SUMMARY
The bacteria-derived tyrosyl-tRNA synthetase (TyrRS)/tRNA pair was first used for unnatural amino acid (Uaa) mutagenesis in eukaryotic cells over 15 years ago. It provides an ideal platform to genetically encode numerous useful Uaas in eukaryotes. However, this pair has been engineered to charge only a small collection of Uaas to date. Development of Uaa-selective variants of this pair has been limited by technical challenges associated with a yeast-based directed evolution platform, which is currently required to alter its substrate specificity. Here we overcome this limitation by enabling its directed evolution in a novel strain of E. coli (ATMY), where the endogenous TyrRS/tRNA pair has been functionally replaced with an archaeal counterpart. The facile E. coli-based selection system enabled rapid engineering of this pair to develop variants that selectively incorporate various Uaas, including p-boronophenylalanine, into proteins expressed in mammalian cells as well as in the ATMY strain of E. coli.
INTRODUCTION
Site-specific incorporation of unnatural amino acids (Uaas) into proteins in living cells provide exciting new ways to understand and engineer their function.(Chin, 2017; Dumas et al., 2015; Italia et al., 2017b; Mukai et al., 2017; Young and Schultz, 2018) To co-translationally incorporate an Uaa, it is encoded by a repurposed nonsense codon, which is suppressed by an orthogonal (i.e., does not cross-react with its host counterparts) Uaa-selective aminoacyl-tRNA synthetase (aaRS)/tRNA pair.(Chin, 2017; Dumas et al., 2015; Italia et al., 2017b; Mukai et al., 2017; Young and Schultz, 2018) Eukaryote or archaea derived aaRS/tRNA pairs are orthogonal in bacteria and used for Uaa incorporation, while those derived from bacteria are typically orthogonal in eukaryotes (Figure 1A).(Chin, 2017; Dumas et al., 2015; Italia et al., 2017b; Mukai et al., 2017; Young and Schultz, 2018) To create Uaa-specific variants of aaRS/tRNA pairs through directed evolution, two cell-based selection systems have been developed, using Escherichia coli(Santoro et al., 2002; Wang et al., 2001) or Saccharomyces cerevisiae (yeast)(Chin et al., 2003a; Chin et al., 2003b) as selection hosts, for engineering pairs that are orthogonal in bacteria or eukaryotes, respectively. Due to its facile nature, the E. coli based selection system has been significantly more successful in creating novel Uaa-selective aaRS/tRNA pairs than its yeast counterpart.(Dumas et al., 2015; Italia et al., 2017a; Italia et al., 2017b) As a result, genetically encoding new Uaas in eukaryotes in the past decade has overwhelmingly relied on the unique pyrrolysyl pair – the only pair that can be engineered using the facile E. coli selection system followed by application in eukaryotes (Figure 1A).(Chin, 2017; Dumas et al., 2015; Italia et al., 2017b; Wan et al., 2014; Young and Schultz, 2018) However, such excessive dependence on a single platform limits the structural diversity of Uaas that can be genetically encoded in eukaryotes.(Dumas et al., 2015; Italia et al., 2017a; Italia et al., 2017b) It also limits the scope of the nascent technology for concurrently incorporating multiple different Uaas into proteins in eukaryotes, each of which must be charged by a distinct orthogonal pair.(Italia et al., 2017b; Xiao et al., 2013; Zheng et al., 2017a; Zheng et al., 2018)
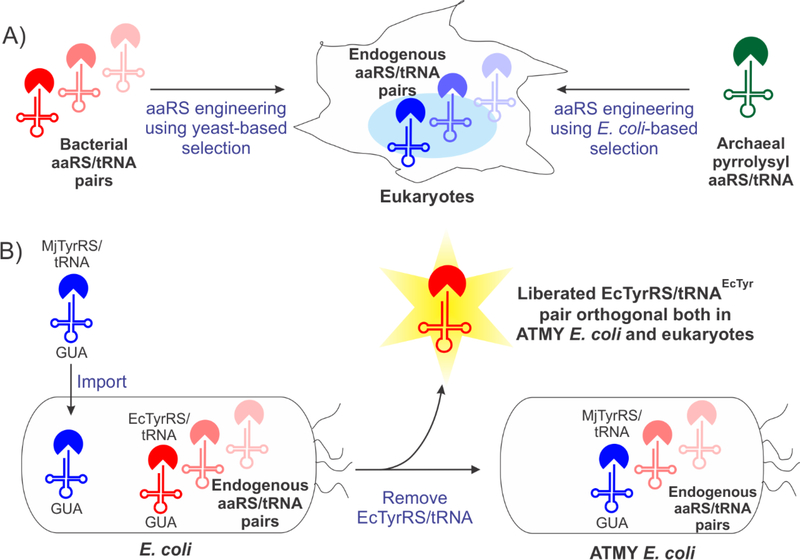
Figure 1. ATMY recombination scheme.
A) Bacteria-derived aaRS/tRNA pairs are typically suitable for expanding the genetic code of eukaryotes, but engineering their substrate specificity must be performed using a yeast-based selection system. The unique pyrrolysyl pair from archaea can be used for Uaa mutagenesis in both bacteria and eukaryotes. Thus, it can be engineered to charge desired Uaas using the E. coli selection system, followed by application in eukaryotes. B) Functionally substituting the endogenous EcTyrRS/tRNA pair of E. coli with an archaeal counterpart liberates it for reintroduction into the resulting ATMY strain as an orthogonal nonsense suppressor, where its substrate specificity can be engineered using the facile E. coli based selection system.
The first demonstrations of site-specific Uaa incorporation in eukaryotic cells was achieved over 15 years ago using a bacteria-derived tyrosyl-tRNA synthetase (TyrRS)/tRNA pair.(Chin et al., 2003a; Sakamoto et al., 2002) However, this pair has been engineered to charge only a small collection of simple tyrosine analogs so far, with no new engineered variants reported in the last decade.(Dumas et al., 2015; Italia et al., 2017a; Italia et al., 2017b) This can be largely attributed to the limitations associated with the aforementioned yeast-based selection system, which is currently needed to engineer its substrate specificity. In contrast, the analogous M. jannaschii (archaea) derived TyrRS (MjTyrRS)/tRNA pair, which is orthogonal in bacteria (but not in eukaryotes), has been engineered using the E. coli selection system to charge over 70 Uaas, including a variety of bioconjugation handles,(Chin et al., 2002b; Seitchik et al., 2012; Wang et al., 2010) photoaffinity probes,(Chin et al., 2002a; Chin et al., 2002b) fluorophores,(Wang et al., 2006) metal-binding groups,(Lee et al., 2009) light-responsive functionalities,(Bose et al., 2006; Deiters et al., 2006) post-translational modifications of tyrosine,(Liu and Schultz, 2006; Luo et al., 2017) etc., many of which cannot currently be incorporated into proteins expressed in eukaryotic cells.(Dumas et al., 2015; Young and Schultz, 2018) The ability to similarly engineer the bacterial TyrRS/tRNA pair using the facile E. coli selections system can facilitate the introduction of such useful Uaas to the eukaryotic genetic code.
Directed evolution of bacteria-derived aaRS/tRNA pairs in E. coli is forbidden due to their cross-reactivity in the host cell. However, functionally replacing an endogenous aaRS/tRNA pair of E. coli with a eukaryotic/archaeal counterpart can enable its reintroduction in the resulting “altered translational machinery” (ATM) strain as an orthogonal nonsense suppressor (Figure 1B).(Englert et al., 2017; Iraha et al., 2010; Italia et al., 2017a) Recently, we demonstrated that such an ATM strain can serve as the selection host for altering the substrate specificity of the “liberated” bacterial tryptophanyl-tRNA synthetase/tRNA pair.(Italia et al., 2017a) The resulting engineered variants of this pair then can be used for Uaa mutagenesis both in eukaryotes and in the engineered E. coli strain.(Italia et al., 2017a) Here, we extend this strategy to liberate the endogenous TyrRS (EcTyrRS)/tRNA pair from E. coli, complemented by an archaeal counterpart (Figure 1B). Furthermore, we demonstrate the feasibility of using the resulting ATM strains to readily evolve variants of the EcTyrRS/tRNA pair, which facilitate the incorporation of several Uaas in both the ATM E. coli strain, as well as in mammalian cells.
RESULTS
Functionally substituting the endogenous EcTyrRS/tRNA pair of E. coli.
Iraha et al. have previously substituted the endogenous tyrosyl pair of E. coli with eukaryotic and archaeal counterparts,(Iraha et al., 2010) corroborating the feasibility of our approach. However, the resulting strains suffer from growth defects of unknown origin.(Iraha et al., 2010) Additionally, whether the resulting E. coli strains can be used as the selection host to alter the substrate specificity of the liberated EcTyrRS/tRNA pair has not been explored. Such efforts are not trivial, as the nonsense suppressing variant of the “liberated” endogenous pair can cross-react with other endogenous pairs.(Italia et al., 2017a; Kleina et al., 1990; Normanly et al., 1990) To better understand the logic of efficiently substituting the endogenous EcTyrRS/tRNA pair with an orthogonal counterpart from a different domain life, we took a systematic approach that involved the removal of the genes for EcTyrRS (tyrS) and tRNAEcTyr (tyrT, tyrU and tyrV) from the genome using several different strategies, and characterization of the resulting strains.
