Abstract
Patients with congestive heart failure (CHF) have increased hospital readmission rates and mortality if they are concomitantly diagnosed with cognitive decline and memory loss. Accordingly, we developed a preclinical model of CHF-induced cognitive impairment with the goal of developing novel protective therapies against CHF related cognitive decline. CHF was induced by ligation of the left coronary artery to instigate a myocardial infarction (MI). By 4- and 8-weeks post-MI, CHF mice had approximately a 50% and 70% decline in ejection fraction as measured by echocardiography. At both 4- and 8-weeks post-MI, spatial memory performance in CHF mice as tested using the Morris water task was significantly impaired as compared with sham. In addition, CHF mice had significantly worse performance on object recognition when compared with shams as measured by discrimination ratios during the novel object recognition NOR task. At 8-weeks post-MI, a subgroup of CHF mice were given Angiotensin (Ang)-(1–7) (50mcg/kg/hr) subcutaneously for 4 weeks. Following 3 weeks treatment with systemic Ang-(1–7), the CHF mice NOR discrimination ratios weres imilar to shams and significantly better than the performance of CHF mice treated with saline. Ang-(1–7) also improved spatial memory in CHF mice as compared with shams. Ang-(1–7) had no effect on cardiac function. Inflammatory biomarker studies from plasma revealed a pattern of neuroprotection that may underlie the observed improvements in cognition. These results demonstrate a preclinical mouse model of CHF that exhibits both spatial memory and object recognition dysfunction. Furthermore, this CHF-induced cognitive impairment is attenuated by treatment with systemic Ang-(1–7).
Keywords: angiotensin-(1–7), congestive heart failure, cognitive impairment, Morris water maze, novel object recognition
Congestive heart failure (CHF) is one of the leading health problems in the United States and worldwide. Estimates show approximately 3% of the adult population in the United States suffers from CHF (Heidenreich et al., 2011). The prevalence of CHF in the United States is projected to increase by 25% and to be accompanied by tripling of direct health care costs from $24.7 to $77.7 billion by the year 2030 (Heidenreich et al., 2011). Part of these costs stem from high hospital readmission rates associated with CHF, estimated to be between 40%−50% within 6 months after initial hospitalization (Krumholz et al., 1997). A likely contributing factor to these high readmission rates and worsening CHF can be attributed to cognitive impairment induced by CHF. Studies have shown that CHF patients diagnosed with cognitive impairment have more difficulty with medication management, are unlikely to participate in outpatient treatment regimens, and are unable to identify early symptoms of cardiovascular complications as well as make satisfactory self-care decisions (Wu et al., 2008). As a consequence, many of these patients are at a greater risk for hospital readmissions and mortality (Zuccalà et al., 2005). Although clinically recognized, recommended treatments or therapies for attenuating cognitive decline in patients suffering from CHF are currently nonexistent.
The specific pathophysiological mechanisms of cognitive decline associated with CHF have not yet been determined. However, studies have shown that changes in the dynamics of cerebral perfusion caused by low systolic blood pressure, low cardiac output (Jefferson, 2010; Jefferson et al., 2011; Zuccalà et al., 2001), and the impairment of cerebrovascular reactivity (Georgia-dis et al., 2000) contribute to cognitive loss in patients with CHF.
One commonality between cognitive function and CHF involves the renin-angiotensin system (RAS). Over the last decade, it has become recognized that RAS involves two separate enzymatic pathways that provide a physiological counterbalance of two related peptides acting at distinct receptors. The well described ACE-AngII-AT1R system is thought to be physiologically opposed and balanced by the ACE2-Ang-(1–7)-Mas system (Ferrario, 2006; Raizada & Ferreira, 2007; Vickers et al., 2002). Functionally, these two separate enzymatic pathways of RAS are thought to be involved in balancing reactive oxygen species (ROS), nitric oxide (NO) production, and inflammation in the brain and in peripheral tissues (Ferrario, 2006; Lazartigues, Feng, & Lavoie, 2007). Increases in AT1 receptor activation are known to increase NAD(P)H oxidase activation and ROS generation which are both known to contribute to abnormal increases of sympathetic nerve activity observed in CHF and hypertension (Lob, Schultz, Marvar, Davisson, & Harrison, 2013; Zimmerman, 2011). The majority of Ang(1–7) is produced from ACE2 cleavage of Ang II and has been shown to decrease ROS production and increase nitric oxide synthase (NOS) in the brain (Polizio, Gironacci, Tomaro, & Peña, 2007; Xu, Sriramula, & Lazartigues, 2011).
The known receptor for Ang-(1–7) is the G-protein-coupled receptor Mas. Recent studies in mice lacking Mas have shown that Ang-(1–7) and Mas are essential for normal object recognition processing and blockade of Mas in the hippocampus impairs object recognition (Lazaroni et al., 2012). Earlier studies have shown that Ang-(1–7) facilitates LTP in CA1 cells and this effect is blocked by antagonism of Mas (Hellner, Walther, Schubert, & Albrecht, 2005). Mas has been shown to have very high expression in the hippocampus (Young, O’Neill, Jessell, & Wigler, 1988). Thus, Ang-(1–7) and activation of Mas may be beneficial or even protective of memory and cognitive function.
The goal of the present study was to (a) develop a mouse model of CHF-induced cognitive impairment, (b) determine if Ang-(1–7) might serve as a potential therapy to attenuate CHF induced cognitive impairment, and (c) identify possible biomarkers associated with Ang-(1–7) treatment. We hypothesized that mice with a permanently ligated left coronary artery inducing a myocardial infarction will develop cognitive impairment concomitant with progressive cardiac dysfunction leading to CHF. In addition, we predicted that treatment with Ang-(1–7) during established cardiac dysfunction will attenuate or reverse the CHF-induced cognitive impairment.
Method
Animal Selection
Animal groups.
A total of 26, male C57Bl/6J adult mice (Harlan, 8–10 weeks old) were used. Behavioral experiments were performed in 2 cohorts. In the first cohort, 10 mice were randomly assigned to either the sham or congestive heart failure (CHF) group with five mice in the sham group and five in the CHF group. This first cohort was only studied for the effects of 4 weeks of CHF on novel object recognition (NOR). The second cohort of mice initially consisted of 10 mice randomly assigned to the CHF group and six mice assigned to the sham group. The higher number of mice assigned to the CHF group was due to the anticipated higher mortality rate among the CHF group over the 12 weeks of testing. However, prior to the end of the first 4 weeks CHF, two mice from the sham group died leaving 10 CHF mice and four sham mice to be tested at 4 and 8 weeks CHF and then again following treatment with Ang-(1–7). All of these mice in the second cohort were tested with both the NOR test and the Morris water maze. Experimental groups are described as follows: sham + Ang-(1–7), CHF + saline, CHF + Ang-(1–7). A third cohort not behaviorally tested was used to determine the inflammatory profile following 1 week of MI and 1 week of Ang-(1–7) treatment.
Animal housing.
The mice were housed together (two to four per container) based on their designated experimental group in a temperature controlled cage rack and maintained on a 12-hr light-dark cycle. Every mouse had access to food and water ad libitum throughout the duration of the experiments. All experiments were performed using protocols that adhered to guidelines and approved by the Institutional Animal Care and Use Committee at the University of Arizona, and to 2012 NIH guidelines for care and use of laboratory animals.
Echocardiography
Transthoracic echocardiography was performed prior to MI surgery and again at 1-, 4-, 8-, and 12-weeks post-MI using a Visual Sonics Vevo 2100 high-resolution imaging system (Visual Sonics, Toronto, ON, Canada) and a 25-MHz transducer. The chests of animals were shaved with a chemical hair remover. The echocardiographic procedure was performed in conscious mice to study cardiac function at more physiological heart rates (Kass, Hare, & Georgakopoulos, 1998) and to eliminate any anesthesia effects.
Two-dimensional M-mode echocardiographic images were obtained from the parasternal short-axis views at the level of the midventricles. Cardiac chamber dimensions and the left ventricular wall thickness were measured. Interventricular septum (IVS), left ventricular posterior wall thickness (LVPW), and internal dimension (LVID) were measured from the M-mode images. Data was analyzed off-line using Vevo 2100 analytic software. The data were obtained in triplicate and averaged.
Analysis
Differences over time within each group were determined by a repeated measures ANOVA. Mean differences among different groups were examined using two-way analysis of variance (Prism, Graphpad Software). Post hoc tests were carried out using Bonferroni t tests.
Mouse Model of Myocardial Infarction (MI)
All mice prior to surgery were weighed and anesthetized. For the CHF mice, MI was induced by ligation of the left coronary artery (LCA) as was previously described (Gao, Dart, Dewar, Jennings, & Du, 2000). Under anesthesia (2.5% isoflurane in a mixture of air and O2) a thoracotomy was performed at the fourth left intercostal space and the LCA permanently ligated to induce a myocardial infarction (MI). Occlusion of the LCA was confirmed by observing blanching, a slight change in color of the anterior wall of the left ventricle downstream of the ligature. Sham mice underwent the same procedure with the exception of ligating the LCA. Echocardiography was performed (as above) at 1-, 4-, 8-, and 12-weeks postsurgery to assess ventricular function, cardiac morphometry and remodeling.
Alzet Pump Implantation With Angiotensin-(1–7)
Mice were anesthetized with isoflurane and an osmotic pump (Alzet, Model 1004, Cupertino, CA) was implanted in the right flank for subcutaneous infusions of 50 mcg/kg/hr of Ang-(1–7) or saline.
Histology
Hearts were rapidly excised following euthanasia, washed, and placed in 10% neutral buffered formalin for 24 hr for fixation prior to histological evaluation. The fixed hearts were processed, embedded in paraffin, sectioned, and stained with hemotoxylin and eosin (H&E).
Biomarker Studies
Cytokine/chemokine plasma assay-second cohort.
