Abstract
The three-dimensional (3D) visualization of dural venous sinuses (DVS) networks is desired by surgical trainers to create a clear mental picture of the neuroanatomical orientation of the complex cerebral anatomy. Our purpose is to document those identified during routine 3D venography created through 3D models using two-dimensional axial images for teaching and learning neuroanatomy. Anatomical data were segmented and extracted from imaging of the DVS of healthy people. The digital data of the extracted anatomical surfaces was then edited and smoothed, resulting in a set of digital 3D models of the superior sagittal, inferior sagittal, transverse, and sigmoid, rectus sinuses, and internal jugular veins. A combination of 3D printing technology and casting processes led to the creation of realistic neuroanatomical models that include high-fidelity reproductions of the neuroanatomical features of DVS. The life-size DVS training models were provided good detail and representation of the spatial distances. Geometrical details between the neighboring of DVS could be easily manipulated and explored from different angles. A graspable, patient-specific, 3D-printed model of DVS geometry could provide an improved understanding of the complex brain anatomy. These models have various benefits such as the ability to adjust properties, to convert two-dimension images of the patient into three-dimension images, to have different color options, and to be economical. Neuroanatomy experts can model such as the reliability and validity of the designed models, enhance patient satisfaction with improved clinical examination, and demonstrate clinical interventions by simulation; thus, they teach neuroanatomy training with effective teaching styles.
Keywords: Dural venous sinuses, Brain imaging, Neurosurgical education, 3D neuroanatomical models, Clinical skills, Minimally invasive neurosurgery, Surgical trainers
Introduction
Neuroanatomy is a discipline where spatial visualization is of importance. While neuroanatomical textbooks and atlases provide 2D static anatomical illustrations, they are of limited value in exposing 3D dynamics of neuroanatomical structures [1, 2]. Learners may find it difficult to visualize 2D images as 3D and understand certain dynamic aspects of functional anatomy. Visual-spatial ability has been defined as the ability to mentally manipulate objectives in three dimensions [3, 4]. Such ability is important for medical students/surgical trainers to understand anatomical structures and is also essential to surgical trainees and surgeons [5, 6]. Therefore, the ability to visualize and mentally manipulate 3D structures and correctly identify them and related structures is an important skill to medical students when the anatomy is presented in various positions [6–8].
3D printing technology creates a physical model from a 3D computerized imaging source file and it is also referred to as rapid prototyping, stereolithographic, or additive manufacturing [6–8]. Considering the importance of 3D learning and teaching anatomy models in medical curricula, understanding the range of models used and the impact of using such 3D strategies on students’ learning necessitate a revision of studies covering 3D models [9, 10].
The benefits of simulation in the acquisition of technical skills, as a means to complement operative training, have been described in a number of studies. Nevertheless, there are no effective studies and functional simulations of dural venous sinuses (DVS) model for the students/surgical trainers to practice minimally invasive neurosurgical procedures before performing these procedures and treatments on actual patients [11–13]. 3D models are functional in surgeries as they provide spatial awareness and let the surgeons experience how the DVS seem, move, and respond during the procedure.
The aim is to evaluate different factors affecting learning by using 3D neuroanatomical models and their impact on the learning process. 3D model of complex DVS anatomy has been a promising tool not only for education but also a guide in treatment strategies, pre-procedural planning, diagnostic work-up, and device testing instructional advancing neurovascular research.
