Abstract
Sepsis is a systemic inflammatory disease resulting from an infection. This disorder affects 750 000 people annually in the United States and has a 62% rehospitalization rate. Septic symptoms range from typical flu-like symptoms (eg, headache, fever) to a multifactorial syndrome known as sepsis-associated encephalopathy (SAE). Patients with SAE exhibit an acute altered mental status and often have higher mortality and morbidity. In addition, many sepsis survivors are also burdened with long-term cognitive impairment. The mechanisms through which sepsis initiates SAE and promotes long-term cognitive impairment in septic survivors are poorly understood. Due to its unique role as an interface between the brain and the periphery, numerous studies support a regulatory role for the blood-brain barrier (BBB) in the progression of acute and chronic brain dysfunction. In this review, we discuss the current body of literature which supports the BBB as a nexus which integrates signals from the brain and the periphery in sepsis. We highlight key insights on the mechanisms that contribute to the BBB’s role in sepsis which include neuroinflammation, increased barrier permeability, immune cell infiltration, mitochondrial dysfunction, and a potential barrier role for tissue non-specific alkaline phosphatase (TNAP). Finally, we address current drug treatments (eg, antimicrobials and intravenous immunoglobulins) for sepsis and their potential outcomes on brain function. A comprehensive understanding of these mechanisms may enable clinicians to target specific aspects of BBB function as a therapeutic tool to limit long-term cognitive impairment in sepsis survivors.
Keywords: Sepsis, sepsis-associated encephalopathy, blood-brain barrier, neuroinflammation, drug delivery, tissue non-specific alkaline phosphatase
Introduction
Sepsis is a debilitating systemic inflammatory process involving multiple organ systems that is preceded by an infection. It is the 10th leading cause of death in the United States with an annual financial burden for patients and survivors that exceeds $20 billion.1 Through mechanisms that remain largely poorly understood, sepsis can induce acute and chronic changes in the central nervous system (CNS), particularly at the blood-brain barrier (BBB). A compromised CNS can lead to sepsis-associated encephalopathy (SAE), a well-characterized state of cognitive impairment and neurological dysfunction often seen in the acute phase of sepsis. Multiple pathways have been investigated for their contribution to the sepsis-associated compromise of the BBB.2,3 This review integrates current clinical knowledge of sepsis with mechanistic insights from both clinical studies and preclinical animal models of sepsis. The overall goal of this review is to understand how sepsis pathophysiology perturbs the integral functions of the cells and proteins that comprise the BBB. Thus, we provide insights to uncover how a compromised BBB may lead to SAE or permanent brain dysfunction in sepsis survivors.
Sepsis Pathophysiology
Clinical sepsis presentation
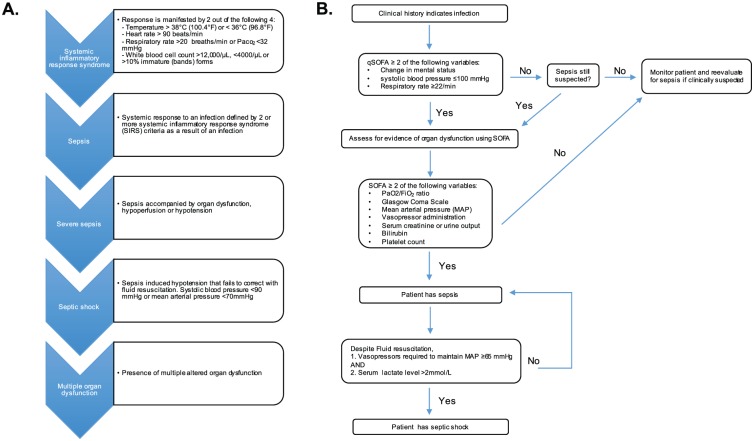
The current definition from the Sepsis-3 consortium describes sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to an infection.4 The most common precipitating sites for sepsis are the respiratory system, genitourinary system, and the abdomen. Sepsis may present as a combination of various non-descript signs and symptoms making early diagnosis difficult. For example, patients may present with fever, cold, pain, delirium, increased heart rate, shortness of breath, diarrhea, and/or low blood pressure. The diagnosis and management of sepsis has changed dramatically over 30 years. The historical definition of sepsis was focused primarily on inflammation and incorrectly portrayed sepsis as a sequential process that eventually ends in septic shock (Figure 1A). In 2016, Sepsis-2 criteria were revised to current Sespis-3 criteria to improve consistency in classification in epidemiological and clinical trials. The revised Sepsis-3 classification shown in Figure 1B focuses on accelerated recognition and management of sepsis.5
Figure 1.
Comparison of the past and present guidelines for diagnosis of sepsis and septic shock. (A) Past guidelines stressed that the diagnosis and progression of sepsis from a systemic inflammatory response to multiple organ dysfunction was sequential rather than multifactorial. (B) The current guidelines for defining sepsis and septic shock stress that multiple linked considerations are necessary for an accurate diagnosis. Both the past and present guidelines involve a clinical screening tool for patients likely to have sepsis that includes a clinical characterization of the severity of the disease. Clinicians have traditionally used a Sequential Organ Failure Assessment (SOFA) system to categorize the severity of organ dysfunction in sepsis, which associates a higher SOFA score with increased mortality.4,6 New sepsis guidelines employ a quick Sequential Organ Failure Assessment (qSOFA) score, which is a modified version of the SOFA score that includes altered mental status, a systolic blood pressure ⩽ 100 mm Hg, and a respiratory rate ⩾ 22/min. Patients with a qSOFA score ⩾ 2 have an overall in-hospital mortality risk greater than 10%.4,7 If warranted, further clinical analysis can be completed using other SOFA criteria.4
Source: Adapted from Singer et al.4
In recent years, the focus in clinical treatment has shifted to severe sepsis and septic shock, which has increased survival in hospitalized patients diagnosed with severe sepsis and critically ill patients with septic shock who have a higher risk of multi-organ failure complications and death.8 This heterogeneous presentation of clinical sepsis makes disease management and appropriate therapeutic interventions difficult. Some challenges associated with the management of sepsis include late diagnoses, poor prognoses, inadequate therapeutics, and post-sepsis complications. These challenges stem from late recognition and difficulties associated with differentiation of sepsis from other illnesses in its early stage. In the later stages of sepsis, recognition becomes easier, yet sepsis is more difficult to treat and often coincides with multi-organ failure.5,8,9
Most sepsis cases are hospital acquired and are often comorbid with prior injury, such as stroke, trauma, or post-surgery. Most cases of hospital-acquired sepsis are treated in the intensive care unit (ICU). However, ICU heterogeneity can make sepsis more common in one ICU versus another. For example, there is a higher incidence of sepsis in a trauma ICU as opposed to a surgical ICU.8 Alternatively, a significant number of sepsis patients are admitted to the hospital or directly to the ICU via the Emergency Department (ED); most of these patients present with community-acquired sepsis from pneumonia or complications from other comorbid conditions such as diabetes.10,11 In addition to the patient setting, the development of sepsis often depends on several risk factors, such as age, where a proportionate relationship exists between increasing age and sepsis acquisition.12,13 Male sex, non-white ethnicity, and preexisting conditions such as Alzheimer disease (AD), HIV, or cancer are also risk factors for acquisition.8,13–15
Current experimental animal models of sepsis
Understanding the mechanisms involved in the pathophysiology of sepsis requires the use of animal models that adequately reproduce several features of the human disorder including both inflammation and infection. The most common animal models are cecal ligation and puncture (CLP), the colon ascendens stent peritonitis (CASP) model, endotoxin injection, and bacterial infusion.16 The CLP model is regarded as the gold standard for human-like sepsis progression in animal models.17 Execution of this model necessitates leakage of polymicrobial feces into the peritoneum after the cecum is punctured with a needle. Disease severity is modeled by controlling needle size and number of punctures; however, a major limitation of this model is the failure to maintain continuous fecal leakage due to abscess formation and necrosis of the punctured bowel. Some investigators also administer antibiotics either at the time of injury or at intervals post injury. Although antibiotic administration is an additional feature which mimics the treatment regimen in human patients, the use of different antibiotic classes and dosing paradigms across laboratories may confound the interpretation of findings when results are compared between laboratories.16,18 The CASP model is a newer model recently introduced to counter the flaws of the CLP model.16,19 The model involves the insertion of a stent at the ascending portion of the colon, allowing continual leakage of feces into the peritoneum.16,20 Despite resemblance to human-like sepsis progression, the drawbacks to this model include animal variation in colon size, fecal content, and the volume of feces that leaks into the peritoneum.16,21 Although these two models have provided remarkable insights to understand the pathophysiology of sepsis in humans, they fail to fully recapitulate the comprehensive clinical progression of sepsis in humans.16
Two alternative sepsis models involve injection or infusion of endotoxin or bacteria. The endotoxin model typically involves injection of lipopolysaccharide (LPS) endotoxin, a component of Gram-negative bacterial cell walls which signals most commonly through toll-like receptor-4 (TLR4). Administration of LPS via different routes (ie, intraperitoneal, intravenous, or intracerebroventricular) initiates a cytokine storm that results in the release of tumor necrosis factor alpha (TNF-α) and numerous interleukins (ILs; IL-1, IL-6, and IL-10). Injection of LPS mimics many classical signs and symptoms of sepsis-induced inflammation, thereby providing a basic understanding of how inflammation activates the immune response in sepsis.16,22 One major limitation of the LPS model is the lack of integration of the infection component. A second limitation is that very large endotoxin doses are required in many rodent models to mimic the pathological profile of the clinical sepsis picture observed in humans.23 Bacterial injection is a less widely used model involving infusion of a bacterium, usually Escherichia coli or Staphylococcus aureus, to initiate both inflammation and infection.16,24,25 Different bacterial strains used for infection present a challenge in this model, as they will produce different patterns of sepsis progression.26 Thus, the characteristics of the sepsis model must be considered when interpreting the effects of sepsis on the CNS and other organ systems.