Measurements of cytokine and chemokine plasma levels in all animals in the second cohort were obtained from 1 ml of blood obtained via cardiac puncture at the end of the experiment prior to euthanasia via halothane anesthesia. Blood was collected in murine EDTA tubes, centrifuged to obtain plasma, and rapidly frozen in liquid nitrogen. Cytokines were assayed using the Proteome Profiler Mouse Cytokine Array Kit, Panel A (RnD Systems, Catalog #ARY006). Dot-blot membranes were blocked with blocking buffer, then incubated over night at 4 °C with 100 μl serum diluted with dilution buffer to 2.0 ml containing the detection antibody cocktail. The membranes were then washed thrice with wash buffer, incubated with 4.0 ml Strepavidin-HRP for 30 min, washed thrice again, and incubated for 1 min with the supplied chemiluminescent substrate. The blot strips were then exposed to Blue-Lite autoradiography (VWR) film for 10 min. The films were then scanned using transmission illumination and blot densities were analyzed using Image J software (NIH). Forty targets were screened including CXL chemokines: CXCL1, CXCL2 (MIP-2), CXCL9, CXCL10, CXCL11, CXCL12 CXCL13; CC chemokines: CCL2 (MCP-1) CCL3, CCL4, CCL5 (RANTES), CCL11, CCL12, CCL17; Interleukins: IL-1α, IL-1β, IL-1ra, IL-2IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-13, IL-12, IL-16, IL-17, IL-23, IL-27; Growth Factors: G-CSF, GM-CSF, M-CSF; and others cytokines I-309, sICAM-1, IFN-γ, TIMP-1, TNF-α, TREM-1, C5/C5a. All samples were run in duplicate. Differences between groups were analyzed and examined using a students-T test. All statistical tests and p values were calculated using MS Excel with Daniel’s XLtoolbox and alpha was set at the 0.05 level. Error bars represent SEM.
Multiplex immunoassay-early disease, 1-week Ang-(1–7) treatment.
To determine if the inflammatory response to myocardial infarction is observed at an earlier time point than 12-weeks post-MI, we employed MILLIPLEX MAP Mouse High Sensitivity Multiplex immunoassay for quantifying inflammatory markers in a separate set of animals with 2-weeks post-MI and 1-week Ang-(1–7) treatment. These animals were not tested for changes in cognition. C57Bl/6J male mice (12-weeks-old) were subjected to MI (permanent ligation of the left coronary as previous) or sham surgery. One-week post-MI or sham, mice were administered (1 mg/kg) Ang-(1–7) or saline by injection (SQ) for 1 week, (4 groups, n = 9 each). Two-weeks post-MI or sham, brains were excised and flash frozen in liquid nitrogen. At the same time, blood was collected in murine EDTA tubes, centrifuged to obtain plasma, and rapidly frozen in liquid nitrogen. Mid brain sections were excised and mechanically disrupted in lysis buffer (Sigma CelLytic MT Mammalian Tissue Lysis Reagent containing Sigma protease inhibitor cocktail and Sigma phosphatase inhibitor cocktail 2). Both brain and plasma cytokines, chemokines, and additional inflammatory analytes were detected and quantified by multiplex immunoassay using a MAGPIX® Multiplexing Instrument and accompanying Milliplex Analyst software (EMD Millipore, MA). This assay allows for the simultaneous measurement of the following cytokines: G-CSF, GM-CSF, IFNg, IL-1a, IL-1B, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12, (p40), IL-12 (p70), IL-13, IL-15, IL-17, IP-10, CXCL1 (KC), CCL2 (MCP-1), CCL3 (MIP1α), CCL4 (MIP1β), CXCL2 (MIP2), CCL5 (RANTES), TNFα. Differences between groups were analyzed by examined using a students-T test. All statistical tests and p values were calculated using MS Excel with Daniel’s XLtoolbox and alpha was set at the 0.05 level. Error bars represent SEM.
Novel Object Recognition (NOR)
Apparatus.
The apparatus consisted of an evenly illuminated Plexiglas box (12 cm × 12 cm × 12 cm) placed on a table inside an isolated observation room. All walls of the apparatus were covered in black plastic, and the floor was gray with a grid that was used to ensure that the location of objects did not change between object familiarization and test phases. The mouse behavior and exploration of objects was recorded with a digital camera. The digital image from the camera was fed into a computer in the adjacent room. Two digital stopwatches were used to track the time the mouse spent interacting with the objects of the test. All data was downloaded to Excel files for analysis. Triplicate sets of distinctly different objects were used for the test.
Procedure.
The novel object recognition task included three phases: habituation phase, familiarization phase, and test phase. For the habituation phase, on the first and second day, mice were brought to the observation room habituated to the empty box for 10 min per day. On the third day, each mouse had a “familiarization” trial with two identical objects followed by a predetermined delay period and then a “test” trial in which one object was identical to the one in the familiarization phase, and the other was novel. All stimuli were available in triplicate copies of each other so that no object needed to be presented twice. Objects were made of glass, plastic, or wood that varied in shape, color, and size. Therefore, different sets of objects were texturally and visually unique. Each mouse was placed into the box the same way for each phase, facing the center of the wall opposite to the objects. To preclude the existence of olfactory cues, the entire box and objects were always thoroughly cleaned with 70% ethanol after each trial and between mice. During the familiarization phase, mice were allowed to explore the two identical objects for 4 min and then returned to their home cages. After a 2-hr delay, the “test phase” commences. The mice were placed back to the same box, where one of the two identical objects presented in the familiarization phase was switched to a novel one and the mouse was allowed to explore these objects for another 4 min. Mouse “exploratory behavior” was defined as the animal directing its nose toward the object at a distance of ~2 cm or less (Ennaceur & Delacour, 1988). Any other behavior, such as resting against the object, or rearing on the object was not considered to be exploration. Exploration was scored by an observer blind to the mouse’s surgical group (CHF vs. Sham). Finally, the positions of the objects in the test phases, and the objects used as novel or familiar, were counterbalanced between the two groups of mice.
Analysis.
Discrimination ratios were calculated from the time spent exploring the novel object minus time spent exploring the familiar object during the test phase divided by the total exploration time. DRatio = (t novel−t familiar)/(t novel + t familiar). Data were analyzed from the first 2 min of the “test phase.” A positive score indicates more time spent with the novel object, a negative score indicates more time spent with the familiar object, and a zero score indicates a null preference. All NOR data was examined using one-way analysis of variance, between subjects (ANOVA). Individual group differences were tested using the post hoc Tukey’s HSD test. In comparisons between groups of different sample sizes, equal variance was tested using a modified Levene’s test. All statistical tests and p values were calculated using MS Excel with Daniel’s XLtoolbox and alpha was set at the 0.05 level. Error bars represent SEM.
Morris Water Task: Testing Spatial Learning and Memory/Visual Test
Apparatus.
The apparatus used was a large circular pool approximately 1.5 m in diameter, containing water at 25 °C made opaque with addition of nontoxic white Crayola paint. An escape platform was hidden just below the surface of the water. Visual, high contrast cues were placed on the walls of the test room. A digital camera connected to a computer in the adjacent room is suspended over the tank to record task progress. For spatial testing prior to MI at 4- and 8-weeks post-MI or sham surgery, the platform was located at different sites in the pool.
Procedure.
During the spatial version of the Morris water task, all animals were given six training trials per day over 4 consecutive days. During these trials, an escape platform was hidden below the surface of water. Mice were released from seven different start locations around the perimeter of the tank, and each animal performed two successive trials before the next mouse was tested. The order of the release locations was pseudorandomized for each mouse such that no mouse was released from the same location on two consecutive trials. Immediately following the 24 spatial trials, the mice performed a probe trial in which the platform was removed and a mouse swam in the pool for 60 s. Following the probe trial the animals were screened for visual ability where the escape platform was raised above the surface of the water but the position of the platform changed between each trial. Performance on the swim task was analyzed with a commercial software application (ANY-maze, Wood Dale, IL). Because different release locations and differences in swimming velocity produce variability in the latency to reach the escape platform, a corrected integrated path length (CIPL) was calculated to ensure comparability of mice performance across different release locations. The CIPL value measures the cumulative distance over time from the escape platform corrected by an animal’s swimming velocity, and is equivalent to the cumulative search error described by Gallagher and colleagues (Gallagher, Burwell, & Burchinal, 1993). Therefore, regardless of the release location, if the mouse mostly swims toward the escape platform the CIPL value will be low. In contrast, the more time a mouse spends swimming in directions away from the platform, the higher the CIPL value.
Analysis.
The primary measure of spatial learning were path length and corrected integrated path length (CIPL). Analysis of Morris water task data was examined using a Welch-t test. All statistical tests and p values were calculated using MS Excel with Daniel’s XLtoolbox and alpha was set at the 0.05 level. Error bars represent SEM.
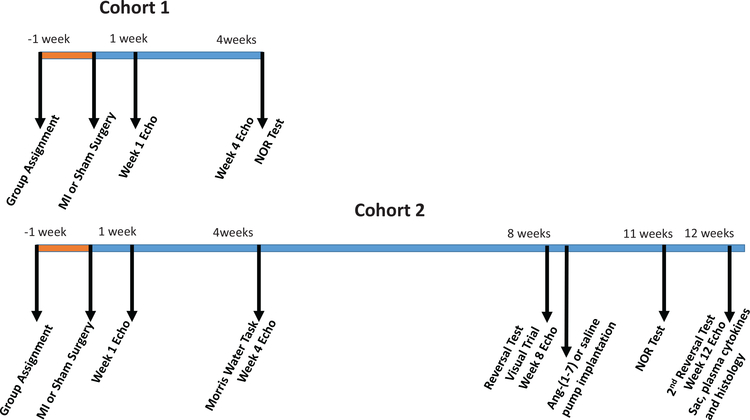
Experimental Timeline
Figure 1 illustrates the experimental timeline. As stated above, two cohorts of mice were tested for changes in cognition. In the first cohort, at 4-weeks post-MI, CHF (n = 5) and sham (n = 5) mice underwent NOR testing. In the second cohort of mice, CHF (n = 10) and sham (n = 4) mice performed the Morris water task at 4-weeks post-MI. At 8-weeks post-MI, these same mice, CHF (n = 9; one CHF mouse died prior to the 8-weeks test) and sham (n = 4) performed a reversal test, in which the hidden platform was placed in a different location compared to earlier tests in order to assess how well mice can learn and adapt to new settings. After the reversal test, the CHF mice were randomly assigned to either the Ang-(1–7) or saline treatment group. Six of the CHF mice were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) and three of the CHF mice were treated with saline via Alzet minipump for 4 weeks. All four of the sham mice were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) via Alzet minipump for 4 weeks. After 3 weeks of Ang-(1–7) treatment, all groups from this cohort performed a NOR test. The following week, all groups from this cohort performed a second Morris water reversal test to examine the effects of Ang-(1–7) treatment on CHF mice for spatial memory and learning.
Figure 1.
Time-line for the course of the experiments for the two cohorts. See the online article for the color version of this figure.
