Abstract
We investigated the characteristics of the choriocapillaris flow voids using optical coherence tomography angiography (OCTA) in 85 patients (164 eyes) with hypertension (mean ± SD age, 56 ± 11 years; 45% women; 20% poorly controlled BP; 16% diabetes) who are without ocular diseases and determined possible correlations with systemic vascular risk factors. Data on 24-hour ambulatory blood pressure (BP), serum creatinine, and urine microalbumin/creatinine ratio (MCR) were collected. Estimated glomerular filtration rate (eGFR) was calculated based on CKD-EPI Creatinine Equation. OCTA imaging (6 × 6 mm scans; AngioVue) with quantitative microvascular analysis of the choriocapillaris was performed. Linear regression was used to investigate the association of systemic risk factors with area (percentage), size (pixels) and number of choriocapillaris flow voids. Diabetes (β = 0.33; 95% CI, 0.02 to 0.63) and daytime systolic BP (β = −0.13; 95% CI, −0.24 to −0.02) were associated with areas of flow voids. Age (β = 0.21; 95% CI, 0.06 to 0.36) and daytime diastolic BP (β = −0.18; 95% CI, −0.34 to −0.02) were associated with size of flow voids. Age (β = −21.21; 95% CI, −31.79 to −10.63) and nighttime diastolic BP (β = 13.89; 95% CI, 0.61 to 27.17) were associated with number of flow voids. Kidney parameters were not associated with any features of flow voids. In patients with hypertension, a higher BP appeared to increase blood flow in the choriocapillaris which needs to be considered when using the OCTA to study eye diseases in hypertensives.
Introduction
Age-related macular degeneration (AMD) is the leading cause of central visual loss in the elderly population1. There is increasing evidence that the choroid plays a crucial pathophysiological role in the development of AMD2. Recent studies using optical coherence tomography (OCTA) showed that eyes with AMD tended to have less perfused choriocapillaris (e.g., larger areas of flow voids)3–6. This is in keeping with two longitudinal studies indicating the low choroidal blood flow as assessed with laser Doppler flowmetry, was associated with an increased risk for developing choroidal neovascularization in AMD7,8.
Systemic hypertension has been shown in various studies to be associated with AMD or choroidal neovascularization (CNV)9–12. This finding has suggested a role of systemic vascular abnormalities in the development and worsening of AMD. Studies correlating blood flow in the choroid and hypertension have yielded discordant conclusions thus far. Spaide et al. showed larger areas of flow voids in eyes of hypertensives compared to non-hypertensives when using OCTA6. Among the non-hypertensives, Polak et al. reported a small, but significant increase in choroidal blood flow in subjects with increasing BP13. It is uncertain whether the observed disparity between these studies were due to the influence of eye diseases instead of systemic disease (inclusion of AMD cases in Spaide’s6 vs eyes that are without any eye diseases in Polak’s13), different imaging technologies (i.e. OCTA vs laser interferometry and color Doppler imaging) or absence of BP measurements in Spaide’s6 or the inaccuracy of one-off office BP measurements in Polak’s13.
Furthermore, chronic kidney disease has also been shown to be associated with AMD14. The choroid and the kidney has the highest perfusion rate per gram tissue15,16. Therefore, systemic vascular influences can presumably affect both the choroid and kidney circulatory parameters. Previous study relied solely on the subjective-based questionnaire and reported no significant associations between history of kidney diseases and choroidal blood flow parameters using laser Doppler flowmetry17. The subjective nature of the questionnaire that was used to elucidate medical histories may be less reliable than the objective measurements of kidney function using biological data (serum creatinine, urine microalbumin/creatinine ratio (MCR), estimated glomerular filtration rate (eGFR).
Despite the widespread use of OCTA technology in studying AMD eyes18, there is limited understanding on the impact of systemic vascular risks on choriocapillaris flow in eyes without eye diseases. In an effort to resolve this issue, we investigated the effect of 24-hour ambulatory BP and renal parameters on flow characteristics of the choriocapillaris using OCTA in adults with treated systemic hypertension. We hypothesized that systemic hypertensive patients with higher levels of BP or poorer kidney function will have larger areas of flow voids that are also increased in size and reduced in number.
Results
Table 1 shows the characteristics of the participants with systemic hypertension, as stratified by their BP levels. The mean ± SD age of participants was 56 ± 11 years and 45% were female (n = 39). In terms of their BP control, 18 (21%) were classified as having intensive BP control, 50 (59%) having standard BP control and 17 (20%) having poor BP control. Among the BP control groups, persons with intensively controlled BP who tended to be on angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers had lower levels of urine MCR and larger areas of flow voids (P < 0.05 each).
Table 1.
Clinical characteristics of treated hypertensive participants, stratified by blood pressure control.
| Intensive BP control | Standard BP control | Poor BP control | *P value | |
|---|---|---|---|---|
| Number. of participants | 18 | 50 | 17 | |
| Number. of eyes | 35 | 95 | 34 | |
| Gender, female (%) | 10 (56%) | 22 (44%) | 7 (41%) | 0.637 |
| Ethnicity, Chinese (%) | 14 (78%) | 43 (86%) | 14 (82%) | 0.870 |
| Smoking Status, Never (%) | 18 (100%) | 45 (90%) | 15 (88%) | 0.575 |
| Diabetes (%) | 6 (33%) | 5 (10%) | 3 (18%) | 0.082 |
| Hyperlipidemia (%) | 10 (56%) | 23 (46%) | 7 (41%) | 0.677 |
| Chronic Kidney Disease (%) | 1 (6%) | 3 (6%) | 1 (6%) | 1.000 |
| Type of antihypertensive medications | ||||
| Beta blockers | 5 (28%) | 22 (44%) | 4 (24%) | 0.219 |
| Calcium channel blockers | 6 (33%) | 28 (56%) | 12 (71%) | 0.080 |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | 16 (89%) | 24 (48%) | 7 (41%) | 0.005 |
| Others (diuretics, alpha 2 adrenergic agonist) | 4 (22%) | 5 (10%) | 2 (12%) | 0.410 |
| Numbers of antihypertensive medications | ||||
| One | 7 (39%) | 29 (58%) | 10 (59%) | 0.620 |
| Two | 8 (44%) | 13 (26%) | 5 (29%) | |
| Three or more | 3 (17%) | 8 (16%) | 2 (12%) | |
| Mean ± SD/median (IQR) | ||||
| Age, years | 55 ± 7 | 57 ± 11 | 56 ± 11 | 0.834 |
| Body mass index, kg/m2 | 27 (25–30) | 26 (24–29) | 25 (24–30) | 0.645 |
| Ambulatory BP, mmHg | ||||
| Systolic BP | 112 ± 4 | 128 ± 5 | 146 ± 9 | <0.001 |
| Diastolic BP | 72 ± 5 | 77 ± 7 | 85 ± 7 | <0.001 |
| Estimated GFR, mL/min/1.73 m2 | 94 (88–102) | 91 (75–101) | 95 (87–105) | 0.191 |
| Creatinine, µmol/L | 67 (55–79) | 77 (59–92) | 73 (62–80) | 0.159 |
| Urine MCR, mg/g | 0.9 (0–2.5) | 0.6 (0–2.2) | 2.3 (0.9–3.7) | 0.022 |
| Choriocapillaries flow voids | ||||
| Area of flow voids, % | 17.2 (16.5–17.8) | 16.8 (16.2–17.2) | 16.7 (16.4–17.1) | 0.017 |
| Size of flow voids, pixels | 10.8 ± 0.9 | 10.6 ± 0.8 | 10.9 ± 0.9 | 0.321 |
| Number of flow voids | 1199 ± 65 | 1191 ± 70 | 1176 ± 65 | 0.305 |
SD = standard deviation; IQR = interquartile range; BP = blood pressure; GFR = glomerular filtration rate; MCR = microalbumin-to-creatinine ratio.