The CNS in sepsis: sickness behavior and SAE
A critical role for the CNS in the pathophysiology of sepsis has emerged over the past 2 decades. Several recent reviews address this topic in excellent detail.13,27–30 One important contribution of the CNS is “sickness behavior.” Sickness behavior is a response seen in sepsis characterized by fever, adaptive behavioral changes, and neuroimmune changes.31 The response is governed primarily by systemic interactions with the vagus nerve (VN) and circumventricular organs (CVOs). The VN is an important mediator of inflammation. Septic mice that underwent a vagotomy (VGX) surgery exhibited an increase in the synthesis of inflammatory cytokines compared with sepsis-only mice.27,32–34 In contrast, stimulation of the VN in septic animals resulted in an overall reduction in the synthesis of inflammatory cytokines, leukocyte recruitment, and endothelial activation.34–36 The VN also relays peripheral information to the medullary autonomic nuclei, whereas the CVOs may serve as sensors for inflammatory mediators, primarily cytokines, and serve as the foci for neuroimmune communication between the peripheral circulation into the brain parenchyma. Many of these neuroimmune communication circuits are well described, but the underlying mechanisms that regulate these pathways remain poorly understood.37,38 For example, activation of the nucleus tractus solitarii and locus coeruleus by inflammatory mediators subsequently activates autonomic nuclei, behavioral, and neuroendocrine centers.39,40 The summative effect can be observed as depression, social withdrawal, increased heart rate, poor blood pressure control, or altered vigilance.18
In addition to sickness behavior, patients with acute sepsis may have changes in brain function that present as delirium, seizures, psychological disorders, abnormal motor movements, and increased mortality.39,41 Changes in brain function are most commonly manifested as delirium. Whereas sepsis-associated delirium usually presents as decreased activity, a hyperactive form associated with agitation may be seen in some patients.39 Tools that can be used to confirm sepsis-associated delirium include medical history, blood chemistry, electrolyte balance, the ICU screening checklist, Confusion Assessment Method, and Glasgow Coma Scale.39,42 Sickness behavior and/or delirium may progress to a more severe phenotype, SAE, which is regarded as a diagnosis of exclusion.43 It is characterized by impaired consciousness, seizures, delirium, coma, focal cognitive deficits, and alterations in electroencephalogram (EEG) patterns.44 Patients with SAE have increased mortality, long-term neurological decline, memory lapse, inattentiveness, disorientation, and verbal difficulties.45
Alterations in EEG wave patterns often predict SAE outcome, and EEG reactivity is associated with mortality even at 1 year post severe sepsis.44,46 For example, a recent study showed resting-state EEG changes in sepsis survivors at 6 to 24 months after hospital discharge, including increased delta and sigma activity compared with control patients.47 Changes in EEG frequencies can be associated with changes in brain function. For example, slowing alpha activity with increased theta activity reflects cortical dysfunction and can occur in patients with mild to moderate encephalopathy. Slowing of delta activity is often associated with more severe neurocognitive decline and indicates impaired function in deeper brain structures, such as the basal ganglia.44 Whereas the evaluation of EEG can be sensitive to SAE diagnosis in the absence of neurological examination abnormalities, it has poor specificity and can be hampered by sedation and analgesia.45,48
Ischemia is another common complication of early sepsis due to drastic changes in systemic blood pressure.41 A number of human clinical studies support the premise of decreased cerebral blood flow (CBF) in acute sepsis.49–52 The abrupt change in blood pressure with added sepsis-associated coagulopathy causes reduced blood flow to neurons. The hippocampal region and watershed areas are affected more often than other brain regions when this occurs. Autopsies in patients who died from septic shock revealed consistent ischemic and hypoxic insults in areas particularly susceptible to low blood flow (eg, amygdala, frontal junctional cortex, etc) and in autonomic centers.53 Furthermore, the autopsies of 7 delirious ICU patients in another study revealed pathological lesions in the hippocampus, striatum, and pons triggered by ischemia or hypoxia.54 The presence of ischemia in post-mortem studies strongly suggest that vascular irregularities and alterations of CBF occur during sepsis. Importantly, multiple clinical observations support the concept that, in the absence of cerebrovascular occlusion (ie, stroke), impaired cerebral autoregulation and hypotension may be the primary drivers of tissue hypoxia and cerebral ischemia observed in sepsis patients.55–57
A recent study in rats conducted by Towner et al58 showed that CBF in the thalamus and cortex is significantly increased 24 hours post LPS injection but significantly reduced 6 weeks post LPS injection when compared with saline controls. Preceding human clinical studies also support the decrease in CBF at 24 hours observed by Towner and colleagues, yet there remains a paucity of literature on how sepsis may affect long-term CBF in human patients. Overall, coincident alterations in cerebral blood and cardiovascular collapse in sepsis emphasize the importance of fluid resuscitation as a crucial component of sepsis management. The ideal type of fluid (colloid vs crystalloids) and ideal composition used to treat septic patients remains controversial. A total of 3 clinical trials demonstrated that colloid use in sepsis treatment failed to show a clear benefit.59–61 In addition to the findings from this study, the restricted accessibility, safety issues, and the expensive value of colloids shifted the debate toward identifying the ideal crystalloid composition (eg, Ringer lactate, Ringer acetate, etc).62 We refer the reader to excellent reviews regarding optimal fluid therapeutic strategies in sepsis.62–64
The utilization of magnetic resonance imaging (MRI) in the diagnosis of SAE offers a unique opportunity in capturing some of the morphologic, ischemic, and metabolic alterations associated with sepsis. A summary of MRI findings in acute sepsis is shown in Table 1. In particular, diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) are 2 MRI modalities currently used in assessing BBB breakdown caused by vasogenic (extracellular) or cytotoxic (intracellular) edema.73 Cytotoxic edema typically caused by ischemia, hypoxia, or vasogenic edema is the most consistently reported MRI change associated with SAE.73–76 Early detection of BBB breakdown by gadolinium (Gd) could establish an adequate therapeutic window for current and future septic treatments, but human studies are limited.77–79 A recent study in rats revealed a significant increase in the infiltration of Gd in the cortex, hippocampus, and thalamus 24 hours and 1 week post LPS injection.58 Gd use may also cause a substantial risk of nephrogenic systemic fibrosis, a risk factor which suggests that Gd-based imaging in the CNS should be evaluated on a case-by-case basis.
Table 1.
MRI imaging studies in patients with sepsis.
