Abstract
Background
Tissue plasminogen activator (t‐PA) is an effective therapy for acute ischemic stroke, but some patients still have poor clinical outcome. In this study, we investigated clinical characteristics of stroke patients and determined predictors for poor clinical outcome in response to t‐PA treatment.
Methods
Clinical data from 247 patients were retrospectively reviewed. Clinical parameters that were associated with survival of patients were analyzed. Areas under receiver operating characteristic curves (ROC) were used to determine the feasibility of using various combinations of the clinical parameters to predict poor clinical response. The clinical outcome was defined according to the changes in Modified Rankin Scale.
Results
Overall, 145 patients had improved/complete recovery, 73 had no change, and 29 had worsening conditions or died during the in‐clinic period. A univariate analysis showed that baseline characteristics including age, CRP, blood glucose level, systolic blood pressure, and admission NIHSS were significantly different (p < 0.05) among patients with different clinical outcome. A further multivariate analysis was then performed. Variables associated with poor clinical outcome (worsening/death) (p < 0.1) were included in the logistic regression model. Four parameters were retained in the model: Age, CRP, Blood glucose level, and Systolic blood pressure (ACBS). To allow a convenient usage of the ACBS classifier, the parameters were put into a scoring system, and the score at 7.7 was chosen as a cut‐off. The ROC curve of this ACBS classifier has an area under the curve (AUC) of 0.7788, higher than other individual parameters. The ACBS classifier provided enhanced sensitivity of 69.2% and specificity of 74.3%.
Conclusion
The ACBS classifier provided a satisfactory power in estimating the patients’ clinical outcome. After further validating, the classifier may provide important information to clinicians for making clinical decisions.
Keywords: outcome, stroke, tissue plasminogen activator
1. INTRODUCTION
Stroke is the leading cause of disability among adults. It is caused by thrombotic or embolic occlusion of a cerebral artery. Each year, about 800,000 strokes occurred; and of all strokes, 87% are ischemic in origin (Roger et al., 2011). The risk of stroke is higher among elderly than youngsters (Rosamond et al., 1999). Many of the stroke survivors continue to experience functional deficits that diminished their quality of life. In Framingham study, almost half of all elderly stroke survivors had moderate to severe neurological deficits (Kelly‐Hayes et al., 2003). Because of an increase in life expectancy, the number of individuals at risk for ischemic stroke is expected to increase, making the management of stroke disability among elderly an important public health concern.
Tissue plasminogen activator (t‐PA) is a US Food and Drug Administration‐approved drug for treatment of ischemic stroke. It is a serine protease that enhances the conversion of inactive plasminogen to active plasmin. Plasmin acts on fibrin clots, resulting in dissolution and lysis. Studies suggested that the administration of t‐PA within 3 hr after the onset of stroke increases the probability of favorable clinical outcome (Wechsler, 2011). However, only a small fraction of potentially eligible stroke patients is receiving t‐PA therapy. In US, it is estimated that the rate of t‐PA use averages not more than 2% (Katzan et al., 2000). In China, the rate of t‐PA usage was only about 1.6% (Dong et al., 2017). The difficulty in predicting the clinical outcome of patients receiving t‐PA treatment is hampering the widespread usage of t‐PA by clinicians.
A prognostic model that would allow an early estimation of clinical outcomes of patients receiving t‐PA treatment is in need. A handy prognostic model should contain variables that are readily available in clinical settings for all patients. To date, there have been limited prognostic models for stroke recovery, and the predictive power was not satisfactory (Counsell & Dennis, 2001). There were other models that relied on imaging variables, which may not be available for all patients (Baird et al., 2001; Johnston, Connors, Wagner, & Haley, 2003; Johnston et al., 2000). In the current study, we investigated different baseline clinical characteristics that may associate with the poor clinical outcome of stroke patients as assessed by Modified Rankin Scale (MRS). These patients likely would not benefit from the t‐PA therapy.
2. PATIENTS AND METHODS
2.1. Patient characteristics
A total of 247 patients with acute ischemic stroke were included in this study, and their clinical data were retrospectively reviewed. These patients were admitted to the Department of Neurology, Yangpu Hospital, Tongji University School of Medicine between January 2016 and December 2017. On arrival in the emergency room, patients underwent standard neurological and cardiological examinations. Blood chemistry, vital signs, and CT scans of the brain were obtained before the start of treatment. The CT scans were reviewed by a neuroradiologist with extensive experience in acute stroke. Previous and concomitant diseases were recorded.