Results
Ventricular Remodeling and Echocardiography
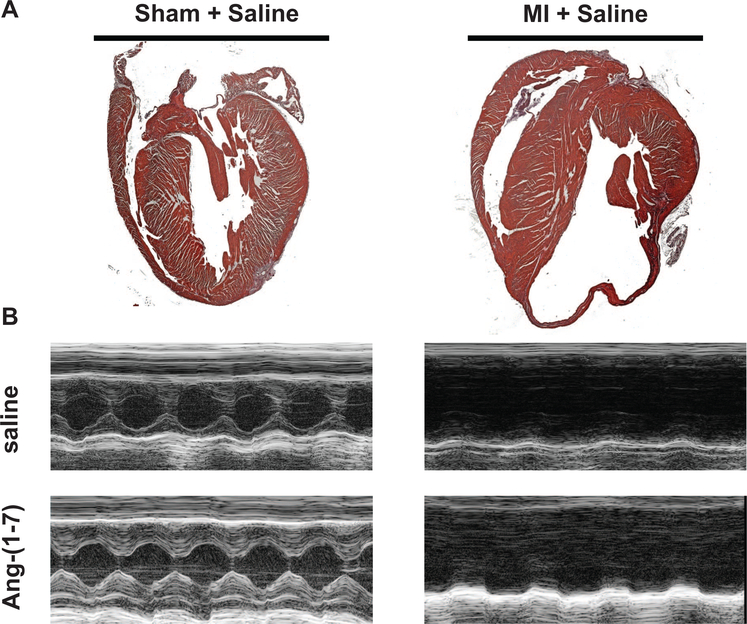
Following completion of the 12-week study period, hearts were excised, fixed, and stained with H&E (see Method section). Representative sections of H&E stained hearts are illustrated in Figure 2A for sham-operated and MI-induced CHF hearts. There was substantial loss of myocardium within the infarcted segment in mice subjected to MI (Figure 2A, right panel) when compared with Sham-operated mice (Figure 2A, left panel). This pattern of wall thinning was evident in all MI operated mice, independent of Ang-(1–7) treatment.
Figure 2.
Cardiac histology and morphometry in sham and congestive heart failure (CHF) mice. (A) Representative images of H&E stained hearts in longitudinal sections of sham and CHF mice. (B) Representative M-mode images of sham and MI mice treated with either saline or Ang-(1–7). From M-mode images, parameters of ventricular chamber dimensions and function were determined. See the online article for the color version of this figure.
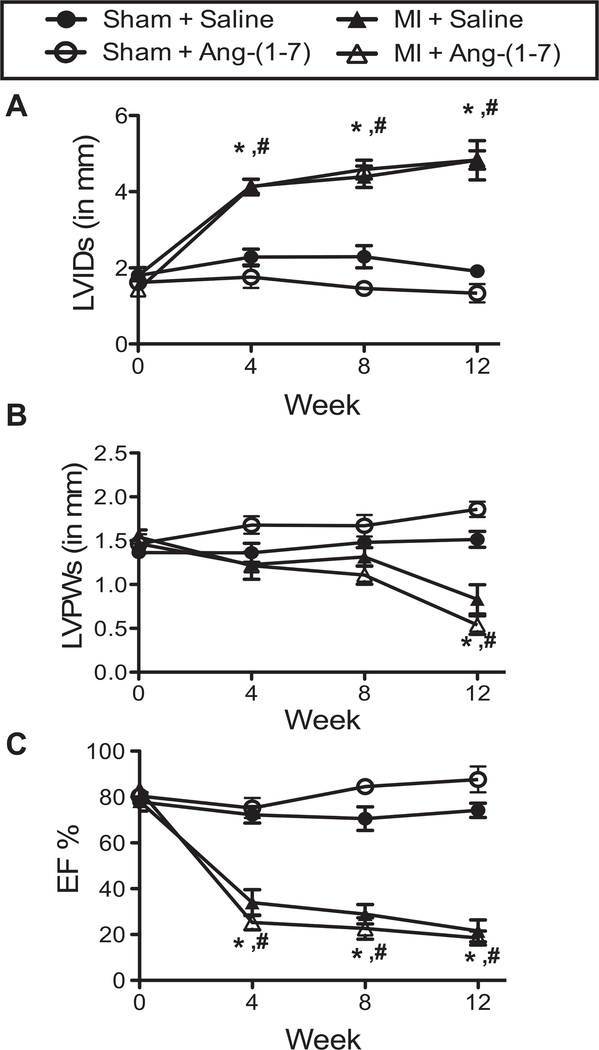
Serial echocardiography was performed at 1-, 4-, 8-, and 12-weeks post-MI in order to monitor the progression of cardiac dysfunction in the MI-induced CHF mice and to correlate the level of cardiac dysfunction with the cognitive impairment. Representative M-mode echocardiographic images from each experimental group are illustrated in Figure 2B. By 4 weeks following MI, the left-ventricular internal dimension during systole (LVIDs) was significantly increased in both MI + saline and MI + Ang-(1–7) hearts over baseline LVIDs and compared with Sham controls (Figure 3A). This elevation in LVIDs persisted throughout the duration of the 12-week study period. From the echocardiographic images, left-ventricular posterior wall dimension during systole (LVPWs) was also monitored (Figure 3B). Interestingly, MI mice maintained LVPWs similar to sham mice up to Week 8 post-MI. During the time period from 8- to 12-weeks post-MI, LVPWs significantly decreased in both MI groups compared with sham controls. This is indicative of ventricular dilation and a notable sign of CHF. The resultant impact of these morphological changes in ventricular chamber dimensions following MI was a decline in cardiac function (ejection fraction; EF%) by 4-weeks post-MI (Figure 3C) that was significantly different from baseline MI mice and sham-operated mice at the same time point. Again, this difference in EF% between MI and sham mice continued until completion of the 12-week study period. Importantly, there was no observed effect of Ang-(1–7) treatment on any of the measured cardiac parameters.
Figure 3.
Echocardiographic parameters of ventricular function and morphometry in sham and congestive heart failure (CHF) mice with and without Ang-(1–7). (A) LVIDs is lateral ventricular internal diameter at end-systole; (B) LVPWs is LV posterior wall thickness at end-systole; and (C) Percent ejection fraction (EF%). Data presented as mean ± S.E.M. Experimental group numbers are as follows, Sham + Saline, n = 5; Sham + Ang-(1–7), n = 7; MI + Saline, n = 8; MI + Ang-(1–7), n = 5. #p < .05 from baseline; #p < .05 from Sham group.)
Novel Object Recognition (NOR) Performance
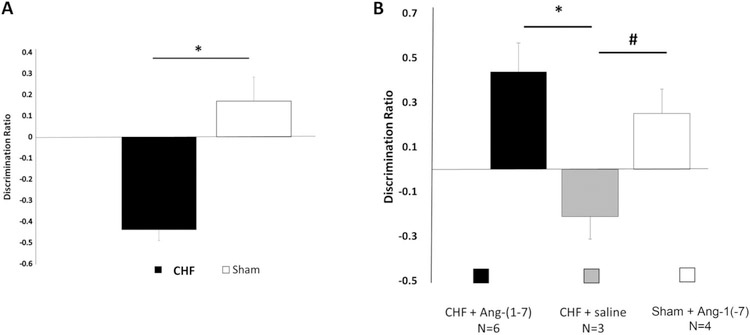
The NOR test is purposed with testing an animal’s ability to discriminate between familiar and novel objects thus examining its object recognition memory. Figure 4A illustrate the results of the NOR test from the first cohort of CHF and sham mice. The mean performance of CHF mice (n = 5) 4-weeks post-MI (M = −.43, SD = .22) was significantly worse as compared with sham mice (n = 5; M = 0.16, SD = .11), F(1, 7) = 27.4, p = .001. Post hoc comparisons using the Tukey’s HSD test indicated that the mean score for CHF was significantly different than the sham, p = .001.
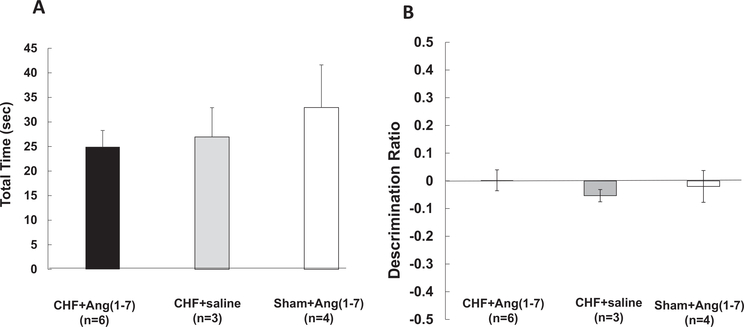
Figure 4.
Novel object recognition (NOR) task performance of sham and congestive heart failure (CHF) mice. (A) Mean discrimination ratios, taken from the first 2 min of “test phase” of CHF and sham mice 4-weeks post-MI. A positive score indicates more time spent with the novel object while a negative score indicates more time spent with the familiar object. A zero score indicates a null preference. CHF mice (n = 5) had significantly lower discriminations compared with shams (n = 5; −.43 ± .05 vs. +0.16 ± .1, F(1, 7) = 27.4, p = .001, ANOVA). * = p < .05. (B) Effects of Ang-(1–7) treatment on novel object recognition (NOR) task performance in CHF and sham mice. Following 3 weeks treatment with systemic Ang-(1–7), CHF mice (n = 6) NOR discrimination ratios were similar to shams (n = 3) and significantly greater than the CHF mice treated with saline (n = 4, * p < .05). CHF saline treated animals DRatios were significantly less that Sham animals (p < .05).
In the second cohort of CHF and sham mice, at 8-weeks-post MI, six CHF mice were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) and three CHF mice were treated with saline via Alzet minipump for 4 weeks. Four sham mice were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) via Alzet minipump for 4 weeks. After 3 weeks of Ang-(1–7) treatment, all groups from this cohort performed a NOR test. As seen in Figure 4B, the mean performance of CHF mice with Ang-(1–7) treatment (n = 6) was similar to sham mice with Ang-(1–7) treatment (n = 3; CHF-Ang-(1–7) M = +0.43, SD = .31 vs. Sham-Ang-(1–7) M = +0.25, SD = .22) and significantly greater in comparison with CHF mice treated with saline (n = 4; M = −.21, SD = .17, F(2, 10) = 6.0,p = .019. Post hoc comparisons using the Tukey’s HSD test indicated that the mean score for CHF-Ang-(1–7) was significantly different than the CHF-saline, p = .015. These results demonstrate that Ang-(1–7) acts to attenuate and even rescue object recognition memory impairment in mice with CHF.
In order to determine if the decreased performance in the CHF mice treated with saline was due to a change in their pattern of exploration as compared to shams, the total time spent exploring each of the identical objects during the “familiarization phase” of the NOR task was determined. As illustrated in Figure 5A, the total time spent exploring the two identical objects was not different between the three groups, suggesting similar levels of interest in environment exploration.