Data are number (%) or mean ± SD or median (interquartile range), as appropriate.
*Test for differences between groups, based on analysis of variance (anova) for normally distributed continuous variables or kwallis for non-normally distributed continuous variables and with chi-square tests or Fisher’s exact test for categorical variables. Bold face indicates statistically significant P value.
Associations between systemic factors and areas of flow voids are shown in Table 2. Univariate linear regression analysis showed that gender, diabetes, hypertensive medications (diuretics, alpha 2 adrenergic agonist), BP control, systolic BP, daytime systolic BP, diastolic BP and daytime diastolic BP were associated with areas of flow voids (all P < 0.10; Table 2). Age and kidney parameters were not significantly associated with areas of flow voids in the univariate model. In the multivariate-adjusted model, diabetes (β = 0.33; 95% CI, 0.02 to 0.63; P = 0.039), lower systolic BP (β = −0.11; 95% CI, −0.22 to −0.01; P = 0.046), and lower daytime systolic BP (β = −0.13; 95% CI, −0.24 to −0.02; P = 0.025; Table 2) were associated with larger areas of flow voids. Figure 1 further illustrates the relation of systolic BP and areas of flow voids showing that a person with lower systolic BP often had larger areas of flow voids compared to a person with higher systolic BP having smaller areas of flow voids. Age, gender, hypertensive medications (diuretics, alpha 2 adrenergic agonist), BP control, and diastolic BP were not significantly associated with areas of flow voids in the multivariate-adjusted model.
Table 2.
Associations of systemic factors with area of flow voids (dependent variable) in hypertensive participants (n = 164 eyes).
| Univariate | Multivariate-adjusted | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | |
| Age, per 10 years | 0.05 | −0.09 to 0.20 | 0.477 | 0.02 | −0.12 to 0.17 | 0.738 |
| Gender | −0.28 | −0.56 to 0.08 | 0.056 | −0.24 | −0.51 to 0.03 | 0.084 |
| Diabetes | 0.36 | −0.01 to 0.73 | 0.050 | 0.33 | 0.02 to 0.63 | 0.039 |
| Type of antihypertensive medications | ||||||
| Beta blockers | −0.01 | −0.30 to 0.28 | 0.959 | — | — | — |
| Calcium channel blockers | −0.11 | −0.41 to 0.19 | 0.469 | — | — | — |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | 0.10 | −0.19 to 0.38 | 0.502 | — | — | — |
| Others (diuretics, alpha 2 adrenergic agonist) | 0.32 | −0.01 to 0.65 | 0.051 | 0.29 | −0.03 to 0.62 | 0.072 |
| Numbers of antihypertensive medications | ||||||
| One | Reference | |||||
| Two | −0.06 | −0.38 to 0.25 | 0.691 | — | — | — |
| Three or more | 0.18 | −0.19 to 0.56 | 0.334 | — | — | — |
| BP control status | ||||||
| Intensive BP control | Reference | Reference | ||||
| Standard BP control | −0.40 | −0.78 to −0.02 | 0.037 | −0.29 | −0.66 to 0.09 | 0.134 |
| Poor BP control | −0.42 | −0.87 to 0.03 | 0.067 | −0.36 | −0.78 to 0.06 | 0.093 |
| Systolic BP | ||||||
| Systolic BP, overall, per 10 mmHg | −0.13 | −0.24 to −0.01 | 0.030 | −0.11 | −0.22 to −0.01 | 0.046 |
| Systolic BP, day, per 10 mmHg | −0.15 | −0.26 to −0.03 | 0.015 | −0.13 | −0.24 to −0.02 | 0.025 |
| Systolic BP, night, per 10 mmHg | −0.05 | −0.14 to 0.075 | 0.365 | — | — | — |
| Diastolic BP | ||||||
| Diastolic BP, overall, per 10 mmHg | −0.19 | −0.37 to −0.02 | 0.032 | −0.13 | −0.31 to 0.06 | 0.173 |
| Diastolic BP, day, per 10 mmHg | −0.20 | −0.37 to −0.03 | 0.019 | −0.15 | −0.33 to 0.04 | 0.115 |
| Diastolic BP, night, per 10 mmHg | −0.10 | −0.26 to 0.07 | 0.238 | — | — | — |
| Kidney Parameters | ||||||
| Estimated GFR, mL/min/1.73 m2 | 0.04 | −0.56 to 0.65 | 0.886 | — | — | — |
| Creatinine | −0.39 | −0.87 to 0.09 | 0.112 | — | — | — |
| Urine MCR, mg/g | 0.03 | −0.12 to 0.18 | 0.709 | — | — | — |
CI = confidence interval; BP = blood pressure; GFR = glomerular filtration rate; MCR = microalbumin-to-creatinine ratio.
Multivariate model - adjusted for age, gender, diabetes, other types of BP medications, BP control and systolic BP.
