Abstract
Postoperative cognitive dysfunction (POCD) is a neurological sequela of surgery and anesthesia. It occurs with high incidence in the aged population. Neuroinflammation is considered one of the least controversial culprits contributing to this postsurgical cognitive impairment, although it is a matter of debate as to why this complication occurs frequently in geriatric individuals. It is unclear how neuroinflammation is activated in aged populations following exposure to anesthesia and surgical procedures. In this study, we investigated the role of sirtuin 1 (SIRT1) in neuroinflammatory priming and cognitive deficits in aged rats after anesthesia and surgery. Our findings demonstrated that the hippocampal expression of SIRT1 decreased with age. The trend of declining SIRT1 expression further deteriorated in aged rats after exposure to anesthesia and surgery. Furthermore, we found that decreased SIRT1 was associated with downregulated expression of DNA methyltransferase 1 (DNMT1) and upregulated acetylated-nuclear factor kappa B (ac-NF-κB) expression, resulting in microglial activation and increased proinflammatory cytokines in the hippocampus of aged rats. Interestingly, our results showed that pretreatment with resveratrol, a SIRT1 agonist, mitigated the neuroinflammatory response and microglial activation and improved cognitive performance in the context fear-conditioning test and Morris water maze. Taken together, our findings suggest that anesthesia and surgery-induced inhibition of hippocampal SIRT1 expression is involved in the activation of neuroinflammation and cognitive impairment in aged rats and that activating SIRT1 might paved a promising path to preventing this postsurgical sequela.
Keywords: Postoperative cognitive dysfunction, SIRT1, neuroinflammation, microglia, aging
Introduction
Postoperative cognitive dysfunction (POCD) is a severe neurological complication characterized by subtle deterioration in memory, attention and information processing speed following anesthesia and surgery [1-4]. It occurs much more frequently in the elderly [5]. As the aging population is burgeoning globally, this age-related postoperative complication presents a worldwide challenge to the medical care system and socioeconomic development [5,6]. However, the pathophysiology of POCD remains under debate, and there are no clinically available measures to prevent and treat it.
There is a growing consensus regarding the role of neuroinflammation in POCD as one of the various putative pathophysiological mechanisms by which anesthesia and surgery induce cognitive dysfunction in aged rodents [7-13]. However, it remains to be elucidated how neuroinflammation is initiated in the aged brain after anesthesia and surgery. Microglia are the primary resident immune cells of the central nervous system (CNS) and are closely associated with neuroinflammatory priming in the brain following multiple exogenous insults and stress. Moreover, the microglia-derived imbalance of the neuroinflammatory response is involved in cognitive deficits in several age-related neurodegenerative diseases [14,15]. Indeed, microglia take on a stronger priming phenotype with aging [16-18]. A growing number of findings show that primed microglia in the aged brain are involved in age-related neuroinflammation and consequently produce deleterious effects on cognitive performance [15-18]. Although mounting evidence supports the etiological role of microglial priming in aged POCD models, the molecular mechanism of microglial priming needs to be further elucidated.
Sirtuin 1 (SIRT1) is a member of the nicotinamide adenine dinucleotide (NAD)+-dependent class III histone deacetylases [19]. Mounting data suggest that SIRT1 plays a vast physiological and pathological role in aging and age-related diseases [20-23]. In the CNS, SIRT1 is suggested to be involved in regulating synaptic plasticity and stress responses and in neurodegenerative injury [21,24-26]. It has been reported that decreased levels of SIRT1 in microglia contribute to the upregulation of IL-1β and further lead to age-related cognitive decline in mice [27]. In addition, nuclear factor kappa B (NF-κB) is one of the substrates of SIRT1, and acetylated NF-κB contributes to the activation of the neuroinflammatory response [28-32]. Interestingly, interventions that activate SIRT1 seem to provide anti-inflammatory and therapeutic effects in several neurodegenerative models [27,33,34]. In summary, solid experimental evidence suggests that the decrement of SIRT1 in the brain is associated with cognitive impairment and that measures focused on increasing SIRT1 might be promising strategies to prevent or treat inflammation-related injury in the brain.
In the present study, we explored a model of abdominal surgery under isoflurane anesthesia in aged rats to examine the role of SIRT1 in microglial activation and the neuroinflammatory response following anesthesia and surgery. We also investigated whether pharmacologic activation of SIRT1 could mitigate primed neuroinflammation and cognitive decline in aged POCD models.
Materials and methods
Animals
Male Sprague-Dawley rats (postnatal day 7, P7; postnatal day 14, P14; postnatal day 57, P57; and 21 months old, M21; weighing 620-670 g at the start of the experiment) were provided by the Center of Experimental Animal of Tongji Medical College. Three to four animals were housed per cage under standard laboratory conditions. All experimental protocols and animal handling procedures were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996, and the experimental protocols were approved by the committee of experimental animals of Tongji Medical College.
Anesthesia, surgery and treatment
Splenectomy was performed as described [35]. Briefly, rats were induced with 3% isoflurane in a 1:1 mix of air and 100% oxygen and maintained with 2% isoflurane in a 1:1 mix of air and 100% oxygen during surgery. A small incision approximately 2-3 cm was made in the upper left quadrant, and then the spleen was visualized, isolated, and removed. The surgical procedure was performed under sterile conditions and lasted approximately 30 min for each rat. After the surgery, the wound was infiltrated with 0.25% bupivacaine. The whole anesthesia plus surgery time for each rat was 2 hours. Animals in the control group were placed in a chamber and exposed to a 1:1 mix of air and 100% oxygen for 2 hours. Resveratrol (Selleck Chemicals, Houston, TX, USA) was prepared by dissolving the powder in a dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, USA)-saline solution and was injected intraperitoneally at 10 mg/kg/day for 7 consecutive days, with the last dose administered 12 hours before surgery. The resveratrol dose was chosen based on a previous study [36]. Animals in the control group were injected with a DMSO-saline solution for 7 consecutive days.