Materials and Methods
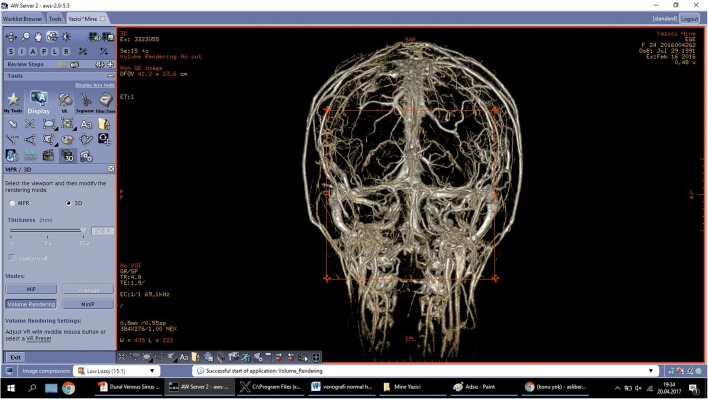
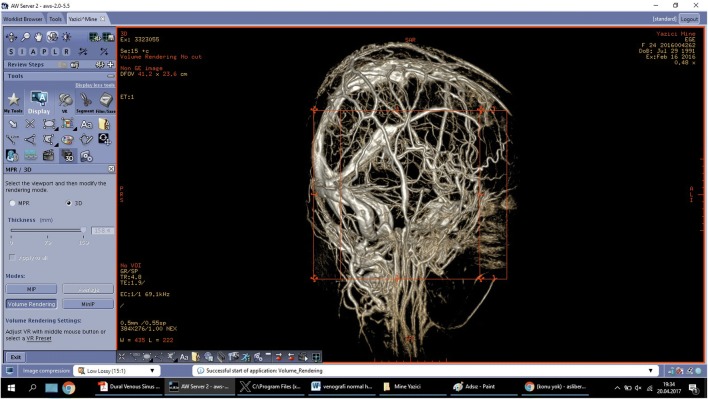
3D MR venographies were used to develop patient-specific DVS training models of the anatomical components (Figs. 1, 2, 3, 4, 5, 6, and 7). Retrospectively collected radiology image data from patients with normal DVS networks and controlled consisted of a 3D MR venography (Figs. 1 and 2). Digital Imaging and Communications in Medicine (DICOM) images were converted into standard 3D file format (StereoLithography file, STL). The STL files were translated into code for 3D printing (Figs. 3, 4, and 7).
Fig. 1.
3D magnetic resonance venography frontal plan image obtained in a 25-year-old woman with normal appearance of dural venous network
Fig. 2.
Computerized tomography venogram of the dural venous network showing normal appearance on sagittal plane
Fig. 3.
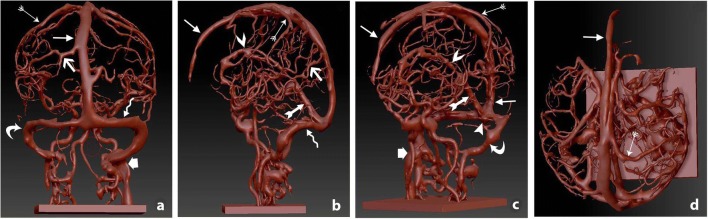
Visualization of the imaging segmentation of the dural venous network, applied on stereolithographic file to remove superimposition of the scalp and skull allowing inspection from a back, b left side, c front side, d superior angles.  superior sagittal sinus,
superior sagittal sinus,  superior anastomotic vein,
superior anastomotic vein,  inferior anastomotic vein,
inferior anastomotic vein,  transverse sinus,
transverse sinus,  straight sinus,
straight sinus,  sigmoid sinus,
sigmoid sinus,  inferior sagittal sinus,
inferior sagittal sinus,  occipital sinus,
occipital sinus,  internal jugular vein
internal jugular vein
Fig. 4.
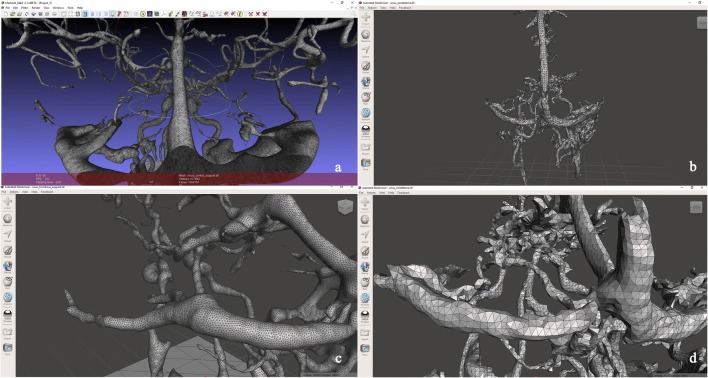
Views of digital model .STL format opened by Autodesk Meshmixer 2017® (version 3.2.37, Autodesk Inc., USA) software before smooth processing (b–d) and after smooth processing (a–c). Smooth processing reduced the number of polygonal triangles and corrected the surface mesh, and the topology of the mesh did not change
Fig. 5.