Figure 5.
Total object exploration time similar in congestive heart failure (CHF) and Sham mice. Total time spent exploring the two identical objects was not different between the three groups suggesting (A) similar levels of interest in environment exploration. (B) During the familiarization phase with two identical objects, there was a null preference for the tw identical objects for CHF + Ang(1–7) mice, the CHF + saline mice and the Sham + Ang(1–7) mice (0.002 ± 0.03, −0.05 ± 0.02, 0.019 ± 0.05, respectively). These results suggest that there was no difference in object preference for identical objects during the familiarization phase.
In addition, the DRatio during the “familiarization phase” was determined for all three treatment groups. The null response, or zero preference DRatio between two identical objects would be expected to be observed during the “familiarization phase” and these data would be expected to be significantly different from the DRatio observed during the “test phase” when the mice were presented with both a familiar and novel object. As seen in Figure 5B, the DRatio obtained during the “familiarization phase” is near zero in all three groups.
Morris Water Task Performance
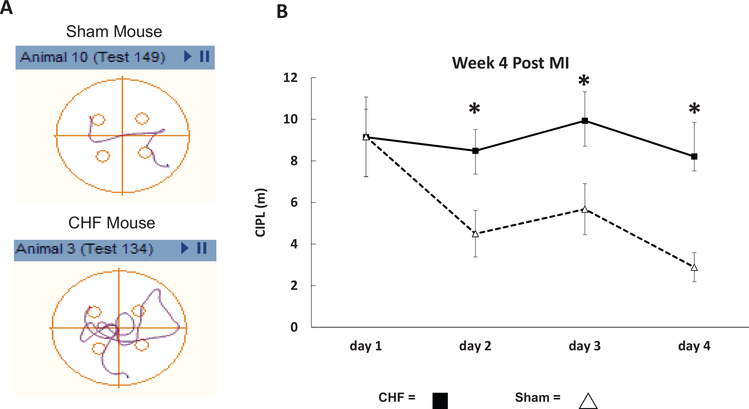
Figure 6A are examples of swim plots for a sham and CHF mouse on Day 3 testing. The sham mouse, as seen in the upper plot, has learned a significantly shorter path to the hidden platform compared to the MI mouse (lower plot). Figure 6B illustrates the averaged CIPL scores for sham (n = 4) and CHF (n = 10) mice over a period of 4 days with six trials per day. The test was administered at 4-weeks post-MI in all of the mice from the second cohort. The sham mice had a significantly lower CIPL on Day 2 (sham: M = 4.5, SD = 9.3 vs. CHF, M = 8.4, SD = 7.9, t(57) = 2.0, p = .01), Day 3 (sham: M = 5.6, SD = 6.0 vs. CHF, M = 9.9, SD = 10.7, t(78) = 1.9, p = .004), and Day 4 (sham: M = 2.8, SD = 3.4, t(75) = 1.9, p = .004) compared with the CHF group. These results suggest that CHF results in a significant impairment of spatial memory in these mice.
Figure 6.
Spatial version of the Morris water task performance of congestive heart failure (CHF) and sham mice. (A) Examples of swim paths for a sham and CHF mouse on Day 3 testing. The sham mouse, as seen in the upper plot, takes a significantly shorter path to the hidden platform compared with the CHF mouse (lower plot). (B) The mean corrected integrated path lengths (CIPL) for CHF (n = 10) and sham (n = 4) mice at 4-weeks post-MI. The paths taken by the CHF mice were significantly longer on Days 2, 3, and 4 compared with the sham group, * p < .05. These results suggest that CHF induces spatial memory loss. See the online article for the color version of this figure.
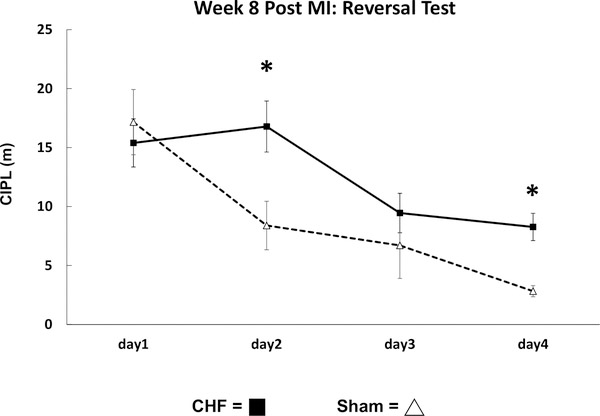
To determine if CHF also affects cognitive flexibility necessary to relearn a new platform location, the performance on the reversal of the Morris swim task was conducted at 8-weeks post-MI. Figure 7, illustrates the averaged CIPL scores for sham (n = 4) and CHF (n = 9) mice over a period of 4 days with six trials per day. The sham mice had a significantly lower CIPL on Day 2 (sham: M = 8.3, SD = 9.8 vs. CHF, M = 16.7, SD = 15.8, t(61) = 1.9, p < .001) and Day 4 (sham: M = 2.8, SD = 2.2 vs. CHF, M = 8.2, SD = 8.4, t(67) = 1.9, p < .001) compared with the CHF group. These results suggest that CHF results in a significant impairment of the ability to flexibly shift a learned response and learn a new place.
Figure 7.
Reversal version of Morris water task performance of congestive heart failure (CHF) and sham mice. The mean corrected integrated path lengths (CIPL) for CHF (n = 10) and sham (n = 4) mice at 8-weeks post-MI. The CIPL in the CHF mice was significantly longer on Days 2 and 4 compared with the sham group. * p < .05.
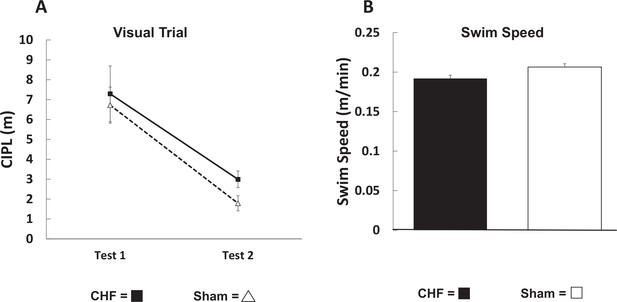
To ensure that the poor performance of CHF mice is due to heart failure induced cognitive impairment rather than changes in visual acuity or physical differences in swim speed, both the sham and CHF groups were tested for visual acuity and their average swim speeds were compared. Figure 8A illustrates the results from the visual trials which were similar between the sham group (n = 4) versus CHF group (n = 10). These results suggest that there were no acuity differences in the two groups of mice. Figure 8B compares the average swim speed of the CHF (n = 10) and sham mice (n = 4) 8-weeks postsurgery. There were no differences is swim speed suggesting that the CHF induced a reduction in performance of the Morris swim task was not due to differences in swimming ability, but rather due to an impairment in spatial memory.
Figure 8.
Visual acuity and swim speed are similar in congestive heart failure (CHF) and sham mice tested with the Morris swim task. (A) There were no differences between the CHF and sham mice in ability to find the elevated platform suggesting that the visual acuity are the same in CHF and sham mice. (B) The average swim speed of the CHF (n = 10, filled histogram) and sham mice (n = 4, open histogram) 8-weeks postsurgery were similar.
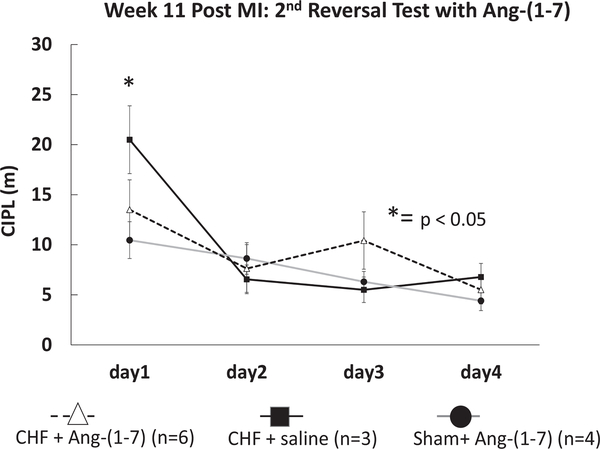
After the reversal test, six of the CHF mice in this cohort were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) and three of the CHF mice were treated with saline via Alzet minipump for 4 weeks. All four of the sham mice from this cohort were treated with subcutaneous 50 mcg/kg/hr Ang-(1–7) via Alzet minipump for 4 weeks. After 4 weeks treatment, a second reversal Morris spatial memory task was conducted. Figure 9 illustrates the mean CIPL of CHF + Ang-(1–7) mice (n = 6), CHF-saline treated mice (n = 3) and Sham + Ang-(1–7) mice (n = 4). The CHF-Ang-(1–7) mice showed significant improvement in spatial memory on the first day of the Morris swim task and performed similarly to the sham mice (n = 4; CHF-Ang-(1–7) M = 10.3, SD = 10.7 vs. Sham-Ang-(1–7) M = 13.5, SD = 14.5). CHF mice treated with saline had a significantly higher CIPL score as compared with CHF-Ang-(1–7) treated mice (CHF-saline: M = 20.4, SD = 13.9 vs. CHF-Ang-(1–7), M = 10.3, SD = 10.7, t(25) = 2.0, p = .01). These results demonstrate that Ang-(1–7) improved cognitive flexibility and memory in the CHF mice.
Figure 9.
Effects of Ang-(1–7) treatment on Morris swim task performance in congestive heart failure (CHF) and sham mice. The mean corrected integrated path lengths (CIPL) for CHF + Ang-(1–7) (n = 6), CHF + saline (n = 3), and sham + Ang-(1–7) (n = 4) mice 12-weeks post-MI. CHF + Ang-(1–7) mice (n = 6) showed significant improvement in spatial memory on the first day of the swim task and performed similarly to sham mice (n = 4), * p < .05 for CHF + Ang-(1–7) compared to CHF-saline and sham.