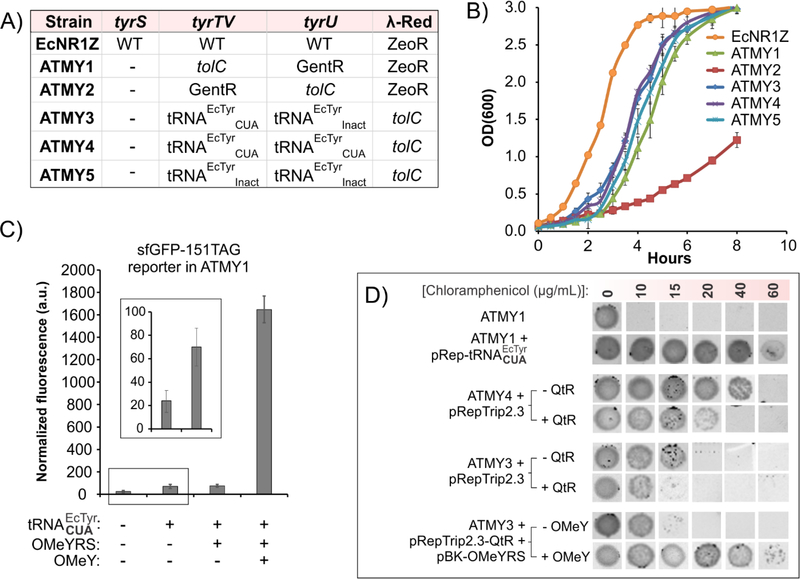
The archaea derived MjTyrRS/tRNA pair represents a good candidate for substituting its E. coli counterpart, as suggested by its extensive use for efficient nonsense suppression in bacteria.(Chatterjee et al., 2013a; Young and Schultz, 2018; Zheng et al., 2016) We encoded the substituting MjTyrRS/tRNA pair in a pUltra vector (pUltraBR-MjY), which has an unusual CloDF13 origin of replication that is compatible with most commonly used plasmids. The K12-derived EcNR1 strain of E. coli, harboring a temperature-inducible λ-Red recombination system, and optimized for efficient genome recombineering, was used as the host.(Wang et al., 2009) To enable the removal of the genes encoding the EcTyrRS/tRNA pair, we either used gene cassettes selectable using antibiotics (gentamycin or zeocin), or the dual-selectable marker tolC that can be repeatedly used to edit multiple loci in the genome (Figure 3A).(Gregg et al., 2014) We started by knocking out tyrS from the genome in the presence of the pUltraBR-MjY plasmid, by first replacing it with tolC, followed by the scarless removal of this dual-selectable marker. From here, we created five different final strains, named ATMY1–5 (Figure 3A, Figure S1, and Table S1), by knocking out the three tRNAEcTyr genes either with different selectable markers, or with its variants that suppress the TAG nonsense codon (tRNAEcTyrCUA) or inactivated by the truncation of the anticodon loop (tRNAEcTyrInact). The λ-Red machinery was also removed from the genome of each strain in the final step. Analysis of their growth (Figure 3B) revealed that replacing the tRNAEcTyr genes with its inactivated or TAG-suppressor variants (ATMY3–5) is tolerated better than the use of selectable markers (ATMY1–2), likely by better preserving the endogenous sequence architecture of these loci, and minimizing perturbation to the expression/processing of genes that these tRNAs are clustered with. Strains ATMY1, and 3–5 exhibited similar but somewhat slower growth rate relative to the progenitor strain. It is possible that the heterologous MjTyrRS/tRNA pair does not interface as efficiently with the E. coli translation system as the native pair, which leads to reduced fitness. However, the viability of these strains was sufficiently robust for their seamless use as selection and expression hosts as described below.
Figure 3. Replacement of the EcTyrRS/tRNA pair of E. coli with MjTyrRS/tRNA, followed by its reintroduction in the resulting strain as an orthogonal nonsense suppressor.
A) Strains ATMY1–5 were created by first scarlessly deleting tyrS in the presence of pUltraBR-MjY, followed by the removal of tyrTV, tyrU and the λ-Red machinery using the indicated cassettes (Also see Figure S1, Table S1). B) Growth rates of the resulting strains. Data represent mean ± SD (n = 3). C) tRNAEcTyrCUA is weakly active in ATMY1 cells in the absence of EcTyrRS, as indicated by a small but significant increase in sfGFP-151TAG reporter expression (magnified in the inset). Co-expression of the OMeY-selective EcTyrRS (OMeYRS) significantly increases TAG suppression levels, leading to strong OMeY-dependent sfGFP-151TAG expression (Also see Figure S2). Expression of full-length sfGFP was measured using OD600-normalized fluorescence of cells suspended in PBS (the normalized fluorescence of ATMY1 cells not harboring a GFP reporter was subtracted from each). Data represent mean ± SD (n = 3). D) The cross-reactivity of tRNAEcTyrCUA in ATMY1 is further confirmed using a CAT-TAG reporter, which leads to cellular survival at up to 60 μg/mL of chloramphenicol. Lowering the tRNAEcTyrCUA levels by expressing it from the genome in ATMY4 and ATMY3 strains reduces this cross-reactivity (survival up to 40 μg/mL and 20 μg/mL, respectively), which can be further attenuated by overexpressing the native tRNAGln from the pRepTrip2.3-QtR plasmid in ATMY4 and ATMY3. Co-expressing OMeYRS in ATMY3 enables OMeY-dependent survival at up to 60 μg/mL, revealing a range where Uaa-selective aaRS-variants can be efficiently selected.
Establishing the EcTyrRS/tRNAEcTyrCUA pair in the ATMY strains as an orthogonal pair.
When we attempted to reintroduce the “liberated” tyrosyl pair as a TAG-suppressor (EcTyrRS/tRNAEcTyrCUA) pair in the ATMY1 strain, we encountered two challenges. 1) Co-expression of this pair from plasmids established for other orthogonal pairs (pBK and pRep for aaRS and tRNA, respectively)(Santoro et al., 2002; Wang et al., 2001) led to a significant level of toxicity. It is likely that the overexpression of the EcTyrRS/tRNAEcTyrCUA pair, which is highly active in E. coli due to its native origin, leads to efficient suppression of endogenous TAG stop codons, resulting in the loss of viability. 2) Expression of the tRNAEcTyrCUA alone (in the absence of the cognate EcTyrRS) resulted in a significant level of TAG suppression activity, observed using both a chloramphenicol-acetyl transferase (CAT-TAG) and a superfolder GFP (sfGFP-151TAG) reporter, each harboring a TAG codon at a permissive site, suggesting that it cross-reacts with another E. coli aaRS (Figure 3C, D). In the presence of the tRNAEcTyrCUA, the sfGFP-expression assay showed a small but significant enhancement in cellular fluorescence (Figure 3C), while in the CAT-expression assay, the cells survived up to 60 μg/mL of chloramphenicol (Figure 3D). Purification of the resulting sfGFP protein followed by its MS analysis (Figure S2) indicated that the tRNAEcTyrCUA is charged by the glutaminyl-tRNA synthetase (GlnRS). Indeed, Söll et al. have previously observed that upon overexpression, E. coli GlnRS is capable of misacylating the tRNAEcTyrCUA.(Swanson et al., 1988) Analogous cross-reactivity of E. coli GlnRS has also been reported with TAG-suppressor variants of several other E. coli-derived tRNAs, driven by its recognition of the non-native CUA anticodon.(Italia et al., 2017a; Jahn et al., 1991; Kleina et al., 1990; Normanly et al., 1990) In this particular case, the degree of the cross-reactivity was weak, and when a previously developed O-methyltyrosine (OMeY, Figure 2) selective EcTyrRS mutant (OMeYRS)(Chatterjee et al., 2013b; Chin et al., 2003a) was co-expressed with tRNAEcTyrCUA, significantly higher level of reporter expression was observed in the presence of OMeY (Figure 3C), and MS analysis of the isolated sfGFP showed a mass consistent with the incorporation of OMeY (Figure S2). Despite its weak nature, the extent of this cross-reactivity was sufficient to jeopardize the selection scheme that is required to engineer the substrate specificity of EcTyrRS in this strain.
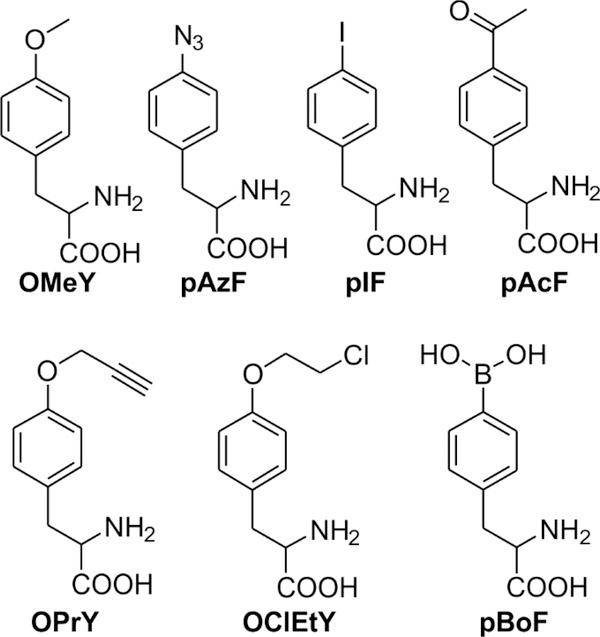
Figure 2.
Uaas used in this study.