Experimental groups
The 21-month-old male rats were randomly assigned to the following groups: (1) CON, the control group rats were injected with the DMSO-saline solution and treated with a 1:1 mix of air and 100% oxygen; (2) ISO+SUR, the anesthesia and surgery group rats were injected with the DMSO-saline solution followed by splenectomy under isoflurane anesthesia; (3) RES, the resveratrol group rats were injected with resveratrol and treated with a 1:1 mix of air and 100% oxygen; and (4) RES+ISO+SUR, rats in this group were injected with resveratrol followed by splenectomy under isoflurane anesthesia.
Fear conditioning test
On days 12-13 after anesthesia and surgery, the animals were subjected to a fear conditioning test (FCT) as described [37]. Each rat was placed in the conditioning chamber and allowed to explore the contextual cues of the chamber for 100 s. Then, in a relatively dark room, the rats were exposed to 3 tone-foot shock pairings (tone: 5000 Hz, 85 db, 30 s; foot shock: 0.8 mA, 2 s) with 1-min intervals and were removed from the chamber 30 s later. Rats were placed back in the same chamber with no cues (tone or shock) 24 hours later for 6 min. Two hours later, the rats were placed in another test chamber that had different contextual cues, including smell, from the first test chamber and were exposed to the tone for 3 cycles (5000 Hz, 85 db, 30 s followed by a 1-min interval, 4.5 min in total) in a relatively light room. Animal behavior was video-recorded in both chambers. A decrease in the percentage of time spent freezing indicated memory impairment.
Morris water maze test
The Morris water maze (MWM) test was performed as previously described 48 hours after the completion of the full fear conditioning protocol [11,38,39]. The training test consisted of 3 trials each day for five consecutive days. During each trial, rats were placed in the water facing the wall of the maze in one of the three quadrants that did not contain the platform. The time spent searching and mounting the platform (escape latency, with a cut-off time of 120 s) and the swim speed were recorded. The probe trial (120 s), in which the platform was removed, was performed 24 hours after the end of the fifth day of training. The percentage of time spent in the target quadrant was considered an indicator of memory performance.
Western blot analysis
Rats were sacrificed 24 hours after anesthesia and surgery for Western blot analysis. Hippocampal tissue was homogenized at 4°C for 30 min in RIPA lysis buffer. The protein levels in the supernatant were determined by a BCA assay kit (Boster, Wuhan, China). Then, these samples were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) by electrophoresis. The membranes were blocked with 5% nonfat skim milk in TBST (0.1% Tween 20 in TBS) for 1 hour at room temperature and then incubated overnight at 4°C with an anti-DNA methyltransferase 1 (DNMT1) (1:1000; Cell Signaling Technology, Beverly, MA), anti-SIRT1 (1:1000; Abcam, Cambridge, UK), anti-IL6 (1:1000, Thermo Fisher Scientific, USA), anti-Acetyl-NF-kappaB (1:1000, Affinity, OH, USA) or anti-β-actin (1:1000, Affinity) antibody. On the second day, the membranes were washed three times with phosphate-buffered saline (PBS, pH 7.4) containing 0.3% Triton X-100 (PBS-T) and then incubated with a horseradish peroxidase (HRP)-conjugated goat anti-mouse or goat anti-rabbit IgG antibody (1:2000; Abbkine, Wuhan, China) for 1.5 hours at room temperature. Labeled proteins were detected with the ChemiDocXRS chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA). Bands were quantified using laboratory imaging software, and the experiments were repeated in triplicate.
Immunofluorescence
Twenty-four hours postoperation, rats were anesthetized with sodium pentobarbital (85 mg/kg) and perfused transcardially with 200 ml of 0.9% saline followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). Brains were harvested, fixed in 4% paraformaldehyde at 4°C for 24 hours and then transferred to a 30% sucrose solution. Ten-micron-thick frozen hippocampal sections were cut from the rat brains using a freezing microtome and serially collected. The tissue sections were incubated with 5% normal donkey serum in PBS for 1 hour, followed by incubation with an anti-ionized calcium-binding adapter molecule 1 (Iba1; 1:500; Wako Chemicals, Japan) antibody at 4°C overnight. After three 5-min rinses in PBS, the sections were incubated with donkey anti-rabbit IgG conjugated to Alexa Fluor®488 (1:200, Abbkine) in the dark for 1 hour at room temperature. Images were acquired with a fluorescence microscope.
TNF-α and IL-1β assays
The protein levels of TNF-α and IL-1β in hippocampal tissues were examined by enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions (R&D Systems, Minneapolis, USA). The hippocampus was homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and centrifuged at 12,000 × g for 15 min to obtain the supernatant. Protein quantification was calculated by BCA assay. The readings were normalized to the amount of standard protein. All samples were assayed in duplicate.
PCR
Hippocampal tissue was harvested 24 hours after surgery, and total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) as previously described [11,40]. Reverse transcription was performed using designed primers and Superscript II Reverse Transcriptase (Takara, Dalian, China). Quantitative real-time PCR was performed using Power SYBR Green PCR Master Mix (Takara). The results were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers for CD86, CD206 and SOCS3 were designed as described and synthesized by Takara (Takara) [41].