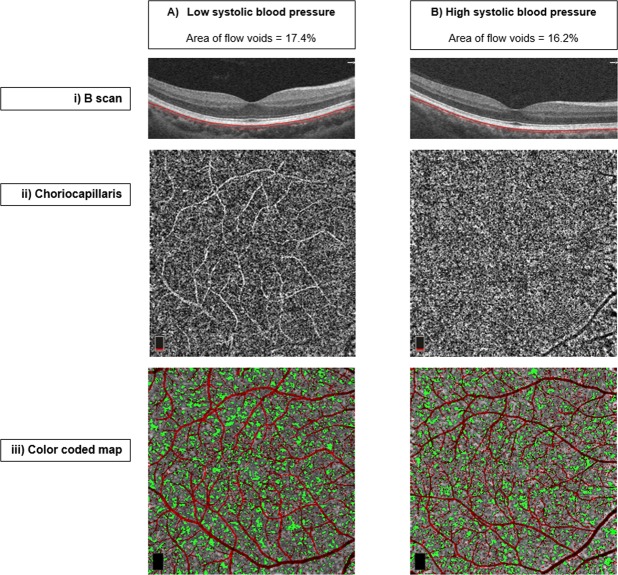
Figure 1.
Area of choriocapillaris flow voids on optical coherence tomography angiography (OCTA) correlated significantly with ambulatory systolic blood pressure. (i) B scan of choriocapillaris. (ii) Choriocapillaris en face plexus. (iii) Color coded map of the flow voids (green) and superficial vascular plexus (red). Choriocapillaris flow voids map showing participant with (A) Low systolic blood pressure (larger area of flow voids) and (B) Low systolic blood pressure (smaller area of flow voids).
Table 3 show the association between systemic factors and size of flow voids. Univariate linear regression analysis showed that age, diabetes, calcium channel blockers, diastolic BP and daytime diastolic BP were associated with size of flow voids (all P < 0.10; Table 3). BP control, systolic BP and kidney parameters were not associated with size of flow voids in the univariate model. In a multivariate model, older age (β = 0.21; 95% CI, 0.06 to 0.36; P = 0.006), calcium channel blockers (β = 0.31; 95% CI, 0.04 to 0.57; P = 0.024), lower diastolic BP (β = −0.19; 95% CI, −0.37 to −0.01; P = 0.034), and lower daytime diastolic BP (β = −0.18; 95% CI, −0.34 to −0.02; P = 0.032; Table 3) were associated with larger size of flow voids. Diabetes was not significantly associated with size of flow voids in the multivariate-adjusted model.
Table 3.
Associations of systemic factors with size of flow voids (dependent variable) in hypertensive participants (n = 164 eyes).
| Univariate | Multivariate-adjusted | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | |
| Age, per 10 years | 0.27 | 0.11 to 0.43 | 0.001 | 0.21 | 0.06 to 0.36 | 0.006 |
| Gender | −0.02 | −0.33 to 0.29 | 0.894 | 0.07 | −0.22 to 0.36 | 0.638 |
| Diabetes | 0.52 | 0.03 to 1.01 | 0.039 | 0.27 | −0.17 to 0.72 | 0.226 |
| Type of antihypertensive medications | ||||||
| Beta blockers | −0.09 | −0.41 to 0.24 | 0.593 | — | — | — |
| Calcium channel blockers | 0.29 | −0.01 to 0.59 | 0.062 | 0.31 | 0.04 to 0.57 | 0.024 |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | −0.02 | −0.32 to 0.29 | 0.921 | — | — | — |
| Others (diuretics, alpha 2 adrenergic agonist) | −0.05 | −0.42 to 0.33 | 0.805 | — | — | — |
| Numbers of antihypertensive medications | ||||||
| One | Reference | Reference | ||||
| Two | 0.07 | −0.28 to 0.41 | 0.696 | — | — | — |
| Three or more | 0.11 | −0.37 to 0.60 | 0.653 | — | — | — |
| BP control status | ||||||
| Intensive BP control | Reference | Reference | ||||
| Standard BP control | −0.15 | −0.53 to 0.23 | 0.433 | — | — | — |
| Poor BP control | 0.11 | −0.38 to 0.60 | 0.666 | — | — | — |
| Systolic BP | ||||||
| Systolic BP, overall, per 10 mmHg | −0.03 | −0.15 to 0.09 | 0.634 | — | — | — |
| Systolic BP, day, per 10 mmHg | −0.04 | −0.15 to 0.08 | 0.534 | — | — | — |
| Systolic BP, night, per 10 mmHg | 0.00 | −0.12 to 0.11 | 0.952 | — | — | — |
| Diastolic BP | ||||||
| Diastolic BP, overall, per 10 mmHg | −0.27 | −0.45 to −0.08 | 0.004 | −0.19 | −0.37 to −0.01 | 0.034 |
| Diastolic BP, day, per 10 mmHg | −0.27 | −0.44 to −0.09 | 0.002 | −0.18 | −0.34 to −0.02 | 0.032 |
| Diastolic BP, night, per 10 mmHg | −0.20 | −0.40 to 0.01 | 0.056 | −0.17 | −0.36 to 0.02 | 0.082 |
| Kidney Parameters | ||||||
| Estimated GFR, mL/min/1.73 m2 | −0.46 | −1.26 to 0.34 | 0.262 | — | — | — |
| Creatinine | −0.15 | −0.71 to 0.42 | 0.604 | — | — | — |
| Urine MCR, mg/g | 0.12 | −0.04 to 0.29 | 0.138 | — | — | — |
CI = confidence interval; BP = blood pressure; GFR = glomerular filtration rate; MCR = microalbumin-to-creatinine ratio.
Multivariate model - adjusted for age, gender, diabetes, calcium channel blockers and diastolic BP.
The association between systemic factors and numbers of flow voids is shown in Table 4. Univariate linear regression analysis showed that age, calcium channel blockers, diastolic BP, daytime diastolic BP and nighttime diastolic BP were associated with numbers of flow voids (all P < 0.10; Table 4). Diabetes, BP control, systolic BP and kidney parameters were not associated with numbers of flow voids in the univariate model. In a multivariate model, younger age (β = −21.21; 95% CI, −31.79 to −10.63; P < 0.001), absence of calcium channel blockers (β = −24.48; 95% CI, −44.43 to −4.53; P = 0.016), and higher nighttime diastolic BP (β = 13.89; 95% CI, 0.61 to 27.17; P = 0.040; Table 4) were significantly associated with greater number of flow voids.
Table 4.