Made of polylactic acid 3D building dural venous network fabricated using MassPortal Pharaox XD printer
Fig. 6.
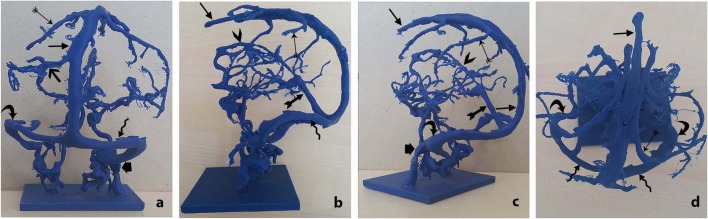
Printed three-dimensional model of dural venous network can be allowed of inspection from all angles. a back, b left side, c front-left side, d superior angles.  superior sagittal sinus,
superior sagittal sinus,  superior anastomotic vein,
superior anastomotic vein,  inferior anastomotic vein,
inferior anastomotic vein,  transverse sinus,
transverse sinus,  straight sinus,
straight sinus,  sigmoid sinus,
sigmoid sinus,  inferior sagittal sinus,
inferior sagittal sinus,  occipital sinus,
occipital sinus,  internal jugular vein
internal jugular vein
Fig. 7.
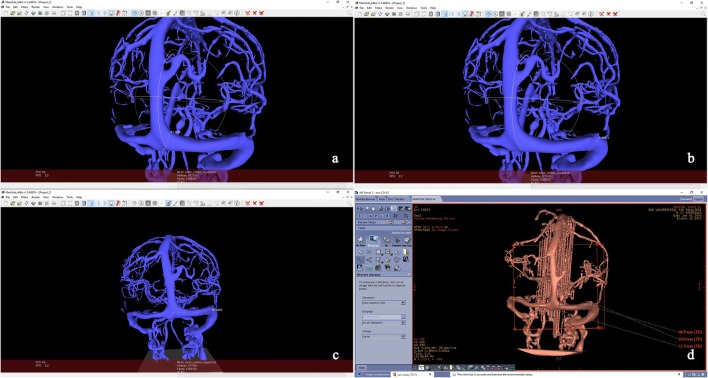
Comparison of proximal, distal diameters, and length of the transverse sinus measured by MeshLab software (a, b, c) and General Electric AW Server software (d) for 3D CT reconstruction of the printed dural venous network model and digital dural venous network in STL format
Data Extraction and Segmentation
Segmentation allowed for surface extraction of all the structures of interest from the MRI scan data. In our work, the method of thresholding is used as a segmentation method. It is one of the simplest ways of segmenting the image. It is also used to convert grayscale images into binary images. Once the degree of graft in anatomical structures of interest has been detected with the help of histograms, they are threshold to highlight the detail of interest in the image to eliminate the remaining details. The new value of a pixel is calculated with the following formula:
Here, the pixels exceeding the threshold T value 1 is assigned as 0, while the new value of pixels remaining below this value is assigned as 0.
The resulting digital model was then edited to repair the artifacts in the polygonal structures and render it printable (Figs. 1, 2, 3, 4, 5, 6, and 7). The digital models in the STL format are arranged by anatomists (M.A.O. and A.B.K.) to remove artifacts that arise. For this, a manual automatic smooth operation was performed using free Autodesk Meshmixer 2017® (version 3.2.37, Autodesk Inc., USA) software. This process took about 2–3 min.
Surface Extraction and Model Processing
The final digital models consisted of a 3D mesh made up of thousands of polygons. Automatic smoothing was applied via an algorithm to the digital surface models to reduce the number of polygons, and improving the appearance of the models is undefined. It modifies the underlying structure by manually editing and then smoothing the text. A comparison before and after changes will help to understand how much the first structure has changed (Fig. 4).