We envisioned that lowering the expression of the highly active tRNAEcTyrCUA, by encoding it in the genome instead of a multi-copy plasmid, may alleviate both the associated toxicity and the extent of the cross-reactivity. Additionally, we thought that the unwanted cross-reactivity could be further attenuated by overexpressing the endogenous tRNAGln, which would presumably outcompete the non-cognate tRNAEcTyrCUA for interaction with GlnRS. We had already created strains ATMY3 and 4, which encode one and two copies of tRNAEcTyrCUA in the genome, respectively (Figure 3A). When subjected to the aforementioned CAT-TAG expression assay, ATMY4 and ATMY3 indeed showed reduced levels of cross-reactivity, surviving up to 40 and 20 μg/mL of chloramphenicol (Figure 3D), respectively. To overexpress the native tRNAGln in these strains, its gene was amplified from the E. coli genome and was incorporated into the multi-copy plasmid pRep to generate pRepTrip2.3-QtR, which also harbors the CAT-TAG reporter. ATMY4 and ATMY3 strains transformed with the pRepTrip2.3-QtR plasmid survived only up to 20 and 15 μg/mL of chloramphenicol (Figure 3D), confirming a significantly attenuated cross-reactivity between GlnRS and tRNAEcTyrCUA in response to the altered expression patterns of these tRNAs. It was also possible to express EcTyrRS from the pBK plasmid in the ATMY3 and ATMY4 strains without compromising their fitness. Owing to the lower degree of background TAG-suppression observed in the ATMY3 strain, it represents the most suitable host for the evolution of EcTyrRS. Expression of the aforementioned OMeYRS from a pBK plasmid enabled ATMY3 to survive up to 60 μg/mL of chloramphenicol in the presence 1 mM OMeY, but only up to 15 μg/mL in its absence (Figure 3D), confirming that the Uaa-selective EcTyrRS mutants can be enriched from their inactive counterparts using this facile antibiotic-based selection in this strain.
Development of a polyspecific EcTyrRS mutant through directed evolution in ATMY3.
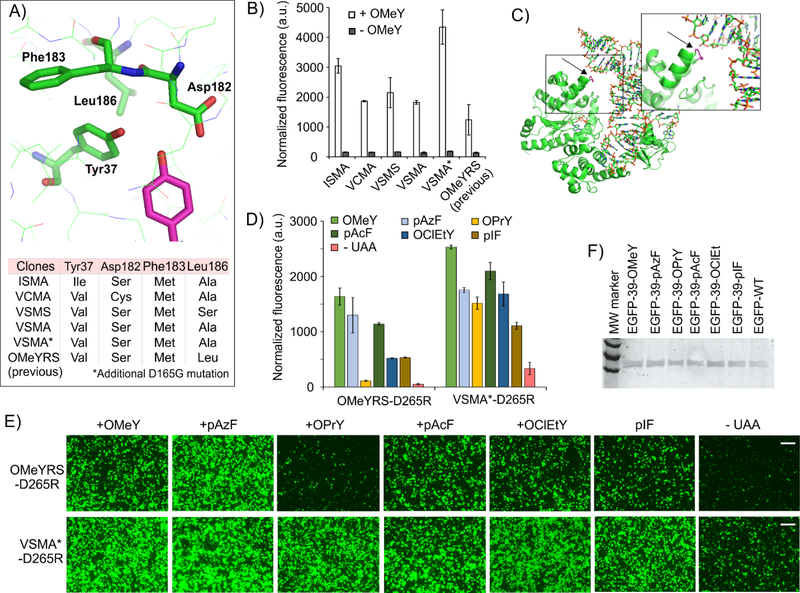
The ability to establish the EcTyrRS/tRNAEcTyrCUA pair as an adequately orthogonal nonsense suppressor in ATMY3 opens the door to allow its directed evolution for generating novel Uaa-selective variants, using the facile double-sieve selection system hosted in E. coli. We have shown that the pRepTrip2.3-QtR plasmid enables the survival of the Uaa-selective EcTyrRS variants in the presence of its cognate Uaa (Figure 3D), providing an efficient mechanism for positive selection. To enable the removal of EcTyrRS variants that charges canonical amino acids (the negative selection),(Wang et al., 2001) we also created the pNeg-QtR plasmid, encoding the TAG-inactivated toxic gene barnase, as well as the tRNAGln expression cassettes. To verify the efficacy of the ATMY3-based selection system, we first sought to develop an OMeY-selective EcTyrRS variant, the feasibility of which has been previously demonstrated using the yeast-based selection system.(Chin et al., 2003a) Based on the available crystal structure (PDB ID: 1X8X),(Kobayashi et al., 2005) 4 key amino acid residues (Tyr37, Asp182, Phe183 and Leu186) in the active site of EcTyrRS were randomized by site-saturation mutagenesis (Figure 4A). This library of mutants, was then subjected to alternating positive and negative selection steps in the ATMY3 strain, in the presence and absence of 1 mM OMeY, respectively. After just one round of each selection, the mutant library exhibited a significant OMeY-dependent survival in the subsequent round of positive selection, indicating successful enrichment of OMeY-selective EcTyrRS variants. Individual colonies were then screened for OMeY-dependent survival under positive-selection conditions, and ten successful clones were characterized by DNA sequencing. Five unique EcTyrRS sequences were identified, which were homologous to each other, as well as to the previously identified OMeYRS (Figure 4A): Tyr37 mutated to hydrophobic residues (Val or Ile), Asp182 to small residues (Ser or Cys), Phe183 to Met, and Leu186 to small residues (Ala or Ser).
Figure 4. Selection and characterization of OMeY-selective EcTyrRS mutants.
A) Active site of EcTyrRS. The bound tyrosine is shown in magenta and the active site residues subjected to randomization are highlighted. Sequence of mutants that show OMeY-dependent survival under positive selection conditions. B) OMeY-selective EcTyrRS mutants show OMeY-dependent sfGFP-151TAG reporter expression in ATMY4 (OD600-normalized fluorescence of suspended cells). The activity of the previously developed OMeYRS is shown for comparison (Also see Table S2). Data represent mean ± SD (n = 3). C) The location of the D165G mutation mapped to T. thermophilus TyrRS/tRNA co-crystal structure (PDB: 1H3E). The VSMA* mutant also facilitates improved OMeY incorporation into the EGFP-39TAG reporter expressed in HEK293T cells, relative to the previously reported OMeYRS, observed by fluorescence microscopy (E), and measuring EGFP fluorescence in clarified cell-free extract (D). Like the previous OMeYRS, VSMA* charges additional Uaas and show higher efficiency (Also see Table S2). Data represent mean ± SD (n = 3). Scale bars: 200 μm. F) SDS-PAGE analysis of EGFP-39TAG reporters incorporating different Uaas, charged by the VSMA* mutant in HEK293T cells.
To evaluate and compare the OMeY-charging efficiency of these mutants relative to the previously developed OMeYRS, we employed the sfGFP-151TAG reporter expression assay in the ATMY4 strain, which was expected to facilitate higher reporter expression (two copies of the tRNAEcTyrCUA in the genome). Each EcTyrRS mutant facilitated robust full-length reporter expression, observed using its characteristic fluorescence, only in the presence of 1 mM OMeY (Figure 4B). Intriguingly, the most efficient mutant (OMeYRS-VSMA*), which facilitated improved reporter expression relative to the original OMeYRS, had a fortuitous Asp165Gly mutation that was found to be beneficial. This Asp residue sits in close proximity with the tRNAEcTyr backbone and its mutation to Gly may enhance the EcTyrRS-tRNA interaction (Figure 4C) through negative charge repulsion. Successful incorporation of OMeY was confirmed by MS analysis (Table S2) of the reporter protein following its isolation using a C-terminal polyhistidine tag by immobilized metal-ion chromatography (IMAC).
To demonstrate that the EcTyrRS mutants developed here would be beneficial for Uaa mutagenesis in mammalian cells, we cloned OMeYRS-VSMA* into the previously described mammalian expression plasmid pAcBac1–16xtRY,(Chatterjee et al., 2013b; Zheng et al., 2017b) also encoding 16 copies of its cognate tRNA and its activity was compared to an otherwise identical plasmid, encoding the previously developed OMeYRS. Additionally, we incorporated the Asp265Arg mutation into each EcTyrRS variant, which confers improved interaction with the tRNAEcTyrCUA.(Takimoto et al., 2009) When co-transfected into HEK293T cells with a plasmid expressing EGFP-39TAG, both plasmids facilitated OMeY-dependent EGFP expression (Figure 4D, E). It was previously found that the original OMeYRS can charge several other para-substituted phenylalanine analogs,(Chatterjee et al., 2013b) including those with bioorthogonal conjugation handles, significantly expanding its utility. We showed that our OMeYRS-VSMA* is also capable of charging these useful Uaas, in some cases (e.g., O-propargyl-tyrosine) with improved efficiency (Figure 4D, E). Successful incorporation of each Uaa was confirmed by the MS analysis (Table S2) of the reporter protein following isolation with excellent yields (60–150 μg per 10 cm dish; 25% - 60% relative to wild-type EGFP reporter). The ability to enrich active OMeY-selective variants from a naïve EcTyrRS library validates the utility of the ATMY3-based selection scheme. Furthermore, the development of a polyspecific EcTyrRS variant with improved efficiency will be useful for single and dual Uaa mutagenesis in mammalian cells.
Engineering EcTyrRS to charge p-boronophenylalanine (pBoF).