All of the patients were treated with t‐PA at a dose of 0.9 mg/kg. National Institute of Health Stroke Scale (NIHSS) score was assessed at various time points during the clinical stay. Patients with admission NIHSS ≥20 were considered as severe, 4–19 were moderate, and <4 represented mild or normal. MRS was assessed at admission and at discharge. We defined the clinical outcome according to the differences in admission MRS and discharge MRS. Patients with a lower MRS (or MRS = 0) at discharge were defined as “improved/complete cure,” those with the same MRS were defined as “no change,” and those with a higher MRS (or died after treatment) were defined as “worsening/death.” Adverse events of hemorrhage, including symptomatic intracerebral hemorrhage, were identified. The study was approved by the Hospital's ethics committee.
2.2. Statistical analyses
All statistical analyses were carried out by SAS9.3. Continuous variables were expressed as mean ± SD, whereas categorical variables were expressed as numbers (percentages). A value of p < 0.05 was considered statistically significant. For comparisons of three or more groups with data normally distributed, one‐way analysis of variance was used; otherwise, Kruskal–Wallis test was used.
A univariate analysis was performed to compare variables among the groups: Improved/complete cure, No change, and Worsening/death. Variables associated with poor clinical outcome (worsening/death) (p < 0.1) were included in the logistic regression model. In the meantime, variable selection was performed to balance the predictive power and complexity of the model. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to determine the feasibility of using clinical parameters as a classifier to predict treatment response of patients. Mann–Whitney test was used to compare the differences of AUC between various parameters. The Youden's Index was used to identify the optimal cut‐off point. Sensitivity, specificity, and confidence interval calculations were performed using standard procedures.
3. RESULTS
We evaluated a total of 247 patients (145 males, 102 female) with acute ischemic stroke; all of them were treated with intravenous t‐PA. Demographic data, baseline clinical findings, and medical history were shown in Table 1. The mean age of the cohort was 69.78 ± 13.62 years. Patients arrived at the emergency department after stroke onset in 1.64 ± 2.21 hr. The time elapsed between symptom onset and t‐PA treatment was 2.72 ± 2.56 hr. Baseline clinical assessment revealed that 75.3% of the patients had hypertension. The median NIHSS score of the cohort at admission was 3.
Table 1.
Baseline characteristics of patients (n = 247) with acute ischemic stroke
| Characteristics | Values | Baseline characteristics | Values |
|---|---|---|---|
| Age (year), mean ± SD | 69.78 ± 13.62 | Medical history | |
| Male, n (%) | 145 (58.70) | Hypertension, n (%) | 186 (75.30) |
| CRP (mg/L), median (IQR) | 0 (0, 6) | Diabetes, n (%) | 71 (28.74) |
| Creatinine (μmol/L), median (IQR) | 73 (60,92) | Atrial fibrillation, n (%) | 41 (16.60) |
| Uric acid (μmol/L), median (IQR) | 293 (211, 371) | Renal insufficiency, n (%) | 11 (4.45) |
| Blood glucose (mmol/L), median (IQR) | 6.76 (5.74, 8.68) | Coronary heart Disease, n (%) | 20 (8.10) |
| Homocysteine (μmol/L), median (IQR) | 16.05 (11.79, 21.00) | Lung infection, n (%) | 1 (0.40) |
| Systolic blood pressure (mmHg), median (IQR) | 154 (142, 170) | Cancer, n (%) | 2 (0.81) |
| Diastolic blood pressure (mmHg), median (IQR) | 86 (76, 95) | Cerebral infraction, n (%) | 12 (4.86) |
| NIHSS, median (IQR) | 3 (2, 7) | Other medical history, n (%) | 35 (14.17) |
NIHSS data were completed at baseline in 247 (100%), at 2 hr in 246 (99.6%), at 24 hr in 232 (93.9%), at 2 days in 221 (89.5%), at 3 days in 211 (85.4%), at 7 days in 196 (79.4%), and at 14 days in 81 (32.8%) patients. MRS data were completed at baseline and at discharge. Higher number of patients had a mild severity (score 0–1) of MRS at discharge (n = 179) than at the baseline (n = 124). Overall, 145 patients had improved/complete recovery, 73 had no changes, and 29 had the outcome of worsening/died.
It has been known that the major complication of thrombolytic therapy for acute stroke is hemorrhage. In the current cohort, the reported treatment‐related hemorrhage included oral (n = 18), gastrointestinal (n = 2), and intracranial hemorrhage (n = 8). Most of the related adverse events (AEs) were mild (n = 14) or moderate (n = 12) in intensity. A total of eight related AEs were severe, including three events of deaths (these three patients had symptomatic hemorrhage).