Biomarker Assays
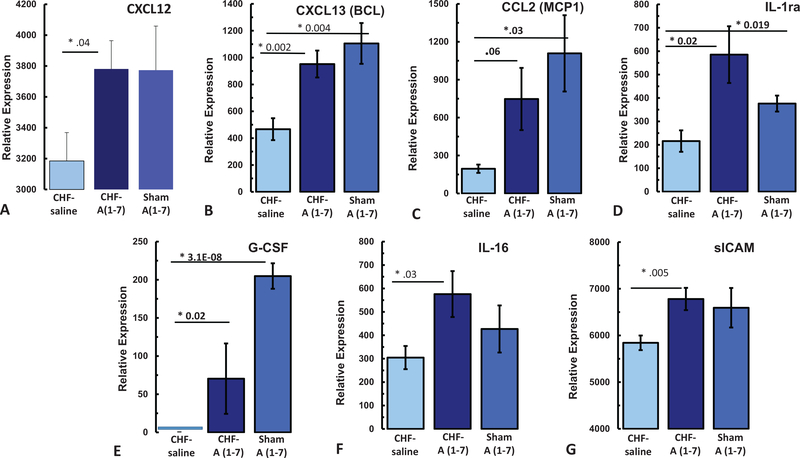
Of the 40 biomarkers assayed with the plasma from the mice from the second cohort, seven showed significant changes in CHF mice treated with Ang-(1–7) as compared with CHF mice treated with saline. One biomarker, CXCL1, showed an effect of CHF alone, independent of treatment. Table 1 and Figure 10 summarize the results in these cytokines from the three groups of mice. With regards to chemokines, CHF-Ang-(1–7) treated mice (n = 6) showed significant increases in CXCL12, CXCL13 as compared with CHF-Sal treated mice (n = 3; CXCL12: CHF-Ang-(1–7) M = 3781.0, SD = 579 vs. CHF-Sal M = 3185, SD = 449, p = .039; CXCL13: CHF-Ang-(1–7) M = 952.0, SD = 317 vs. CHF-Sal M = 466.8, SD = 199, p = .002). This Ang-(1–7) induced increase in CXCL12 and CXCL13 was also observed in the sham-Ang-(1–7) mice suggesting the change in CXCL12 and CXCL13 is due to the Ang-(1–7) treatment. Ang-(1–7) also increased CCL2 in both CHF and sham animals, but this increase in the CHF-Ang-(1–7) animals did not reach statistical significance. With regards to growth factors, the CHF-Saline treated mice had no detectable levels of G-CSF. Treatment with Ang-(1–7) significantly increased G-CSF in both the sham and the CHF animals (CHF-Ang-(1–7) M = 70.3, SD = 130, p = 3.15E-08 *; Sham-Ang-(1–7) M = 204.9., SD = 40, p = .004). Of all the interleukins tested, only IL-16 was increased by Ang-(1–7) in the CHF-Ang-(1–7) animals as compared with CHF-saline treated (CHF-Ang-(1–7) M = 576.0, SD = 310 vs. CHF-Sal M = 304.8, SD = 121, p = .03). Lastly, Ang-(1–7) increased sICAM and IL-1ra in both the CHF and sham mice (sICAM: CHF-Ang-(1–7) M = 6781.0, SD = 754; CHF-Saline: M = 5841.9, SD = 384, p = .005; IL-1ra: CHF-Ang-(1–7) M = 585.3, SD = 342; CHF-Saline M = 215.9, SD = 1112, p = .02).
Table 1.
Relative Expression of Plasma Cytokines From 12-Week CHF Mice Following 3 Weeks Treatment With Ang-(1–7)
| Cytokine, relative expression |
p values |
|||||
|---|---|---|---|---|---|---|
| CHF-saline | CHF-A(1–7) | Sham-A(1–7) | Sham-A(1–7) vs. | CHF-Sal vs. | Sham-A(1–7) vs | |
| Cytokine | (n = 3) | (n = 6) | (n = 4) | CHF-sal | CHF-A(1–7) | CHF-A(1–7) |
| CCL2 (MCP1) | 195.4 ± 33 | 747.3 ± 246 | 1108.5 ± 302 | .029* | .060 | .374 |
| CXCL1 (KC) | 813.4 ± 410 | 1065.3 ± 264 | 406.3 ± 82 | .373 | .618 | .038* |
| CXCL13 | 466.8 ± 82 | 952.0 ± 100 | 1105.6 ± 152 | .004* | .002* | .415 |
| CXCL12 | 3185.0 ± 183 | 3781.2 ± 184 | 3773.6285 | .110 | .039* | .982 |
| G-CSF | 0 | 70.3 ± 46 | 204.9 ± 17 | .004* | 3.15E-08* | .009* |
| sICAM (CD54) | 5841.9 ± 156 | 6781.0 ± 238 | 6593.4 ± 423 | .131 | .005* | .707 |
| IL-1ra | 215.9 ± 46 | 585.3 ± 121 | 376.2 ± 34 | .020* | .019* | .135 |
| IL-16 | 304.8 ± 43 | 576.0 ± 38 | 427.0 ± 100 | .302 | .029* | .305 |
p<.0.5.
Figure 10.
Effects of Ang-(1–7) treatment on serum inflammatory biomarkers in the 2nd Cohort. (A) CXCL12, (B) CXCL13, (C) CCL2, (D) IL-1ra, (E) G-CSF, (F) IL-16, (G) sICAM. * p < .05. See the online article for the color version of this figure.
Multiplex Immunoassay 2-Weeks Postmyocardial Infarction and 1-Week Ang-(1–7)
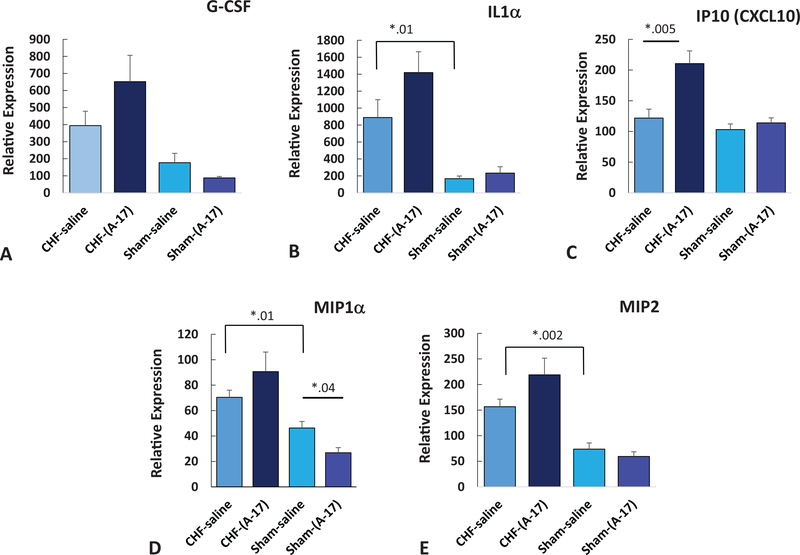
Effect of early disease of 2-weeks post-MI in the absence of Ang-(1–7) (CHF-saline) resulted in a significant increase in IL1α, MIP1α, and MIP2 as compared with sham-saline treated animals (see Table 2, Figure 11; IL1α: CHF-saline, M = 889.2, SD = 556; sham-saline: M = 167.8, SD = 69, p = .01; MIP1α: CHF-saline, M = 70.4, SD = 14; sham-saline: M = 46.3, SD = 11.4, p = .01; MIP2: CHF-saline, M = 156.6, SD = 39.2; sham-saline: M = 73.7, SD = 27, p = .002). Of the 25 cytokines assayed in the plasma from the 1-week CHF-mice treated with Ang-(1–7) for 1 week only IP-10 showed a significant effect with Ang-(1–7) treatment in the CHF mice (CHF-Ang-(1–7): M = 210.5, SD = 61; CHF-Saline: M = 121.8, SD = 38, p = .002). None of the samples from the brain showed any significant effect following 1-week treatment with Ang-(1–7).
Table 2.
Relative Values of Plasma Cytokines From Early Disease, 2 Weeks Postmyocardial Infarction and Following 1-Week Treatment With Ang-(1–7)
| Cytokine, relative expression |
p values |
||||||
|---|---|---|---|---|---|---|---|
| Cytokine | CHF-Saline (n = 9) | CHF-A(1–7) (n = 9) | Sham-saline (n = 9) | Sham-A(1–7) (n = 9) | Sham-saline vs. CHF-sal | CHF-sal vs. CHF-A(1–7) | Sham-sal vs. sham-A(1–7) |
| IL1α | 889.2 ± 210 | 1419.7 ± 245 | 167.8 + 31 | 406.3 ± 82 | .01* | .12 | .44 |
| IP10 | 121.8 ± 14 | 210.5 ± 20 | 103.1 ± 9 | 114.0 ± 8 | .34 | .005* | .39 |
| MIP1α (CCL3) | 70.4 ± 5 | 90.6 ± 15 | 46.3 ± 5 | 26.8 ± 4 | .01* | .28 | .04* |
| MIP2 | 156.6 ± 15 | 218.8 ± 32 | 73.7 ± 12 | 59.4 ± 9 | .002* | .13 | .37 |
Note.CHF = congestive heart failure; IL 1 = Interleukin-1; CCL3 = Chemokine ligand 3.
p<.0.5.
Figure 11.
Effects of Ang-(1–7) treatment on serum inflammatory biomarkers in early disease at 2-weeks post-MI and 1-week Ang-(1–7) treatment. (A) G-CSF, (B) IL1α, (C) IP10, (D) MIP1α, (E) MIP2. * p < .05. See the online article for the color version of this figure.
Discussion
The results from this study are the first to show congestive heart failure (CHF) induced cognitive impairment in an animal model. Further, these results support the hypothesis that Ang-(1–7) treatment attenuates cognitive impairment induced by CHF.
Mechanisms thought to contribute to cognitive impairment in both the present animal model of CHF and in patients with CHF include changes in cerebral blood flow, micro emboli, and inflammation (Dardiotis et al., 2012; Gruhn et al., 2001; Woo, Kumar, Macey, Fonarow, & Harper, 2009). In studies by Gruhn et al. (2001) cerebral blood flow was measured with single-photon emission computed tomography (SPECT) and found to be reduced by 30% in patients with severe heart failure. The causes for decreased cerebral perfusion in CHF have been attributed to low cardiac output, low blood pressure, and altered cerebrovascular reactivity (Georgiadis et al., 2000; Zuccalà et al., 2001). Studies have also suggested that in heart failure there may be an increased frequency of multiple cortical or subcortical infarcts, small vessel diseases with white-matter lesions, and lacunar infarcts with cerebral embolisms due to hypoperfusion (Vogels, Scheltens, Schroeder-Tanka, & Weinstein, 2007; Pullicino et al., 2008).
The mouse has been used as a preclinical model of CHF in a number of different studies and has recently been reviewed (Breckenridge, 2010). However, it should be recognized that the mouse heart has a number of features including heart rate, metabolism, calcium handling, to name a few, which are quite different from the human heart. While no animal model is a perfect model for human disease, the decision in the present study to use the mouse was chosen due, in part, to the relative affordability of the mouse, the common use of the mouse in behavioral tests, the experience of our research group in using ligation of the LAD to produce MI, and the general recognition that LAD ligation results in a postoperative change in the myocardium, dilated cardiomyopathy, and a reasonable approximation of the effects seen in humans following myocardial infarction (Breckenridge, 2010).