Statistical analysis
All data are expressed as the mean ± SD, and data were analyzed by GraphPad Prism 7.0 software. Statistical evaluation between 2 groups was performed using the unpaired Student’s t test. Statistical evaluation between >2 groups ware performed using a one-way ANOVA followed by Bonferroni’s post hoc test. Data collected from the escape latency in the MWM test were analyzed using two-way ANOVA with repeated measures followed by Bonferroni’s post hoc test to compare the four groups. P<0.05 was considered significant.
Results
Anesthesia and surgery aggravates the age-related decrease in SIRT1 expression in the hippocampus of aged rats
Given the evidence that older patients are more likely to develop POCD and that sirtuins potentially play a role in aging and neurodegenerative disease [25], we first evaluated the expression of SIRT1 in the hippocampus of male rats at four different ages: P7, P14, P57 and M21. SIRT1 expression in the hippocampus was shown to decrease in the aged rats (P<0.05, Figure 1A, 1C). Then, we examined the hippocampal expression of SIRT1 after anesthesia and surgery in the aged rats. As shown in Figure 1D, anesthesia and surgery led to a significant decrease in SIRT1 expression in the hippocampus (P<0.05, Figure 1B, 1D).
Figure 1.
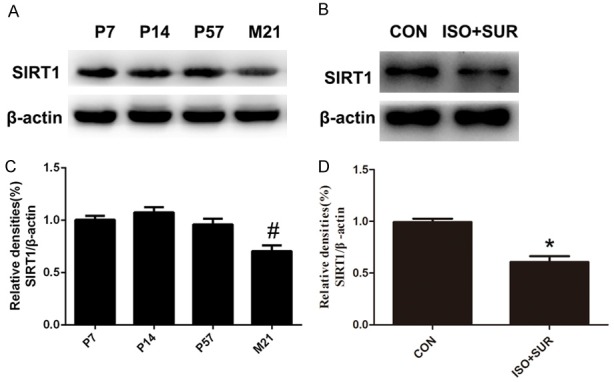
Anesthesia and surgery exacerbate the age-related downregulation of SIRT1 expression in the hippocampus. A. Representative immunoblot bands of SIRT1 expression at four different ages. B. The corresponding densitometry analysis of SIRT1 normalized to β-actin. Statistical analysis with Student’s t test showed that SIRT1 expression decreased in aged rats. C. A representative immunoblot band of SIRT1 expression in the CON and ISO+SUR groups. D. The corresponding densitometry analysis of SIRT1 normalized to β-actin. Statistical analysis with Student’s t test showed that anesthesia and surgery decreased SIRT1 expression in aged rats. Data are presented as the mean ± SD from 6 rats per group. *P<0.05, ISO+SUR group rats versus CON group rats; #P<0.05, M21 group rats versus P7, P14, and P57 group rats.
Anesthesia and surgery induces microglial overactivation in the hippocampus of aged rats
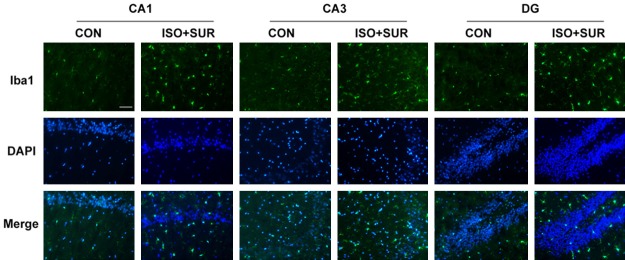
To explore the effects of anesthesia and surgery on microglial activation, we used immunostaining to detect Iba1, a classic microglial marker. Microglia are considered to be the critical resident immune cells of the brain. Studies have indicated that microglial activation in the aging brain contributes to neuronal damage and age-related cognitive decline in neurodegenerative diseases [42,43]. We observed a significant increase in the number of Iba1-positive cells in the CA1, CA3 and DG regions of the hippocampus of the ISO+SUR group compared with those in the CON group (Figure 2).
Figure 2.
Effects of anesthesia and surgery on microglial activation in the hippocampus. Immunostaining of Iba1 (green) and DAPI (blue) in the CON and ISO+SUR groups. Double staining showed that anesthesia and surgery significantly increased the number of Iba1-positive cells in the hippocampus of aged rats. Scale bar =50 μm.