A univariate analysis was performed for the available variables, and the results showed that baseline characteristics including age, CRP, blood glucose level, systolic blood pressure, and admission NIHSS were significantly different (p < 0.05) among patients with different clinical outcome (Table 2). A further multivariate analysis was then performed. Variables associated with poor clinical outcome (worsening/death) (p < 0.1) were included in the logistic regression model. In the meantime, variable selection was performed. After balancing the predictive power and complexity of the model, four parameters were retained: Age, CRP, Blood glucose level, and Systolic blood pressure (ACBS). These parameters were statistically significant among groups, and their predictive power was high when used in combination. Also, these were objective parameters (when compared to NIHSS) and could be obtained easily in clinical setting. Recognizing the relatively wide 95% CI of the data, several models were established (Table 3).
Table 2.
Univariate analysis of baseline characteristics and clinical outcome
| Baseline characteristics | Improved/complete cure, n = 145 | No change, n = 73 | Worsening/death | p |
|---|---|---|---|---|
| Age (year), mean ± SD | 69.17 ± 13.24 | 68.05 ± 14.57 | 77.14 ± 10.79 | 0.001 |
| 80 ≤ age | 38 (26.39) | 21 (28.77) | 15 (51.72) | 0.029 |
| 70 ≤ age < 80 | 27 (18.75) | 13 (17.81) | 5 (17.24) | |
| 60 ≤ age < 70 | 46 (31.94) | 16 (21.92) | 8 (27.59) | |
| Age <60 | 33 (22.92) | 23 (31.51) | 1 (3.45) | |
| Gender, n (%) | ||||
| Male | 86 (59.31) | 40 (54.79) | 19 (65.52) | 0.595 |
| Female | 59 (40.69) | 33 (45.21) | 10 (34.48) | |
| Hypertension, n (%) | 105 (72.41) | 59 (80.82) | 22 (75.86) | 0.396 |
| Diabetes, n (%) | 41 (28.28) | 19 (26.03) | 11 (37.93) | 0.479 |
| Atrial fibrillation, n (%) | 22 (15.17) | 16 (21.92) | 3 (10.34) | 0.283 |
| Renal insufficiency, n (%) | 5 (3.45) | 2 (2.74) | 4 (13.79) | 0.072 |
| Coronary heart disease, n (%) | 13 (8.97) | 5 (6.85) | 2 (6.90) | 0.837 |
| Lung infection, n (%) | 0 (0.00) | 1 (1.37) | 0 (0.00) | 0.413 |
| Cancer, n (%) | 0 (0.00) | 1 (1.37) | 1 (3.45) | 0.083 |
| Cerebral Infraction, n (%) | 6 (4.14) | 4 (5.48) | 2 (6.90) | 0.753 |
| Other medical history, n (%) | 17 (11.72) | 10 (13.70) | 8 (27.59) | 0.081 |
| Admission CRP, median, (IQR) | 0 (0,6) | 0 (0,6) | 5 (0,20.89) | 0.005 |
| 16.5 ≤ CRP | 12 (8.45) | 6 (8.45) | 9 (31.03) | 0.030 |
| 7 ≤ CRP < 16.5 | 15 (10.56) | 8 (11.27) | 2 (6.90) | |
| 0 ≤ CRP < 7 | 115 (80.99) | 57 (80.28) | 18 (62.07) | |
| Creatinine (μmol/L), median (IQR) | 74 (62,95) | 72 (54,84) | 72.5 (58.5,89) | 0.206 |
| Uric acid (μmol/L), median (IQR) | 290 (205,371) | 309 (236,381) | 305 (54.84,369) | 0.655 |
| Blood Glucose (mmol/L), median (IQR) | 6.47 (5.64,8.63) | 6.77 (5.65,8.23) | 8.36 (6.62,10.93) | 0.008 |
| 9 ≤ glucose | 30 (21.58) | 13 (18.31) | 12 (44.44) | 0.054 |
| 7.5 ≤ glucose < 9 | 15 (10.79) | 9 (12.68) | 4 (14.81) | |
| 0 < glucose < 7.5 | 94 (67.63) | 49 (69.01) | 11 (40.74) | |
| Homocysteine (μmol/L), median (IQR) | 16.46 (11.64,21) | 14.6 (10.82,18.58) | 20 (14.43,26.98) | 0.058 |
| Systolic blood pressure (mmHg), median (IQR) | 151 (140,166) | 154.5 (143,170) | 166 (148,177.5) | 0.049 |
| 165 ≤ systolic blood pressure | 37 (25.87) | 22 (31.43) | 16 (57.14) | 0.024 |
| 155 ≤ systolic blood pressure < 165 | 25 (17.48) | 13 (18.57) | 4 (14.29) | |
| 0 < systolic blood pressure < 155 | 81 (56.64) | 35 (50.00) | 8 (28.57) | |
| Diastolic blood pressure (mmHg), median (IQR) | 85 (76,93) | 88 (77,98) | 85.5 (75.5,95.5) | 0.337 |
| Admission NIHSS, median (IQR) | 3 (2,7) | 3 (2,5) | 4 (3,9) | 0.032 |
| 20 ≤ NIHSS | 8 (5.52) | 4 (5.48) | 4 (13.79) | 0.082 |
| 4 ≤ NIHSS < 20 | 58 (40.00) | 26 (35.62) | 16 (55.17) | |
| 0 ≤ NIHSS < 4 | 79 (54.48) | 43 (58.90) | 9 (31.03) | |
Table 3.