In the present study, systemic treatment with Ang-(1–7) starting 8 weeks following coronary ligation and CHF induction did not have any effect on ejection fraction or measures of cardiac function. Thus, the ability of Ang-(1–7) to improve cognition was not related any Ang-(1–7) effects on ejection fraction. This is in contrast to what has been observed by others when Ang-(1–7) formulation was given orally to rats one day prior to infarction and following 60 days (Marques et al., 2012). In this study, Ang-(1–7) was clearly cardioprotective. The main difference between the present study and this study was that in the present study, CHF was clearly already developed and significant damage had already occurred to the cardiac tissue by the time Ang-(1–7) was administered 8-weeks post-MI.
The role of Ang-(1–7) in the modulation of hypertension, blood pressure, and heart failure have recently been reviewed (van Twist et al., 2013; Patel et al., 2014; Lee, Lloyd, Dearden, & Wong, 2013). It is well known that increased activation of renin-angiotensin-aldosterone system (RAAS) in heart failure is a compensatory mechanism. Further increases in ACE2 and Ang-(1–7) has been shown to be beneficial as an antihypertensive. (Patel et al., 2014) and has vasodilatory actions linked to increases in NO production. In preclinical studies, Ang-(1–7) has been shown to be cardioprotective following MI (Wang et al., 2010) which may be mediated by inhibition of oxidative stress (Liao et al., 2011). In the present studies, we did not see any effect of systemic Ang-(1–7) on cardiac function following 3-weeks post-MI on measures of cardiac function. However, we did not measure any potential changes in blood pressure due to Ang-(1–7) treatment. Given that Ang-(1–7) has been shown in some models to result in small decreases in blood pressure (Patel et al., 2014), a drop in blood pressure would not be expected to improve cerebral circulation or cognition. However, future studies are needed to test this hypothesis.
Congestive Heart Failure on Novel Object Recognition Performance
Object recognition memory is a process that relies on the function of perirhinal cortical circuits that support recognition of familiar objects (Burke, Ryan, & Barnes, 2012; Burke, Maurer et al., 2012; Bussey, Muir, & Aggleton, 1999; Mumby & Pinel, 1994). Several studies suggest that the perirhinal cortex of the parahippocampal gyrus plays a crucial role in object recognition memory (Burke, Ryan, et al., 2012; Burke, Maurer et al., 2012; Winters, Saksida, & Bussey, 2008; Mumby & Pinel, 1994). The poor performance of CHF mice 4-weeks post-MI in the NOR task indicates a disturbance of temporal lobe networks that are involved in discriminating between complex stimulus features and guiding decisions on whether an object is familiar or novel. The preference for the familiar object observed in the CHF mice is similar to that reported in studies of rats following perirhinal cortex damage (Mumby, Glenn, Nesbitt, & Kyriazis, 2002). It has been suggested that damage to the perirhinal cortex may alter memory reconsolidation and identification of the familiar object thus resulting in increased time spent with the familiar object at the expense of time spent with the novel object (Ennaceur, 2010). Possible pathophysiological mechanisms involves the possibility that perirhinal cortex may be particularly vulnerable to gray matter loss from reduced cerebral blood flow (Woo, Macey, Fonarow, Hamilton, & Harper, 2003) and this could be one explanation for the object recognition memory deficits observed in CHF mice.
Congestive Heart Failure on Morris Spatial Memory Task Performance
The hippocampus and prefrontal cortex are known to have an important role in spatial learning, memory, and cognitive flexibility across mammalian species (Euston, Gruber, & McNaughton, 2012; Kesner & Churchwell, 2011; Moser, Moser, Forrest, Andersen, & Morris, 1995; Wilson, Munn, Ross, Harding, & Wright, 2009). This form of memory can be effectively tested using navigational tasks such as the Morris swim task, which can be used in a spatial configuration that relies on distal visual landmarks to guide accurate location of an escape platform located under the surface of the water. It can also be used in a cued version in which the platform is visible above the water surface. The former configuration of the swim task interrogates spatial memory, while the latter can be used to evaluate sensory, motor or motivational differences between groups. Lesions of the hippocampus disrupt the spatial components of the task, leaving performance on the cued version of the task intact. Thus, both hippocampus-dependent and independent functions can be tested. Interestingly, NMDA receptor blockade, at levels that also block induction of long-term potentiation, also affects the spatial version of this behavioral task (Redish et al., 2001). The overall decreased performance of the CHF mice in the spatial configuration of the Morris swim task may suggest that CHF induces hippocampal dysfunction.
Reversal learning, or the ability to flexibly shift a learned response and learn a new place, can also be tested with the swim task. The reversal Morris swim task configuration involves the relocation of the hidden platform to a different location compared with earlier tests. Completion of this task requires retrieval of previous spatial memories as well relearning the new platform location (Vorhees & Williams, 2006). The prefrontal cortex is thought to be important for memory retrieval and cognitive flexibility (Euston et al., 2012; Kesner & Churchwell, 2011). The decreased performance of the CHF mice on the 8-week post-MI reversal test may suggest that CHF may also induce dysfunction within the prefrontal cortex.
Ang-(1–7) Effects on Spatial and Object Recognition Memory
After 8-weeks post-MI, CHF mice were treated for 3 weeks with 50mcg/kg/hr of systemic Ang-(1–7) and retested of both the NOR task and the reversal Morris spatial memory task. In the NOR test, treatment with Ang-(1–7) essentially normalized the overall mean performance of CHF mice to that observed in sham mice. In the second reversal test of Morris spatial memory task, treatment with Ang-(1–7) normalized the first day of testing of the CHF to that seen with the sham animals. The mechanisms underlying these effects might be anticipated to involve Mas receptors in the regions of the perirhinal cortex, hippocampus, and the prefrontal cortex. Ang-(1–7) activation of these receptors might be expected to involve actions of Ang-(1–7) at both the level of the brain microvasculature and inside the blood-brain barrier at the level of neurons and glia.
As previously described, Ang-(1–7) is a heptapeptide product of the less commonly known enzymatic pathway RAS identified as the ACE2-Ang-(1–7)-Mas receptor system (Höcht et al., 2008; Santos, Campagnole-Santos, & Andrade, 2000). Within the brain, the Mas receptor is known to be expressed on neurons, microglia and vascular endothelial cells (Regenhardt et al., 2014). Further, all three of these key components that make up the “neurovascular unit” (neurons, microglia, and endothelial cells) are central players in neurogenic hypertension, CHF-induced increases in brain inflammation and ROS production (Zubcevic, Waki, Raizada, & Paton, 2011). Both CHF and hypertension increase circulating cytokines promoting ROS production and inhibition nitric oxide production within the “neurovascular unit.” The end-result of this feed-forward cascade could reasonably be hypothesized to result in neuronal dysfunction within temporal lobe circuits resulting in cognitive impairment. Thus, one potential mechanisms of action for Ang-(1–7) protection of cognitive function may be due to Ang-(1–7), acting at the Mas receptors at both endothelial cells and neurons to inhibit neurovascular ROS production and mitigating the brain inflammatory cascade and related cognitive impairment.
Central administration of Ang-(1–7) has been shown to stimulate nitric oxide release and upregulate the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats (Saavedra, Sánchez-Lemus, & Benicky, 2011). In addition, Ang-(1–7)’s reduction of NOS expression may reduce the amount of ROS production and inflammation that causes neuronal damage (Jiang et al., 2013; Mecca et al., 2011). These combined actions of Ang-(1–7) provide some explanation as to how CHF mice, after 3-weeks treatment with Ang-(1–7) were able to recover their cognitive abilities. Future studies are needed to fully test the proposed mechanisms underlying Ang-(1–7) cognitive protection. Future studies are needed to determine which brain regions are impacted or spared following CHF, and to fully test the proposed mechanisms underlying Ang-(1–7) cognitive protection.
Effects of Ang-(1–7) Treatment on Inflammatory Biomarkers
There is a critical unmet need to identify reliable biomarkers related to disease and related cognitive impairment. In addition, if Ang-(1–7) is to someday be a therapy to treat or prevent cardiac disease related cognitive impairment, then a surrogate biomarker for Ang-(1–7)’s effectiveness in altering the inflammatory milieu involved in the progression of cardiac disease related cognitive impairment is needed. Patients with CHF typically have elevated proinflammatory cytokines including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-a (TNF-α; Braunwald, 2008; Mann, 2015). The patterns of general inflammation and specific cytokine and chemokine expression are known to change over the course of cardiac disease (Dick & Epelman, 2016). Following the initial, acute injury to the heart and the activation of repair mechanisms the physiological immune response is activated which is thought to be required for repair and reestablishing cardiac homeostasis. As the cardiac disease progresses, the inflammatory response becomes pathophysiological leading to advanced myocardial disease and ventricular dysfunction (Mann, 2015). This dual role for the inflammatory system in both physiology and pathology was first described by Elie Metchnikoff (Gordon, 2008) and also informs us as to how the inflammatory milieu in our CHF mouse model may influence cognition (Daulatzai, 2016).
Recent work demonstrates a significant correlation between inflammatory markers and cognitive impairment in CHF patients (Athilingam et al., 2013). Importantly, it has been well established that increases in circulating chemokines and cytokines can have significant effects on all components of the neurovascular interface and increase the permeability of the blood-brain barrier thus allowing the circulating cytokines and chemokines to have effects in both the cerebral vasculature and in the brain peryikaria (Le Thuc, Blondeau, Nahon, & Rovère, 2015; Le Thuc et al., 2016; Vilar-Bergua et al., 2016). Because inflammation has significant effects on hippocampal function, a region of the brain known to be critical for learning and memory (Le Thuc et al., 2015; Yirmiya & Goshen, 2011) we measured plasma inflammatory analytes in both the late disease CHF state following 3-weeks treatment with Ang-(1–7) and also an earlier in disease only 2-weeks post-MI and following 1 week of Ang-(1–7) treatment. In plasma samples from early stage cardiac disease in our mice, myocardial infarction alone, without any treatment with Ang-(1–7) resulted in increases in in IL1α, MIP1α, and MIP2 as compared with sham-saline treated animals. These results are consistent with what has been reported in humans with heart failure (Aukrust et al., 2008; Dick & Epelman, 2016; Ueland et al., 2015). Of the 26 cytokines and chemokines analyzed, 1-week treatment with Ang-(1–7) in the CHF animals showed a significant increase only in IP10 (CXCL10). These results from the early disease state mice suggest that this CHF mouse model follows, generally, the inflammatory profile observed in humans. The effects on IP10 may suggest a role for Ang-(1–7) in modulation of this chemokine but further studies are needed to fully understand this early effect of Ang-(1–7) on the inflammatory profile.