Anesthesia and surgery decreases DNMT1 expression and increases ac-NF-κB and IL-6 expression in the hippocampus
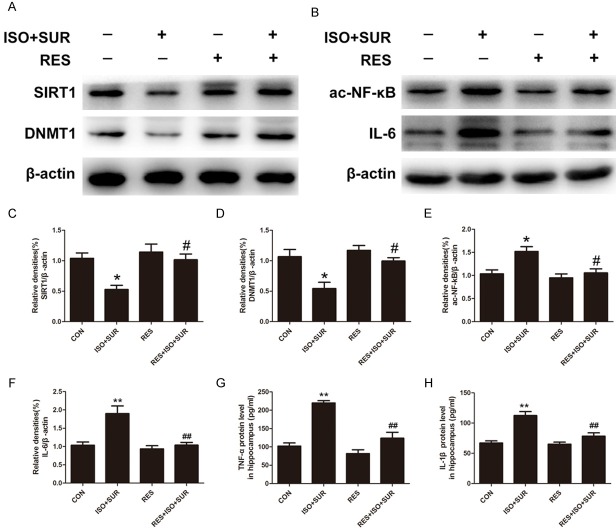
DNA methylation, one of the most studied epigenetic processes, is maintained through replication by the enzyme DNMT1. SIRT1 directly deacetylates DNMT1, alters DNMT1 activity and thereby enhances the epigenetic mechanism that underlies transcriptional suppression of proinflammatory cytokines [27,28,44,45]. We extended our experiments to examine whether DNMT1 expression was changed after anesthesia and surgery. As expected, the expression of DNMT1 in the hippocampus of rats that underwent anesthesia and surgery was significantly decreased compared with that of control rats (P<0.05, Figure 3A, 3B). NF-κB is a transcription factor that can be activated by cellular damage and stress. Its activation has been found to be involved in aging and aging-related chronic diseases [46]. However, SIRT1 regulates the acetylation level of NF-κB (ac-NF-κB) and thus represses NF-κB transcriptional activity, ultimately suppressing NF-κB-dependent gene expression, including that of proinflammatory cytokines [28]. Then, we determined whether anesthesia and surgery upregulated ac-NF-κB expression. The protein level of ac-NF-κB in the hippocampus was notably increased after anesthesia and surgery (P<0.05, Figure 3A, 3C). Next, we determined whether anesthesia and surgery caused upregulation of proinflammatory cytokines. Consistent with previous findings [10], we found that the expression of IL-6 was significantly increased in the hippocampus (P<0.01, Figure 3A, 3D).
Figure 3.
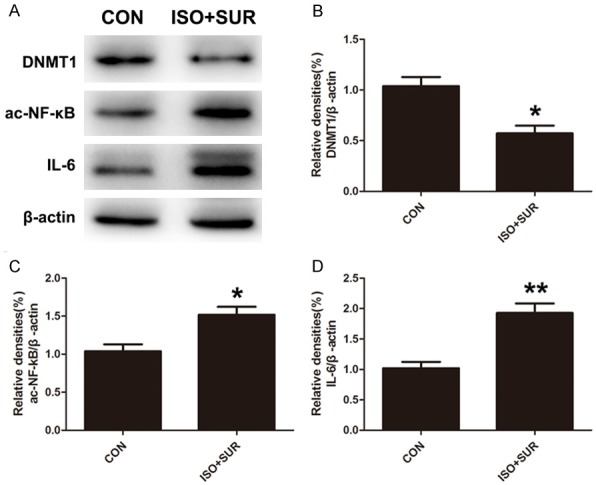
Effects of anesthesia and surgery on the levels of DNMT1 and hippocampal neuroinflammation. A. Representative immunoblot bands of DNMT1, ac-NF-κB and IL-6 expression in the hippocampus in CON and ISO+SUR groups. B-D. The corresponding densitometry analysis of DNMT1, ac-NF-κB and IL-6 expression normalized to β-actin. Statistical analysis with Student’s t test showed that anesthesia and surgery significantly decreased DNMT1 expression and increased ac-NF-κB and IL-6 expression in the hippocampus of aged rats. Data are presented as the mean ± SD from 6 rats per group. *P<0.05, **P<0.01, ISO+SUR group rats versus CON group rats.
Activation of SIRT1 inhibits microglial overactivation in the hippocampus of aged rats exposed to anesthesia and surgery
Resveratrol is an activator of SIRT1 [47]. We examined the effect of resveratrol on anesthesia and surgery-induced microglial overactivation. As shown before, anesthesia and surgery led to a notable increase in microglial activation in the hippocampus. The rats pretreated with resveratrol showed a significant decrease in the density of Iba1-positive cells in the CA1, CA3, and DG regions of the hippocampus compared with that of rats in the ISO+SUR group (Figure 4A). To further observe the effect of anesthesia and surgery on the phenotypic change of microglia in the hippocampus, phenotypic markers of microglia were tested. Anesthesia and surgery significantly increased the expression of CD86, a marker of a cytotoxic M1 phenotype in microglia, and SOCS3, a marker of an immunomodulatory M2b phenotype in microglia, while decreasing the expression of CD206, a marker of a repair/regeneration M2a phenotype in microglia (P<0.01, Figure 4B-D). However, pretreatment with resveratrol resulted in a significant decrease in the expression of CD86 and SOCS3 but an increase in the expression of CD206 in the aged rats compared with that in rats in the ISO+SUR group (P<0.01, Figure 4B-D).
Figure 4.