Laplacian algorithm is used to reduce polygon numbers with automatic smooth operation. A natural problem with the formation of automatic triangular surface braids is that the resulting polygonal surface is faceted, and it contains single triangles with very small angles. The original Gaussian surface of the STL format digital model is smooth. However, the resulting network may roughly rough the surface. Additionally, singular triangles can cause difficulties in calculating the BEM (boundary element method) and corresponding volume network formation. For this reason, a smooth method is required to improve the qualities of the surface networks. The Laplacian smooth algorithm is an algorithm used to correct triangular surface mesh. This algorithm changes the position of the nodes without changing the network topology. In the Laplacian smooth process, each corner moves to a new position defined by taking the average position of its neighbors:
In the formula where Ni is the number of adjacent vertices of the node, qj is the adjacent vertex position. To control the smooth ratio, the current position of qi is included in the calculation of the new position of pi and the new formula was calculated as
The β value is the parameter that controls the softening rate. Generally, β takes a value between [0, 1].
The digital model representing the DVS required minimal manual editing, apart from minor smoothing and polygon error repair, whereas the digital models representing the DVS network as well as sagittal, sigmoid, transverse sinuses, and internal jugular veins had a significant number of artifacts, including gaps in the polygonal wall and an irregular block-like appearance. Once the models were rendered free of errors, the data were converted to a STL—a format compatible with 3D printers (Figs. 3 and 6). Once mesh editing was applied to the extracted surface models, they appeared more realistic and resembled the anatomical structures in the MRI scans.
3D Printing
The STL file of the final digital dataset was delivered electronically to the Laboratory of Digital Imaging and 3D Modeling at the Ege University, Izmir, where the 3D printing was carried out using the Mass Portal PHARAOH XD printer. The DVS networks were reproduced and anatomically embedded into the training models (Fig. 5). Each model was printed en bloc, surrounded by supports to protect over-hanging parts of the model during the printing process. Once printed, the models underwent post-manufacture processing, which included removal of the support structures.
A questionnaire was applied to 10 neurosurgical residents to evaluate the 3D model’s perception. An Objective Structured Assessment of Technical Skills (OSATS) scale was used in the questionnaire to provide information about the competence and sensitivity of the anatomical model as a specialty training tool.
Pearson correlation coefficients and kappa statistics were used to assess the rating for OSATS questions. After CT scan of the anatomical models was measured, the life-size, patient-specific model was proven to be individualized. SPSS (18.0) program was used for statistical analysis, and the threshold value for statistical significance was calculated as 0.05.
Results
The reconstructed models of DVS network can be rotated into 360° at any angle and the patient’s position, which helped in a better understanding of the orientation of vessels. The DVS model demonstrated the exact anatomy to understand the variable configuration in space. Geometrical DVS network anomalies can be clearer in control and variable cases. All reconstructed 3D-printed models described here can be displayed in 3D presentation in a life-size specimen. It remains a true anatomical representation. The external shape of the DVS network and their dimensions are measurable (Fig. 7). Surgical satisfaction of the model proved to be measurable during training and planning surgical procedures. The evaluation revealed that the model was remarked as an actual data in terms of teaching, learning, surgical training, and preoperative planning (95%).
Some opinions were obtained according to the survey results.
The rapid development of 3D pressure has created a new earning and teaching tool for medical education.
New and cheap 3D printing technology has been combined with the production of patient-specific models from DICOM data obtained during CT, MRI, or ultrasound scanning.
Depending on the area of interest, these printed models show anatomical and structural conformity consistent with the patient’s actual illness process.
This eligibility allows students to see and understand rough pathology and structural relationships before surgery.
Improved visualization permits surgical teams to plan interventions more precisely and limit resection, model-appropriate implant sizes.
The superior sagittal sinus run convex, was narrow anteriorly, ran backwards, and showed gradual widening (Figs. 1, 2, 3b–d, and 6b–d). Primary tributaries from cortical veins of the frontal lobes were received (Figs. 1, 2, 3a–d, and 6a–d). The superior sagittal sinuses was deviated, usually to the right, and continued as a transverse sinus (Figs. 1, 3a,d, and 6a–d). Its dilated posterior end was the confluence of the sinuses. It also connected with the occipital and contralateral transverse sinus (Figs. 1 ,2, 3, and 6). The size and degree of communication of the channels meeting at the confluence were highly variable.