Multivariate analysis of occurrence of worsening/death after treatment in patients with different clinical outcome
| Model | Independent variables | Levels (risk factors) | Odds ratio | 95% CI | p | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Model 1 | Gender | Male versus female | 1.141 | 0.400 | 3.254 | 0.805 |
| Renal insufficiency | Absence versus presence | 0.220 | 0.035 | 1.398 | 0.109 | |
| Cancer | Absence versus presence | 0.507 | 0.013 | 19.172 | 0.714 | |
| Age | 80 ≤ age versus age <60 | 5.772 | 0.650 | 51.238 | 0.273 | |
| 70 ≤ age < 80 versus age <60 | 5.014 | 0.474 | 53.045 | 0.532 | ||
| 60 ≤ age < 70 versus age <60 | 5.662 | 0.630 | 50.909 | 0.316 | ||
| CRP | 16.5 ≤ CRP versus 0 ≤ CRP < 7 | 5.694 | 1.649 | 19.665 | 0.008 | |
| 7 ≤ CRP < 16.5 versus 0 ≤ CRP < 7 | 0.868 | 0.166 | 4.548 | 0.227 | ||
| Blood glucose | 9 ≤ blood glucose versus 0 < blood glucose < 7.5 | 2.309 | 0.780 | 6.835 | 0.521 | |
| 7.5 ≤ blood glucose < 9 versus 0 < blood glucose < 7.5 | 2.631 | 0.660 | 10.498 | 0.411 | ||
| SBP | 165 ≤ SBP versus 0 < SBP < 155 | 5.906 | 1.869 | 18.667 | 0.006 | |
| 155 ≤ SBP < 165 versus 0 < SBP <155 | 1.662 | 0.393 | 7.025 | 0.570 | ||
| NIHSS | 20 ≤ NIHSS versus 0 ≤ NIHSS < 4 | 1.867 | 0.334 | 10.445 | 0.678 | |
| 4 ≤ NIHSS < 20 versus 0 ≤ NIHSS < 4 | 1.770 | 0.601 | 5.211 | 0.644 | ||
| Model 2 | Age | Unit = 1 | 1.044 | 1.007 | 1.082 | 0.019 |
| CRP | Unit = 1 | 1.013 | 0.999 | 1.027 | 0.068 | |
| Blood glucose | Unit = 1 | 1.091 | 0.967 | 1.231 | 0.158 | |
| SBP | Unit = 1 | 1.024 | 1.004 | 1.044 | 0.016 | |
| Model 3 | Age | 80 ≤ age versus age <60 | 6.039 | 0.719 | 50.746 | 0.253 |
| 70 ≤ age < 80 versus age <60 | 6.207 | 0.637 | 60.431 | 0.326 | ||
| 60 ≤ age < 70 versus age <60 | 5.536 | 0.628 | 48.843 | 0.394 | ||
| CRP | 16.5 ≤ CRP versus 0 ≤ CRP < 7 | 6.732 | 2.108 | 21.503 | 0.002 | |
| 7 ≤ CRP < 16.5 versus 0 ≤ CRP < 7 | 0.912 | 0.179 | 4.638 | 0.200 | ||
| Blood glucose | 9 ≤ blood glucose versus 0 < blood glucose < 7.5 | 2.512 | 0.914 | 6.902 | 0.352 | |
| 7.5 ≤ blood glucose < 9 versus 0 < blood glucose < 7.5 | 2.387 | 0.620 | 9.192 | 0.531 | ||
| SBP | 165 ≤ SBP versus 0 < SBP < 155 | 5.654 | 1.888 | 16.931 | 0.006 | |
| 155 ≤ SBP < 165 versus 0 < SBP < 155 | 1.783 | 0.428 | 7.422 | 0.664 | ||
Model 1, the characteristic variables of p < 0.1 in the univariate analysis were all used as independent variables, and the logistic regression model was established by using clinical outcome of worsening/death after treatment. Model 2: Age, CRP, blood glucose, SBP were selected as independent variables. Model 3: Age, CRP, blood glucose, and SBP were selected as an independent variable.