| Publication | Subject | Acute MRI findings |
|---|---|---|
| Hollinger et al65 | Adult human | T2-weighted hypersensitivity with altered DWI showing ring enhancement |
| Sharshar et al66 | Adult human | Multiple ischemic strokes with predominant white matter lesions |
| Jackson et al67 | Adult human | Bilateral diffuse hyperintense areas in the white matter of the cortex and cerebellum |
| Kondo et al68 | Pediatric human | Restricted diffusion in the basal ganglia and subcortical white matter of the frontal and occipital lobes; brain edema was also present |
| Morandi et al69 | Adult human | White matter hyperintensities (WMHs) |
| Bozza et al70 | Rodent | Accumulation of the vasogenic edema fluid at the base of the brain |
| Suchyta et al71 | Adult human | Atrophy, WMH, edema, and localized bilateral hemorrhage in the cortex and subcortical structures |
| Polito et al57 | Adult human | Leukoencephalopathy and ischemic stroke |
| Luitse et al72 | Adult human | Acute abnormal hypointensity of the white matter and edema; subacute WMH |
| Towner et al58 | Rodent | Increased MRI intensities in the cortex, Perirhinal cortex, and hippocampus; increased gadolinium infiltration into various brain regions |
DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Sedatives are often administered in the ICU when treating sepsis. A study conducted by Qiao et al80 in rats showed that the application of dexmedetomidine and midazolam improved survival and reduced cytokine levels and splenic apoptosis in septic mice. Another systematic review by Zamani et al81 emphasized the importance of the kind of sedative used in treating sepsis; the findings from this study revealed that dexmedetomidine improved short-term mortality when compared with other sedatives. It is widely thought that the neuroprotective effects of dexmedetomidine result from neuronal death prevention, suppression of inflammatory cytokines, and modulation of neurotransmitters released in the sympathetic nervous system.81–84 However, a limitation noted by Zamani et al was the small sample size included in the clinical studies. It is important to note that the tools used in the confirmation of sepsis-associated delirium are not helpful in ICU-sedated patients who may otherwise exhibit signs of delirium.43
Sepsis also affects long-term neurological outcomes. The greatest risk factor for long-term impairment is the duration of delirium in the acute phase of sepsis and the increased ventricle-to-brain ratio as calculated by MRI.39,41,85,86 A seminal study published by Iwashyna et al87 suggested that up to 70% of sepsis survivors may exhibit lasting neurological impairment, including alterations in mood, cognition, and motor function. Cognitive, motor, and mood impairments are three of the most common long-term neurological outcomes in septic patients.41 Current evidence also suggests an increased susceptibility to other neurodegenerative disorders such as stroke or AD post sepsis insult.88 Thus, patient populations that are the most vulnerable to long-term neurologic decline post sepsis are the elderly and patients with preexisting neurodegenerative diseases.12,13,39,88 The consequences of sepsis on both acute and chronic neurological outcomes demonstrate a critical need to understand the mechanisms involved in SAE development. Harnessing this knowledge will provide essential therapeutic avenues to limit SAE progression and protect against long-term neurological impairment or dysfunction. The remainder of this review will focus on the role of the BBB in sepsis-associated cognitive dysfunction, as preclinical and clinical investigations have uncovered 3 primary BBB-linked mechanisms that contribute to the cause of SAE and the associated short-term and long-term cognitive dysfunction: (1) activation of neuroinflammation, (2) microcirculatory dysfunction, and (3) increased neuronal excitotoxicity.39,89
Mechanisms of BBB Dysfunction in Sepsis
BBB overview
This section will provide a brief overview of the cell biology and physiology of the BBB, as the focus of this review is the BBB in sepsis. BBB and other associated cell types within the neurovascular unit that are affected by sepsis are shown in Table 2.39,107 The BBB is a highly selective, dynamic, and semipermeable biological interface between the brain parenchyma and cerebral circulation. Preservation of BBB integrity protects normal brain function and is dependent on maintaining a precise cerebral homeostasis driven, in large part, by ion and gas concentrations and nutrient availability. The BBB’s unique structure is composed of endothelial cells, astrocytes, pericytes, and a basal lamina. The endothelial cells are joined by tight junctions (TJs), and surrounding pericytes and astrocytes, which associate with the basal lamina to protect the brain parenchyma. Endothelial cells are sealed or joined together by TJs which are composed of occludin, claudin, and cadherin proteins. Because paracellular movement of compounds around and between endothelial cells is highly restricted, active transport is required to move polar solutes and nutrients across the BBB into the brain parenchyma.108 However, water, small gases, and small- to moderate-sized lipid-soluble compounds can enter the brain passively.109,110 Primary efflux transporters include permeability glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which actively pump compounds out of the brain and back into circulation.108 Often, these transporters further restrict the permeability of compounds and drugs which may otherwise have the molecular characteristics to passively cross the BBB.111 Readers are referred to many excellent reviews on the BBB for a more in-depth discussion of these and other topics pertaining to the BBB.37,88,109,112,113
Table 2.
BBB cell types affected by sepsis and consequential outcomes.
| Cell types | Cellular pathology | Consequences | Refs |
|---|---|---|---|
| Astrocytes | Astrogliosis | Increased BBB permeability Neuroinflammation |
90–94 |
| Endothelial cells | Endothelial activation | Increased BBB permeability Microthrombus formation Ischemia |
95–97 |
| Microglia | Microgliosis | Increased iNOS expression Neuroinflammation Cytokine production |
98–100 |
| Pericytes | Unknown | Increased BBB permeability | 101,102 |
| Neurons | Excitotoxicity and neuronal dysfunction | Cognitive dysfunction | 87,103–106 |
BBB, blood-brain barrier.
Neuroinflammation and BBB permeability
A probable starting point of sepsis-induced acute brain dysfunction is the initiation of neuroinflammation, but the mechanism by which this occurs is not well understood.51 Neuroinflammation is a response to CNS disruption or dysfunction and is typically found in all neurological disorders.114 Current literature suggests that neuroinflammation in sepsis begins when immune cells recognize foreign pathogen-associated molecular patterns (PAMPs) such as LPS, flagellin, fimbriae, peptidoglycan, heat shock proteins, and DNA fragments, which are encoded as “danger signals” to the host. Recognition of PAMPs causes the release of proinflammatory cytokines in the periphery.115 Inflammatory mediators may enter the brain by numerous mechanisms that include transcellular diffusion, solute carrier proteins, receptor-mediated transcytosis, and adsorptive transcytosis.37 Many cytokines enter the brain through receptor-mediated endocytosis on brain endothelial cells. For example, during inflammation, TNF-α is upregulated and its transportation from blood to brain parenchyma is increased, primarily through receptor-mediated endocytosis of its receptors, tumor necrosis factor receptor 1 (TNFR1) and tumor necrosis factor receptor 2 (TNFR2).116,117 Molecules originating in peripheral or CNS tissues may activate vascular endothelium and various leukocytes to produce hormones that facilitate their entry into the brain. For example, Nishijima et al118 observed that prostaglandin E2 enhanced transport of serum-insulin-like growth factor 1 across the BBB.
Cytokine production contributes to neuronal dysfunction in sepsis in addition to many other neurological disorders. Cytokine infiltration enhances the activation of endothelial cells and microglia, which ultimately leads to loss of neuronal function. Activation of the endothelium leads to enhanced activity of the coagulation cascade, microthrombus formation, and ischemia, which, in turn, promotes increased BBB permeability and leukocyte infiltration. This process triggers neuronal damage, apoptosis, and brain edema.119,120 Cytokine-mediated microglial activation occurs simultaneously with endothelial cell activation. Although the normal microglial response is to phagocytose-injured neuronal cells and clear debris, sustained and dysregulated microglial activation is highly detrimental to specific regions of the CNS. Thus, persistent microglial activation enhances the production of inflammatory cytokines and reactive oxygen species (ROS), which perpetuates a vicious cycle of increased BBB permeability coupled with neuronal damage and apoptosis.116,119 Collectively, neuronal apoptosis and microglial activation are 2 primary mechanisms that increase the activity of inducible nitric oxide synthase (iNOS) activity and generation of nitric oxide (NO). Neuronal apoptosis is further exacerbated due to neuronal sensitivity from increased levels of NO produced by activated microglia.121,122 Intriguingly, iNOS levels are elevated in sepsis and are highest in deceased septic patients.70,100 This increased iNOS activity could also be responsible for the cardiovascular collapse seen in sepsis.53,100,117 It is likely that this cardiovascular collapse also affects the cerebral microcirculation and leads to subsequent sepsis-associated brain dysfunction.
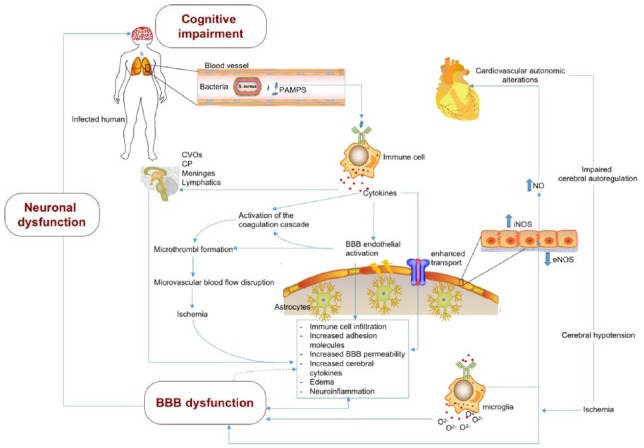
Sepsis, particularly Gram-negative sepsis, has been shown to upregulate caveolin-1 at the endothelial membrane.123 Increased caveolin-1 has recently been shown to increase the amount of peripheral immune infiltration into the brain.124 The mechanisms by which this occurs are not completely understood, but new preclinical studies have shed light on some prevailing theories. Wu et al125 found that caveolin-1 facilitates T-cell trafficking into the CNS via intercellular adhesion molecule 1 (ICAM-1)-mediated signaling. Caveolin-1 causes acid sphingomyelinase to interact with ICAM-1 increasing the binding affinity for peripheral immune cells.126 Once activated, ICAM-1 facilitates peripheral immune cell diapedesis into the brain. This process occurs via Src phosphorylation within endothelial cells and a subsequent conformational change to ICAM-1, which directly induces the transcellular migration.127 The leaky BBB enhances the entire process during sepsis. After entering the brain, T cells are recruited toward damaged glia via cytokine release. Recent evidence suggests that IL-17A aids this migration process.128 In addition, T cells are helped by astrocytes to re-cross the leaky BBB and carry information about the status of the brain to the rest of the body.129 It is postulated that the peripheral immune cells also release cytokines that maintain the leakiness of the BBB as they exit the brain. When and how long this cross-talk between microglia and peripheral immune cells persists remains to be elucidated. The feedback loop has, however, been implemented in non-autonomous neuronal death.130 The brain regions most susceptible are the nigra-striatal pathway and hippocampus.131 Future studies are warranted to further characterize the brain/immune communication network and, in particular, where the peripheral immune cells ultimately reside after exiting the brain. Collectively, these mechanisms represent the complicated and multifactorial mechanisms that must be involved in sepsis at the BBB. An integrated overview of the brain and peripheral mechanisms found in acute sepsis is shown in Figure 2.