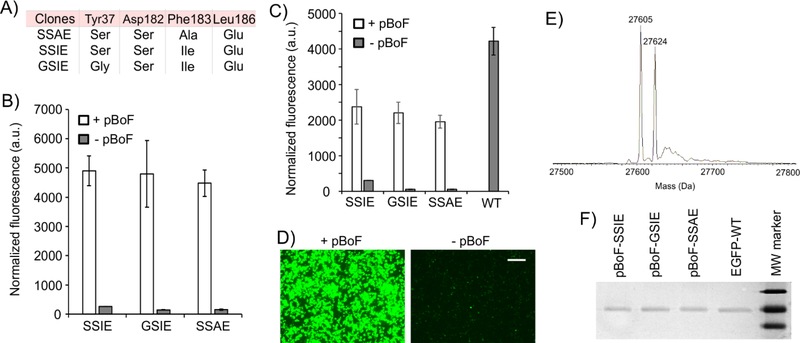
The MjTyrRS/tRNA pair has been engineered to genetically encode pBoF (Figure 2) in E. coli,(Brustad et al., 2008) where it has facilitated several enabling applications including the development of engineered carbohydrate-binding proteins,(Brustad et al., 2008; Liu et al., 2009) genetically encoded fluorescent sensors for physiologically relevant reactive oxygen species (e.g., hydrogen peroxide, peroxynitrite),(Chen et al., 2013; Chen et al., 2016; Wang et al., 2012) novel bioconjugation strategies,(Yang et al., 2014) etc. The ability to engineer EcTyrRS to charge pBoF with high fidelity and efficiency will extend the utility of this versatile chemical handle for application in eukaryotic cells. We subjected the aforementioned EcTyrRS mutant library in the ATMY3-based selection system to enrich potential mutants capable of charging pBoF. Numerous colonies were identified after just two rounds of positive and a round of negative selection, which exhibited pBoF-dependent survival under positive selection conditions. Characterization by DNA sequencing identified three distinct but convergent clones, where Tyr37 mutates to Gly or Ser, Asp182 to Ser, Phe183 to Ala or Ile, and Leu186 to Asp (Figure 5A). The hydrophilic nature of these engineered active sites are in agreement with the need to accommodate the polar boronic acid functionality. The aforementioned sfGFP-151TAG reporter expression assay in ATMY4 was used to confirm that all three mutants charge pBoF with high fidelity and efficiency (Figure 5B). MS analysis of the reporter protein confirmed the expected mass along with a dehydrated peak, characteristic of phenyl boronic acids (Figure 5E, Table S2).(Bandyopadhyay and Gao, 2016; Brustad et al., 2008; Cal et al., 2012; Zheng et al., 2016) All three mutants were cloned into the mammalian expression vector described above, further incorporating the beneficial Asp265Arg mutation. Co-transfection of HEK293T cells with the EGFP-39TAG reporter resulted in efficient reporter expression only in the presence of 1 mM pBoF (Figure 5C, D; Figure S3A). The reporter protein in each case was isolated in good yield (140–160 μg per 10 cm dish; 58% to 65% relative to the wild-type EGFP reporter; Table S2). Taking further advantage of this excellent efficiency, we demonstrated the ability to incorporate pBoF into two different sites in the EGFP-39TAG-151TAG reporter expressed in HEK293T cells with only a minor loss in the yield (Figure S3B, C). Such efficient multi-site incorporation of pBoF can facilitate the development of novel carbohydrate binding proteins (e.g., antibodies), strategically employing multiple boronate handles.
Figure 5. Selection and activity of pBoF-selective EcTyrRS mutants from a naïve mutant library.
A) Sequence of mutants that show pBoF-dependent survival under positive selection conditions. B) Each of three mutants facilitate pBoF-dependent sfGFP-151TAG reporter expression in ATMY4 (Also see Table S2). C) These mutants also charge pBoF into the EGFP-39TAG reporter expressed in HEK293T cells monitored by EGFP-fluorescence in cell-free extract. Expression of the wild-type EGFP reporter is also shown for reference, demonstrating high efficiency of pBoF incorporation (Also see Table S2). Data represent mean ± SD (n = 3). D) Fluorescence microscopy images of HEK293T cells showing pBoF-dependent EGFP-39TAG expression facilitated by the GSIE-mutant (Also see Figure S3). Scale bar: 200 μm. E) MS-analysis of the reporter protein confirm pBoF incorporation (expected mass: 27626 Da, additional dehydration peak is an established behavior of phenylboronates). F) SDS-PAGE of pBoF-incorporating reporter proteins.
DISCUSSION
The great potential of the TyrRS/tRNA pair for driving the genetic code expansion technology has been highlighted by the remarkable success of the MjTyrRS/tRNA pair in E. coli.(Dumas et al., 2015; Young and Schultz, 2018) However, the use of the EcTyrRS/tRNA pair to similarly expand the eukaryotic genetic code has been significantly limited by the challenges associated with the eukaryote-hosted selection system required to engineer its substrate specificity.(Dumas et al., 2015; Italia et al., 2017a; Italia et al., 2017b) Recently, the universally orthogonal pyrrolysyl pair has been engineered using the E. coli selection system to charge several hydrophobic phenylalanine derivatives, providing an alternative platform for genetically encoding such Uaas in eukaryotes.(Dumas et al., 2015; Wan et al., 2014) However, it has been particularly difficult to use this platform to genetically encode hydrophilic amino acids such as pBoF. The ability to readily engineer the EcTyrRS/tRNA pair using the E. coli based selection scheme overcomes these limitations, paving the way for potentially introducing numerous other highly sought-after Uaas, such as those mimicking post-translational modifications of tyrosine (sulfation, phosphorylation, etc.),(Liu and Schultz, 2006; Luo et al., 2017) into the genetic code of eukaryotes. Additionally, the EcTyrRS/tRNA pair have been used together with the pyrrolysyl pair to site-specifically incorporate two distinct Uaas into proteins expressed in mammalian cells.(Xiao et al., 2013; Zheng et al., 2017a) The scope of this technology would be further expanded with the ability to incorporate a broader set of useful Uaas using this pair.
Our work also reveals several important insights for successful functional replacement of aaRS/tRNA pairs in E. coli with evolutionarily distant counterparts. We found that removing endogenous tRNA genes using large selectable markers may lead to growth defects. The tRNA genes are often clustered with other tRNAs and other genes, which are co-transcribed and the resulting transcript is subsequently processed. It is possible that the insertion of large genetic elements may perturb these processes. Replacing the tRNA genes with their inactivated or nonsense-suppressing counterpart can alleviate these concerns. It is possible that the minor growth defects of the ATMY strains are a consequence of the suboptimal performance of the substituting MjTyrRS/tRNA pair. This heterologous pair – the tRNA in particular – did not evolve to optimally interface with the E. coli translation machinery. Furthermore this pair may not be fully orthogonal in E. coli. Indeed, MjTyrRS was recently shown to be capable of charging the endogenous prolyl-tRNA of E. coli at very low levels.(Javahishvili et al., 2014) Consequently, it may be possible to further overcome the minor residual growth defects of ATMY strains through optimizing the performance of the substituting MjTyrRS/tRNA pair.
The cross-reactivity of the nonsense-suppressing variants of endogenous tRNAs with other host synthetases represents a major challenge in our strategy to enable directed evolution of endogenous aaRS/tRNA pairs in E. coli. GlnRS appears to be a prevalent source of such cross-reactivity, particularly with TAG-suppressor tRNAs.(Italia et al., 2017a; Jahn et al., 1991; Kleina et al., 1990; Normanly et al., 1990; Swanson et al., 1988) We have previously shown that misacylation by GlnRS can be avoided by using a TGA-suppressor tRNAEcTrp instead of its TAG-suppressing counterpart.(Italia et al., 2017a) This strategy did not work for tRNAEcTyr, due to its poor TGA suppression efficiency. However, lowering its expression, coupled with overexpression of endogenous tRNAGln sufficiently attenuated the degree of this misacylation. To further eliminate the residual cross-reactivity, a more orthogonal tRNAEcTyrCUA can be engineered in the future through its directed evolution in the ATMY strains.
Although the MjTyrRS/tRNA pair has already been well established for Uaa mutagenesis in E. coli, the EcTyrRS/tRNAEcTyrCUA pair provides an alternative platform in ATMY strains. The very high efficiency of this native pair in E. coli, which enables high level of TAG suppression from just one or two tRNAEcTyrCUA genes encoded in the E. coli genome, represents a unique advantage of this system. In contrast, the MjTyrRS/tRNA pair must be heavily overexpressed from optimized multi-copy vectors to provide good suppression efficiency.(Amiram et al., 2015; Chatterjee et al., 2013a; Zheng et al., 2016)
In conclusion, here we further demonstrate the utility of “liberating” the endogenous aaRS/tRNA pairs of E. coli, and their use for genetic code expansion of both E. coli and eukaryotes. The ability to readily engineer the substrate specificity of the EcTyrRS/tRNA pair using the facile E. coli selection system resurrects its untapped potential to further expand the catalog of useful Uaas genetically encoded in eukaryotes.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Abhishek Chatterjee (abhishek.chatterjee@bc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains
The E. coli strain DH10b (Life Technologies) was used for cloning and propagation. The bacterial EcNR1 progenitor strain was a gift from Prof. George M. Church. Strains were propagated at 37 °C or 30 °C for desired time while shaking at 250 RPM in sterile culture tubes. The strains were grown on LB-agar plates and liquid LB medium with the following antibiotic concentrations unless noted otherwise: 95 μg/mL spectinomycin, 50 μg/mL chloramphenicol, 10 μg/mL gentamycin, 100 μg/mL ampicillin, 15 μg/mL zeocin, 12 μg/mL tetracycline, 30 μg/mL kanamycin.
Mammalian cell lines
The HEK293T cell line was used as a representative mammalian cell line after purchase from ATCC and was maintained as recommended by the supplier in DMEM, high glucose media, in the presence of Penicillin/Streptomycin. Cells were maintained at 37 °C, 100% humidity, 5% CO2.
METHODS DETAILS
λ-Red recombination
All strains were derived from EcNR1, and the previously described recombination protocol was followed.(Italia et al., 2017a) EcNR1 was additionally engineered to create a new precursor strain EcNR1GT (EcNR1 ΔgalK ΔtolC) to take advantage of dual selectable markers galK and tolC. A list of all oligonucleotides and other DNA sequences used for recombination is included in a supplementary document.