The four variables, age, CRP, blood glucose, and SBP, were continuous variables and might not be convenient to use in a regression model. The parameters were thus put into a scoring system (Table 4). The levels were first assigned on the basis of the cut‐off of the single parameter, and then adjusted in combination with other parameters until the predictive power of the classifier became satisfactory (higher predictive value than the single parameter). The score at 7.7 was chosen as a cut‐off, as it has the highest Youden's Index. Risk of the poor clinical outcome increased with higher scores.
Table 4.
ACBS scoring system
| Characteristics | Criteria | Score |
|---|---|---|
| Age (year) | <60 | 0 |
| 60–69 | 1 | |
| 70–79 | 2 | |
| ≥80 | 3 | |
| CRP (mg/L) | <7 | 1 |
| 7–16.4 | 2 | |
| ≥ 16.5 | 3 | |
| Blood glucose level (mmol/L) | <7.5 | 1 |
| 7.5–8.9 | 2 | |
| ≥9 | 3 | |
| Systolic blood pressure (mmHg) | <155 | 1 |
| 155–164 | 2 | |
| ≥165 | 3 |
The ROC curve of this ACBS classifier has an AUC of 0.7788, while other individual parameters had smaller AUC, including age (AUC = 0.6617), CRP (AUC = 0.6337), blood glucose grade (AUC = 0.6371), SBP grade (AUC = 0.6753), admission NIHH (AUC = 0.6510), and admission MRS (AUC = 0.6173) (Table 5 and Figure 1). The ACBS classifier provided enhanced sensitivity of 69.2% and specificity of 74.3%.
Table 5.
Comparisons of AUC between ACBS classifier and various parameters
| Indicators | AUC | 95% CI | Difference* | p * | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| ACBS score | 0.7788 | 0.6831 | 0.8744 | – | – |
| Age | 0.6617 | 0.5667 | 0.7567 | −0.1451 | 0.0020 |
| CRP | 0.6337 | 0.5267 | 0.7406 | −0.1417 | 0.0182 |
| Blood glucose | 0.6371 | 0.5306 | 0.7435 | −0.1035 | 0.0109 |
| SBP | 0.6753 | 0.5702 | 0.7803 | −0.1278 | 0.0453 |
| NIHSS of admission | 0.6510 | 0.5541 | 0.7479 | −0.1615 | 0.0358 |
| MRS of admission | 0.6173 | 0.4991 | 0.7355 | −0.2788 | 0.0153 |
| Reference line | 0.5000 | 0.5000 | 0.5000 | −0.1451 | <0.0001 |
The differences in AUC and p‐value compared to ACBS.
Figure 1.
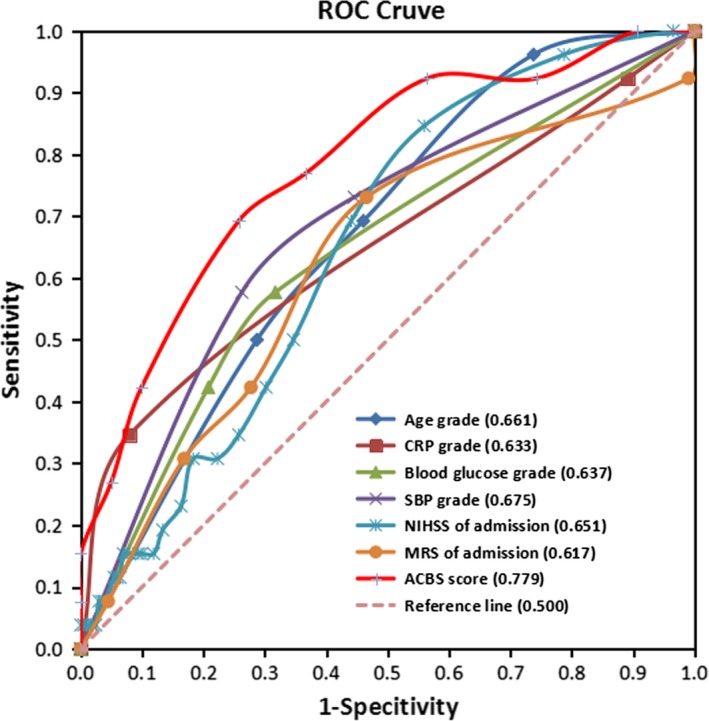
Receiver operating characteristic curves for age, CRP, blood glucose grade, SBP grade, baseline NIHSS, baseline MRS, and ACBS classifier with respect to clinical outcome
4. DISCUSSION
The current study showed that the combination of age, CRP, baseline SBP, and blood glucose was a classifier that could predict patients with high risk of poor response to t‐PA treatment. Comparing to other predictive models (Baird et al., 2001; Johnston et al., 2003, 2000), the ACBS scoring system is simple to use. It is based on commonly assessed clinical parameters for stroke patients.