Figure 2.
Complex neuroinflammatory processes promote BBB dysfunction in early sepsis which enhance neuronal dysfunction and trigger cognitive impairment. Highly complex and multifactorial mechanisms transduce systemic inflammatory signals to the brain in early sepsis, resulting in neuronal dysfunction and subsequent acute and chronic cognitive impairment. The BBB serves as both a nexus and an interface for these signals. Sepsis begins with systemic infection that evokes an exaggerated host immune response from the recognition of pathogen-associated molecular patterns (PAMPs).132 In this example, sepsis is triggered by a local lung infection (eg, pneumonia). Systemic proliferation of the infection initiates a hyperinflammatory response that stimulates the production of immune cells, cytokines, and other inflammatory mediators. These inflammatory mediators initiate a cascade of events that either directly or indirectly impact the peripheral microcirculation via cardiovascular autonomic alterations, or activation of the coagulation cascade, and converge on cerebral microvessels and their component BBB endothelial cells. Cerebral hypotension and microthrombus formation also initiate ischemia. The convergence of these events lead to BBB dysfunction, including immune cell infiltration, upregulation of adhesion molecules, increased BBB permeability, activation of cerebral cytokines, cerebral edema, and enhanced neuroinflammation.2,37,88,121 Positive feedback of the disrupted BBB on neuroinflammation and potential amplification of this feedback is indicated by the solid bidirectional arrow (direct feedback) and the dotted arrow (indirect feedback).
BBB, blood-brain barrier; CP, choroid plexus; CVOs, circumventricular organs; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
Mitochondrial dysfunction
Mitochondrial dysfunction is a common consequence of sepsis. It has been described in a number of studies with substantial evidence pointing toward oxidative stress as a contributing factor. This literature is summarized in several excellent reviews.133–136 The abnormality in the function of the mitochondria plays a role in the development of post-sepsis behavioral, psychological, and cognitive dysfunctions such as SAE.137–139 At the cellular level, reactive nitrogen species (RNS), like NO, and ROS, such as peroxynitrite (ONOO−), inhibit complexes I and IV of the electron transport chain (ETC). This inhibition produces a subsequent decrease in oxygen consumption and permits the buildup of O2− species and the eventual leakage of this species across the ETC along with other ROS/RNS.140–142 The leaked species activate uncoupling proteins that cause an increased H+ (proton) permeability from the inner mitochondria into the mitochondrial matrix to form oxide ions. Ultimately, these ions are converted to water without any adenosine triphosphate (ATP) generation.143 This process may seem to decrease the number of reactive species from the reaction described above, but the downside of continual proton leakage is an induction of cytopathic hypoxia, a condition whereby mitochondria are unable to use oxygen irrespective of the presence or absence of oxygen.144 In addition to cytopathic hypoxia, ONOO− causes single-strand DNA breaks at the genomic level; this further exacerbates mitochondrial dysfunction because of the high reliance of oxygen in the reparative process of the damaged DNA.145 Also, some ROS/RNS enhance both endoplasmic reticulum and mitochondrial membrane permeability, which permits the leakage of calcium and proapoptotic proteins into the cytoplasm.146–148
The course of infection, type and amount of ROS/RNS, and the brain regions where oxidative stress occurs are important when investigating the timing of oxidative damage leading to mitochondrial dysfunction. Recent studies in rats using thiobarbituric acid and protein carbonyls as markers of lipid and protein oxidation, respectively, have suggested that lipid peroxidation is consistent and widely distributed in the hippocampus, cerebellum, and cortex 6 hours post CLP, whereas oxidative damage due to protein oxidation was largely restricted to the hippocampus. Investigators also identified a concomitant imbalance in the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT). They found that the SOD activity increased in the first 6 hours post sepsis, whereas both SOD and CAT activity levels were decreased compared with sham-injured mice at 12 to 96 hours.138 Taken together, these findings suggest that oxidative damage in the CNS occurs much earlier than expected in sepsis. Expanding on these findings, Barichello et al used N-acetylcysteine (NAC) and deferoxamine (DFX) antioxidants as a therapeutic intervention in male rats at 6 hours post CLP. They found that the combined administration of NAC and DFX reduced oxidative hippocampal damage, but not when administered separately.149 These results further emphasize the importance of all CNS antioxidant systems and signify that multiple targets are required for adequate therapeutic efficacy in sepsis.
Overall, the systemic immune response in sepsis accelerates the increased generation of ROS/RNS, which, in turn, promotes lipid peroxidation in the cerebrovasculature and brain parenchyma. The continued assault from the periphery perpetuates a vicious cycle of ROS/RNS generation between the brain and the periphery. As the overproduction of ROS/RNS overwhelms the capacity of the antioxidant system, the end results manifest as neuroinflammation, ischemia, and increased BBB permeability. Most importantly, the vicious cycle promotes an impaired oxidative metabolism which persists throughout the duration of sepsis and likely continues after recovery.150 Thus, sustained production of ROS/RNS after recovery is hypothesized to be another mechanism that contributes to long-term neurological impairment post sepsis.
Putative role of tissue non-specific alkaline phosphatase at the BBB
The identification of unexplored membrane proteins may be key to better understanding the specific barrier functions of the BBB in disease states such as sepsis. In turn, this knowledge may provide novel therapeutic targets for intervention. One potential therapeutic target localized primarily to the surface of brain endothelial cells is the non-specific isoform of alkaline phosphatase (AP). The enzyme AP has been shown to play an integral role in the regulation of inflammation and can be found either as a soluble form in the peripheral circulation or as a membrane-bound form on brain endothelium, as well as numerous other cell types in the periphery. There are 4 isoforms of AP in humans encoded by 4 separate genes (gene names are in italics): intestinal alkaline phosphatase (IAP; ALPI), placental alkaline phosphatase (PLAP; ALPP), germinal alkaline phosphatase (GCAP; ALPPL2), and tissue non-specific alkaline phosphatase (TNAP; ALP).151,152 TNAP, also known as bone/liver/kidney AP, is the most abundant AP isoform in humans and rodents. TNAP is the only isoenzyme of AP detected in the human brain and has long been used as a marker of brain endothelium, although its presence has also been detected in neurons.153,154
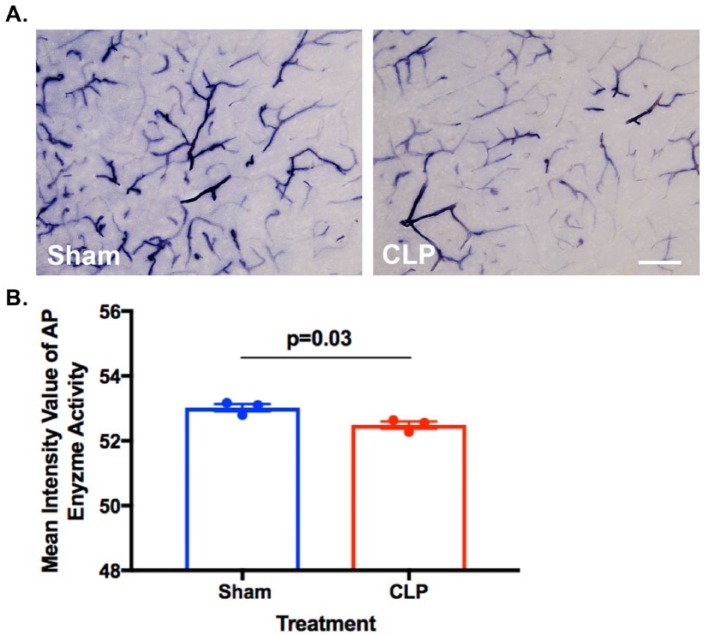
The cellular and molecular mechanisms underlying TNAP’s functional role in brain endothelium and BBB are unclear; however, results from numerous studies across several species strongly suggest that TNAP plays a role in the transport of specific classes of compounds across the BBB.155 Brain endothelial cell TNAP protein may also help facilitate cross-talk between the BBB and other cell types; in addition, a number of molecules, including cyclic adenosine monophosphate (cAMP) and IL-6, have been shown to modulate TNAP expression. Deracinois et al153 found that TNAP expression was increased in brain endothelial cells, and that the inhibition of AP activity using levamisole, a non-specific AP inhibitor, increased brain endothelial cell permeability. We speculate that TNAP’s regulatory phosphatase activity on a number of BBB endothelial proteins may play an important role in maintaining BBB integrity, thereby alleviating septic encephalopathy or long-term brain dysfunction. As shown in Figure 3 of our in vivo study, TNAP enzyme activity appears to be upregulated in CLP-injured mice compared with their sham-injured counterparts. However, the mechanistic function of TNAP in the BBB remains to be elucidated in sepsis and is currently being investigated in our lab.
Figure 3.