Genomic recombination to build ATMY1–5
Strategy common to ATMY1–5:
EcNR1GT was transformed with pUltraBR-MjY. To genomically remove the E. coli tyrosyl-tRNA synthetase (tyrS), the gene encoding tolC was PCR amplified using primers EcYS.tolC-F2 and EcYS.tolC-R2 to generate the PCR product tyrS::tolC. 50 ng of the tyrS::tolC PCR cassette was transformed to facilitate recombination following the aforementioned protocol,(Italia et al., 2017a) and the resulting strain was plated on LB-Agar plates supplemented with 0.005% SDS. The resulting colonies were characterized via colony PCR, as well as DNA sequencing the colony PCR products. The intermediate strain was stocked with genotype EcNR1GT pUltraBR-MjY tyrS::tolC. Subsequently, dual selectable tolC was deleted from the genome using the 90 bp oligo tyrS.90 del and plating on LB-Agar plates supplemented with colicin and 30 μg/ml Vancomycin, resulting in intermediate strain EcNR1GT pUltraBR MjY ΔtyrS. This intermediate strain was used to build the five variants of tyrosyl ATM strains.
ATMY1:
Wild-type E. coli contains three copies of the tRNATyrGUA, localized in two genomic locations. The two tRNAs tyrT and tyrV are clustered in the same cassette, while tyrU is in a different genomic location. To build ATMY1, tyrTV was deleted by recombination using the tolC selectable marker. The tolC marker was amplified with EcY_TV.tolC-F and EcY_TV.tolC-R, resulting in PCR cassette tyrTV::tolC. The tyrTV::tolC PCR product was transformed in the recombination following the aforementioned protocol and plated on 0.005% SDS. Colonies were verified, resulting in intermediate strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::tolC. To replace the E. coli tRNATyrCCA (tyrU), antibiotic resistance gene GentR was amplified using tyrU.KO.gentR-RBS-F and tyrU.KO.gentR-R to create tyrU::GentR and used with the recombination protocol, creating intermediate EcNR1GT pUltraBR MjY ΔtyrS tyrTV::tolC tyrU::GentR. In order to delete the λ-RED genes from the final strain, a second antibiotic resistance gene ZeoR was amplified with primers dlambda-ZtattB-F and dlambda-ZtattB-R to create PCR product λ-RED::ZeoR. The PCR product was transformed and recombinant hits were selected on LB-Agar supplemented with Zeocin. Confirmed screening resulted in final strain ATMY1 (EcNR1GT pUltraBR MjY ΔtyrS tyrTV::tolC tyrU::GentR λ-RED::ZeoR).
ATMY2:
To build ATMY2, tyrTV was deleted using a GentR selectable marker. The GentR marker was amplified with tyrTV.KOgent-F and tyrTV.KOgent-R, resulting in PCR cassette tyrTV::GentR. The tyrTV::GentR PCR product was transformed in the recombination following the aforementioned protocol and plated on gentamycin. Colonies were verified, resulting in intermediate strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::GentR. To replace the E. coli tRNATyrCCA (tyrU), the tyrU::tolC cassette was amplified using tyrU.KO.tolC-RBS-F and tyrU-tolC.R and used with the recombination protocol with cells plated on LB supplemented with 0.005% SDS, creating intermediate EcNR1GT pUltraBR MjY ΔtyrS tyrTV::GentR tyrU::tolC. The λ-RED genes were removed from the final strain using a ZeoR cassette as described above. Colony screening by PCR and DNA sequencing confirmed the generation of the final strain ATMY2 (EcNR1GT pUltraBR MjY ΔtyrS tyrTV::GentR tyrU::tolC λ-RED::ZeoR).
The second generation of strains, ATMY3–5, were generated from EcNR1GT pUltraBR MjY ΔtyrS using the dual-selectable marker tolC. Cells were plated on either LB-Agar supplemented with 0.005% SDS to select for the presence of tolC or LB-Agar supplemented with colicin+vancomycin to select for the absence of tolC. In the second generation of strains, the tyrosyl-tRNA genes encoded in the tyrTV and tyrU locus are replaced with either tRNATyrCUA, its TAG-suppressing counterpart, or tRNATyr (Inact), which contains an inactivated tRNA that was generated by deleting two residues in the anticodon (GUA→XXA).
ATMY3:
The previously built intermediate strain (EcNR1GT pUltraBR-MjY ΔtyrS) was used to build ATMY3. The tolC cassette was amplified with primers EcY_TV.tolC-F and EcY_TV.tolC-R to create tyrTV::tolC. Next, tolC was replaced from the tyrTV location with PCR cassette tyrTV::CUA, which was amplified from a gblock (IDT) using tyrTV.lhm-F and tyrTV.rhm-F, and was designed to introduce the tRNATyrCUA into this locus expressed from the endogenous tyrTV promoter. This resulted in the intermediate strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::CUA. Next, tyrU was replaced by tolC, using the tyrU::tolC cassette (amplified with primers tyrU.KO.tolC-RBS-F and tyrU-tolC.R), to develop strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::CUA tyrU::tolC. Afterwards, the tolC cassette at the tyrU locus was replaced with the tRNATyrInact cassette amplified from a tyrU.inact-gblock with primers tyrU.lh-F and tyrU.rh-R via tolC negative selection, resulting in strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::CUA tyrU::Inact. Finally, the λ-RED genes were deleted using the PCR cassette λ-RED::tolC, amplified with primers dlambda.tolC-F and dlambda.tolC-R (tolC positive selection). Final screening resulted in ATMY3 strain: EcNR1GT pUltraBR MjY ΔtyrS tyrTV::CUA tyrU::Inact λ-RED::tolC.
ATMY4:
The recombinations to build ATMY4 are identical to ATMY3, but only differ in the tyrU genomic location. Instead of replacing the tyrU locus with a tRNATyrInact, a tRNATyrCUA, cassette was used for constructing ATMY4, resulting in two copies of tRNATyrCUA in its genome. To replace the tolC cassette at the tyrU locus, the PCR cassette tyrU::CUA was amplified with primers tyrU.lh-F and tyrU.rh-R from tyrU.CUA-gblock. Subsequent λ-RED recombinations were performed as described with ATMY3, resulting in a final ATMY4 strain with genotype: EcNR1GT pUltraBR MjY ΔtyrS tyrTV::CUA tyrU::CUA λ-RED::tolC.
ATMY5:
We also built a strain where both tyrU and tyrTV loci were replaced with tRNATyrInact, thus creating a strain with no amber suppressor tRNAs. This strain was generated in the same order as ATMY3 and ATMY4, but used inactivated tRNA gblocks for tolC deletion at tyrU and tyrTV loci. From EcNR1GT pUltraBR MjY ΔtyrS tyrTV::tolC, tolC was removed using tyrTV::Inact PCR cassette, containing one copy of the E.coli tRNATyrInact, amplified from tyrTV.inact-gblock.orig with primers tyrTV.lhm-F and tyrTV.rhm-R, resulting in strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::Inact. Next tyrU was replaced with tolC to build EcNR1GT pUltraBR MjY ΔtyrS tyrTV::Inact tyrU::tolC, and the tolC was subsequently removed using tyrU::Inact PCR cassette (containing one copy of the E.coli tRNATyrInact) from tyrU.inact-gblock with primers tyrU.lh-F and tyrU.rh-R, resulting in strain EcNR1GT pUltraBR MjY ΔtyrS tyrTV::Inact tyrU::Inact. The λ-RED recombination was performed as described with ATMY3, resulting in a final ATMY5 strain containing two genomically expressed inactviated tyrosyl tRNAs with genotype: EcNR1GT pUltraBR MjY ΔtyrS tyrTV::Inact tyrU::Inact λ-RED::tolC.
Growth Comparison
5 mL starter cultures of EcNR1Z, ATMY1–5 strains were grown to saturation overnight (16 hrs) in LB with all strain-dependent antibiotics. The starter cultures were diluted to an initial OD600 of 0.02 in three identical cultures of 50 mL LB with Spectinomycin and grown in 250 mL sterile Erlenmeyer flasks at 37 °C, with shaking (250 rpm). A 10 mm cuvette was used to monitor OD600 every 30 minutes.
Assessment of aaRS/tRNA activity using the CAT reporter
The reporter plasmid used for chloramphenicol acetyltransferase (CAT) assays varied based on the strain used (wild-type or ATMY1–5). Refer to Supplementary sequences for plasmid sequences.
Strains containing no genomically incorporated TAG-suppressor tRNA (ATMY1, ATMY2, ATMY5):
These strains were transformed with any of the following variants of pRep: pRepTrip2.3-EcY-TAG (expresses tRNATyrCUA) or pRepTrip2.3-TAG (does not express tRNATyrCUA). To co-express the EcTyrRS, a pBK plasmid encoding the appropriate EcTyrRS mutant was also co-transformed into these strains. As a control for no-aaRS co-expression, empty pBK plasmids were used. Cells containing intended plasmids were grown to saturation overnight in LB media supplemented with spectinomycin, tetracyclin and kanamycin (if pBK is present), diluted to an OD600 of 0.1, and 3 μL was spot-plated on LB-agar plates supplemented with kanamycin (+pBK plates), spectinomycin, tetracycline, ampicillin, and varying concentrations of chloramphenicol. Growth was recorded after 24 or 48 hrs of incubation at 37 °C.
Strains containing genomically incorporated TAG-suppressor tRNA (ATMY3 and ATMY4):
The strains were transformed with any of the following variants of pRep: pRepTrip2.3-TAG (does not express tRNATyrCUA) or pRepTrip2.3P-EcQtR-2x TAG (does not express tRNATyrCUA, but contains two copies of the E. coli tRNAGln expressed from the proK promoter). Strains containing pRep variants were further co-transformed with pBK-EcTyrRS variants (if EcTyrRS co-expression is desired). The chloramphenicol resistance of these strains were assayed as described above.