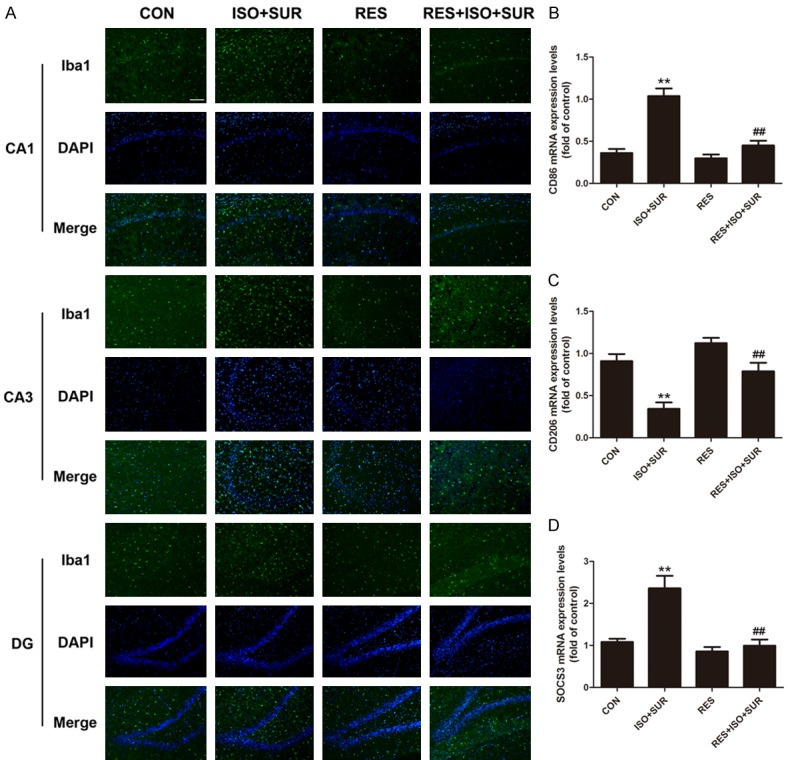
Effect of SIRT1 activation on microglial overactivation caused by anesthesia and surgery in the hippocampus of aged rats. A. Immunostaining of Iba1 (green) and DAPI (blue). Double staining showed that pretreatment with resveratrol significantly reduced Iba1-positive cells in the hippocampus of aged rats following anesthesia and surgery. Scale bar =100 μm. B. CD86 mRNA expression levels. C. CD206 mRNA expression levels. D. SOCS3 mRNA expression levels. Statistical analysis with one-way ANOVA followed by Bonferroni’s post hoc test showed that anesthesia and surgery significantly increased CD86 mRNA and SOCS3 mRNA expression and decreased CD206 mRNA expression in the hippocampus of aged rats, while pretreatment with resveratrol decreased CD86 mRNA and SOCS3 mRNA expression and increased CD206 mRNA expression in the hippocampus of aged rats. Data are presented as the mean ± SD from 6 rats per group. **P<0.01, ISO+SUR group rats versus CON group rats; ##P<0.01, RES+ISO+SUR group rats versus ISO+SUR group rats.
Activation of SIRT1 increases DNMT1 expression and decreases ac-NF-κB and proinflammatory cytokine expression in the hippocampus of aged rats after anesthesia and surgery
As previously tested, DNMT1 was downregulated in the hippocampus after anesthesia and surgery, consistent with SIRT1 expression. Interestingly, Western blot analysis revealed increased expression of DNMT1 and SIRT1 in the RES+ISO+SUR group compared with that in the ISO+SUR group (P<0.05, Figure 5A, 5C, 5D). Next, we tested the effect of resveratrol on ac-NF-κB expression in the hippocampus by Western blot. The activation of SIRT1 decreased ac-NF-κB expression in the hippocampus of rats that underwent anesthesia and surgery compared with that in rats in the ISO+SUR group (P<0.05, Figure 5B, 5E). Because the level of ac-NF-κB is positively associated with the transcription of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, we investigated whether upregulating SIRT1 could alleviate the neuroinflammatory response in the hippocampus. As reported previously, the levels of IL-6, IL-1β, and TNF-α were significantly increased in the aged rats following exposure to anesthesia and surgery in our study. However, resveratrol pretreatment remarkably decreased the levels of IL-6, IL-1β and TNF-α compared with those in the ISO+SUR group (P<0.01, Figure 5B, 5F-H).
Figure 5.
Effects of SIRT1 activation on the decreased levels of DNMT1 and increased hippocampal neuroinflammation caused by anesthesia and surgery. A and B. Representative immunoblot bands of SIRT1, DNMT1, ac-NF-κB and IL-6 expression in the hippocampus. C-F. The corresponding densitometry analysis of SIRT1, DNMT1, ac-NF-κB and IL-6 expression normalized to β-actin. Statistical analysis with one-way ANOVA followed by Bonferroni’s post hoc test showed that pretreatment with resveratrol significantly increased SIRT1 and DNMT1 expression and decreased ac-NF-κB and IL-6 expression in the hippocampus of aged rats. G and H. Protein levels of TNF-α and IL-1β detected by ELISA. Statistical analysis with one-way ANOVA followed by Bonferroni’s post hoc test showed that anesthesia and surgery significantly increased the protein levels of TNF-α and IL-1β in the hippocampus, while pretreatment with resveratrol significantly decreased the protein levels of TNF-α and IL-1β in aged rats. Data are presented as the mean ± SD from 6 rats per group. *P<0.05, **P<0.01, ISO+SUR group rats versus CON group rats; #P<0.05, ##P<0.01, RES+ISO+SUR group rats versus ISO+SUR group rats.