In plasma samples following 12-weeks CHF and 3-weeks Ang-(1–7) treatment, of the chemokines tested, we found Ang-(1–7) treatment significantly increased CXCL12, CXCL13, and CCL2 in the CHF-Ang-(1–7) compared with the CHF-saline treated animals. Sham-Ang-(1–7) animals also showed an increase in CXCL12, CXCL13, and CCL2 compared with CHF-saline animals, but because we did not have a sham-saline treated group it is difficult to interpret the effects of Ang-(1–7) in the sham animals. The correlation between Ang-(1–7) related increases in plasma CXCL12, CXCL13, and CCL2 improvements in cognition following 3 weeks of Ang-(1–7) treatment may involve the known role for CXCL13 and CXCL12 in neurogenesis. While a number of studies have shown that increases in CXCL13 associated the recruitment of B cells to the brain during initial neuroinflammation, stroke, and neuroinflammatory disease (Kowarik et al., 2012; Le Thuc et al., 2015), later stages of disease have suggested that these chemokines may be involved in neuroregeneration and neuroprotection (Chapman et al., 2015; Conductier, Blondeau, Guyon, Nahon, & Rovère, 2010). For example, disruption of CXCL1, CXCL12, and CCL2 via selective knockout have been shown to reduce the infarct size postischemic effects of middle cerebral artery occlusion (Dénes, Ferenczi, Halász, Környei, & Kovács, 2008; Dimitrijevic, Stamatovic, Keep, & Andjelkovic, 2007; Ruscher et al., 2013; Soriano et al., 2002). However, both CCL2 and CXCL12 have been suggested to be involved in neuroregeneration and maintenance after an ischemic event and promote migration of neural precursor cells (Chapman et al., 2015; Lindvall & Kokaia, 2015; Liu et al., 2007; Mao et al., 2014). The present study also found that 3 weeks of Ang-(1–7) treatment in the CHF model resulted in a significant increase in plasma IL1ra, G-CSF, IL-16, and sICAM. Interleukin-1 receptor antagonist (IL1ra) is an endogenous competitive antagonist at the interleukin-1 type-1 receptor (IL-1R) known to show neuroprotection in animal models of head injury (Clausen et al., 2009; Tehranian et al., 2002) and in humans (Helmy et al., 2016). G-CSF has also been shown to be neuroprotective in animal model of brain ischemia. Granulocyte-colony stimulating factor (G-CSF) is a member of the hematopoietic growth factor family. G-CSF effector is expressed in the brain and is induced in response to ischemia (Schneider et al., 2005) and has been shown to be neuroprotective (Solaroglu, Cahill, Tsubokawa, Beskonakli, & Zhang, 2009; Sugiyama et al., 2011).
These results on the effects of Ang-(1–7) treatment on the inflammatory profile in our CHF mouse model suggest a complex interplay between the physiological and pathological immune system during both CHF and Ang-(1–7) treatment. The cellular mechanisms underlying how treatment with Ang-(1–7) might modulate the expression of these chemokines is currently unknown and will require further study. Future studies will be aimed at the identification of a biomarker “thumbprint” for Ang-(1–7) cognitive protection.
Conclusion/Significance
The development of a preclinical model of CHF induced cognitive impairment is a critical step to the advancement of potential treatments to prevent cognitive loss during heart disease. The results of the present study show that the preclinical mouse model of heart failure exhibits both spatial learning and object recognition memory deficits. Importantly, results from this study show novel effects of treatment with systemic Ang-(1–7) to attenuate and even rescue CHF induced cognitive impairment in mice and that these effects of Ang-(1–7) on cognition are correlated with neuroprotective biomarkers. These exciting results suggest a novel role for Ang-(1–7) as a potential therapeutic agent for cognitive impairment as a result of heart disease.
Acknowledgments
These data were presented in part as a poster presentation at the Society for Neuroscience meeting in 2013. This work was supported by the NIH and the McKnight Brain Research Foundation. NIH: JK R01HL098256, KO2HL105799, CB McKnight Brain Research Foundation.
Meredith Hay is the founder and a major stockholder of ProNeurogen, Inc. ProNeurogen holds exclusive licensing rights from UA to the technology discussed herein.
Contributor Information
Meredith Hay, Department of Physiology and Evelyn F. McKnight, Brain Institute, University of Arizona.
Todd W. Vanderah, Department of Pharmacology, University of Arizona
Farmin Samareh-Jahani, Department of Physiology, University of Arizona.
Eleni Constantopoulos, Department of Physiology, University of Arizona.
Ajay R. Uprety, Evelyn F. McKnight Brain Institute, University of Arizona
Carol A. Barnes, Evelyn F. McKnight Brain Institute and Department of Psychology, University of Arizona
John Konhilas, Department of Physiology and Sarver Heart Center, University of Arizona..
References
- Athilingam P, Moynihan J, Chen L, D’Aoust R, Groer M, & Kip K (2013). Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure. Congestive Heart Failure, 19, 92–98. 10.1111/chf.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, . . . Damås JK (2008). Chemokines and cardiovascular risk. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1909–1919. 10.1161/ATVBAHA.107.161240 [DOI] [PubMed] [Google Scholar]
- Braunwald E (2008). Biomarkers in heart failure. The New England Journal of Medicine, 358, 2148–2159. 10.1056/NEJMra0800239 [DOI] [PubMed] [Google Scholar]
- Breckenridge R (2010). Heart failure and mouse models. Disease Models & Mechanisms, 3, 138–143. 10.1242/dmm.005017 [DOI] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Hartzell AL, Nematollahi S, Uprety A, Wallace JL, & Barnes CA (2012). Representation of three-dimensional objects by the rat perirhinal cortex. Hippocampus, 22, 2032–2044. 10.1002/hipo.22060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Ryan L, & Barnes CA (2012). Characterizing cognitive aging of recognition memory and related processes in animal models and in humans. Frontiers in Aging Neuroscience, 4, 15 10.3389/fnagi.2012.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, & Aggleton JP (1999). Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. The Journal of Neuroscience, 19, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KZ, Ge R, Monni E, Tatarishvili J, Ahlenius H, Arvidsson A, . . . Kokaia Z (2015). Inflammation without neuronal death triggers striatal neurogenesis comparable to stroke. Neurobiology of Disease, 83, 1–15. 10.1016/j.nbd.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Clausen F, Hånell A, Björk M, Hillered L, Mir AK, Gram H, & Marklund N (2009). Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. The European Journal of Neuroscience, 30, 385–396. 10.1111/j.1460-9568.2009.06820.x [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, & Rovère C (2010). The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. Journal of Neuroimmunology, 224, 93–100. 10.1016/j.jneuroim.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Dardiotis E, Giamouzis G, Mastrogiannis D, Vogiatzi C, Skoularigis J, Triposkiadis F, & Hadjigeorgiou GM (2012). Cognitive impairment in heart failure. Cardiology Research and Practice, 2012, 1–9. 10.1155/2012/595821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulatzai MA (2016). Fundamental role of pan-inflammation and oxidative-nitrosative pathways in neuropathogenesis of Alzheimer’s disease. American Journal of Neurodegenerative Disease, 5, 1–28. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dénes A, Ferenczi S, Halász J, Környei Z, & Kovács KJ (2008). Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. Journal of Cerebral Blood Flow and Metabolism, 28, 1707–1721. 10.1038/jcbfm.2008.64 [DOI] [PubMed] [Google Scholar]
- Dick SA, & Epelman S (2016). Chronic heart failure and inflammation: What do we really know? Circulation Research, 119, 159–176. 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, & Andjelkovic AV (2007). Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke, 38, 1345–1353. 10.1161/01.STR.0000259709.16654.8f [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2010). One-trial object recognition in rats and mice: Methodological and theoretical issues. Behavioural Brain Research, 215, 244–254. 10.1016/j.bbr.2009.12.036 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, & Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. Behavioural Brain Research, 31, 47–59. 10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, & McNaughton BL (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76, 1057–1070. 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM (2006). Angiotensin-converting enzyme 2 and angiotensin- (1–7): An evolving story in cardiovascular regulation. Hypertension, 47, 515–521. 10.1161/01.HYP.0000196268.08909.fb [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, & Burchinal M (1993). Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behavioral Neuroscience, 107, 618–626. 10.1037/0735-7044.107.4.618 [DOI] [PubMed] [Google Scholar]
- Gao XM, Dart AM, Dewar E, Jennings G, & Du XJ (2000). Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovascular Research, 45, 330–338. 10.1016/S0008-6363(99)00274-6 [DOI] [PubMed] [Google Scholar]
- Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, & Werdan K (2000). Cerebrovascular reactivity is impaired in patients with cardiac failure. European Heart Journal, 21, 407–413. 10.1053/euhj.1999.1742 [DOI] [PubMed] [Google Scholar]
- Gordon S (2008). Elie Metchnikoff: Father of natural immunity. European Journal of Immunology, 38, 3257–3264. 10.1002/eji.200838855 [DOI] [PubMed] [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, & Aldershvile J (2001). Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke, 32, 2530–2533. 10.1161/hs1101.098360 [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, . . . Woo YJ (2011). Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation, 123, 933–944. 10.1161/CIR.0b013e31820a55f5 [DOI] [PubMed] [Google Scholar]
- Hellner K, Walther T, Schubert M, & Albrecht D (2005). Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Molecular and Cellular Neurosciences, 29, 427–435. http://dx.doi.org/10.10167j.mcn.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, & Hutchinson PJ (2016). Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 36, 1434–1448. 10.1177/0271678X15620204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höcht C, Gironacci MM, Mayer MA, Schuman M, Bertera FM, & Taira CA (2008). Involvement of angiotensin-(1–7) in the hypothalamic hypotensive effect of captopril in sinoaortic denervated rats. Regulatory Peptides, 146, 58–66. http://dx.doi.org/10.1016Zj.regpep.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Jefferson AL (2010). Cardiac output as a potential risk factor for abnormal brain aging. Journal of Alzheimer’s Disease, 20, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, . . . Benjamin EJ (2011). Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). The American Journal of Cardiology, 108, 1346–1351. 10.1016/j.amjcard.2011.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Gao L, Shi J, Lu J, Wang Y, & Zhang Y (2013). Angiotensin-(1–7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacological Research, 67, 84–93. 10.1016/j.phrs.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Kass DA, Hare JM, & Georgakopoulos D (1998). Murine cardiac function: A cautionary tail. Circulation Research, 82, 519–522. 10.1161/01.RES.82.4.519 [DOI] [PubMed] [Google Scholar]