Inferior sagittal sinus’ size was increased in size posteriorly and terminated in the straight sinus (Figs. 2, 3b, c, and 6b, c). Each sigmoid sinus curved inferomedially as continuations of the transverse sinuses and turned forward to the internal jugular vein (Figs. 1, 2, 3a–c, and 6a–c).
The straight sinuses were observed in each control case. The straight sinus lies in the junction of the falx cerebri with the tentorium cerebelli and is also triangular in cross-section (Figs. 2, 3b, c, and 6b, c). Postero-inferiorly, the sinus runs as a continuation of the inferior sagittal sinus.
The occipital sinus was the smallest of the sinuses, situated in the attached margin of the falx cerebelli and is occasionally paired (Figs. 1, 2, and 6). Cavernous sinus was totally normal in the control group (Figs. 2, 3b, d, 6b, and c). Internal jugular veins were symmetrical and dominant-side of cases (Figs. 1, 2, 3, and 6). Measurements of model also were evaluated in ten separate parameters, including diameter, length, height, angle, and shapes (Fig. 7). After CT scan of the anatomical models was measured, the life-size, patient-specific model was proven to be individualized. The difference was not significant in the distances between models and CT scans of the models.
Discussion
The venous sinuses are often neglected when considering cerebral disease [14, 15]. The neuroanatomy of the DVS system is particularly eloquent and poorly understood [16, 17]. The spatial visualization of neurodural anatomy is of primary importance. Knowledge of variable anatomical structure of DVS is also essential. A critical element of neurosurgical training is based on the analysis of neuroanatomy and the ability to safely and precisely navigate surgical instruments through restrictive corridors without resulting in collateral damage to the surrounding tissue [15, 18, 19]. Text-books of neuroanatomy are often used in surgical training, but the images present in those books provide 2D, static snapshots of the real 3D anatomy [20–22]. Cadaver dissection guide surgeons in neuroanatomy, but they cannot provide tactile feedback and dynamic properties of real living tissue [23, 24]. Live animal surgeries are also used in understanding dynamics, but the animal anatomy might significantly differ from the anatomy of the human brain [25, 26]. With all these drawbacks, surgical models are progressively distinguished as powerful, cost-efficient alternatives allowing errors without cadaver or animal involvement and procedure rehearsal [27–29]. Model size, visual appearance, and neurosurgical anatomy were perceived as very realistic [30–32].
3D models have become popular rapidly especially in orthopedic surgery, plastic surgery, and vascular surgery [9–12, 15, 24, 27]. Due to its ability to demonstrate complex anatomy of structures and networks, 3D printing is preferred in catheter-based interventions and interventional neurosurgery. Reduction in operation and recovery duration, reduced blood loss, and better resection margins are some of the potential advantages [30, 31]. Manufacture of 3D-printed models of the brain in this article was based on CT and MRI data which were unique to the patient with variations of dural sinuses (Figs. 1, 2, 3, 4, 5, and 6). To our best knowledge, this is the first full-sized DVS model prepared with innovatory approach described in this article, which allows forming complex neuroanatomical models with use of low-cost fused deposition modeling printing technique (Fig. 6a–d). So far, the only cost-effective DVS models that have been created have represented variation DVS or internal jugular vein, not showing them as a whole anatomy of the area (Fig. 6a–d).
In terms of production, time and cost our model fabrication is unequivocal. The total cost of each model where the manufacturing is fully automated is US$50, and no additional implant is printed on the model. It only takes 12 h to print. On the average, 2 h for segmentation, 2 h for regulation, and 2–3 min for smooth is required for the process. Accordingly, the total time is about 16 h. With these images, patient-specific 3D anatomical models are quickly printable, easy, and cost-effective.