Alkaline phosphatase (AP) activity in the brain and BBB. (A) Histological staining for AP activity shows decreased TNAP enzyme activity in the cortex of septic male mice (10-15 months old) subjected to the cecal ligation and puncture (CLP) model of experimental sepsis. C57BL/6J mice were subjected to CLP or a sham injury and brains were harvested 24 hours later. (B) Graph shows the quantification of cortical AP enzyme activity in CLP (n = 3, 52.49 ± 0.1094) versus sham (n = 3, 53 ± 0.1142) mice (sections = 3 per mouse; data represented as mean ± SEM, *P < .05, t(4) = 3.384, unpaired Student’s t-test, scale bar = 115 µm). AP activity was assessed in 35-µm brain sections with the BCIP/NBT AP Substrate Kit (Vector Laboratories, Burlingame, CA) following previously published methods.156
BBB, blood-brain barrier; TNAP, tissue non-specific alkaline phosphatase.
Other brain-specific functions of TNAP have been described as having a role in proliferation and migration in the developing nervous system, control of axonal growth formation and maturation of synapse, and dephosphorylation of extracellular phospho-tau in AD.154,157–159 Despite the absence of a clearly elucidated mechanism for TNAP in brain endothelium and neurons, emerging data suggest that the manipulation of the AP activity can influence disease outcomes. For example, pretreatment of experimental autoimmune encephalomyelitis (EAE) mice with bovine intestinal AP reduced the disease severity through a mechanism that caused a reduction in neuroinflammation and autoreactive T regulatory cell proliferation.160 Increased levels of AP have also been detected in blood of epileptic patients, suggesting its applicability as a potential biomarker for many neurological diseases.161
Pharmaceutical Interventions in Sepsis
Antimicrobial delivery across the BBB
There is a current shift in the literature regarding specific pathogens that play a role in the inflammatory response associated with sepsis. Knowing the microbe implicated in sepsis best dictates which appropriate antibiotic or other type of treatment is required. Whereas older studies implicate Gram-negative bacteria necessitating antibiotic intervention in sepsis, newer studies have begun to reveal that other pathogens like Gram-positive bacteria, fungi, and viruses can also stimulate the inflammatory response associated with sepsis. According to recent epidemiologic studies, Gram-positive bacteria cause approximately 50 000 more cases of sepsis every year in the United States compared with Gram-negative bacteria.8,14,162 Because factors such as the type of inciting pathogen and the site of infection are good predictors of patient mortality, it is essential that the antimicrobial agent be used to effectively treat the disease as well as any subsequent side effects such as neurological impairment. Currently, this is often difficult and impractical in the hospital, as the time from diagnosis to initiation of treatment is critical. In addition, the sepsis field has faced many difficulties in developing effective therapeutics to treat sepsis. Currently, there are no Food and Drug Administration (FDA)-approved drugs used to treat sepsis as there have been numerous clinical trial failures over the past 15 years.163–166 This difficulty stems from our incomplete knowledge about the mechanisms that underlie the disease pathology associated with sepsis.
Initial suspicion of sepsis necessitates the use of non-specific broad-spectrum antibiotics against Gram-positive (eg, vancomycin) and Gram-negative (eg, imipenem) bacteria before blood cultures become available. Following pathogen identification, the initial antibiotic regimen is often narrowed to a single agent.45,167 Although numerous human and animal studies have shown an increase in survival following antibiotic administration, limited published data are available on whether or how antibiotics are able to penetrate the BBB. More importantly, the effects of antibiotics and other antimicrobials on brain function and sepsis-associated neurological impairment are not well studied.168–173 Thus, a complete knowledge of drug mechanisms in the CNS is essential for identifying an appropriate drug regimen and therapeutic approach to treat the neurological impairment associated with sepsis.174,175
The most common drug classes used to treat sepsis are shown in Table 3. Drugs belonging to the fluoroquinolone and sulfonamide classes, along with rifampin, metronidazole, and chloramphenicol, readily enter the brain regardless of disease state. Antimicrobials that do not normally penetrate the brain may readily cross into the BBB, or into the cerebrospinal fluid (CSF), due to opening of TJs and reduced P-gp activity.186 In contrast, more hydrophilic and larger drugs such as vancomycin and members of the β-lactam class of antibiotics do not readily enter the CSF or brain unless the meninges are inflamed.187
Table 3.
Antimicrobials used in sepsis treatment and their corresponding CNS penetration.
| Drug class | Example(s) | Lipophilicity | CNS penetration | Refs |
|---|---|---|---|---|
| β-lactam | Penicillin, piperacillin | ++ | ++ | 176,177 |
| Fluoroquinolones | Ciprofloxacin, moxifloxacin | +++ | +++ | 178 |
| Tetracyclines | Doxycycline | −− | 0 | 179 |
| Glycylcycline tigecycline | −− | ++ | 180 | |
| Glycopeptides | Vancomycin | −− | + | 181 |
| Anti-tuberculosis | Pyrazinamide, isoniazid | − | +++ | 182 |
| Anti-fungal | Amphotericin B | −− | ++ | 183 |
| Anti-parasitic | Pyrimethamine, albendazole | ++ | + | 184,185 |
CNS, central nervous system.
indicates higher, − indicates lower, and 0 indicates neutral or no change.
Intravenous immunoglobulin administration
Active advancement in understanding the pathophysiology of sepsis has led to the use of emerging immunomodulatory adjuvants to target septic encephalopathy. The acute phase of sepsis is embodied by a diminished production of immunoglobulin G (IgG) because the immune system takes 1 to 2 weeks to generate sufficient IgG levels needed to respond to an infection.188 Therefore, the observed reduction in IgG levels has warranted the use of immunomodulatory intravenous immunoglobulins (IVIgs) in sepsis patients.188,189 The mechanisms by which IVIg operates are complex and remain unclear.190,191 However, it has been proposed that the IVIg polyclonal IgG domains exert an immunomodulatory function by binding Fc receptors (FcγRs) found on many immune cell types (ie, microglia, endothelial, leukocyte). IVIg binding is thought to neutralize endotoxins/cytokines, inhibit complement activation, and block leukocyte adhesion molecule binding.90,192,193
Several studies have demonstrated the efficacy of IVIg treatment in sepsis. Esen et al193 showed that the administration of IVIg enriched with IgA and IgM improved BBB permeability, reduced sickness behavior, and improved mortality in CLP-induced rats. Further investigations by the same group revealed that the improvement in BBB integrity, neuronal destruction, and amelioration of septic encephalopathy is mediated by the inhibition of complement 5a (C5a).90 A small number of clinical meta-analytical studies have reported a decrease in mortality of sepsis patients administered IVIg; this finding complements the results observed by Esen et al. 193,194–196 In contrast, a larger double-blinded randomized control trial conducted in the International Neonatal Immunotherapy Study (INIS) showed no significant differences in mortality following IVIg administration.192,197 Note that the studies included in the meta-analysis showing a decrease in mortality after IVIg administration consisted of relatively small patient populations, which suggests that these studies may not have been sufficiently powered to detect meaningful differences in mortality.191,192,198,199
Taken together, the apparent effects of IVIg in sepsis treatment appear to be promising yet inconclusive. The high cost of treatment combined with unknown mechanism(s) of action and limited efficacy in a number of publications have made it difficult for organizations like the FDA and the Surviving Sepsis Campaign Guidelines (SSCG) to recommend IVIg as an adjuvant in sepsis treatment.188,190,192
Conclusions
The heterogeneous presentation and causes of sepsis are profoundly linked to its variable clinical outcomes. Chronic neurological impairment is an increasingly common yet poorly understood clinical outcome. Understanding the mechanistic determinants of BBB integrity during sepsis is critically important for sepsis diagnosis and implementation of treatment options to ensure a positive prognosis. Importantly, long-term prognosis in sepsis survivors is linked to both transient and permanent alterations in BBB permeability and function. Thus, targeting the BBB should be incorporated as part of a short- and long-term therapeutic strategy in all sepsis patients. The development of therapies that inhibit BBB dysfunction and stimulate normal BBB function will limit mortality, suppress neuroinflammation, and improve neurological outcomes in sepsis survivors. Equally important for effective sepsis treatment is a better understanding of how antimicrobials and other drugs (eg, IVIgs or vasopressors) used in treating sepsis readily cross the BBB, and whether there are any additional unknown impacts on brain function. Taken together, the identification of cellular and molecular mechanisms that preserve BBB function in the face of sepsis will provide valuable therapeutic targets to treat numerous inflammatory disorders that target both the brain and the periphery—ranging from AD and stroke to diabetes and cardiovascular disease.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: DCN and CMB contributed to manuscript design, compilation of manuscript, and creation of figures and tables. ALB wrote section on tissue non-specific alkaline phosphatase (TNAP). ASM contributed to the table on antimicrobials used in sepsis. JG contributed to the section on BBB overview. BPL-W contributed to the section on neuroinflammation and BBB permeability. SAB performed tissue alkaline phosphatase (TNAP) enzyme stain and image analysis. WJG contributed to the section on neuroinflammation and BBB permeability. PRL contributed to section on pharmacological intervention in sepsis. All authors reviewed the final documents and provided comments.