Assessment of tRNA/aaRS activity using the sfGFP-151TAG reporter
Strains containing no genomically incorporated TAG-suppressor tRNA (ATMY1):
ATMY1 harboring pEvolT5-EcY-sfGFP151TAG or pEvolT5-sfGFP151TAG (to assess background nonsense suppression in the absence of the suppressor tRNA) with or without pBK-EcTyrRSwt, pBK-EcOMeYRS, pBK-Empty, or newly evolved pBK-EcTyrRS variants were grown to saturation overnight in LB supplemented with spectinomycin, kanamycin and chloramphenicol. The starter cultures were diluted 100× in LB supplemented with required antibiotics. Cultures were grown at 37 °C until 0.55 OD600 and induced with a final concentration of 1 mM IPTG. Unnatural amino acids (UAA) were also added during induction to a final concentration of 1 mM. Cultures were grown for an additional 16 hours at 37 °C. For fluorescence measurements, cells were pelleted at 5000 ×g, resuspended in PBS, diluted 10-fold, and transferred to a 96-well clear-bottom plate. Fluorescence was measured by using a SpectraMAX M5 (Molecular Devices) (Ex. 488 nm; Em. 534 nm) and normalized by OD600. A culture of ATMY1 with no sfGFP reporter was also grown similarly and used to assess the background fluorescence, which was subtracted from all measurements.
Strains containing genomically incorporated TAG-suppressor tRNA:
For ATMY4, assessment of the tRNA/aaRS activity was accomplished with the same methods as above except strains harbored pEvolT5-sfGFP151TAG instead of its counterpart that encodes the suppressor tRNA.
Protein purification
Protein expression was performed using the same strain, plasmid combinations, and methods as described above (Assessment of tRNA/aaRS activity using a sfGFP151 reporter). Overnight expression cultures (10 mL) were centrifuged and resuspended in lysis buffer: B-PER Bacterial Protein Extraction Reagent (Thermo Scientific) + 1X Halt Protease Inhibitor Cocktail (Thermo Scientific) + 0.01% Pierce Universal Nuclease (Thermo Scientific). After 30 min incubation at room temperature, the lysate was clarified by centrifuging at 22,000 ×g for 5 min. The soluble C-terminally polyhistidine-tagged sfGFP was purified from the supernatant using a HisPur Ni-NTA resin (Thermo Scientific) following manufacturer’s protocol. Protein purity was confirmed by SDS-PAGE and protein molecular weight was confirmed by ESI-MS (Agilent Technologies 1260 Inifinity ESI-TOF).
Construction of the EcTyrRS library
To construct the library, four residues were randomized in tyrosyl tRNA-synthetase (pBK-EcYRS1a): Y37-FLIMVSTAYHCG, D182-NST, F183-NNK, L186-NNK. The EcYRS1a library was built via sequential overlap extension of three gel purified PCR products. N-terminal piece A was amplified from the endogenous EcYRS using primers pBK seqT-F and EcYRS-35oR. Piece B was amplified with primers EcYRS-Y37mut-F and EcYRS-181oR, which was subsequently overlap extended to join piece AB using the terminal primers: pBK seqT-F and EcYRS-181oR. Lastly, C-terminal piece C was amplified using EcYRS-D182.F193.L186-NST.NNK.NNK-F and pBK MCS JIsqR. Piece AB was joined with piece C via overlap extension with terminal primers pBK MCS JIsqF and pBK MCS JIsqR. These inserts were digested with NdeI/NcoI (NEB) and ligated by T4 DNA Ligase (NEB) into the pBK vector, cut with the same restriction enzymes. The ligation mixture was ethanol precipitated with Yeast-tRNA (Ambion) and transformed into electrocompetent DH10b cells. The library was covered using >107 transformants.
OMeY and pBoF Synthetase Selection in ATMY3
ATMY3 was co-transformed with the pBK-EcYRS1a library and the positive selection reporter plasmid pRepTrip2.3P-EcQtR-2x. The reporter plasmid harbors two copies of proK-promoted E. coli tRNAGln in its genomic context, a CAT reporter containing TAG codon (Q98TAG), an ampicillin reporter containing one TAG codon (3TAG) an arabinose-inducible T7 RNA polymerase containing two TAG nonsense codons (at positions 8 and 114), and a wild-type GFPuv reporter expressed from the t7 promoter. Suppression of CAT leads to chloramphenicol resistance, and suppression of T7 RNA polymerase drives expression of a t7-promoted GFPuv. 9.2×107 cfu (colony forming units) were plated on LB + 0.5× each of spectinomycin, tetracyclin, and kanamycin + 0.02% arabinose + chloramphenicol (30, 50 μg/mL) + 60 μg/ml Amp in the presence of 1 mM OMeY or pBoF for 36 hrs at 37 °C.
Colonies from the 30 and 50 μg/mL chloramphenicol positive selection plates were harvested, and the pBK plasmids harboring mutant EcTyrRS were isolated. These were co-transformed into ATMY1 harboring the negative selection plasmid pNeg2–2xQtR, which contains an arabinose induced barnase gene with two stop TAG codons (3 and 45). 3×107 cfu were plated on LB + ampicillin + 0.5× kanamycin and incubated for 12 hrs at 37 °C. Cells were harvested and library pBK plasmid was purified.
Isolated pBK plasmids from the negative selection were transformed back into ATMY3 pRepTrip2.3P-EcQtR-2x, and 106 cfu were plated on LB + 0.5× each of spectinomycin, tetracyclin, and kanamycin + 0.02% arabinose + chloramphenicol (30, 50 μg/mL) + 60 μg/ml Amp in the presence or absence of 1 mM OMeY or pBoF for 18 hrs at 37 °C. 96 colonies from the +UAA plates were picked into 1 mL LB supplemented with Spec/Tet/Kan in deep 96-well polypropylene plates and grown overnight. Overnight cultures were diluted to 100× and 3 μL of each was spot plated on LB/Agar plates supplemented with spectinomycin/tetracyclin/kanamycin, and chloramphenicol (30 or 50 μg/mL) in the presence or absence of the UAA. 10 clones showing the most prominent UAA-dependent growth were picked, sequenced, and spot-plated again to confirm the growth phenotype.
Assessment of aaRS/tRNA activity in HEK293T cells
Cell maintenance and transfections were performed as previously described.(Italia et al., 2017a) For these transfections PEI (Sigma) was used; 250 ng of pAcBac EGFP39* was co-transfected with 250 ng of pAcBac-EcYRS (WT, OMeYRS, pBoFRS variants)-16xtRY-TAG into 24 well dishes. UAAs were added to the culture medium to a final concentration of 1 mM at the time of transfection. Fluorescence images were taken at 48 hr post-transfection using a Zeiss Axio Observer fluorescence microscope. EGFP39*-expression data was obtained as previously described.(Italia et al., 2017a).
The EGFP reporter was expressed and purified from HEK293T cells as previously described using either pAcBac-EGFP-TAG or pAcBac-GFP-2xTAG reporters with the tRNA/aaRS plasmid.(Italia et al., 2017a)
Plasmid construction
Complementation plasmid pUltraBR MjY:
To generate pUltraBR MjY, previously reported pUltra Hit14,(Chatterjee et al., 2013c) was used to generate additional derivatives. A previously developed but selection incompatible complementation plasmid, pBR MjY,(Iraha et al., 2010) was used to amplify the MjYRS and MjY-tRNA with primers pBR.MjY-XbaI-F and MjYtR_RS.SphI-R. The full length PCR product containing the MjYRS/tRNA was cloned into pUltra Hit14 via XbaI/NcoI (NEB), producing pUltraBR MjY.
Selection plasmid construction:
To generate the positive selection plasmid containing an ampicillin reporter, pRep-ScW14 was digested with SpeI.(Italia et al., 2017a) The 5’ fragment of the ampicillin resistance cassette (amplified from a pAcBac vector with Amp_NheI-F and Amp-2.3.InsTAG-R) was overlap-extended with the 3’ fragment (amplified with Amp-2.3.InsTAG-F and pacbac1-Amp_SpeI-R), and amplified using terminal primers Amp_NheI-F and pacbac1-Amp_SpeI-R. This was digested and cloned with SpeI into pRep-ScW14, creating pRepTrip2.3-ScW14.
To generate the tRNATyrCUA containing positive selection plasmid, pRepTrip2.3-EcY-TAG, pRepTrip2.3-ScW14 was digested with SpeI/BglII (NEB) the encoded tRNA. The lpp promoted tRNATyrCUA was amplified from lpp-EcYtR Gblock with pRep-SpeI-F and EcY-Gblock-AB-R and cloned into the digested vector. To create the positive selection plasmid containing no tRNAs, pRepTrip2.3-EcY-TAG was digested with SpeI/AvrII and was allowed to re-ligate, cutting out the tRNA and producing pRepTrip2.3 TAG.
To generate the positive selection plasmid containing E. coli tRNAGln, pRepTrip2.3-EcY-TAG was digested with SpeI/AvrII (NEB). The proK promoter was amplified from pEvol vector using proK-SpeI-F and proK-QtR-2x-oR, which was then overlap-extended with the QtR-2x cassette amplified from Top10 genomic DNA using primers QtR-2x-oF and QtR-2x-Gen-AvrII-R. The PCR cassette was digested with SpeI/AvrII and ligated into the vector.
The negative selection plasmid pNeg2–2xQtR was cloned by digesting vector pNeg and an insert (encoding glutaminyl-tRNAs) amplified from pRepTrip2.3P-EcQtR-2x using QtR-2x-EcoRI-F and QtR-Gen-EcoRI-R with EcoRI. The pNeg2-2xQtR contains two stop codons in the toxic barnase gene, as well as two E. coli tRNAGln expressed from the proK promoter.