Development of 3D printing technologies enables faster, easier, cheaper, and more accurate models [6, 25, 26, 28, 29]. The advancements in this area such as optimal imaging and improved volumetric software will soon allow us to circumvent one of the 3D manufacturing steps and print the PLA directly from medical images. 3D modeling is the technology of the future due to the fact that it leads to personalized surgical initiatives, the development of doctor-patient relationships, and the visual contribution to intern education. One of the most intriguing work areas of recent times is that 3D printing is an attractive, powerful, and versatile technology that has the potential to be accessible to everyone. With 3D printing materials in education, young neurosurgeons are actively involved in the learning of sight and touch. Effective learning is provided by seeing the organ structures as a whole. In educational establishments, cost-effective production and widespread use of 3D printing materials is important, as the number of students, the financial resources of the institution, and the significance given to the applied training in the training program, and the continuity of production of 3D printing materials.
For this, 3D printing material unit and its team should be involved in training management. In pre-operative planning unlike traditional practicing on live animals, surgeons can now learn with organs and tissues created with 3D print. 3D biodegraded neuroanatomical models can be useful for neurosurgeons to reflect lesions and normal brain structures, cranial nerves, vessels, and the relationship between the skull and the brain structures. These 3D bioprint models can help to identify safe surgical corridors in brain surgery.
Newly developed 3D printing technologies can recreate patient-specific anatomy, but the stiffness of the materials limits fidelity to real-life surgical situations (Figs. 1,2, 3, 4, 5, 6, and 7). 3D models can be used to visualize the pathology and variation of a neuroanatomical structure in neurosurgical education.
In the work done, 3D-printed models have shown that they can improve the performance of the residents and the learning speed. They significantly improve the knowledge, management, and confidence of the young neurosurgeons regardless of their area of expertise. In surgical intervention, physical interaction with the model has proven to be the key to gaining the necessary motor skills to improve the results of the operating room. 3D digital reconstruction of the surgical anatomy was complementary with 3D physical models. In addition, in neurosurgical education, the majority of assistants preferred kinesthetic learning (tactile learning) in the display of anatomical variation. The physical interactions or activities between the 3D-printed models and the students are among the reasons why models are indispensable in medical education while providing better understanding of the forms and spatial relationships between the structures.
A full-scale reproduction including external physical details and brain imaging was developed via a unique collaboration of a group of special anatomists, neuroradiologists, and neurosurgeons.
The uses of neurovascular 3D printing can be summarized as follows:
Neurosurgical education requires analysis of the normal structure of the organs as well as anatomical variations. Following the analysis, the variations can be highlighted, digitized, modeled, and manipulated using 3D imaging.
Visual inspection, direct manipulation of hand-held models of neuroanatomy, variations, and pathology can be carried out with medical 3D printing fabrication of neurovascular structures, typically gathered from volumetric medical image data.
Advanced modern imaging techniques such as CT and MR are combined with dedicated 3D printing software and hardware as part of neurosurgical 3D printing.
Neurosurgical 3D printing enables diagnostic work-up of complex dural venous structure in addition to surgical and interventional procedural planning and simulation.
3D printing develops the relation between the patient and the surgeon and improves the communication with the patient and their families as it guides them in understanding their own diseases and participating in their own decision-making.
Currently, clinical practice and the use of neurosurgical 3D bio-printing are not widespread. Nevertheless, it holds great promise for the future.
Conclusion
An actual 3D neuroanatomical model including venous sinuses has been created. We believe that this model can be used both in medical education and pre-operative surgical planning stage for pathology regarding the cranium. 3D model of complex dural sinuses anatomy has been a promising tool not only for education but also a guide in treatment strategies, pre-procedural planning, diagnostic work-up, and device testing instructional advancing neurodural research and DVS disease interventions.
Acknowledgements
Special thanks to Asst. Prof. Deniz Tanır, Faculty of Economics and Administrative Sciences, Management Information Systems, Kafkas University for sincere efforts and assistances.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
This article has not been submitted or published elsewhere in part or in whole and that it is original work.