ORCID iD: Candice M Brown  https://orcid.org/0000-0001-5845-0221
https://orcid.org/0000-0001-5845-0221
References
- 1. Martin AB, Hartman M, Benson J, Catlin A; National Health Expenditure Accounts Team. National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood). 2016;35:150–160. [DOI] [PubMed] [Google Scholar]
- 2. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557–566. [DOI] [PubMed] [Google Scholar]
- 3. Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. [DOI] [PubMed] [Google Scholar]
- 4. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laszlo I, Trasy D, Molnar Z, Fazakas J. Sepsis: from pathophysiology to individualized patient care. J Immunol Res. 2015;2015:510436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 7. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahapatra S, Heffner AC. Shock, Septic (Sepsis). Treasure Island, FL: StatPearls; 2018. [Google Scholar]
- 10. Macdonald SP, Williams JM, Shetty A, et al. Review article: sepsis in the emergency department—part 1: definitions and outcomes. Emerg Med Australas. 2017;29:619–625. [DOI] [PubMed] [Google Scholar]
- 11. Morr M, Lukasz A, Rubig E, Pavenstadt H, Kumpers P. Sepsis recognition in the emergency department—impact on quality of care and outcome? BMC Emerg Med. 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischmann C, Thomas-Rueddel DO, Hartmann M, et al. Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int. 2016;113:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 15. Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kingsley SM, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep. 2016;18:26. [DOI] [PubMed] [Google Scholar]
- 17. Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22–30. [DOI] [PubMed] [Google Scholar]
- 18. Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. [DOI] [PubMed] [Google Scholar]
- 19. Zantl N, Uebe A, Neumann B, et al. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Traeger T, Koerner P, Kessler W, et al. Colon ascendens stent peritonitis (CASP)—a standardized model for polymicrobial abdominal sepsis. J Vis Exp. 2010;46:2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michie HR, Manogue KR, Spriggs DR, et al. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. [DOI] [PubMed] [Google Scholar]
- 23. Wyler F, Neutze JM, Rudolph AM. Effects of endotoxin on distribution of cardiac output in unanesthetized rabbits. Am J Physiol. 1970;219:246–251. [DOI] [PubMed] [Google Scholar]
- 24. Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49:186–196. [DOI] [PubMed] [Google Scholar]
- 25. Fink MP, Fiallo V, Stein KL, Gardiner WM. Systemic and regional hemodynamic changes after intraperitoneal endotoxin in rabbits: development of a new model of the clinical syndrome of hyperdynamic sepsis. Circ Shock. 1987;22:73–81. [PubMed] [Google Scholar]
- 26. Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. [DOI] [PubMed] [Google Scholar]
- 27. Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–475. [DOI] [PubMed] [Google Scholar]
- 28. Barichello T, Sayana P, Giridharan VV, et al. Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol. 2019;56:186–251. [DOI] [PubMed] [Google Scholar]
- 29. Polat G, Ugan RA, Cadirci E, Halici Z. Sepsis and septic shock: current treatment strategies and new approaches. Eurasian J Med. 2017;49:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirsten TB, Galvao MC, Reis-Silva TM, Queiroz-Hazarbassanov N, Bernardi MM. Zinc prevents sickness behavior induced by lipopolysaccharides after a stress challenge in rats. PLoS ONE. 2015;10:e0120263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kessler W, Diedrich S, Menges P, et al. The role of the vagus nerve: modulation of the inflammatory reaction in murine polymicrobial sepsis. Mediators Inflamm. 2012;2012:467620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitsui T, Fukatsu K, Yanagawa M, et al. Truncal vagotomy temporarily decreases the pro- and anti-inflammatory cytokine levels in the small intestine. Surg Today. 2014;44:1123–1127. [DOI] [PubMed] [Google Scholar]
- 34. Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. [DOI] [PubMed] [Google Scholar]
- 35. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 36. Li N, Li Z, Xiang H, Wang X, Zhang X, Li J. Protective effects of vagus nerve stimulation on rats with sepsis-associated encephalopathy. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:509–513. [PubMed] [Google Scholar]
- 37. Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev. 2018;70:278–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quan N. In-depth conversation: spectrum and kinetics of neuroimmune afferent pathways. Brain Behav Immun. 2014;40:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mazeraud A, Pascal Q, Verdonk F, Heming N, Chretien F, Sharshar T. Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med. 2016;37:333–345. [DOI] [PubMed] [Google Scholar]
- 40. Reyes EP, Abarzua S, Martin A, Rodriguez J, Cortes PP, Fernandez R. LPS-induced c-Fos activation in NTS neurons and plasmatic cortisol increases in septic rats are suppressed by bilateral carotid chemodenervation. Adv Exp Med Biol. 2012;758:185–190. [DOI] [PubMed] [Google Scholar]
- 41. Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3:61–69. [DOI] [PubMed] [Google Scholar]
- 42. Pun BT, Dunn J. The sedation of critically ill adults: part 1: assessment. The first in a two-part series focuses on assessing sedated patients in the ICU. Am J Nurs. 2007;107:40–48; quiz 49. [DOI] [PubMed] [Google Scholar]
- 43. Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22:283–287. [DOI] [PubMed] [Google Scholar]
- 44. Hosokawa K, Gaspard N, Su F, Oddo M, Vincent JL, Taccone FS. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: a systematic review. Crit Care. 2014;18:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaudhry N, Duggal AK. Sepsis associated encephalopathy. Adv Med. 2014;2014:762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med. 2015;41:686–694. [DOI] [PubMed] [Google Scholar]
- 47. Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84:62–69. [DOI] [PubMed] [Google Scholar]
- 48. Piazza O, Russo E, Cotena S, Esposito G, Tufano R. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99:518–521. [DOI] [PubMed] [Google Scholar]
- 49. Sinclair JF, Balakrishnan G, Skeoch CH, Hallworth D. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit Care Med. 1990;18:684. [DOI] [PubMed] [Google Scholar]
- 50. Brassard P, Kim YS, van Lieshout J, Secher NH, Rosenmeier JB. Endotoxemia reduces cerebral perfusion but enhances dynamic cerebrovascular autoregulation at reduced arterial carbon dioxide tension. Crit Care Med. 2012;40:1873–1878. [DOI] [PubMed] [Google Scholar]
- 51. Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taccone FS, Su F, Pierrakos C, et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sharshar T, Annane D, de la Grandmaison GL, Brouland JP, Hopkinson NS, Francoise G. The neuropathology of septic shock. Brain Pathol. 2004;14:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janz DR, Abel TW, Jackson JC, Gunther ML, Heckers S, Ely EW. Brain autopsy findings in intensive care unit patients previously suffering from delirium: a pilot study. J Crit Care. 2010;25:538.e7-538.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crippa IA, Subira C, Vincent JL, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care. 2018;22:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goodson CM, Rosenblatt K, Rivera-Lara L, Nyquist P, Hogue CW. Cerebral blood flow autoregulation in sepsis for the intensivist: why its monitoring may be the future of individualized care. J Intensive Care Med. 2018;33:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Polito A, Eischwald F, Maho AL, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Towner RA, Saunders D, Smith N, et al. Assessing long-term neuroinflammatory responses to encephalopathy using MRI approaches in a rat endotoxemia model. Geroscience. 2018;40:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 60. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. [DOI] [PubMed] [Google Scholar]
- 61. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 62. Avila AA, Kinberg EC, Sherwin NK, Taylor RD. The use of fluids in sepsis. Cureus. 2016;8:e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Semler MW, Rice TW. Sepsis resuscitation: fluid choice and dose. Clin Chest Med. 2016;37:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vincent JL, Gottin L. Type of fluid in severe sepsis and septic shock. Minerva Anestesiol. 2011;77:1190–1196. [PubMed] [Google Scholar]
- 65. Hollinger P, Zurcher R, Schroth G, Mattle HP. Diffusion magnetic resonance imaging findings in cerebritis and brain abscesses in a patient with septic encephalopathy. J Neurol. 2000;247:232–234. [DOI] [PubMed] [Google Scholar]
- 66. Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. [DOI] [PubMed] [Google Scholar]
- 67. Jackson JC, Hopkins RO, Miller RR, Gordon SM, Wheeler AP, Ely EW. Acute respiratory distress syndrome, sepsis, and cognitive decline: a review and case study. South Med J. 2009;102:1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kondo A, Sugiura C, Fujii Y, Inoue T, Maegaki Y, Ohno K. Fulminant sepsis-associated encephalopathy in two children: serial neuroimaging findings and clinical course. Neuropediatrics. 2009;40:157–161. [DOI] [PubMed] [Google Scholar]
- 69. Morandi A, Gunther ML, Vasilevskis EE, et al. Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont). 2010;7:28–33. [PMC free article] [PubMed] [Google Scholar]
- 70. Bozza FA, Garteiser P, Oliveira MF, et al. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suchyta MR, Jephson A, Hopkins RO. Neurologic changes during critical illness: brain imaging findings and neurobehavioral outcomes. Brain Imaging Behav. 2010;4:22–34. [DOI] [PubMed] [Google Scholar]