Bacterial suppression plasmid construction:
pEvoltac MjY-sfGFP151TAG was previously cloned in our lab,(Italia et al., 2017a) which is an expression plasmid containing an amber suppressor tRNA and T5.lac promoted sfGFP-151TAG reporter. This plasmid contains a p15a origin of replication and a chloramphenicol resistance marker, compatible with the ATMY strains. The proK-promoted E. coli tRNATyrCUA was amplified from ProK-EcY-gBlock using primers EcWtR PstI-F and pEvol-tR_SphI-R. The insert and vector were digested with PstI/SphI (NEB) and ligated to produce pEvolT5 EcY sfGFP151TAG.
To create a corresponding expression plasmid with no suppressor tRNAs for use in ATMY3 or ATMY4, pEvolT5 EcY sfGFP151TAG was digested with ProK/SphI and blunt ligated with NEB blunt ligation kit to create pEvolT5 sfGFP151TAG.
To create pBK-EcYRS, pBK-MjYRS was used as a vector. Top10 genomic DNA was used as the template to amplify the E. coli TyrRS using EcYRS-NdeI-F and EcYS-NcoI-R, which was subsequently cloned with NdeI/NcoI to create pBK-EcYRS. All other tyrosyl pBK variants were cloned with these terminal primers and subjected to site-directed mutagenesis (for other mutant variants) if necessary.
Mammalian suppression plasmid construction:
Previously reported pAcBac1 variant pB1U-OMeYRS-noWPRE-16xtRY-TAG was used to generate mammalian reporter and suppression plasmids.(Chatterjee et al., 2013b; Zheng et al., 2017b) To build pB1U plasmids containing the variety of EcYRS mutants, the synthetases cloned into pBK were amplified with terminal primers EcYRS-NheI-F and EcYRS-XhoI-R. These PCR products were cloned into pB1U-OmeYRS-noWPRE-16xtRY-TAG via NheI/XhoI.
QUANTIFICATION AND STATISTICAL ANALYSIS
The growth rate analysis was performed in triplicate, with the final OD600 reported as the mean. Error bars represent standard deviation. Expression analysis in bacteria (sfGFP) or HEK293T cells (EGFP) were reported as the mean of three independent replicates. Error bars represent standard deviation. In our experience, this provides adequate levels of accuracy.
DATA AND SOFTWARE AVAILABILITY
We are willing to disclose or deposit any required data. We have not developed any software.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N/A | ||
| Bacterial and Virus Strains | ||
| ATMY1 | This paper | N/A |
| ATMY2 | This paper | N/A |
| ATMY3 | This paper | N/A |
| ATMY4 | This paper | N/A |
| ATMY5 | This paper | N/A |
| EcNR1Z | This paper | N/A |
| EcNR1GT | This paper | N/A |
| EcNR1 | George Church/Addgene | Cat#26930 |
| E.coli DH10b/Top10 | ThermoFisher Scientific | Cat#18297010 |
| Biological Samples | ||
| N/A | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| O-methyltyrosine | Fisher Scientific | Cat#AAH6309606 |
| 4-azido-L-phenylalanine | Chem-Impex | CAT#06162 |
| o-propargyltyrosine | Syntides | CAT#11252961 |
| p-acetylphenylalanine | Chem-Impex | CAT#24756 |
| p-iodophenylalanine | Chem-Impex | CAT#03376 |
| p-boronophenylalanine | Fisher Scientific | Cat#AC358932500 |
| Critical Commercial Assays | ||
| N/A | ||
| Deposited Data | ||
| N/A | ||
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | Cat#CRL-1573 |
| Experimental Models: Organisms/Strains | ||
| N/A | ||
| Oligonucleotides | ||
| See Supplementary Table S3 for all primers/oligos | N/A | N/A |
| Recombinant DNA | ||
| See supplementary information for all recombinant DNA sequences | N/A | N/A |
| Software and Algorithms | ||
| N/A | ||
| Other | ||
| N/A | ||
SIGNIFICANCE.
The unnatural amino acid (Uaa) mutagenesis technology holds much potential to probe and manipulate the biology of eukaryotic cells. In general, bacteria-derived aaRS/tRNA pairs are orthogonal and suitable to execute Uaa mutagenesis in eukaryotes. However, these pairs must be engineered in yeast to charge desired Uaas, and the technically challenging nature of this platform has limited its success. For example, the bacteria-derived tyrosyl-tRNA synthetase (TyrRS)/tRNA pair was one of the first to be used for eukaryotic genetic code expansion over 15 years ago, but has been used to incorporate only a handful of simple Uaas thus far. However, this pair represents an ideal platform to genetically encode several Uaas that are currently unavailable for incorporation in eukaryotes, including models for various post-translational modifications of tyrosine. To overcome this limitation, herein we created novel E. coli strains (ATMY1–5), where the endogenous TyrRS/tRNA pair was functionally replaced with an archaeal counterpart. We demonstrated that the liberated TyrRS/tRNA pair can be used in the resulting ATMY strains as an orthogonal nonsense suppressor, and its substrate specificity can then be engineered using the facile E. coli based directed evolution platform. Using this strategy, we developed several mutants of the bacterial TyrRS/tRNA pair which can be used for Uaa incorporation both in the ATMY E. coli strains and mammalian cells. The ability to readily engineer this pair should enable us to genetically encode Uaas that were previously inaccessible in eukaryotes.
Acknowledgement.
The EcNR1 strain was a kind gift from Prof. G.M. Church (Harvard). This work was supported by NIH award R01GM124319 to A.C.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and two tables and can be found with this article online.
ADDITIONAL RESOURCES
This section is not applicable to our work.
References.
- Amiram M, Haimovich AD, Fan C, Wang Y-S, Aerni H-R, Ntai I, Moonan DW, Ma NJ, Rovner AJ, and Hong SH (2015). Evolution of translation machinery in recoded bacteria enables multi-site incorporation of nonstandard amino acids. Nature biotechnology 33, 1272 DOI: 10.1038/nbt.3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, and Gao J (2016). Iminoboronate-Based Peptide Cyclization That Responds to pH, Oxidation, and Small Molecule Modulators. Journal of the American Chemical Society 138, 2098–2101. DOI: 10.1021/jacs.5b12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M, Groff D, Xie J, Brustad E, and Schultz PG (2006). The incorporation of a photoisomerizable amino acid into proteins in E. coli. Journal of the American Chemical Society 128, 388–389. DOI: 10.1021/ja055467u [DOI] [PubMed] [Google Scholar]
- Brustad E, Bushey ML, Lee JW, Groff D, Liu W, and Schultz PG (2008). A genetically encoded boronate-containing amino acid. Angewandte Chemie (International ed in English) 47, 8220–8223. DOI: 10.1002/anie.200803240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal PM, Vicente JB, Pires E, Coelho AV, Veiros LF, Cordeiro C, and Gois PM (2012). Iminoboronates: a new strategy for reversible protein modification. Journal of the American Chemical Society 134, 10299–10305. DOI: 10.1021/ja303436y [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Sun SB, Furman JL, Xiao H, and Schultz PG (2013a). A versatile platform for single- and multiple-unnatural amino acid mutagenesis in Escherichia coli. Biochemistry 52, 1828–1837. DOI: 10.1021/bi4000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Xiao H, Bollong M, Ai HW, and Schultz PG (2013b). Efficient viral delivery system for unnatural amino acid mutagenesis in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America 110, 11803–11808. DOI: 10.1073/pnas.1309584110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Xiao H, Yang PY, Soundararajan G, and Schultz PG (2013c). A tryptophanyl-tRNA synthetase/tRNA pair for unnatural amino acid mutagenesis in E. coli. Angewandte Chemie (International ed in English) 52, 5106–5109. DOI: 10.1002/anie.201301094 [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Ren W, Wright QE, and Ai HW (2013). Genetically encoded fluorescent probe for the selective detection of peroxynitrite. Journal of the American Chemical Society 135, 14940–14943. DOI: 10.1021/ja408011q [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Tian Z, Kallio K, Oleson AL, Ji A, Borchardt D, Jiang DE, Remington SJ, and Ai HW (2016). The N-B Interaction through a Water Bridge: Understanding the Chemoselectivity of a Fluorescent Protein Based Probe for Peroxynitrite. Journal of the American Chemical Society 138, 4900–4907. DOI: 10.1021/jacs.6b01285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JW (2017). Expanding and reprogramming the genetic code. Nature 550, 53–60. DOI: 10.1038/nature24031 [DOI] [PubMed] [Google Scholar]
- Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang Z, and Schultz PG (2003a). An expanded eukaryotic genetic code. Science (New York, NY) 301, 964–967. DOI: 10.1126/science.1084772 [DOI] [PubMed] [Google Scholar]
- Chin JW, Cropp TA, Chu S, Meggers E, and Schultz PG (2003b). Progress toward an expanded eukaryotic genetic code. Chemistry & biology 10, 511–519. DOI: 10.1016/S1074-5521(03)00123-6 [DOI] [PubMed] [Google Scholar]
- Chin JW, Martin AB, King DS, Wang L, and Schultz PG (2002a). Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proceedings of the National Academy of Sciences of the United States of America 99, 11020–11024. DOI: 10.1073/pnas.172226299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JW, Santoro SW, Martin AB, King DS, Wang L, and Schultz PG (2002b). Addition of p-azido-L-phenylalanine to the genetic code of Escherichia coli. Journal of the American Chemical Society 124, 9026–9027. DOI: 10.1021/ja027007w [DOI] [PubMed] [Google Scholar]