References
- 1.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21(1):74–78. [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil MK, Payer AF, Johnson TE. Effectiveness of using cross-sections in the recognition of anatomical structures in radiological images. Anat Rec B New Anat. 2005;283(1):9–13. doi: 10.1002/ar.b.20053. [DOI] [PubMed] [Google Scholar]
- 3.Liauw L, van Buchem MA, Spilt A, de Bruïne FT, van den Berg R, Hermans J, Wasser MNJM. MR Angiography of the Intracranial Venous System. Radiology. 2000;214(3):678–682. doi: 10.1148/radiology.214.3.r00mr41678. [DOI] [PubMed] [Google Scholar]
- 4.Estevez ME, Lindgren KA, Bergethon PR. A novel three-dimensional tool for teaching human neuroanatomy. Anat Sci Educ. 2010;3(6):309–317. doi: 10.1002/ase.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller R. Approaches to learning spatial relationships in gross anatomy: perspective from wider principles of learning. Clin Anat. 2000;13(6):439–443. doi: 10.1002/1098-2353(2000)13:6<439::AID-CA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Raikos A, Smith JD. Anatomical variations: How do surgical and radiology training programs teach and assess them in their training curricula? Clin Anat. 2015;28(6):717–724. doi: 10.1002/ca.22560. [DOI] [PubMed] [Google Scholar]
- 7.Trelease RB, Rosset A. Transforming clinical imaging data for virtual reality learning objects. Anat Sci Educ. 2008;1(2):50–55. doi: 10.1002/ase.13. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen N, Mulla A, Nelson AJ, Wilson TD. Visuospatial anatomy comprehension: the role of spatial visualization ability and problem-solving strategies. Anat Sci Educ. 2014;7(4):280–288. doi: 10.1002/ase.1415. [DOI] [PubMed] [Google Scholar]
- 9.Govsa F, Yagdi T, Ozer MA, Eraslan C, Alagoz AK. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur Arch Oto-Rhino-Laryngology. 2017;274(2):1097–1102. doi: 10.1007/s00405-016-4355-0. [DOI] [PubMed] [Google Scholar]
- 10.Meier LM, Meineri M, Qua Hiansen J, Horlick EM. Structural and congenital heart disease interventions: the role of three-dimensional printing. Netherlands Heart J. 2017;25(2):65–75. doi: 10.1007/s12471-016-0942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu A, Wilson T, Ladak H, et al. Evaluation of a three-dimensional educational computer model of the larynx: voicing a new direction. J Otolaryngol Head Neck Surg. 2010;39(3):315–322. [PubMed] [Google Scholar]
- 12.Javan R, Herrin D, Tangestanipoor A. Understanding spatially complex segmental and branch anatomy using 3D printing: Liver, lung, prostate, coronary arteries, and circle of Willis. Acad Radiol. 2016;23(9):1183–1189. doi: 10.1016/j.acra.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Madurska MJ, Poyade M, Eason D, Rea P, Watson AJM. Development of a patient-specific 3D-printed liver model for preoperative planning. Surg Innov. 2017;24(2):145–150. doi: 10.1177/1553350616689414. [DOI] [PubMed] [Google Scholar]
- 14.Akamatsu Y, Sato K, Endo H, et al. Single-session hematoma removal and transcranial coil embolization for a cavernous sinus dural arteriovenous fistula: A technical case report. World Neurosurg. 2017;104:1046.e7–1046.e12. doi: 10.1016/j.wneu.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Weinstock P, Rehder R, Prabhu SP, Forbes PW, Roussin CJ, Cohen AR. Creation of a novel simulator for minimally invasive neurosurgery: fusion of 3D printing and special effects. J Neurosurg Pediatr. 2017;20(1):1–9. doi: 10.3171/2017.1.PEDS16568. [DOI] [PubMed] [Google Scholar]
- 16.San Millán Ruíz D, Gailloud P, Rüfenacht DA, et al. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol. 2002;23(9):1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Park KS, Kang DH, Hwang JH, Hwang SK. Stereotactic radiosurgery for dural carotid cavernous sinus fistulas. World Neurosurg. 2017;106:836–843. doi: 10.1016/j.wneu.2017.04.143. [DOI] [PubMed] [Google Scholar]