Associations of systemic factors with number of flow voids (dependent variable) in hypertensive participants (n = 164 eyes).
| Univariate | Multivariate-adjusted | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | |
| Age, per 10 years | −22.82 | −34.51 to −11.13 | <0.001 | −21.21 | −31.79 to −10.63 | <0.001 |
| Gender | −8.25 | −31.67 to 15.17 | 0.490 | −15.08 | −0.22 to 0.36 | 0.164 |
| Diabetes | −20.42 | 57.86 to 17.03 | 0.285 | −3.00 | −35.32 to 29.33 | 0.856 |
| Type of antihypertensive medications | ||||||
| Beta blockers | 9.30 | −17.66 to 36.26 | 0.499 | — | — | — |
| Calcium channel blockers | −19.12 | −41.53 to 3.30 | 0.095 | −24.48 | −44.43 to −4.53 | 0.016 |
| Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers | 4.02 | −18.86 to 26.90 | 0.731 | — | — | — |
| Others (diuretics, alpha 2 adrenergic agonist) | 7.99 | −21.24 to 37.23 | 0.592 | — | — | — |
| Numbers of antihypertensive medications | ||||||
| One | Reference | Reference | ||||
| Two | −6.67 | −32.27 to 18.92 | 0.609 | — | — | — |
| Three or more | 3.14 | −40.48 to 46.76 | 0.653 | — | — | — |
| BP control status | ||||||
| Intensive BP control | Reference | Reference | ||||
| Standard BP control | −5.34 | −32.54 to 21.87 | 0.701 | — | — | — |
| Poor BP control | −24.48 | −55.62 to 6.66 | 0.123 | — | — | — |
| Systolic BP | ||||||
| Systolic BP, overall, per 10 mmHg | −1.46 | −10.85 to 7.94 | 0.761 | — | — | — |
| Systolic BP, day, per 10 mmHg | −1.79 | −10.69 to 7.10 | 0.693 | — | — | — |
| Systolic BP, night, per 10 mmHg | −0.05 | −8.56 to 8.47 | 0.992 | — | — | — |
| Diastolic BP | ||||||
| Diastolic BP, overall, per 10 mmHg | 16.40 | 3.71 to 29.10 | 0.011 | 12.16 | −0.03 to 24.36 | 0.051 |
| Diastolic BP, day, per 10 mmHg | 15.19 | 28.62 to 27.53 | 0.016 | 10.13 | −1.35 to 21.61 | 0.084 |
| Diastolic BP, night, per 10 mmHg | 15.15 | 1.27 to 29.03 | 0.032 | 13.89 | 0.61 to 27.17 | 0.040 |
| Kidney Parameters | ||||||
| Estimated GFR, mL/min/1.73 m2 | 49.16 | −7.53 to 105.86 | 0.089 | — | — | — |
| Creatinine | −4.39 | −45.13 to 36.35 | 0.833 | — | — | — |
| Urine MCR, mg/g | −8.56 | −23.87 to 6.76 | 0.274 | — | — | — |
CI = confidence interval; BP = blood pressure; GFR = glomerular filtration rate; MCR = microalbumin-to-creatinine ratio.
Multivariate model - adjusted for age, gender, diabetes, calcium channel blockers and diastolic BP.
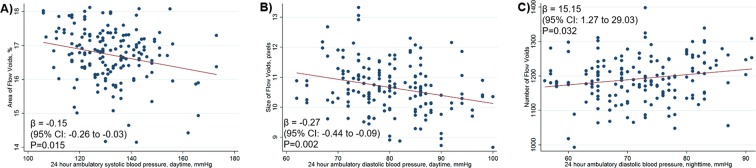
Figure 2 further illustrates the association observed between BP with varying features (by area, size and number) of choriocapillaris flow voids. In patients with hypertension, a higher BP appeared to have smaller area of flow voids (β = −0.15; 95% CI, −0.26 to −0.03; P = 0.015; Fig. 2A), that are also reduced in size (β = −0.27; 95% CI, −0.44 to −0.09; P = 0.002; Fig. 2B) and greater in numbers (β = 15.15; 95% CI, 1.27 to 29.03; P = 0.032; Fig. 2B).
Figure 2.
Scatterplot showing negative correlations of (A) area of choriocapillaris flow voids with 24-hour ambulatory daytime systolic blood pressure and (B) size of choriocapillaris flow voids with 24-hour ambulatory daytime diastolic blood pressure.
Discussion
Certain features of choriocapillaris flow voids were significantly correlated with 24-hour ambulatory BP, presence of diabetes and use of calcium channel blockers in systemic hypertensive patients. Our results suggest increased choroidal blood circulation in patients with uncontrolled hypertension, which supports the potential application of using OCTA technology to detect alterations of choroidal perfusion in systemic disease.
The present data advance our knowledge on the impact of BP on the choroidal blood flow in several ways. First, systemic hypertensive patients having higher BP are more likely to have smaller area of flow voids. Also, these flow voids are generally smaller in average size and more numerous, compared to those with lower BP. This suggests that in hypertensive patients, the increasing levels of BP would lead to an increase in blood flow within the choriocapillaris. This finding is contrary to that reported by Spaide et al.6. He studied the flow characteristics of the choriocapillaris in 80 patients aged 24–99 years old and showed larger area of flow void in individuals with systemic hypertension6. In his study, there was no mention on the BP levels among the controls and hypertension group, which can result in biasness. Instead, our study is in good agreement with Polak’s previous published work. She investigated choroidal flow using laser interferometry and color Doppler imaging in 318 middle-aged male volunteers who did not receive any systemic medication and found a significant correlation between higher levels of BP and an increase in choroidal blood flow13.
The impact of BP was in the opposition direction for the retinal vessels. In the same cohort of patients with systemic hypertension, we observed a sparser retinal capillary density with increasing systolic BP, potentially associated with hypertension-induced retinal capillary dropout19. Previous studies on the association between retinal blood flow and different techniques to assess retinal blood flow did not show an association between BP and retinal perfusion20,21. Autoregulation is known as the ability of a vascular bed to keep blood flow relatively constant despite alterations in perfusion pressure. The choroid has long been assumed to be a passive vascular bed, that is, one that does not show autoregulation. Indeed, early studies have indicated if perfusion pressure is increased to the choroid, there is a linear increase in blood flow22. Contrary to this notion, the choroid has shown some regulatory compensation response when the ocular perfusion pressure was experimentally modified in later studies23–25. In such experiments23–25, the effect of acute changes in perfusion pressure is studied and results can not necessarily be extrapolated to systemic hypertension, which represents the persistent elevation of systemic BP. Our results indicate that choroidal flow was greater in those with higher BP. The slope of the regression line is, however, flat indicating a non-linear relation between BP and choroidal flow. Therefore, the data support the concept of choroidal autoregulation, but the choroidal vasculature appears to have lesser autoregulatory capacity than the retinal vasculature26.
The mechanism underlying the impact of hypertension on the ocular microvasculature is unclear based on the present data. The posterior tissues of the eye are supplied by two vascular supply with distinct properties26. For the retinal vasculature, it has been shown that chronic systemic hypertension is associated with narrower blood vessels27–29. This indicates that an elevated vascular resistance is associated with a dysfunctional autoregulation of the retinal vasculature30. In the choroid, however, no study has yet shown that hypertension is associated with arteriolar vasoconstriction. Whether an increase in choroidal perfusion is protective against AMD remains unclear. It is plausible that a higher blood flow in the choroid may prevent hypoxia in the outer retina31. On the contrary, choroidal overperfusion may cause oxidative stress to the outer retina, which has been postulate to contribute to the development of AMD32.