Several analyses from observational studies as well as randomized clinical trials reported the predictive value of age (Fiorelli et al., 1995; Generalized efficacy of t‐PA for acute stroke, 1997; Johnston et al., 2003). In the current study, patients with worsening/death outcome after treatment were significantly older. Age was also a variable in the ACBS classifier predicting the clinical outcome. The DRAGON score, which includes age in the scoring system, predicts the functional outcome of patients as assessed by MRS as well (Kent, 2012). Indeed, age was also a variable in different models that predict the risk of hemorrhage after t‐PA (Lou et al., 2008; Lyden, 2012; Menon et al., 2012). Overall, the data suggested that age is an important characteristic that has to be considered for clinical decision.
Our findings indicated that high blood glucose level was associated with poor clinical outcome of patients. Several studies also showed that hyperglycemia was related to poor outcome of acute ischemic stroke patients (Fuentes, 2010; Stead et al., 2009; Williams et al., 2002). The relationship between high blood glucose level and severity of ischemic stroke can be explained by the increase of lactate production in the ischemic region. The production lead to generation of hydrogen ions and disruption of intracellular pH homeostasis. As a result, some reactions and enzyme systems that are essential to cellular viability were interrupted (Lindsberg & Roine, 2004; Pulsinelli, Waldman, Rawlinson, & Plum, 1982; Rehncrona, Rosen, & Siesjo, 1981). In the current cohort, about 35% of patients had high blood glucose level (>7.5 mmol/L). Initiation of intensive insulin therapy has been suggested for stroke patients, but currently available data fail to identify the clinical benefits of the therapy, for example, the UK Glucose Insulin in Stroke Trial (GIST‐UK) showed no significant clinical benefit associated with insulin therapy in 933 patients with stroke (Gray et al., 2007).
High blood pressure is common in acute stroke patients. Several studies suggested a U‐shaped relationship between baseline blood pressure and poor outcome of ischemic stroke (Leonardi‐Bee, Bath, Phillips, & Sandercock, 2002; Vemmos et al., 2004). Both high blood pressure and low blood pressure were prognostic for poor outcome. The analyses of data from the thrombolysis implementation and monitor of AIS in China (TIMS‐China) showed that a higher first 2 hr systolic blood pressure was related to symptomatic intracerebral hemorrhage. A proper control of systolic blood pressure for the first 2 hr was thus recommended to decrease the risk of hemorrhage (Wu, 2016). Our study also supported the notion that high systolic blood pressure at admission was related to poor clinical outcome among ischemic stroke subjects.
Several studies have reported the predictive value of plasma CRP concentrations. Elevated CRP concentration predicted poor survival and poor functional outcome of patients with acute ischemic stroke (Mazaheri, Reisi, Poorolajal, & Ghiasian, 2018; Muir, Weir, Alwan, Squire, & Lees, 1999). It was suggested that CRP concentration may correlate with the degree of inflammation directly consequent to cerebral infraction. There were some studies, however, reported that baseline CRP failed to predict clinical outcomes (Karlinski et al., 2014; Topakian, Strasak, Nussbaumer, Haring, & Aichner, 2008). Our results supported the usefulness of baseline CRP as a predictive marker of poor clinical outcome.
Our study has some limitations. The study populations of the current study represent hospital‐based cohorts; unselected patients in different clinical settings are needed for validating the model. Also, the number of patients is limited for establishing a prognostic model. This is a retrospective study, therefore our findings await replication in a prospective cohort to determine the best approach to manage patients with high score in the ACBS scoring system. The cut‐off criteria for the clinical variables will need to be further verified. In summary, we evaluated the baseline clinical characteristics of stroke patients associated with patient outcome as assessed by MRS. The ACBS classifier provided a satisfactory sensitivity and specificity in estimating the patients’ response to t‐PA treatment. After further validating, the classifier may provide important information to clinicians when discussing treatment options with patients and their families.
5. CONCLUSION
The ACBS classifier provided a satisfactory power in estimating the patients’ clinical outcome. After further validating, the classifier may provide important information to clinicians for making clinical decisions.
INFORMED CONSENT
All the patients gave their written information consent.
ETHICAL APPROVAL
The study was approved by the Hospital's ethics committee.
CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
Yue Y, Li Z, Hu L, et al. Clinical characteristics and risk score for poor clinical outcome of acute ischemic stroke patients treated with intravenous thrombolysis therapy. Brain Behav. 2019;9:e01251 10.1002/brb3.1251
Yun‐hua Yue, Zhi‐zhang Li, and Liang Hu contribute equally to this work.