- 18.Selden NR, Origitano TC, Hadjipanayis C, et al. Model-based simulation for early neurosurgical learners. Neurosurgery. 2013;73(Suppl 1):15–24. doi: 10.1227/NEU.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 19.Shui W, Zhou M, Chen S, Pan Z, Deng Q, Yao Y, Pan H, He T, Wang X. The production of digital and printed resources from multiple modalities using visualization and three-dimensional printing techniques. Int J Comput Assist Radiol Surg. 2017;12(1):13–23. doi: 10.1007/s11548-016-1461-9. [DOI] [PubMed] [Google Scholar]
- 20.Tam MDBS. Building virtual models by postprocessing radiology images: A guide for anatomy faculty. Anat Sci Educ. 2010;3(5):261–266. doi: 10.1002/ase.175. [DOI] [PubMed] [Google Scholar]
- 21.Tan S, Hu A, Wilson T, Ladak H, Haase P, Fung K. Role of a computer-generated three-dimensional laryngeal model in anatomy teaching for advanced learners. J Laryngol Otol. 2012;126(4):395–401. doi: 10.1017/S0022215111002830. [DOI] [PubMed] [Google Scholar]
- 22.Tabernero Rico RD, Juanes Méndez JA, Prats GA. New generation of three-dimensional tools to learn anatomy. J Med Syst. 2017;41(5):88. doi: 10.1007/s10916-017-0725-4. [DOI] [PubMed] [Google Scholar]
- 23.Moore CW, Wilson TD, Rice CL. Digital preservation of anatomical variation: 3D-modeling of embalmed and plastinated cadaveric specimens using uCT and MRI. Ann Anat. 2017;209:69–75. doi: 10.1016/j.aanat.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Sergovich A, Johnson M, Wilson TD. Explorable three-dimensional digital model of the female pelvis, pelvic contents, and perineum for anatomical education. Anat Sci Educ. 2010;3(3):127–133. doi: 10.1002/ase.135. [DOI] [PubMed] [Google Scholar]
- 25.Randazzo M, Pisapia J, Singh N, et al. 3D printing in neurosurgery: A systematic review. Surg Neurol Int. 2016;7(34):801. doi: 10.4103/2152-7806.194059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan JR, Almefty KK, Nakaji P, Frakes DH. Cerebral aneurysm clipping surgery simulation using patient-specific 3D printing and silicone casting. World Neurosurg. 2016;88:175–181. doi: 10.1016/j.wneu.2015.12.102. [DOI] [PubMed] [Google Scholar]
- 27.Govsa F, Ozer MA, Sirinturk S, Eraslan C, Alagoz AK. Creating vascular models by postprocessing computed tomography angiography images: a guide for anatomical education. Surg Radiol Anat. 2017;39(8):905–910. doi: 10.1007/s00276-017-1822-2. [DOI] [PubMed] [Google Scholar]
- 28.Ryan JR, Chen T, Nakaji P, Frakes DH, Gonzalez LF. Ventriculostomy simulation using patient-specific ventricular anatomy, 3D printing, and hydrogel casting. World Neurosurg. 2015;84(5):1333–1339. doi: 10.1016/j.wneu.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Ploch CC, Mansi CSSA, Jayamohan J, Kuhl E. Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg. 2016;90:668–674. doi: 10.1016/j.wneu.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 30.Govsa F, Karakas AB, Ozer MA, Eraslan C. Development of life-size patient-specific 3D-printed dural venous models for preoperative planning. World Neurosurg. 2018;110:e141–e149. doi: 10.1016/j.wneu.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Chen M, Song Y, Li H, Lu B. Quality improvement of surface triangular mesh using a modified Laplacian smoothing approach avoiding intersection. PLoS One. 2017;12(9):e0184206. doi: 10.1371/journal.pone.0184206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Despotovic I, Goossens B, Philips W. MRI segmented of the human brain: Challenges, methods, and applications. Computational and Mathematical Methods in Medicine. 2015;2015:1–23. doi: 10.1155/2015/450341. [DOI] [PMC free article] [PubMed] [Google Scholar]