The presence of microvascular changes in the eye, such as an increased in size and lesser in numbers of flow voids using OCTA, was independently associated with older age. This is in keeping with what Spaide had already published on age and flow voids6. Using statistical modeling, he demonstrated that the appearance of choriocapillaris flow voids followed a power-law distribution, initially with many small flow voids and progressively fewer larger flow voids. This is in keeping with a variety of other studies using magnetic resonance imaging33, laser Doppler flowmetry34, or laser interferometry35. Whether this age-related decline in choroidal perfusion is due to reduced metabolic demand or a risk factor for eye disease is unknown.
We also saw that individuals taking calcium channel blockers had choriocapillaris flow voids that were larger in average size and lesser in numbers. Calcium channels are known to regulate smooth muscles, which may also impact the regulation of blood flow in the choroid. Calcium channel blockers relax and widen blood vessels and trigger the release of nitric oxide36. Nifedipine, an L-type calcium channel blocker, altered the regulation of choroidal blood flow during isometric exercise in 15 healthy men37. Various studies using animal models, have also indicated that calcium channel blockers may increase ocular blood flow38–40. Verapamil, however, reversed the downward shift induced by nitric oxide synthase inhibition in the choroidal pressure/flow relationship in the rabbit41. The relatively small sample size of persons taking different types of calcium channel blockers prevented post-hoc analysis of specific calcium channel blockers on choroidal flow voids.
What is the immediate clinical relevance of our study? Systemic parameters such as BP levels, diabetes status and hypertensive medications can impact the choriocapillaris and should be considered by future studies. Current studies have mostly defined their controls as individuals without overt eye diseases3–5,42, with the exception of one study which further excluded participants if they had diabetes or hypertension43. When using the OCTA to examine the role of altered choroidal microvasculature in eye diseases, clinicians should account for patients’ systemic health status and medication information, which can thereby lead to a more valid conclusion.
One of the major strength of the current study is the usage of reliable and objective clinical tests, which include 24-hr ambulatory BP monitoring, urine MCR and eGFR. There are several limitations that need to be emphasized. First, the flow voids that we have visualized within the choriocapillaris could also be a result of an extremely slow flow rate. Currently, OCTA visualizes blood flow within the vessels by detecting motion contrast from blood flow. A vessel having very low blood flow rate, one that is below the detection threshold of the OCTA device, will not be elucidated44. For this reason, the term “flow void” refers to the signal loss occurring within the flowing blood. Second, even though we report smaller areas of flow voids that are reduced in size and greater in numbers among those with higher BP, this was only a cross-sectional study. OCTA was recently made available in the clinic and follow-up study is in progress. Third, smoking is known to reduce blood flow of the choriocapillaris within the macular region by the acute effects of nicotine in cigarettes, as evaluated by OCTA45. Moreover, abnormal choroidal blood flow regulation in response to modifications in ocular perfusion pressure was seen in smokers46. However, we did not assess this relation of smoking on choriocapillaris flow due to a paucity of smokers. Fourth, the lack of ocular parameters such as intraocular pressure47 and axial length48 may confound the measurements of flow voids as these ocular factors are known to have an effect on flow voids. Last, we did not calculate the repeatability of our measurements. However, a previous publication reported the repeatability of flow voids measurements to be excellent3.
In conclusion, we have demonstrated the impact of ambulatory BP on choriocapillaris flow voids in adults with treated systemic hypertension. Choriocapillaris flow voids measured using the OCTA may mirror systemic microvascular dysfunction due to systemic hypertension. This suggests the possible use of the OCTA as a device to detect systemic microvascular dysfunction. Future studies are needed to comprehend the impact that eye diseases such as AMD have on the choroidal vasculature.
Methods
Study Participants
We conducted a prospectively planned observational cross-sectional study including 164 eyes from 85 from participants with essential hypertension enrolled in the Response of the Myocardium to Hypertrophic Conditions in the Adult Population (REMODEL; Response of the myocardium to hypertrophic conditions in the adult population; NCT02670031)49,50. Briefly, Asians with essential hypertension on antihypertensive medications, aged 18 years and older, were recruited from a tertiary cardiac centre and primary care clinics in Singapore, from January 2017 to February 2018. Participants with secondary causes of hypertension, any on-going unstable medical conditions, previously diagnosed significant coronary artery disease (defined as previous myocardial infarction, more than 70% coronary stenosis on invasive coronary angiography or positive cardiac stress tests), strokes, atrial fibrillation and women who are pregnant or breast feeding were excluded from the study49.
Study was approved by the SingHealth Centralized Institutional Review Board, conducted in accordance to the Declaration of Helsinki, where written informed consents were obtained from participants.
Examination procedures
Detailed interviewer-administered questionnaire was used to collect demographic data, lifestyle risk factors, medical history and medication use51. Ethnicities were set by the Singapore census52. 24-hour ambulatory BP (systolic blood pressure, SBP and diastolic blood pressure, DBP) were measured in all participants. In the current analysis, daytime ambulatory BP was measured at intervals of every 20 minutes from 6 am to 10 pm and nighttime ambulatory BP was measured at intervals of every 30 minutes from 10 pm to 6 am. Hypertensive patients were stratified into three groups based on the Systolic Blood Pressure Intervention Trial (SPRINT)53: Intensive BP control defined as systolic BP <120 mmHg, standard BP control as systolic BP 120–139 mmHg and poor BP control as systolic BP ≥140 mmHg. Diabetes was defined based on self-reported physician diagnosed diabetes or glucose-lowering medications. Hyperlipidemia was defined based on clinical history of elevated cholesterol or lipid-lowering medications. Participants’ height was measured using a wall-mounted measuring tape and weight was measured using a digital scale (SECA, model 782 2321009, Germany)54. Body mass index (BMI) was calculated as body weight (in kilograms) divided by body height (in meters) squared. Smoking status was defined as those never smoked, current smokers and past smokers.
Blood and mid-stream urine samples were collected for analysis of serum creatinine and urine microalbumin/creatinine ratio (MCR). Bio-specimens were processed in an accredited laboratory at the Singapore General Hospital. eGFR (in mL/min/1.73 m2) was calculated from plasma creatinine using the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation55. MCR was measured using immunoassay. Normal MCR range was 0.2 to 3.3 mg/mmol creatinine whilst values >33.9 mg/mmol creatinine implied clinical albuminuria.