REFERENCES
- Baird, A. E. , Dambrosia, J. , Janket, S.‐J. , Eichbaum, Q. , Chaves, C. , Silver, B. , … Warach, S. (2001). A three‐item scale for the early prediction of stroke recovery. Lancet, 357(9274), 2095–2099. 10.1016/S0140-6736(00)05183-7 [DOI] [PubMed] [Google Scholar]
- Counsell, C. , & Dennis, M. (2001). Systematic review of prognostic models in patients with acute stroke. Cerebrovascular Disease, 12(3), 159–170. 10.1159/000047699 [DOI] [PubMed] [Google Scholar]
- Dong, Q. , Dong, Y. i. , Liu, L. , Xu, A. , Zhang, Y. , Zheng, H. , & Wang, Y. (2017). The Chinese Stroke Association scientific statement: Intravenous thrombolysis in acute ischaemic stroke. Stroke and Vascular Neurology, 2(3), 147–159. 10.1136/svn-2017-000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli, M. , Alperovitch, A. , Argentino, C. , Sacchetti, M. l. , Toni, D. , Sette, G. , … Fieschi, C. (1995). Prediction of long‐term outcome in the early hours following acute ischemic stroke. Italian Acute Stroke Study Group. Archives of Neurology, 52(3), 250–255. 10.1001/archneur.1995.00540270038017 [DOI] [PubMed] [Google Scholar]
- Fuentes, B. , Ortega‐Casarrubios, M. A. , & SanJosé, B., Castillo, J., Leira, R., Serena, J., … Díez‐Tejedor, E. (2010). Persistent hyperglycemia >155 mg/dL in acute ischemic stroke patients: How well are we correcting it?: Implications for outcome. Stroke, 41(10), 2362–2365. [DOI] [PubMed] [Google Scholar]
- Generalized efficacy of t‐PA for acute stroke Subgroup analysis of the NINDS t‐PA Stroke Trial. (1997). Stroke, 28(11), 2119–2125. [DOI] [PubMed] [Google Scholar]
- Gray, C. S. , Hildreth, A. J. , Sandercock, P. A. , O'Connell, J. E. , Johnston, D. E. , Cartlidge, N. E. F. , … Alberti, K. G. M. M. (2007). Glucose‐potassium‐insulin infusions in the management of post‐stroke hyperglycaemia: The UK Glucose Insulin in Stroke Trial (GIST‐UK). The Lancet Neurology, 6(5), 397–406. 10.1016/S1474-4422(07)70080-7 [DOI] [PubMed] [Google Scholar]
- Johnston, K. C. , Connors, A. F. , Wagner, D. P. , & Haley, E. C. (2003). Predicting outcome in ischemic stroke: External validation of predictive risk models. Stroke, 34(1), 200–202. 10.1161/01.STR.0000047102.61863.E3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, K. C. , Connors, A. F. , Wagner, D. P. , Knaus, W. A. , Wang, X.‐Q. , & Haley, E. C. (2000). A predictive risk model for outcomes of ischemic stroke. Stroke, 31(2), 448–455. 10.1161/01.STR.31.2.448 [DOI] [PubMed] [Google Scholar]
- Karlinski, M. , Bembenek, J. , Grabska, K. , Kobayashi, A. , Baranowska, A. , Litwin, T. , & Czlonkowska, A. (2014). Routine serum C‐reactive protein and stroke outcome after intravenous thrombolysis. Acta Neurologica Scandinavica, 130(5), 305–311. 10.1111/ane.12227 [DOI] [PubMed] [Google Scholar]
- Katzan, I. L. , Furlan, A. J. , Lloyd, L. E. , Frank, J. I. , Harper, D. L. , Hinchey, J. A. , … Sila, C. A. (2000). Use of tissue‐type plasminogen activator for acute ischemic stroke: The Cleveland area experience. JAMA, 283(9), 1151–1158. 10.1001/jama.283.9.1151 [DOI] [PubMed] [Google Scholar]
- Kelly‐Hayes, M. , Beiser, A. , Kase, C. S. , Scaramucci, A. , D’Agostino, R. B. , & Wolf, P. A. (2003). The influence of gender and age on disability following ischemic stroke: The Framingham study. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 12(3), 119–126. 10.1016/S1052-3057(03)00042-9 [DOI] [PubMed] [Google Scholar]
- Kent, T. A. (2012). Predicting outcome of IV thrombolysis‐treated ischemic stroke patients: The DRAGON score. Neurology, 78(17), 1368. [DOI] [PubMed] [Google Scholar]
- Leonardi‐Bee, J. o. , Bath, P. M. W. , Phillips, S. J. , & Sandercock, P. A. G. (2002). Blood pressure and clinical outcomes in the International Stroke Trial. Stroke, 33(5), 1315–1320. 10.1161/01.STR.0000014509.11540.66 [DOI] [PubMed] [Google Scholar]
- Lindsberg, P. J. , & Roine, R. O. (2004). Hyperglycemia in acute stroke. Stroke, 35(2), 363–364. 10.1161/01.STR.0000115297.92132.84 [DOI] [PubMed] [Google Scholar]
