, Wang J, Wu P, Tandra N, Liang Z, Ji C, Yin L, Hu X, Yan Y, Mao F, Zhang X, Yu J, Xu W, Qian H. HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res. 2018;10:101–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Lu S, Fan HW, Li K, Fan XD. Suppression of Elp2 prevents renal fibrosis and inflammation induced by unilateral ureter obstruction (UUO) via inactivating Stat3-regulated TGF-β1 and NF-κB pathways. Biochem Biophys Res Commun. 2018;501:400–7. doi: 10.1016/j.bbrc.2018.04.227. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW, Zhang YY, Zhang DY, Wei GH. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton) 2018;23:728–736. doi: 10.1111/nep.13099. [DOI] [PubMed] [Google Scholar]
- 41.Liu B, Ding F, Hu D, Zhou Y, Long C, Shen L, Zhang Y, Zhang D, Wei G. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9:1–14. doi: 10.1186/s13287-017-0760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Wang J, Ying GS, Shen L, Zhang Z. Diabetic retinopathy and renal function in Chinese type 2 diabetic patients. Int Urol Nephrol. 2014;46:1375–81. doi: 10.1007/s11255-014-0675-4. [DOI] [PubMed] [Google Scholar]
- 43.Qi W, Lyu S, Liu G, Cheng J, Song Y, Ming T, Guan G. Human umbilical cord mesenchymal stem cells co-culture ameliorates podocytic apoptosis: a possible role of HGF. Chinese Journal Nephrology. 2014;30:933–8. [Google Scholar]
- 44.Huang D, Yi Z, He X, Mo S, Dang X, Wu X. Distribution of infused umbilical cord mesenchymal stem cells in a rat model of renal interstitial fibrosis. Ren Fail. 2013;35:1146–50. doi: 10.3109/0886022X.2013.815109. [DOI] [PubMed] [Google Scholar]
- 45.Velnar T, Bailey T, Smrkoli V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 46.Bakhtyar N, Jeschke MG, Mainville L, Herer E, Amini-Nik S. Acellular gelatinous material of human umbilical cord enhances wound healing: a candidate remedy for deficient wound healing. Front Physiol. 2017;8:1–10. doi: 10.3389/fphys.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kizil C, Kyritsis N, Brand M. Effects of inflammation on stem cells: together they strive? EMBO Rep. 2015;16:416–26. doi: 10.15252/embr.201439702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt TK, Hopf H, Hussain Z. Physiology of wound healing. Adv Skin Wound Care. 2000;13:6–11. [PubMed] [Google Scholar]
- 49.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–9. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etzrodt M, Cortez-Retamozo V, Newton A, Zhao J, Ng A, Wildgruber M, Romero P, Wurdinger T, Xavier R, Geissmann F, Meylan E, Nahrendorf M, Swirski FK, Baltimore D, Weissleder R, Pittet MJ. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 2012;1:317–24. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates miR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrestha C, Zhao L, Chen K, He H, Mo Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol. 2013;2013:592454. doi: 10.1155/2013/592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–68. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 54.Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, Fu H, Yan Y, Zhang X, Wang M, Zhu W, Qian H, Xu W. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485–2500. doi: 10.1002/stem.2432. [DOI] [PubMed] [Google Scholar]
- 55.Shi H, Xu X, Zhang B, Xu J, Pan Z, Gong A, Zhang X, Li R, Sun Y, Yan Y, Mao F, Qian H, Xu W. 3,3’-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing. Theranostics. 2017;7:1674–88. doi: 10.7150/thno.18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Zgheib C, Hodges MM, Caskey RC, Hu J, Liechty KW. Mesenchymal stem cells correct impaired diabetic wound healing by decreasing ECM proteolysis. Physiol Genomics. 2017;49:541–8. doi: 10.1152/physiolgenomics.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–39. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabapathy V, Sundaram B, VM S, Mankuzhy P, Kumar S. Human Wharton’s Jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9:e93726. doi: 10.1371/journal.pone.0093726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32:504–13. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Y, Xu W, Qian H, Si Y, Zhu W, Cao H, Zhou H, Mao F. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356–65. doi: 10.1111/j.1478-3231.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 61.Yan Y, Zhu Y, Sun F, Zhang B, Li L, Sun Z, Li W, Qian H, Zhu W, Xu W. Extracellular regulated protein kinases 1/2 phosphorylation is required for hepatic differentiation of human umbilical cord-derived mesenchymal stem cells. Exp Biol Med. 2015;240:534–45. doi: 10.1177/1535370214548996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Y, Qian H, Zhu W, Mao F, Li T, Xu W. Hepatocyte growth factor modified human umbilical cord mesenchymal stem cells accelerate the recovery of mouse hepatic injury. Rom Biotechnol Lett. 2012;17:7673–83. [Google Scholar]