- 72. Luitse MJ, van Asch CJ, Klijn CJ. Deep coma and diffuse white matter abnormalities caused by sepsis-associated encephalopathy. Lancet. 2013;381:2222. [DOI] [PubMed] [Google Scholar]
- 73. Stubbs DJ, Yamamoto AK, Menon DK. Imaging in sepsis-associated encephalopathy—insights and opportunities. Nat Rev Neurol. 2013;9:551–561. [DOI] [PubMed] [Google Scholar]
- 74. Abe S, Okumura A, Fujii T, et al. Sepsis associated encephalopathy in an infant with biliary atresia. Brain Dev. 2008;30:544–547. [DOI] [PubMed] [Google Scholar]
- 75. Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res. 2009;34:1289–1292. [DOI] [PubMed] [Google Scholar]
- 76. Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–2190. [PMC free article] [PubMed] [Google Scholar]
- 77. Bhaskaran A, Kashyap P, Kelly B, Ghera P. Nephrogenic systemic fibrosis following acute kidney injury and exposure to gadolinium. Indian J Med Sci. 2010;64:33–36. [PubMed] [Google Scholar]
- 78. Schlaudecker JD, Bernheisel CR. Gadolinium-associated nephrogenic systemic fibrosis. Am Fam Physician. 2009;80:711–714. [PubMed] [Google Scholar]
- 79. Chien CC, Wang HY, Wang JJ, et al. Risk of acute kidney injury after exposure to gadolinium-based contrast in patients with renal impairment. Ren Fail. 2011;33:758–764. [DOI] [PubMed] [Google Scholar]
- 80. Qiao H, Sanders RD, Ma D, Wu X, Maze M. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care. 2009;13:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zamani MM, Keshavarz-Fathi M, Fakhri-Bafghi MS, et al. Survival benefits of dexmedetomidine used for sedating septic patients in intensive care setting: a systematic review. J Crit Care. 2016;32:93–100. [DOI] [PubMed] [Google Scholar]
- 82. Ma D, Hossain M, Rajakumaraswamy N, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. [DOI] [PubMed] [Google Scholar]
- 83. Maes M, Lin A, Kenis G, Egyed B, Bosmans E. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res. 2000;96:245–253. [DOI] [PubMed] [Google Scholar]
- 84. Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–1326. [DOI] [PubMed] [Google Scholar]
- 85. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 89. Tauber SC, Eiffert H, Bruck W, Nau R. Septic encephalopathy and septic encephalitis. Expert Rev Anti Infect Ther. 2017;15:121–132. [DOI] [PubMed] [Google Scholar]
- 90. Esen F, Orhun G, Ozcan PE, et al. Neuroprotective effects of intravenous immunoglobulin are mediated through inhibition of complement activation and apoptosis in a rat model of sepsis. Intensive Care Med Exp. 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. [DOI] [PubMed] [Google Scholar]
- 92. Cardoso FL, Herz J, Fernandes A, et al. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation. 2015;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Catalao CHR, Santos-Junior NN, da Costa LHA, Souza AO, Alberici LC, Rocha MJA. Brain oxidative stress during experimental sepsis is attenuated by simvastatin administration. Mol Neurobiol. 2017;54:7008–7018. [DOI] [PubMed] [Google Scholar]
- 95. Skibsted S, Jones AE, Puskarich MA, et al. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gando S, Kameue T, Matsuda N, Hayakawa M, Hoshino H, Kato H. Serial changes in neutrophil-endothelial activation markers during the course of sepsis associated with disseminated intravascular coagulation. Thromb Res. 2005;116:91–100. [DOI] [PubMed] [Google Scholar]
- 97. Reis PA, Estato V, da Silva TI, et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog. 2012;8:e1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zrzavy T, Hoftberger R, Berger T, et al. Pro-inflammatory activation of microglia in the brain of patients with sepsis [published online ahead of print May 27, 2018]. Neuropathol Appl Neurobiol. doi: 10.1111/nan.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Singer BH, Newstead MW, Zeng X, et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS ONE. 2016;11:e0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharshar T, Gray F, Lorin de, la Grandmaison G, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–1805. [DOI] [PubMed] [Google Scholar]
- 101. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. [DOI] [PubMed] [Google Scholar]
- 102. Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2alpha/Notch3 pathways. Sci Rep. 2016;6:20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fang J, Lian Y, Xie K, Cai S, Wen P. Epigenetic modulation of neuronal apoptosis and cognitive functions in sepsis-associated encephalopathy. Neurol Sci. 2014;35:283–288. [DOI] [PubMed] [Google Scholar]
- 104. Heming N, Mazeraud A, Verdonk F, Bozza FA, Chretien F, Sharshar T. Neuroanatomy of sepsis-associated encephalopathy. Crit Care. 2017;21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wei H, Cao X, Zeng Q, et al. Ghrelin inhibits proinflammatory responses and prevents cognitive impairment in septic rats. Crit Care Med. 2015;43:e143–e150. [DOI] [PubMed] [Google Scholar]
- 106. Messaris E, Memos N, Chatzigianni E, et al. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med. 2004;32:1764–1770. [DOI] [PubMed] [Google Scholar]
- 107. De Luca C, Colangelo AM, Alberghina L, Papa M. Neuro-immune hemostasis: homeostasis and diseases in the central nervous system. Front Cell Neurosci. 2018;12:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. [DOI] [PubMed] [Google Scholar]
- 109. Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. [DOI] [PubMed] [Google Scholar]
- 110. Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR. Molecular determinants of blood-brain barrier permeation. Ther Deliv. 2015;6:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Adkins CE, Mittapalli RK, Manda VK, et al. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front Pharmacol. 2013;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282:4067–4079. [DOI] [PubMed] [Google Scholar]
- 114. Ransohoff RM, Schafer D, Vincent A, Blachere NE, Bar-Or A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics. 2015;12:896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005;365:63–78. [DOI] [PubMed] [Google Scholar]
- 116. Akrout N, Sharshar T, Annane D. Mechanisms of brain signaling during sepsis. Curr Neuropharmacol. 2009;7:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pan W, Kastin AJ. TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. [DOI] [PubMed] [Google Scholar]
- 118. Nishijima T, Piriz J, Duflot S, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. [DOI] [PubMed] [Google Scholar]
- 119. Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what’s the cause of all this confusion? Curr Opin Crit Care. 2012;18:518–526. [DOI] [PubMed] [Google Scholar]
- 120. Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heneka MT, Loschmann PA, Gleichmann M, et al. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-alpha/lipopolysaccharide. J Neurochem. 1998;71:88–94. [DOI] [PubMed] [Google Scholar]
- 122. Leist M, Volbracht C, Kuhnle S, Fava E, Ferrando-May E, Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med. 1997;3:750–764. [PMC free article] [PubMed] [Google Scholar]
- 123. Sowa G. Role of caveolin proteins in sepsis. Pediatr Ther. 2012;2012:001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang X, Ren X, Wang Y, et al. Traumatic brain injury research and expression of caveolin-1 and its relationship with disease prognosis. Pak J Pharm Sci. 2017;30:997–1000. [PubMed] [Google Scholar]
- 125. Wu H, Deng R, Chen X, et al. Caveolin-1 is critical for lymphocyte trafficking into central nervous system during experimental autoimmune encephalomyelitis. J Neurosci. 2016;36:5193–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lopes Pinheiro MA, Kroon J, Hoogenboezem M, et al. Acid sphingomyelinase-derived ceramide regulates ICAM-1 function during T cell transmigration across brain endothelial cells. J Immunol. 2016;196:72–79. [DOI] [PubMed] [Google Scholar]
- 127. Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. [DOI] [PubMed] [Google Scholar]
- 128. Zimmermann J, Krauthausen M, Hofer MJ, Heneka MT, Campbell IL, Muller M. CNS-targeted production of IL-17A induces glial activation, microvascular pathology and enhances the neuroinflammatory response to systemic endotoxemia. PLoS ONE. 2013;8:e57307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xie L, Yang SH. Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res. 2015;1623:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Crisafulli SG, Brajkovic S, Cipolat Mis MS, Parente V, Corti S. Therapeutic strategies under development targeting inflammatory mechanisms in amyotrophic lateral sclerosis. Mol Neurobiol. 2018;55:2789–2813. [DOI] [PubMed] [Google Scholar]
- 131. Ambrosi G, Kustrimovic N, Siani F, et al. Complex changes in the innate and adaptive immunity accompany progressive degeneration of the nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine in the rat. Neurotox Res. 2017;32:71–81. [DOI] [PubMed] [Google Scholar]
- 132. Rajaee A, Barnett R, Cheadle WG. Pathogen- and danger-associated molecular patterns and the cytokine response in sepsis. Surg Infect (Larchmt). 2018;19:107–116. [DOI] [PubMed] [Google Scholar]
- 133. Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. [DOI] [PubMed] [Google Scholar]
- 135. Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev. 2017;2017:5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bar-Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: are there clues to better treatment? Redox Report. 2015;20:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hopkins RO. Sepsis, oxidative stress, and brain injury. Crit Care Med. 2007;35:2233–2234. [DOI] [PubMed] [Google Scholar]
- 138. Barichello T, Fortunato JJ, Vitali AM, et al. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34:886–889. [DOI] [PubMed] [Google Scholar]