Activation of SIRT1 ameliorates cognitive impairment in aged rats after anesthesia and surgery
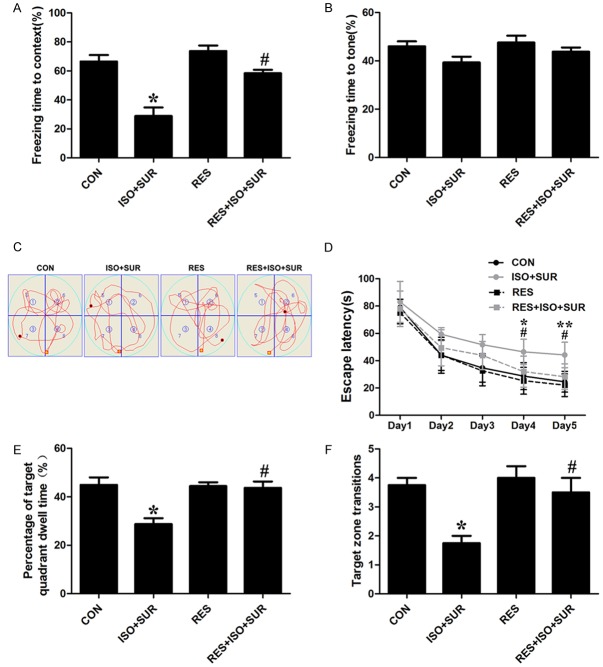
We investigated whether SIRT1 activation could prevent anesthesia and surgery-related cognitive impairment in aged rats. The FCT was performed to assess associative learning and memory. The FCT studies showed that rats that underwent anesthesia and surgery exhibited a decrease in freezing times in the context test compared with rats in the CON group (P<0.05, Figure 6A), while the tone test of the FCT did not show significant differences between the CON group and the ISO+SUR group. Next, we found that the rats pretreated with resveratrol showed longer freezing times compared with rats in the ISO+SUR group in the context test of the FCT (P<0.05, Figure 6A). However, the tone test of the FCT did not show any significance among experimental groups, indicating that anesthesia and surgery selectively impaired associative learning and memory in the aged rats and that this impairment was rescued by resveratrol. We also evaluated spatial learning and memory performance using the MWM on days 15-20 after anesthesia and surgery. Significant differences were observed in the escape latency among the various groups. On the fifth training day, the rats in the ISO+SUR group spent more time locating the hidden platform compared with rats in the CON group (P<0.01, Figure 6D), indicating that anesthesia and surgery induced spatial learning impairment in the aged rats. Moreover, the rats pretreated with resveratrol spent a shorter time finding the platform compared with rats in the ISO+SUR group (P<0.05, Figure 6D), indicating that resveratrol attenuated anesthesia and surgery-induced spatial learning impairment in the aged rats. In the probe trial, the rats in the ISO+SUR group showed a reduction in the percentage of time spent in the target quadrant and a decrease in the frequency of target zone transitions, suggesting that anesthesia and surgery also caused spatial memory impairment in the aged rats (P<0.05, Figure 6E, 6F). Interestingly, resveratrol reversed anesthesia and surgery-induced spatial memory impairments in the aged rats, as revealed by the increased percentage of time spent in the target quadrant and the frequency of target zone transitions (P<0.05, Figure 6E, 6F).
Figure 6.
Effects of SIRT1 activation on cognitive function in aged rats following anesthesia and surgery. A. Freezing time in the fear conditioning context test. B. Freezing time in the fear conditioning tone test. C. Representative swim paths obtained on the fourth day of training in the MWM. D. Escape latency in the MWM in five training days. E. The percentage of time spent in the target quadrant in the probe trial of the MWM. F. The number of target zone transitions in the probe trial of the MWM. Statistical evaluation was performed using one-way ANOVA followed by Bonferroni’s post hoc test except for escape latency in the MWM test, which was analyzed using two-way ANOVA with repeated measures followed by Bonferroni’s post hoc test. Statistical analysis showed that pretreatment with resveratrol significantly mitigated the cognitive deficits induced by anesthesia and surgery in aged rats. Data are presented as the mean ± SD from 10 rats per group. *P<0.05, **P<0.01, ISO+SUR group rats versus CON group rats; #P<0.05, RES+ISO+SUR group rats versus ISO+SUR group rats.
Discussion
In the present study, our findings showed that decreased hippocampal expression of SIRT1 was involved in anesthesia and surgery-induced neuroinflammation and cognitive decline in aged rats. The decreased DNMT1 and increased ac-NF-κB levels underlay the activation of neuroinflammation resulting from postsurgical SIRT1 inhibition in the hippocampus. Pretreatment with resveratrol increased the expression of SIRT1, changed the trend of DNMT1 and ac-NF-κB expression induced by anesthesia and surgery and consequently mitigated the cognitive impairment of aged rats in the FCT and the MWM test. These findings suggest that SIRT1 modulates hippocampal neuroinflammation by regulating the expression of DNMT1 and ac-NF-κB in our aged POCD model.
A wealth of evidence indicates that individuals exposed to anesthesia and surgery show a high risk of cognitive decline after such procedures. This risk increases with age [2,28,29]. A vast range of molecular mechanisms have been proposed to explain this aged-related postoperative complication, but few of them are universally accepted. However, there has been great interest in neuroinflammation and its role in aged POCD models because aging is accompanied by neuroinflammatory priming [48-51]. Hence, we investigated whether age-related neuroinflammatory changes are involved in POCD and its relevant signaling pathways. As previously reported, we found increased microglial activation and the release of inflammatory cytokines in the aged hippocampus [15,52-54]. Our next step was to examine the mechanism involved in neuroinflammation activation in aged rats.
SIRT1 is a deacetylase that has been shown to regulate diverse cellular processes, including brain development, aging, stress, inflammation and cancer [20,21,55]. In the adult brain, SIRT1 is associated with modulating synaptic plasticity and memory formation [24,26]. Given its important role in healthy brain aging and calorie restriction, SIRT1 has also been found to be significantly inhibited in a number of neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease [22,23,56,57]. A delicate study found that microglial expression of SIRT1 decreased with aging. Moreover, this decrease was associated with aged-related cognitive decline [27]. In the present study, we tested hippocampal SIRT1 levels in P7, P14, P57 and M21 rats, showing that SIRT1 expression decreased with age. Then, we investigated whether anesthesia and surgery influenced hippocampal SIRT1 expression in aged rats. Interestingly, we found that SIRT1 expression was significantly reduced after anesthesia and surgery. These results suggest that anesthesia and surgery aggravate the age-dependent decrease of SIRT1 in the hippocampus of aged rats. However, it is unclear whether this change in SIRT1 is involved in microglial activation in POCD.