- 63.Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. doi: 10.1155/2018/6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. HucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–79. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xuan J, Feng W, An ZT, Yang J, Xu HB, Li J, Zhao ZF, Wen W. Anti-TGFβ-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through TGFβ-1/Smad pathway. Mol Cell Biochem. 2017;429:113–22. doi: 10.1007/s11010-017-2940-1. [DOI] [PubMed] [Google Scholar]
- 67.Manabe T, Fukuda K. Regenerative medicine in cardiology. Nihon Geka Gakkai Zasshi. 2004;105:454–8. [PubMed] [Google Scholar]
- 68.Hollweck T. Cardiac differentiation of human Wharton’s Jelly stem cells- experimental comparison of protocols. Open Tissue Engineering & Regenerative Medicine Journal. 2011;4:95–102. [Google Scholar]
- 69.Qian Q, Qian H, Zhang X, Zhu W, Yan Y, Ye S, Peng X, Li W, Xu Z, Sun L, Xu W. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev. 2012;21:67–75. doi: 10.1089/scd.2010.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Zhou G, Chen Y, Liu S, Chen F, Xie L, Wang W, Zhang Y, Wang T, Lai X, Ma L. Human umbilical cord mesenchymal stem cells alleviate interstitial fibrosis and cardiac dysfunction in a dilated cardiomyopathy rat model by inhibiting TNF-α and TGF-β1/ERK1/2 signaling pathways. Mol Med Rep. 2018;17:71–8. doi: 10.3892/mmr.2017.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:761643. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao W, Sun L, Zhao Y, Ma J, Sun X, Zhan J, Qian H, Xu W, Zhu W. Human umbilical cord mesenchymal stem cell derived exosomes upregulates Smad7 expression for repairing acute myocardial injury in rats. Chinese Journal of Clinical Laboratory Science. 2015;33:527–31. [Google Scholar]
- 73.Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681–92. doi: 10.3892/mmr.2016.5416. [DOI] [PubMed] [Google Scholar]
- 74.Huang ZW, Liu N, Li D, Zhang HY, Wang Y, Liu Y, Zhang LL, Ju XL. Angiopoietin-1 modified human umbilical cord mesenchymal stem cell therapy for endotoxin-induced acute lung injury in rats. Yonsei Med J. 2017;58:206–16. doi: 10.3349/ymj.2017.58.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Zhu Y, Li Z, Fan X, Liu Y, Xu Y, Zhang J. Effect of human umbilical cord mesenchymal stem cell-derived exosomes on endotoxin induced acute lung injury in rats. Journal of Chinese Practical Diagnosis and Therapy. 2017;31:628–31. [Google Scholar]
- 76.Hao Y, Ran Y, Lu B, Li J, Zhang J, Feng C, Fang J, Ma R, Qiao Z, Dai X, Xiong W, Liu J, Zhou Q, Hao J, Li R, Dai J. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells on canine radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2018;102:407–16. doi: 10.1016/j.ijrobp.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 77.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, Gao F, Yan Y, Ruan Z, Liu Z. Combination therapy with human umbilical cord mesenchymal stem cells and angiotensin-converting enzyme 2 is superior for the treatment of acute lung ischemia-reperfusion injury in rats. Cell Biochem Funct. 2015;33:113–20. doi: 10.1002/cbf.3092. [DOI] [PubMed] [Google Scholar]
- 79.Min F, Gao F, Li Q, Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. 2015;11:2387–96. doi: 10.3892/mmr.2014.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. 2007;23:272–9. [PubMed] [Google Scholar]
- 81.Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613–28. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 82.Börger V, Bremer M, Görgens A, Giebel B. Mesenchymal stem/stromal cell-derived extracellular vesicles as a new approach in stem cell therapy. Isbt Science. 2016;11:228–34. [Google Scholar]
- 83.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–42. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 84.Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharm Bull. 2016;6:293–9. doi: 10.15171/apb.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7:105–15. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]