Ocular examinations
Participants underwent a questionnaire regarding their ocular history (e.g. intraocular surgery or glaucoma). To mitigate the risk of angle-closure glaucoma, we performed a pre-dilation check. Intraocular pressure was measured using noncontact tonometry (Auto Non-Contact Tonometer, NT-3000; Nidek, Gamagori, Japan)19. Persons having an intraocular pressure reading of less than 21 mmHg went on to receive pupillary dilation and imaging scans. Conversely, those having an intraocular pressure reading of 21 mmHg and above exited from the study. Fundus photography and OCTA were performed approximately 30 minutes after topical instillation of 2 drops of 1% tropicamide, given 5 minutes apart. Fundus photography was performed using a retinal camera (Canon CR-DGi with a 10-DSLR back; Canon, Tokyo, Japan) to ascertain the presence of eye disease56. Patients with eye diseases (e.g. glaucoma, vascular or nonvascular retinopathies, and age-related macular degeneration) were excluded from the study56.
Optical coherence tomography angiography
The OCTA imaging system provides a non-invasive method for visualizing the choriocapillaris (AngioVue; Optovue, Inc., Fremont, California USA)57. The AngioVue OCTA employs the split-spectrum amplitude decorrelation angiography (SSADA) algorithm to acquire flow signal58 and allows a high-resolution 3-dimensional visualization of perfused vasculature57,59. The device has an A-scan rate of 70,000 scans per second and 2 successive B-scans were taken at the same location. Each imaging cube consisted of 2 repeated volumes (304 B-scans × 304 A-scans). For this study, we used choriocapillaris flow measurements within the macula, in scans with a 6.0 × 6.0 mm2 field of view centered on the fovea59. Each scan was automatically segmented by the AngioVue software (version 2016.2.0.35) so as to visualize the superficial vascular plexus and choriocapillaris.
Method for measuring choriocapillaris flow voids
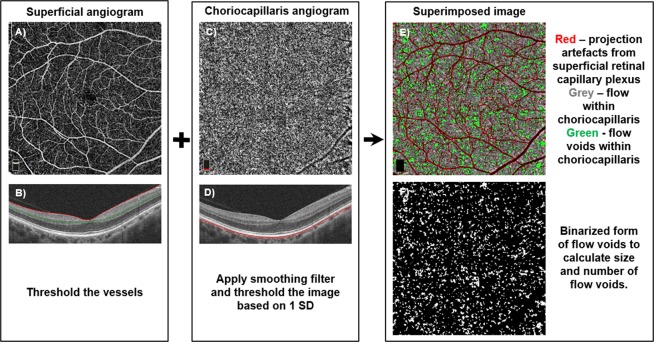
We calculated the area of choriocapillaris flow voids based on an image-processing algorithm as described previously by Nesper et al. (Fig. 3)3. Briefly, the en face angiograms of the (1) superficial retinal capillary plexus (Fig. 3A) (3 μm below the inner limiting membrane to 16 μm below inner plexiform layer; Fig. 3B) and (2) choriocapillaris (Fig. 3C) (31 μm to 59 μm below the RPE reference; Fig. 3D) were segmented from the OCTA device3. The images of the superficial capillary plexus and the choriocapillaris slabs were then imported into MATLAB (MathWorks, Inc., Natick, Massachusetts)3.
Figure 3.
Algorithm for artifact removal and calculation of features of choriocapillaris flow voids.
Superficial retinal capillary plexus left noticeable projection artefacts on the choriocapillaris which may confound the calculation of flow voids3. To remove the shadow artifacts on the choriocapillaris, we thresholded the superficial retinal vascular plexus image to create a binary mask (red color; Fig. 3E). We then overlaid the mask on the choriocapillaris image, which helped us to identify the artifacts on the choriocapillaris image. Regions in the choriocapillaris that were caused by projection artifacts of the superficial retinal vascular plexus were excluded in the calculation of the percentage of flow voids.
To identify the flow voids in the choriocapillaris image, we first applied a Gaussian smoothing filter (σ = 20 μm) to reduce the speckle noise in the choriocapillaris image and then thresholded the choriocapillaris image, as described previously by Zhang et al.42:
Imean represents the average pixel intensity of the choriocapillaris image while 𝜂 denotes a positive number that was multiplied against the standard deviation of the pixel intensity of the choriocapillaris image. A value of 1 SD was used because Zhang et al. showed that it provided the best repeatability42. Pixels that fell below this threshold were considered as flow voids (green color; Fig. 3E).
The area of choriocapillaris flow voids was defined as a percentage between the region that is absent from flow and the total scanned region, as follows (Fig. 3E)42:
The size and number of flow voids were calculated from a binarized image of the choriocapillaris flow voids (Fig. 3F). The binarized image was imported into ImageJ software (National Institutes of Health, Bethesda, MD; available at https://imagej.nih.gov/ij/) for analysis5. The image was threshold to select the flow voids and the “Distribution” command was then applied with the parameter set as “Area” to obtain the average size of the flow voids60. The “Analyze Particles” command was employed to count the number of flow voids present.
A trained grader masked to the participants’ characteristics reviewed the quality of all OCTA scans. Poor quality scans were excluded from the analysis if one of the following criteria were met: (1) poor clarity images; (2) local weak signal caused by artifacts such as floaters; (3) residual motion artifacts visible as irregular vessel patterns on the en face angiogram and (4) scans with segmentation failure59.
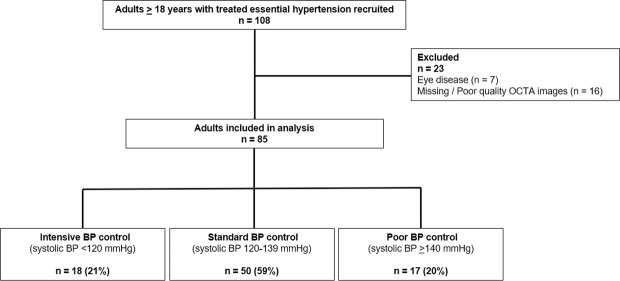
Of the 108 participants, we excluded participants with eye diseases (n = 7) and missing or poor quality OCTA images (n = 16), leaving 85 participants for analysis (Fig. 4). Comparisons between included and excluded participants revealed no differences in terms of their age (P = 0.09), systolic BP (P = 0.24) and diastolic BP (P = 0.56).
Figure 4.
Identification of study participants. Of the 85 participants with systemic hypertension, 17 (20%) had poorly controlled blood pressure.