- Kesner RP, & Churchwell JC (2011). An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of Learning and Memory, 96, 417–431. 10.1016/j.nlm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, . . . Hemmer B (2012). CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. Journal of Neuroinflammation, 9, 93 10.1186/1742-2094-9-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, & Hennen J (1997). Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Archives of Internal Medicine, 157, 99–104. 10.1001/archinte.1997.00440220103013 [DOI] [PubMed] [Google Scholar]
- Lazaroni TL, Raslan AC, Fontes WR, de Oliveira ML, Bader M, Alenina N, . . . Pereira GS (2012). Angiotensin-(1–7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiology of Learning and Memory, 97, 113–123. 10.1016/j.nlm.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Lazartigues E, Feng Y, & Lavoie JL (2007). The two fACEs of the tissue renin-angiotensin systems: Implication in cardiovascular diseases. Current Pharmaceutical Design, 13, 1231–1245. 10.2174/138161207780618911 [DOI] [PubMed] [Google Scholar]
- Lee VC, Lloyd EN, Dearden HC, & Wong K (2013). A systematic review to investigate whether angiotensin-(1–7) is a promising therapeutic target in human heart failure. International Journal of Peptides, 2013, 260346 10.1155/2013/260346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thuc O, Blondeau N, Nahon JL, & Rovere C (2015). The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Annals of the New York Academy of Sciences, 1351, 127–140. 10.1111/nyas.12855 [DOI] [PubMed] [Google Scholar]
- Le Thuc O, Cansell C, Bourourou M, Denis RG, Stobbe K, Devaux N, . . . Rovere C (2016). Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Reports, 17, 1738–1752. 10.15252/embr.201541499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Wang L, Yang C, He J, Wang X, Guo R, . . . Ma H (2011). Cyclooxygenase mediates cardioprotection of angiotensin-(1–7) against ischemia/reperfusion-induced injury through the inhibition of oxidative stress. Molecular Medicine Reports, 4, 1145–1150. [DOI] [PubMed] [Google Scholar]
- Lindvall O, & Kokaia Z (2015). Neurogenesis following stroke affecting the adult brain. Cold Spring Harbor Perspectives in Biology, 7, a019034 10.1101/cshperspect.a019034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Wang L, Yier T, & Chopp M (2007). Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. Journal of Neuroscience Research, 85, 2120–2125. 10.1002/jnr.21359 [DOI] [PubMed] [Google Scholar]
- Lob HE, Schultz D, Marvar PJ, Davisson RL, & Harrison DG (2013). Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension, 61, 382–387. 10.1161/HYPERTENSIONAHA.111.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL (2015). Innate immunity and the failing heart: The cytokine hypothesis revisited. Circulation Research, 116, 1254–1268. 10.1161/CIRCRESAHA.116.302317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Huang M, Chen SC, Li YN, Xia YP, He QW, . . . Hu B (2014). Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neuroscience & Therapeutics, 20, 460–468. 10.1111/cns.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques FD, Melo MB, Souza LE, Irigoyen MC, Sinisterra RD, de Sousa FB, . . . Santos RA (2012). Beneficial effects of long-term administration of an oral formulation of angiotensin-(1–7) in infarcted rats. International Journal of Hypertension, 2012, 1–12. 10.1155/2012/795452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ, & Sumners C (2011). Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Experimental Physiology, 96, 1084–1096. 10.1113/expphysiol.2011.058578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, & Morris RG (1995). Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 92, 9697–9701. 10.1073/pnas.92.21.9697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Glenn MJ, Nesbitt C, & Kyriazis DA (2002). Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behavioural Brain Research, 132, 215–226. 10.1016/S0166-4328(01)00444-2 [DOI] [PubMed] [Google Scholar]
- Mumby DG, & Pinel JP (1994). Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience, 108, 11–18. 10.1037/0735-7044.108.1.11 [DOI] [PubMed] [Google Scholar]
- Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, & Burrell LM (2014). From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Frontiers in Physiology, 5, 227 10.3389/fphys.2014.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizio AH, Gironacci MM, Tomaro ML, & Peña C (2007). Angiotensin-(1–7) blocks the angiotensin II-stimulated superoxide production. Pharmacological Research, 56, 86–90. 10.1016/j.phrs.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Pullicino PM, Wadley VG, McClure LA, Safford MM, Lazar RM, Klapholz M, . . . Howard G (2008). Factors contributing to global cognitive impairment in heart failure: Results from a population-based cohort. Journal of Cardiac Failure, 14, 290–295. 10.1016/j.cardfail.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada MK, & Ferreira AJ (2007). ACE2: A new target for cardiovascular disease therapeutics. Journal of Cardiovascular Pharmacology, 50, 112–119. 10.1097/FJC.0b013e3180986219 [DOI] [PubMed] [Google Scholar]
- Redish AD, Battaglia FP, Chawla MK, Ekstrom AD, Gerrard JL, Lipa P, . . . Barnes CA (2001). Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. The Journal of Neuroscience, 21, RC134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, . . . Sumners C (2014). Centrally administered angiotensin-(1–7) increases the survival of stroke-prone spontaneously hypertensive rats. Experimental Physiology, 99, 442–453. 10.1113/expphysiol.2013.075242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Kuric E, Liu Y, Walter HL, Issazadeh-Navikas S, Englund E, & Wieloch T (2013). Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. Journal of Cerebral Blood Flow and Metabolism, 33, 1225–1234. 10.1038/jcbfm.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Sánchez-Lemus E, & Benicky J (2011). Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology, 36, 1–18. http://dx.doi.org/10.1016Zj.psyneuen.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RA, Campagnole-Santos MJ, & Andrade SP (2000). Angiotensin-(1–7): An update. Regulatory Peptides, 91, 45–62. 10.1016/S0167-0115(00)00138-5 [DOI] [PubMed] [Google Scholar]
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, . . . Schäbitz WR (2005). The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. The Journal of Clinical Investigation, 115, 2083–2098. 10.1172/JCI23559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, & Zhang JH (2009). Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurological Research, 31, 167–172. 10.1179/174313209X393582 [DOI] [PubMed] [Google Scholar]
- Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, . . . Pan Y (2002). Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. Journal of Neuroimmunology, 125, 59–65. 10.1016/S0165-5728(02)00033-4 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, & Kitagawa K (2011). Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke, 42, 770–775. 10.1161/STROKEAHA.110.597799 [DOI] [PubMed] [Google Scholar]
- Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, . . . Iverfeldt K (2002). Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. Journal of Neurotrauma, 19, 939–951. 10.1089/089771502320317096 [DOI] [PubMed] [Google Scholar]
- Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, & Askevold ET (2015). Inflammatory cytokines as biomarkers in heart failure. Clinica Chimica Acta, 443, 71–77. 10.1016/j.cca.2014.09.001 [DOI] [PubMed] [Google Scholar]
- van Twist DJ, Houben AJ, de Haan MW, Mostard GJ, Kroon AA, & de Leeuw PW (2013). Angiotensin-(1–7)-induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and angiotensin II co-infusion. Hypertension, 62, 789–793. 10.1161/HYPERTENSIONAHA.113.01814 [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, . . . Tummino P (2002). Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. The Journal of Biological Chemistry, 277, 14838–14843. 10.1074/jbc.M200581200 [DOI] [PubMed] [Google Scholar]
- Vilar-Bergua A, Riba-Llena I, Nafría C, Bustamante A, Llombart V, Delgado P, & Montaner J (2016). Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. Journal of Cerebral Blood Flow and Metabolism, 36, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels RL, Scheltens P, Schroeder-Tanka JM, & Weinstein HC (2007). Cognitive impairment in heart failure: A systematic review of the literature. European Journal of Heart Failure, 9, 440–449. 10.1016/j.ejheart.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Vorhees CV, & Williams MT (2006). Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols, 1, 848–858. 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, . . . Walther T (2010). Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection after myocardial infarction. Circulation: Heart Failure, 3, 286–293. 10.1161/CIRCHEARTFAILURE.109.905968 [DOI] [PubMed] [Google Scholar]
- Wilson WL, Munn C, Ross RC, Harding JW, & Wright JW (2009). The role of the AT4 and cholinergic systems in the nucleus basalis magnocellularis (NBM): Effects on spatial memory. Brain Research, 1272, 25–31. 10.1016/j.brainres.2009.03.025 [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, & Bussey TJ (2008). Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and Biobehavioral Reviews, 32, 1055–1070. 10.1016/j.neubiorev.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Woo MA, Kumar R, Macey PM, Fonarow GC, & Harper RM (2009). Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. Journal of Cardiac Failure, 15, 214–223. 10.1016/jxardfail.2008.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Fonarow GC, Hamilton MA, & Harper RM (2003). Regional brain gray matter loss in heart failure. Journal of Applied Physiology, 95, 677–684. 10.1152/jappl-physiol.00101.2003 [DOI] [PubMed] [Google Scholar]
- Wu JR, Moser DK, Lennie TA, Peden AR, Chen YC, & Heo S (2008). Factors influencing medication adherence in patients with heart failure. Heart & Lung, 37, 8–16. 10.1016/j.hrtlng.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Xu P, Sriramula S, & Lazartigues E (2011). ACE2/ANG-(1–7)/Mas pathway in the brain: The axis of good. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 300, R804–R817. 10.1152/ajpregu.00222.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, & Goshen I (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity, 25, 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Young D, O’Neill K, Jessell T, & Wigler M (1988). Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proceedings of the National Academy of Sciences of the United States of America, 85, 5339–5342. 10.1073/pnas.85.14.5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC (2011). Angiotensin II and angiotensin-1–7 redox signaling in the central nervous system. Current Opinion in Pharmacology, 11, 138–143. 10.1016/jxoph.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Waki H, Raizada MK, & Paton JF (2011). Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension, 57, 1026–1033. 10.1161/HYPERTENSIONAHA.111.169748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccalà G, Marzetti E, Cesari M, Lo Monaco MR, Antonica L, Cocchi A, & Bernabei R (2005). Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. American Journal of Medicine, 118, 496–502. [DOI] [PubMed] [Google Scholar]
- Zuccalà G, Onder G, Pedone C, Carosella L, Pahor M, Bernabei R, & Cocchi A (2001). Hypotension and cognitive impairment: Selective association in patients with heart failure. Neurology, 57, 1986–1992. 10.1212/WNL.57.11.1986 [DOI] [PubMed] [Google Scholar]