- 139. Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. 2000;28:3019–3024. [DOI] [PubMed] [Google Scholar]
- 140. Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Brookes PS, Bolanos JP, Heales SJ. The assumption that nitric oxide inhibits mitochondrial ATP synthesis is correct. FEBS Lett. 1999;446:261–263. [DOI] [PubMed] [Google Scholar]
- 142. Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. [DOI] [PubMed] [Google Scholar]
- 143. d’Avila JC, Santiago AP, Amancio RT, Galina A, Oliveira MF, Bozza FA. Sepsis induces brain mitochondrial dysfunction. Crit Care Med. 2008;36:1925–1932. [DOI] [PubMed] [Google Scholar]
- 144. Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219–237. [DOI] [PubMed] [Google Scholar]
- 145. Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. [DOI] [PubMed] [Google Scholar]
- 146. Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. [DOI] [PubMed] [Google Scholar]
- 147. Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. [DOI] [PubMed] [Google Scholar]
- 148. Zhan RZ, Fujiwara N, Shimoji K. Regionally different elevation of intracellular free calcium in hippocampus of septic rat brain. Shock. 1996;6:293–297. [DOI] [PubMed] [Google Scholar]
- 149. Barichello T, Machado RA, Constantino L, et al. Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med. 2007;35:2186–2190. [DOI] [PubMed] [Google Scholar]
- 150. Berg RM, Moller K, Bailey DM. Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab. 2011;31:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pettengill M, Matute JD, Tresenriter M, et al. Human alkaline phosphatase dephosphorylates microbial products and is elevated in preterm neonates with a history of late-onset sepsis. PLoS ONE. 2017;12:e0175936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Buchet R, Millan JL, Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol Biol. 2013;1053:27–51. [DOI] [PubMed] [Google Scholar]
- 153. Deracinois B, Duban-Deweer S, Pottiez G, Cecchelli R, Karamanos Y, Flahaut C. TNAP and EHD1 are over-expressed in bovine brain capillary endothelial cells after the re-induction of blood-brain barrier properties. PLoS ONE. 2012;7:e48428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Brun-Heath I, Ermonval M, Chabrol E, et al. Differential expression of the bone and the liver tissue non-specific alkaline phosphatase isoforms in brain tissues. Cell Tissue Res. 2011;343:521–536. [DOI] [PubMed] [Google Scholar]
- 155. Deracinois B, Lenfant AM, Dehouck MP, Flahaut C. Tissue non-specific alkaline phosphatase (TNAP) in vessels of the brain. Subcell Biochem. 2015;76:125–151. [DOI] [PubMed] [Google Scholar]
- 156. De Jong AS, Van Kessel-van Vark M, Raap AK. Sensitivity of various visualization methods for peroxidase and alkaline phosphatase activity in immunoenzyme histochemistry. Histochem J. 1985;17:1119–1130. [DOI] [PubMed] [Google Scholar]
- 157. Hanics J, Barna J, Xiao J, Millan JL, Fonta C, Negyessy L. Ablation of TNAP function compromises myelination and synaptogenesis in the mouse brain. Cell Tissue Res. 2012;349:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Diez-Zaera M, Diaz-Hernandez JI, Hernandez-Alvarez E, Zimmermann H, Diaz-Hernandez M, Miras-Portugal MT. Tissue-nonspecific alkaline phosphatase promotes axonal growth of hippocampal neurons. Mol Biol Cell. 2011;22:1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Narisawa S, Hasegawa H, Watanabe K, Millan JL. Stage-specific expression of alkaline phosphatase during neural development in the mouse. Dev Dyn. 1994;201:227–235. [DOI] [PubMed] [Google Scholar]
- 160. Huizinga R, Kreft KL, Onderwater S, et al. Endotoxin- and ATP-neutralizing activity of alkaline phosphatase as a strategy to limit neuroinflammation. J Neuroinflammation. 2012;9:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Razazizan N, Mirmoeini M, Daeichin S, Ghadiri K. Comparison of 25-hydroxy vitamin D, calcium and alkaline phosphatase levels in epileptic and non-epileptic children. Acta Neurol Taiwan. 2013;22:112–116. [PubMed] [Google Scholar]
- 162. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wang X, Li S. Effect of small-dose levosimendan on mortality rates and organ functions in Chinese elderly patients with sepsis. Clin Interv Aging. 2017;12:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA. 2017;317:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Vincent JL, Marshall JC, Dellinger RP, et al. Talactoferrin in severe sepsis: results from the phase II/III oral talactoferrin in severe sepsis trial. Crit Care Med. 2015;43:1832–1838. [DOI] [PubMed] [Google Scholar]
- 167. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. [DOI] [PubMed] [Google Scholar]
- 168. Kim RY, Ng AM, Persaud AK, et al. Antibiotic timing and outcomes in sepsis. Am J Med Sci. 2018;355:524–529. [DOI] [PubMed] [Google Scholar]
- 169. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. [DOI] [PubMed] [Google Scholar]
- 170. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Coopersmith CM, Amiot DM, 2nd, Stromberg PE, et al. Antibiotics improve survival and alter the inflammatory profile in a murine model of sepsis from Pseudomonas aeruginosa pneumonia. Shock. 2003;19:408–414. [DOI] [PubMed] [Google Scholar]
- 172. Choudhury S, Kannan K, Pule Addison M, et al. Combined treatment with atorvastatin and imipenem improves survival and vascular functions in mouse model of sepsis. Vascul Pharmacol. 2015;71:139–150. [DOI] [PubMed] [Google Scholar]
- 173. Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1048–R1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203. [DOI] [PubMed] [Google Scholar]
- 175. Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41:1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Dickinson GM, Droller DG, Greenman RL, Hoffman TA. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob Agents Chemother. 1981;20:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Karlsson M, Hammers-Berggren S, Lindquist L, Stiernstedt G, Svenungsson B. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology. 1994;44:1203–1207. [DOI] [PubMed] [Google Scholar]
- 178. Alffenaar JW, de Vries PM, Luijckx GJ, van Soolingen D, van der Werf TS, van Altena R. Plasma and cerebrospinal fluid pharmacokinetics of moxifloxacin in a patient with tuberculous meningitis. Antimicrob Agents Chemother. 2008;52:2293–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yim CW, Flynn NM, Fitzgerald FT. Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis. Antimicrob Agents Chemother. 1985;28:347–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Lengerke C, Haap M, Mayer F, et al. Low tigecycline concentrations in the cerebrospinal fluid of a neutropenic patient with inflamed meninges. Antimicrob Agents Chemother. 2011;55:449–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Albanese J, Leone M, Bruguerolle B, Ayem ML, Lacarelle B, Martin C. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob Agents Chemother. 2000;44:1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ellard GA, Humphries MJ, Allen BW. Cerebrospinal fluid drug concentrations and the treatment of tuberculous meningitis. Am Rev Respir Dis. 1993;148:650–655. [DOI] [PubMed] [Google Scholar]
- 183. Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol. 2007;3:573–581. [DOI] [PubMed] [Google Scholar]
- 184. McLeod R, Mack D, Foss R, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Toxoplasmosis Study Group. Antimicrob Agents Chemother. 1992;36:1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Takayanagui OM, Bonato PS, Dreossi SA, Lanchote VL. Enantioselective distribution of albendazole metabolites in cerebrospinal fluid of patients with neurocysticercosis. Br J Clin Pharmacol. 2002;54:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. [DOI] [PubMed] [Google Scholar]
- 187. Shin SH, Kim KS. Treatment of bacterial meningitis: an update. Expert Opin Pharmacother. 2012;13:2189–2206. [DOI] [PubMed] [Google Scholar]
- 188. Hamano N, Nishi K, Onose A, et al. Efficacy of single-dose intravenous immunoglobulin administration for severe sepsis and septic shock. J Intensive Care. 2013;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. [DOI] [PubMed] [Google Scholar]
- 190. Soares MO, Welton NJ, Harrison DA, et al. Intravenous immunoglobulin for severe sepsis and septic shock: clinical effectiveness, cost-effectiveness and value of a further randomised controlled trial. Crit Care. 2014;18:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hartung HP, Mouthon L, Ahmed R, Jordan S, Laupland KB, Jolles S. Clinical applications of intravenous immunoglobulins (IVIg)—beyond immunodeficiencies and neurology. Clin Exp Immunol. 2009;158:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Di Rosa R, Pietrosanti M, Luzi G, Salemi S, D’Amelio R. Polyclonal intravenous immunoglobulin: an important additional strategy in sepsis? Eur J Intern Med. 2014;25:511–516. [DOI] [PubMed] [Google Scholar]
- 193. Esen F, Senturk E, Ozcan PE, et al. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med. 2012;40:1214–1220. [DOI] [PubMed] [Google Scholar]
- 194. Pildal J, Gotzsche PC. Polyclonal immunoglobulin for treatment of bacterial sepsis: a systematic review. Clin Infect Dis. 2004;39:38–46. [DOI] [PubMed] [Google Scholar]
- 195. Alejandria MM, Lansang MA, Dans LF, Mantaring JB., 3rd Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013;9:CD001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35:2677–2685. [PubMed] [Google Scholar]
- 197. Darenberg J, Ihendyane N, Sjolin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:333–340. [DOI] [PubMed] [Google Scholar]
- 198. Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med. 2007;35:2686–2692. [PubMed] [Google Scholar]
- 199. Hentrich M, Fehnle K, Ostermann H, et al. IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med. 2006;34:1319–1325. [DOI] [PubMed] [Google Scholar]