Statistical analyses
Primary outcomes were features (by area, size and number) of choriocapillaris flow voids. The normality of the distribution of the continuous variables was assessed using Shapiro-Wilk test. To compare continuous variables among groups, a 1-way analysis of variance (ANOVA) was performed for normally distributed variables whereas Kruskal-Wallis test was used for non-normally distributed variables. Continuous variables that were normally distributed are presented as mean ± standard deviation (SD) whereas non-normally distributed variables are presented as median (interquartile range [IQR]). Chi-square test or Fisher’s exact test were used for categorical variables. There was a strong correlation between the right and left eyes for area (Pearson correlation coefficient, r = 0.65; P < 0.001) and size (r = 0.65; P < 0.001) of flow voids. However, the correlation was weaker for number of flow voids (r = 0.49; P < 0.001). Hence, generalized estimating equations were used to account for inter-eye correlation since data from both eyes were used61,62. Associations between systemic factors (independent variables) with features of choriocapillaris flow voids (dependent variable) were assessed using univariate and multivariate linear regression models with generalized estimating equations63. In addition to biologically plausible factors such as age, gender, and diabetes, we also used the univariate analysis as an initial step to select covariates for further consideration in the multivariate regression, factors with P < 0.10 in the univariate model were included in the multivariate model63. Data were analyzed with statistical software (STATA, version 13.1; StataCorp LP).
Acknowledgements
This work was funded by grants from the National Medical Research Council (NMRC CG/C010A/2017 and STaR/0016/2013) and the Duke-NUS-KP (Coll)/2018/0009A, Singapore. The sponsor or funding organization had no role in the design or conduct of this research.
Author Contributions
J.C., C.W.L.C., T.Y.W. and L.S. conceived and designed the study. J.C. and T.T.L. collected the data. J.C., B.T., S.H.W., K.D. and M.A. analyzed and interpreted the data. J.C., S.H.W., B.T. and L.S. wrote the main manuscript text. All authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the terms of consent to which the participants agreed but are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bourne RR, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. The Lancet. Global health. 2013;1:e339–349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen. American journal of ophthalmology. 2017;174:42–55. doi: 10.1016/j.ajo.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Investigative ophthalmology & visual science. 2017;58:4792–4798. doi: 10.1167/iovs.17-22360. [DOI] [PubMed] [Google Scholar]
- 5.Borrelli, E. et al. Reduced Choriocapillaris Flow in Eyes with Type 3 Neovascularization and Age-Related Macular Degeneration. Retina, 10.1097/IAE.0000000000002198 (2018). [DOI] [PubMed]
- 6.Spaide RF. Choriocapillaris Flow Features Follow a Power Law Distribution: Implications for Characterization and Mechanisms of Disease Progression. American journal of ophthalmology. 2016;170:58–67. doi: 10.1016/j.ajo.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Metelitsina TI, et al. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Investigative ophthalmology & visual science. 2008;49:358–363. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boltz A, et al. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Investigative ophthalmology & visual science. 2010;51:4220–4225. doi: 10.1167/iovs.09-4968. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2003;110:636–643. doi: 10.1016/S0161-6420(02)01448-3. [DOI] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research, G Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/S0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung CM, et al. Prevalence, racial variations, and risk factors of age-related macular degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology. 2014;121:1598–1603. doi: 10.1016/j.ophtha.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Lambert NG, et al. Risk factors and biomarkers of age-related macular degeneration. Progress in retinal and eye research. 2016;54:64–102. doi: 10.1016/j.preteyeres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polak K, et al. Choroidal blood flow and arterial blood pressure. Eye. 2003;17:84–88. doi: 10.1038/sj.eye.6700246. [DOI] [PubMed] [Google Scholar]
- 14.Nitsch D, Evans J, Roderick PJ, Smeeth L, Fletcher AE. Associations between chronic kidney disease and age-related macular degeneration. Ophthalmic Epidemiol. 2009;16:181–186. doi: 10.1080/09286580902863064. [DOI] [PubMed] [Google Scholar]
- 15.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 16.Friedman E, Kopald HH, Smith TR. Retinal and Choroidal Blood Flow Determined with Krypton-85 Anesthetized Animals. Invest Ophthalmol. 1964;3:539–547. [PubMed] [Google Scholar]
- 17.Xu W, et al. Association of risk factors for choroidal neovascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am J Ophthalmol. 2010;150:40–47 e42. doi: 10.1016/j.ajo.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, et al. Optical Coherence Tomographic Angiography Imaging in Age-Related Macular Degeneration. Ophthalmology and eye diseases. 2017;9:1179172116686075. doi: 10.1177/1179172116686075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua, J. et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens, 10.1097/HJH.0000000000001916 (2018). [DOI] [PMC free article] [PubMed]
- 20.Fuchsjager-Mayrl G, et al. Retinal blood flow and systemic blood pressure in healthy young subjects. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:673–677. doi: 10.1007/s004170100333. [DOI] [PubMed] [Google Scholar]
- 21.Garhofer G, Werkmeister R, Dragostinoff N, Schmetterer L. Retinal blood flow in healthy young subjects. Investigative ophthalmology & visual science. 2012;53:698–703. doi: 10.1167/iovs.11-8624. [DOI] [PubMed] [Google Scholar]
- 22.Armaly MF, Araki M. Effect of ocular pressure on choroidal circulation in the cat and Rhesus monkey. Invest Ophthalmol. 1975;14:584–591. [PubMed] [Google Scholar]
- 23.Luksch A, et al. Role of NO in choroidal blood flow regulation during isometric exercise in healthy humans. Investigative ophthalmology & visual science. 2003;44:734–739. doi: 10.1167/iovs.02-0177. [DOI] [PubMed] [Google Scholar]
- 24.Schmidl D, et al. Comparison of choroidal and optic nerve head blood flow regulation during changes in ocular perfusion pressure. Investigative ophthalmology & visual science. 2012;53:4337–4346. doi: 10.1167/iovs.11-9055. [DOI] [PubMed] [Google Scholar]
- 25.Schmidl D, et al. Factors Associated With Choroidal Blood Flow Regulation in Healthy Young Subjects. Investigative ophthalmology & visual science. 2016;57:5705–5713. doi: 10.1167/iovs.16-20225. [DOI] [PubMed] [Google Scholar]