- Lou, M. , Safdar, A. , Mehdiratta, M. , Kumar, S. , Schlaug, G. , Caplan, L. , … Selim, M. (2008). The HAT score: A simple grading scale for predicting hemorrhage after thrombolysis. Neurology, 71(18), 1417–1423. 10.1212/01.wnl.0000330297.58334.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden, P. D. (2012). Stroke: Haemorrhage risk after thrombolysis—The SEDAN score. Nature Reviews Neurology, 8(5), 246–247. 10.1038/nrneurol.2012.66 [DOI] [PubMed] [Google Scholar]
- Mazaheri, S. , Reisi, E. , Poorolajal, J. , & Ghiasian, M. (2018). C‐reactive protein levels and clinical outcomes in stroke patients: A prospective cohort study. Archives of Iranian Medicine, 21(1), 8–12. [PubMed] [Google Scholar]
- Menon, B. K. , Saver, J. L. , Prabhakaran, S. , Reeves, M. , Liang, L. i. , Olson, D. W. M. , … Smith, E. E. (2012). Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue‐type plasminogen activator. Stroke, 43(9), 2293–2299. 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- Muir, K. W. , Weir, C. J. , Alwan, W. , Squire, I. B. , & Lees, K. R. (1999). C‐reactive protein and outcome after ischemic stroke. Stroke, 30(5), 981–985. 10.1161/01.STR.30.5.981 [DOI] [PubMed] [Google Scholar]
- Pulsinelli, W. A. , Waldman, S. , Rawlinson, D. , & Plum, F. (1982). Moderate hyperglycemia augments ischemic brain damage: A neuropathologic study in the rat. Neurology, 32(11), 1239–1246. 10.1212/WNL.32.11.1239 [DOI] [PubMed] [Google Scholar]
- Rehncrona, S. , Rosen, I. , & Siesjo, B. K. (1981). Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. Journal of Cerebral Blood Flow and Metabolism, 1(3), 297–311. 10.1038/jcbfm.1981.34 [DOI] [PubMed] [Google Scholar]
- Roger, V. L. , Go, A. S. , Lloyd‐Jones, D. M. , Adams, R. J. , Berry, J. D. , Brown, T. M. , … … C. S. (2011). Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation, 123(4), e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond, W. D. , Folsom, A. R. , Chambless, L. E. , Wang, C.‐H. , McGovern, P. G. , Howard, G. , … Shahar, E. (1999). Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke, 30(4), 736–743. 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- Stead, L. G. , Gilmore, R. M. , Bellolio, M. F. , Mishra, S. , Bhagra, A. , Vaidyanathan, L. , … Brown, R. D. (2009). Hyperglycemia as an independent predictor of worse outcome in non‐diabetic patients presenting with acute ischemic stroke. Neurocritical Care, 10(2), 181–186. 10.1007/s12028-008-9080-0 [DOI] [PubMed] [Google Scholar]
- Topakian, R. , Strasak, A. M. , Nussbaumer, K. , Haring, H.‐P. , & Aichner, F. T. (2008). Prognostic value of admission C‐reactive protein in stroke patients undergoing iv thrombolysis. Journal of Neurology, 255(8), 1190–1196. 10.1007/s00415-008-0866-y [DOI] [PubMed] [Google Scholar]
- Vemmos, K. N. , Tsivgoulis, G. , Spengos, K. , Zakopoulos, N. , Synetos, A. , Manios, E. , … Mavrikakis, M. (2004). U‐shaped relationship between mortality and admission blood pressure in patients with acute stroke. Journal of Internal Medicine, 255(2), 257–265. 10.1046/j.1365-2796.2003.01291.x [DOI] [PubMed] [Google Scholar]
- Wechsler, L. R. (2011). Intravenous thrombolytic therapy for acute ischemic stroke. New England Journal of Medicine, 364(22), 2138–2146. 10.1056/NEJMct1007370 [DOI] [PubMed] [Google Scholar]
- Williams, L. S. , Rotich, J. , Qi, R. , Fineberg, N. , Espay, A. , Bruno, A. , … Tierney, W. R. (2002). Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology, 59(1), 67–71. 10.1212/WNL.59.1.67 [DOI] [PubMed] [Google Scholar]
- Wu, W. , Huo, X. , Zhao, X. , Liao, X. , Wang, C. , Pan, Y. , … TIMS‐CHINA investigators . (2016). Relationship between blood pressure and outcomes in acute ischemic stroke patients administered lytic medication in the TIMS‐China study. PLoS ONE, 11(2), e0144260. [DOI] [PMC free article] [PubMed] [Google Scholar]