- Deiters A, Groff D, Ryu Y, Xie J, and Schultz PG (2006). A genetically encoded photocaged tyrosine. Angewandte Chemie (International ed in English) 45, 2728–2731. DOI: 10.1002/anie.200600264 [DOI] [PubMed] [Google Scholar]
- Dumas A, Lercher L, Spicer CD, and Davis BG (2015). Designing logical codon reassignment–Expanding the chemistry in biology. Chemical science 6, 50–69. DOI: 10.1039/C4SC01534G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert M, Vargas-Rodriguez O, Reynolds NM, Wang YS, Soll D, and Umehara T (2017). A genomically modified Escherichia coli strain carrying an orthogonal E. coli histidyl-tRNA synthetase*tRNA(His) pair. Biochimica et biophysica acta 1861, 3009–3015. DOI: 10.1016/j.bbagen.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg CJ, Lajoie MJ, Napolitano MG, Mosberg JA, Goodman DB, Aach J, Isaacs FJ, and Church GM (2014). Rational optimization of tolC as a powerful dual selectable marker for genome engineering. Nucleic acids research 42, 4779–4790. DOI: 10.1093/nar/gkt1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraha F, Oki K, Kobayashi T, Ohno S, Yokogawa T, Nishikawa K, Yokoyama S, and Sakamoto K (2010). Functional replacement of the endogenous tyrosyl-tRNA synthetase-tRNATyr pair by the archaeal tyrosine pair in Escherichia coli for genetic code expansion. Nucleic acids research 38, 3682–3691. DOI: 10.1093/nar/gkq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italia JS, Addy PS, Wrobel CJ, Crawford LA, Lajoie MJ, Zheng Y, and Chatterjee A (2017a). An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes. Nature Chemical Biology 13, 446–450. DOI: 10.1038/nchembio.2312 [DOI] [PubMed] [Google Scholar]
- Italia JS, Zheng Y, Kelemen RE, Erickson SB, Addy PS, and Chatterjee A (2017b). Expanding the genetic code of mammalian cells. Biochemical Society Transactions 45, 555–562. DOI: 10.1042/BST20160336 [DOI] [PubMed] [Google Scholar]
- Jahn M, Rogers MJ, and Soll D (1991). Anticodon and acceptor stem nucleotides in tRNA(Gln) are major recognition elements for E. coli glutaminyl-tRNA synthetase. Nature 352, 258–260. DOI: 10.1038/352258a0 [DOI] [PubMed] [Google Scholar]
- Javahishvili T, Manibusan A, Srinagesh S, Lee D, Ensari S, Shimazu M, and Schultz PG (2014). Role of tRNA orthogonality in an expanded genetic code. ACS chemical biology 9, 874–879. DOI: 10.1021/cb4005172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleina LG, Masson JM, Normanly J, Abelson J, and Miller JH (1990). Construction of Escherichia coli amber suppressor tRNA genes. II. Synthesis of additional tRNA genes and improvement of suppressor efficiency. Journal of molecular biology 213, 705–717. DOI: 10.1016/S0022-2836(05)80257-8 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Takimura T, Sekine R, Kelly VP, Kamata K, Sakamoto K, Nishimura S, and Yokoyama S (2005). Structural snapshots of the KMSKS loop rearrangement for amino acid activation by bacterial tyrosyl-tRNA synthetase. Journal of molecular biology 346, 105–117. DOI: 10.1016/j.jmb.2004.11.034 [DOI] [PubMed] [Google Scholar]
- Lee HS, Spraggon G, Schultz PG, and Wang F (2009). Genetic incorporation of a metal-ion chelating amino acid into proteins as a biophysical probe. Journal of the American Chemical Society 131, 2481–2483. DOI: 10.1021/ja808340b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Mack AV, Brustad EM, Mills JH, Groff D, Smider VV, and Schultz PG (2009). Evolution of proteins with genetically encoded “chemical warheads”. Journal of the American Chemical Society 131, 9616–9617. DOI: 10.1021/ja902985e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, and Schultz PG (2006). Recombinant expression of selectively sulfated proteins in Escherichia coli. Nature biotechnology 24, 1436–1440. DOI: 10.1038/nbt1254 [DOI] [PubMed] [Google Scholar]
- Luo X, Fu G, Wang RE, Zhu X, Zambaldo C, Liu R, Liu T, Lyu X, Du J, Xuan W, Yao A, Reed SA, Kang M, Zhang Y, Guo H, Huang C, Yang PY, Wilson IA, Schultz PG and Wang F (2017). Genetically encoding phosphotyrosine and its nonhydrolyzable analog in bacteria. Nature chemical biology 13, 845 DOI: 10.1038/nchembio.2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T, Lajoie MJ, Englert M, and Soll D (2017). Rewriting the Genetic Code. Annual review of microbiology 71, 557–577. DOI: 10.1146/annurev-micro-090816-093247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Kleina LG, Masson JM, Abelson J, and Miller JH (1990). Construction of Escherichia coli amber suppressor tRNA genes. III. Determination of tRNA specificity. Journal of molecular biology 213, 719–726. DOI: 10.1016/S0022-2836(05)80258-X [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Hayashi A, Sakamoto A, Kiga D, Nakayama H, Soma A, Kobayashi T, Kitabatake M, Takio K, Saito K, et al. (2002). Site-specific incorporation of an unnatural amino acid into proteins in mammalian cells. Nucleic acids research 30, 4692–4699. DOI: 10.1093/nar/gkf589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SW, Wang L, Herberich B, King DS, and Schultz PG (2002). An efficient system for the evolution of aminoacyl-tRNA synthetase specificity. Nature biotechnology 20, 1044–1048. DOI: 10.1038/nbt742. [DOI] [PubMed] [Google Scholar]
- Seitchik JL, Peeler JC, Taylor MT, Blackman ML, Rhoads TW, Cooley RB, Refakis C, Fox JM, and Mehl RA (2012). Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. Journal of the American Chemical Society 134, 2898–2901. DOI: 10.1021/ja2109745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Hoben P, Sumner-Smith M, Uemura H, Watson L, and Soll D (1988). Accuracy of in vivo aminoacylation requires proper balance of tRNA and aminoacyl-tRNA synthetase. Science (New York, NY) 242, 1548–1551. DOI: 10.1126/science.3144042 [DOI] [PubMed] [Google Scholar]
- Takimoto JK, Adams KL, Xiang Z, and Wang L (2009). Improving orthogonal tRNA-synthetase recognition for efficient unnatural amino acid incorporation and application in mammalian cells. Molecular bioSystems 5, 931–934. DOI: 10.1039/b904228h [DOI] [PubMed] [Google Scholar]
- Wan W, Tharp JM, and Liu WR (2014). Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool. Biochimica et biophysica acta 1844, 1059–1070. DOI: 10.1016/j.bbapap.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Niu W, Guo J, and Schultz PG (2012). Unnatural amino acid mutagenesis of fluorescent proteins. Angewandte Chemie (International ed in English) 51, 10132–10135. DOI: 10.1002/anie.201204668 [DOI] [PubMed] [Google Scholar]
- Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, and Church GM (2009). Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898. DOI: 10.1038/nature08187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie J, and Schultz PG (2006). A genetically encoded fluorescent amino acid. Journal of the American Chemical Society 128, 8738–8739. DOI: 10.1021/ja062666k [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang W, Song W, Wang Y, Yu Z, Li J, Wu M, Wang L, Zang J, and Lin Q (2010). A biosynthetic route to photoclick chemistry on proteins. Journal of the American Chemical Society 132, 14812–14818. DOI: 10.1021/ja104350y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brock A, Herberich B, and Schultz PG (2001). Expanding the genetic code of Escherichia coli. Science (New York, NY) 292, 498–500. DOI: 10.1126/science.1060077 [DOI] [PubMed] [Google Scholar]
- Xiao H, Chatterjee A, Choi SH, Bajjuri KM, Sinha SC, and Schultz PG (2013). Genetic incorporation of multiple unnatural amino acids into proteins in mammalian cells. Angewandte Chemie (International ed in English) 52, 14080–14083. DOI: 10.1002/anie.201308137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li J, and Chen PR (2014). Transition metal-mediated bioorthogonal protein chemistry in living cells. Chemical Society reviews 43, 6511–6526. DOI: 10.1039/C4CS00117F [DOI] [PubMed] [Google Scholar]
- Young DD, and Schultz PG (2018). Playing with the molecules of life. ACS chemical biology 13, 854–870. DOI: 10.1021/acschembio.7b00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Addy P, Mukherjee R, and Chatterjee A (2017a). Defining the current scope and limitations of dual noncanonical amino acid mutagenesis in mammalian cells. Chemical science 8, 7211–7217. DOI: 10.1039/C7SC02560B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Lajoie MJ, Italia JS, Chin MA, Church GM, and Chatterjee A (2016). Performance of optimized noncanonical amino acid mutagenesis systems in the absence of release factor 1. Molecular bioSystems 12, 1746–1749. DOI: 10.1039/c6mb00070c. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Lewis TL Jr., Igo P, Polleux F, and Chatterjee A (2017b). Virus-Enabled Optimization and Delivery of the Genetic Machinery for Efficient Unnatural Amino Acid Mutagenesis in Mammalian Cells and Tissues. ACS synthetic biology 6, 13–18. DOI: 10.1021/acssynbio.6b00092 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Mukherjee R, Chin MA, Igo P, Gilgenast MJ, and Chatterjee A (2018). Expanding the Scope of Single-and Double-Noncanonical Amino Acid Mutagenesis in Mammalian Cells Using Orthogonal Polyspecific Leucyl-tRNA Synthetases. Biochemistry 57, 441–445. DOI: 10.1021/acs.biochem.7b00952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We are willing to disclose or deposit any required data. We have not developed any software.