- 26.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Progress in retinal and eye research. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhargava M, et al. Prevalence and risk factors for retinopathy in persons without diabetes: the Singapore Indian Eye Study. Acta Ophthalmol. 2014;92:e602–609. doi: 10.1111/aos.12446. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima S, Nakagami T, Suto C, Hirose A, Uchigata Y. Prevalence of retinopathy and its risk factors in a Japanese population. Journal of diabetes investigation. 2013;4:349–354. doi: 10.1111/jdi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeganathan VS, et al. Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay Eye Study. Archives of ophthalmology. 2010;128:40–45. doi: 10.1001/archophthalmol.2009.330. [DOI] [PubMed] [Google Scholar]
- 30.Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow - relevance for glaucoma. Experimental eye research. 2011;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Progress in retinal and eye research. 2017;58:115–151. doi: 10.1016/j.preteyeres.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Progress in retinal and eye research. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emeterio Nateras OS, et al. Choroidal blood flow decreases with age: an MRI study. Current eye research. 2014;39:1059–1067. doi: 10.3109/02713683.2014.892997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Archives of ophthalmology. 1998;116:150–154. doi: 10.1001/archopht.116.2.150. [DOI] [PubMed] [Google Scholar]
- 35.Dallinger S, et al. Age dependence of choroidal blood flow. Journal of the American Geriatrics Society. 1998;46:484–487. doi: 10.1111/j.1532-5415.1998.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 36.Motro M, Shemesh J, Grossman E. Coronary benefits of calcium antagonist therapy for patients with hypertension. Curr Opin Cardiol. 2001;16:349–355. doi: 10.1097/00001573-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Schmidl D, et al. Effect of nifedipine on choroidal blood flow regulation during isometric exercise. Investigative ophthalmology & visual science. 2012;53:374–378. doi: 10.1167/iovs.11-8536. [DOI] [PubMed] [Google Scholar]
- 38.Harino S, Riva CE, Petrig BL. Intravenous nicardipine in cats increases optic nerve head but not retinal blood flow. Invest Ophthalmol Vis Sci. 1992;33:2885–2890. [PubMed] [Google Scholar]
- 39.Shimazawa M, et al. Effect of lomerizine, a new Ca(2+)channel blocker, on the microcirculation in the optic nerve head in conscious rabbits: a study using a laser speckle technique. Exp Eye Res. 1999;69:185–193. doi: 10.1006/exer.1999.0689. [DOI] [PubMed] [Google Scholar]
- 40.Tomita K, Araie M, Tamaki Y, Nagahara M, Sugiyama T. Effects of nilvadipine, a calcium antagonist, on rabbit ocular circulation and optic nerve head circulation in NTG subjects. Invest Ophthalmol Vis Sci. 1999;40:1144–1151. [PubMed] [Google Scholar]
- 41.Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69:413–429. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Investigative ophthalmology & visual science. 2018;59:203–211. doi: 10.1167/iovs.17-22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Sheikh M, et al. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investigative ophthalmology & visual science. 2017;58:2063–2069. doi: 10.1167/iovs.16-21289. [DOI] [PubMed] [Google Scholar]
- 44.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayhan Z, Kaya M, Ozturk T, Karti O, Hakan Oner F. Evaluation of Macular Perfusion in Healthy Smokers by Using Optical Coherence Tomography Angiography. Ophthalmic surgery, lasers & imaging retina. 2017;48:617–622. doi: 10.3928/23258160-20170802-03. [DOI] [PubMed] [Google Scholar]
- 46.Wimpissinger B, et al. Effects of isometric exercise on subfoveal choroidal blood flow in smokers and nonsmokers. Investigative ophthalmology & visual science. 2003;44:4859–4863. doi: 10.1167/iovs.03-0391. [DOI] [PubMed] [Google Scholar]
- 47.Akahori T, Iwase T, Yamamoto K, Ra E, Terasaki H. Changes in Choroidal Blood Flow and Morphology in Response to Increase in Intraocular Pressure. Investigative ophthalmology & visual science. 2017;58:5076–5085. doi: 10.1167/iovs.17-21745. [DOI] [PubMed] [Google Scholar]
- 48.Mo J, Duan A, Chan S, Wang X, Wei W. Vascular flow density in pathological myopia: an optical coherence tomography angiography study. BMJ open. 2017;7:e013571. doi: 10.1136/bmjopen-2016-013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goh, V. J. et al. Novel Index of Maladaptive Myocardial Remodeling in Hypertension. Circulation. Cardiovascular imaging10, 10.1161/CIRCIMAGING.117.006840 (2017). [DOI] [PMC free article] [PubMed]
- 50.Lim EH, et al. Importance of Sex-Specific Regression Models to Estimate Synthetic Hematocrit and Extracellular Volume Fraction. JACC Cardiovasc Imaging. 2018;11:1366–1367. doi: 10.1016/j.jcmg.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 51.Chua, J. et al. Inter-relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in Asians: 6-year longitudinal study. The British journal of ophthalmologyApr 9, 10.1136/bjophthalmol-2018-311897 (2018). [DOI] [PMC free article] [PubMed]
- 52.Department of Statistics, M. o. T. a. I., Republic of Singapore. Table 4–6 Chinese, Malays and Indians Resident Population by Age Group, Dialect Group and Sex (ed Department of Statistics) (Singapore, 2001).
- 53.Group SR, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cackett P, et al. Smoking, cardiovascular risk factors, and age-related macular degeneration in Asians: the Singapore Malay Eye Study. American journal of ophthalmology. 2008;146:960–967 e961. doi: 10.1016/j.ajo.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Levey AS, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chua J, et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. Journal of hypertension. 2019;37:572–580. doi: 10.1097/HJH.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mastropasqua R, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Normal-Tension Glaucoma and Chronic Nonarteritic Anterior Ischemic Optic Neuropathy. Current eye research. 2018;43:778–784. doi: 10.1080/02713683.2018.1438630. [DOI] [PubMed] [Google Scholar]
- 58.Jia Y, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Optics express. 2012;20:4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoji, T. et al. Progressive Macula Vessel Density Loss in Primary Open Angle Glaucoma: A Longitudinal Study. American journal of ophthalmologyJul 19, 10.1016/j.ajo.2017.07.011 (2017). [DOI] [PMC free article] [PubMed]
- 60.Uji A, et al. Choriocapillaris Imaging Using Multiple En Face Optical Coherence Tomography Angiography Image Averaging. JAMA ophthalmology. 2017;135:1197–1204. doi: 10.1001/jamaophthalmol.2017.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ying GS, Maguire MG, Glynn R, Rosner B. Tutorial on Biostatistics: Linear Regression Analysis of Continuous Correlated Eye Data. Ophthalmic epidemiology. 2017;24:130–140. doi: 10.1080/09286586.2016.1259636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic epidemiology. 2012;19:159–165. doi: 10.3109/09286586.2012.674614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chua J, et al. Ethnic differences of intraocular pressure and central corneal thickness: the Singapore Epidemiology of Eye Diseases study. Ophthalmology. 2014;121:2013–2022. doi: 10.1016/j.ophtha.2014.04.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the terms of consent to which the participants agreed but are available from the corresponding author on reasonable request.