As a sensor of brain injury and aging, microglia constantly survey changes to the cerebral microenvironmental due to endogenous to exogenous insults [58]. Therefore, we examined whether SIRT1 inhibition was associated with microglial activation in the hippocampus of our aged POCD model. As expected, immunofluorescent detection showed that microglia were activated in the CA1, CA3, and DG regions of the hippocampus. Whether microglial activation mediates neuroprotective or neurotoxic effects depends on the subphenotype of the microglia [59]. Remarkably, our findings showed that the neurotoxic subphenotypes M1 and M2b were significantly increased, but the neuroprotective subphenotype M2a was notably decreased after anesthesia and surgery. Pretreatment with the SIRT1 agonist resveratrol enhanced the expression of SIRT1 in the hippocampus and inhibited microglial activation in our aged POCD model. The changes in microglial phenotypes caused by anesthesia and surgery were also reversed by resveratrol pretreatment. These results suggest that anesthesia and surgery-induced SIRT1 downregulation is closely correlated with microglial activation.
In the context of exploring the role of neuroinflammation in POCD, a growing body of research has attributed the upregulated neuroinflammatory response to the shuttling of the p65 subunit of NF-κB to the nucleus, thus further promoting the transcription of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α [11,13,60,61]. Inhibition of the p65 NF-κB signaling pathway relieves POCD after sevoflurane anesthesia [62]. SIRT1 physically interacts with the p65 subunit of NF-κB and inhibits transcription by deacetylating p65 at lysine 310 [63]. It has been reported that a boost in SIRT1 promotes the deacetylation of p65, thereby suppressing transcriptional activation by NF-κB in SH-SY5Y cells [64]. In our study, the level of ac-NF-κB was increased following anesthesia and surgery. The activity of SIRT1 was enhanced by resveratrol pretreatment, resulting in a reduction in ac-NF-κB in our model. DNA methylation is a common epigenetic signal that inhibits the transcription of various factors, including proinflammatory cytokines. Recently, epigenetic alterations, in particular alterations in DNA methylation, have been observed during the inflammatory response [65]. DNMT1 activity has been suggested to affect the methylation status of IL-6, TNF-α and IL-1β promoters and thereby affect the expression of IL-6, TNF-α and IL-1β [27,45]. Surprisingly, SIRT1 has been found to directly modify DNMT1 activity via deacetylation of DNMT1 at specific lysine residues, thereby enhancing its methyltransferase activity and reducing its transcriptional repressive activity [66]. Consistent with the previous study, our data showed the downregulation of DNMT1 in the hippocampus after anesthesia and surgery, while pretreatment with the SIRT1 agonist resveratrol reversed DNMT1 expression in our aged POCD model. In summary, these findings suggest that an exacerbated SIRT1 decrease is associated with dysregulated hippocampal neuroinflammation via modulation of the deacetylation of p65 NF-κB and DNMT1 in our aged POCD model.
In rodents, the presence of POCD can be identified by the detection of deficits in fear learning and spatial-based working memory. The fear conditioning test is a widely used experimental tool for exploring the neurobiological basis of fear learning in many species [37]. In the present study, we found that anesthesia and surgery impaired fear learning and that pharmacological activation of SIRT1 with resveratrol alleviated this cognitive impairment in aged rats. The results were then confirmed in the MWM test. Taken together, these results show that cognitive dysfunction in our aged POCD model is strongly correlated with SIRT1 decrease in the hippocampus and that pharmacological enhancement of SIRT1 provides a potentially neuroprotective effect.
Conclusions
In summary, the present study demonstrated that SIRT1 mediated the activation of the neurotoxic phenotype of microglia and neuroinflammation partially via regulation of the expression of ac-NF-κB and DNMT1 in the hippocampus of an aged POCD model. Consequently, these changes resulted in impairment in cognitive function. However, pretreatment with the SIRT1 agonist resveratrol increased the expression of SIRT1, inhibited the neuroinflammatory response in the hippocampus and improved cognitive function in aged models. Therefore, SIRT1 is a promising therapeutic target for preventing and treating POCD in the aged population.
Acknowledgements
The present work was supported by grants from the National Natural Science Foundation of China (grant NO. 81400882 to Shiyong Li, grant NOs. 81771159, 81571047 and 81271233 to Ailin Luo, grant NO. 8150051085 to Yilin Zhao), Science and Technology Projects of Wuhan (grant number 2015060101010036 to Ailin Luo) and also supported by 2010 Clinical Key Disciplines Construction Grant from the Ministry of Health of China (grant to Anesthesiology Disciplines of Tongji Medical College).
Disclosure of conflict of interest
None.
References
- 1.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 4.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 6.Silbert B, Evered L, Scott DA. Cognitive decline in the elderly: is anaesthesia implicated? Best Pract Res Clin Anaesthesiol. 2011;25:379–393. doi: 10.1016/j.bpa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L. Post-Operative Cognitive Dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Feng X, Valdearcos M, Lutrin D, Uchida Y, Koliwad SK, Maze M. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–545. doi: 10.1016/j.bja.2017.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li SY, Xia LX, Zhao YL, Yang L, Chen YL, Wang JT, Luo AL. Minocycline mitigates isoflurane-induced cognitive impairment in aged rats. Brain Res. 2013;1496:84–93. doi: 10.1016/j.brainres.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 17.Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- 19.Dillin A, Kelly JW. Medicine. The yin-yang of sirtuins. Science. 2007;317:461–462. doi: 10.1126/science.1146585. [DOI] [PubMed] [Google Scholar]
- 20.Guarente L. Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 21.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, Seredenina T, Arbez N, Zhu S, Sommers K, Qian J, Zhang J, Mori S, Yang XW, Tamashiro KL, Aja S, Moran TH, Luthi-Carter R, Martin B, Maudsley S, Mattson MP, Cichewicz RH, Ross CA, Holtzman DM, Krainc D, Duan W. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2011;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008;58:10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho SH, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, Krabbe G, Sohn PD, Lo I, Minami S, Devidze N, Zhou Y, Coppola G, Gan L. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci. 2015;35:807–818. doi: 10.1523/JNEUROSCI.2939-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing H, Lin H. Sirtuins in epigenetic regulation. Chem Rev. 2015;115:2350–2375. doi: 10.1021/cr500457h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 30.Natoli G. When sirtuins and NF-kappaB collide. Cell. 2009;136:19–21. doi: 10.1016/j.cell.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in aging and disease. Aging Dis. 2011;2:449–465. [PMC free article] [PubMed] [Google Scholar]
- 32.Lanzillotta A, Pignataro G, Branca C, Cuomo O, Sarnico I, Benarese M, Annunziato L, Spano P, Pizzi M. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol Dis. 2013;49:177–189. doi: 10.1016/j.nbd.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol Res. 2013;67:60–67. doi: 10.1016/j.phrs.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, Liao Y, Tong JB, Terrando N, Ouyang W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. doi: 10.1111/cns.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milad MR, Igoe S, Orr SP. Fear conditioning in rodents and humans. 2011;50:111–132. [Google Scholar]
- 38.Tan L, Chen X, Wang W, Zhang J, Li S, Zhao Y, Wang J, Luo A. Pharmacological inhibition of PTEN attenuates cognitive deficits caused by neonatal repeated exposures to isoflurane via inhibition of NR2B-mediated tau phosphorylation in rats. Neuropharmacology. 2017;114:135–145. doi: 10.1016/j.neuropharm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao YL, Xiang Q, Shi QY, Li SY, Tan L, Wang JT, Jin XG, Luo AL. GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons’ exposure to isoflurane. Anesth Analg. 2011;113:1152–1160. doi: 10.1213/ANE.0b013e318230b3fd. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S, Qian Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hefendehl JK, Neher JJ, Suhs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014;13:60–69. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 44.Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu HS, Hung SC. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer. 2015;136:547–559. doi: 10.1002/ijc.29033. [DOI] [PubMed] [Google Scholar]
- 45.Shen J, Liu Y, Ren X, Gao K, Li Y, Li S, Yao J, Yang X. Changes in DNA methylation and chromatin structure of pro-inflammatory cytokines stimulated by LPS in broiler peripheral blood mononuclear cells. Poult Sci. 2016;95:1636–1645. doi: 10.3382/ps/pew086. [DOI] [PubMed] [Google Scholar]
- 46.Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, Kaeberlein M, Tryggvason K, Zhou Z. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16:738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Gemma C. Neuroimmunomodulation and aging. Aging Dis. 2010;1:169–172. [PMC free article] [PubMed] [Google Scholar]
- 49.Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 50.Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Z, Zhao Y, Ruan L, Zhu L, Jin K, Zhuge Q, Su DM, Zhao Y. Impact of aging immune system on neurodegeneration and potential immunotherapies. Prog Neurobiol. 2017;157:2–28. doi: 10.1016/j.pneurobio.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33:1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan H, Cao J, Zhang J, Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. J Neuroinflammation. 2014;11:93. doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satoh A, Imai SI, Guarente L. The brain, sirtuins, and ageing. Nat Rev Neurosci. 2017;18:362–374. doi: 10.1038/nrn.2017.42. [DOI] [PubMed] [Google Scholar]
- 56.Theendakara V, Patent A, Peters Libeu CA, Philpot B, Flores S, Descamps O, Poksay KS, Zhang Q, Cailing G, Hart M, John V, Rao RV, Bredesen DE. Neuroprotective Sirtuin ratio reversed by ApoE4. Proc Natl Acad Sci U S A. 2013;110:18303–18308. doi: 10.1073/pnas.1314145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. 2013;110(Suppl 1):i82–91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602. doi: 10.1016/j.bbrc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 61.Li Z, Liu F, Ma H, White PF, Yumul R, Jiang Y, Wang N, Cao X. Age exacerbates surgery-induced cognitive impairment and neuroinflammation in Sprague-Dawley rats: the role of IL-4. Brain Res. 2017;1665:65–73. doi: 10.1016/j.brainres.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Zheng JW, Meng B, Li XY, Lu B, Wu GR, Chen JP. NF-kappaB/P65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:394–407. [PubMed] [Google Scholar]
- 63.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nopparat C, Sinjanakhom P, Govitrapong P. Melatonin reverses H2 O2 -induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-kappaB. J Pineal Res. 2017;63 doi: 10.1111/jpi.12407. [DOI] [PubMed] [Google Scholar]
- 65.Hartnett L, Egan LJ. Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis. 2012;33:723–731. doi: 10.1093/carcin/bgs006. [DOI] [PubMed] [Google Scholar]
- 66.Peng L, Yuan Z, Ling H, Fukasawa K, Robertson K, Olashaw N, Koomen J, Chen J, Lane WS, Seto E. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol Cell Biol. 2011;31:4720–4734. doi: 10.1128/MCB.06147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]