Abstract
BACKGROUND & AIMS:
Patients with nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) often require histologic assessment via liver biopsy. Magnetic resonance imaging (MRI)-based methods for measuring liver triglycerides based on proton density fat fraction (PDFF) are increasingly used as a noninvasive tool to identify patients with hepatic steatosis and to assess for change in liver fat over time. We aimed to determine whether MRI-PDFF accurately reflects a variety of liver histology features in patients with NAFLD or NASH.
METHODS:
We performed a retrospective analysis of pooled data from 3 phase 2a trials of pharmacotherapies for NAFLD or NASH. We collected baseline clinical, laboratory, and histopathology data on all subjects who had undergone MRI analysis in 1 of the trials. We assessed the relationship between liver PDFF values and liver histologic findings using correlation and area under the receiver operating characteristic (AUROC) analyses. As an ancillary analysis, we also simulated a clinical trial selection process and calculated subject exclusion rates and differences in population characteristics caused by PDFF inclusion thresholds of 6% to 15%.
RESULTS:
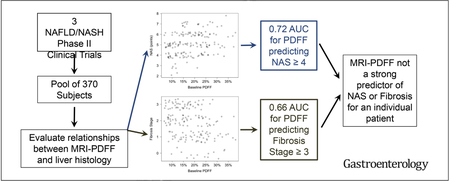
In 370 subjects, the mean baseline PDFF was 17.4% ± 8.6%. Baseline PDFF values correlated with several histopathology parameters, including steatosis grade (r = 0.78; P < .001), NAFLD activity score (NAS, r = 0.54; P < .001), and fibrosis stage (r = −0.59; P < .001). However, PDFF did not accurately identify patients with NAS ≥ 4 (AUROC = 0.72) or fibrosis stage ≥3 (AUROC = 0.66). In a theoretical trial of these subjects, exclusion rates increased as PDFF minimum threshold level increased. There were no significant differences in cohort demographics when PDFF thresholds ranging from 6% to 15% were used, and differences in laboratory and histopathology data were small.
CONCLUSIONS:
In an analysis of patients with NAFLD or NASH, we determined that although The MRI-PDFF correlated with steatosis grade and NAS, and inversely with fibrosis stage, it was suboptimal in identification of patients with NAS >4 or advanced fibrosis. Although MRI-PDFF is an important imaging biomarker for continued evaluation of this patient population, liver biopsy analysis is still necessary.
Keywords: Risk, Prognostic Factor, Diagnostic, Quantification
Graphical Abstract
Nonalcoholic fatty liver disease (NAFLD) affects approximately 20% of the general population and has emerged as a leading cause of chronic liver disease in the United States.1 Nonalcoholic steatohepatitis (NASH), characterized by hepatic steatosis with features of necroinflammation and ballooned hepatocytes on liver biopsy, represents the more severe phenotype of NAFLD and can progress to long-term hepatic dysfunction, cirrhosis, and hepatocellular carcinoma.2–5 NASH afflicts approximately 3% to 12% of the US population. Given its prevalence and association with progressive NAFLD, accurate, safe, noninvasive methods for patient evaluation are urgently needed.
Currently, percutaneous liver biopsy remains the gold standard for assessing NASH. However, invasive procedures carry associated risks and costs, and judicious use of biopsy means performing it only when strictly necessary.6 To reduce the need for biopsy, various imaging biomarkers have emerged as potential noninvasive diagnostic tools for diagnosis and monitoring of patients with NASH. In particular, magnetic resonance imaging (MRI)-based methods for measuring liver triglyceride content in terms of the proton density fat fraction (PDFF) have been shown to be accurate and reproducible in quantifying hepatic steatosis.7–10 In fact, based in part on observations that changes in hepatic steatosis may be correlated with changes in other histological endpoints,11,12 PDFF is now being used in some early-phase clinical trials both for population enrichment before biopsy and as a primary or secondary endpoint for assessing for improvements in NAFD/NASH.13,14
Given the increasing evidence supporting MRI-PDFF as a reliable biomarker of liver fat, we sought to better understand the relationship between MRI-PDFF and additional liver histopathological parameters. The purpose of our study was to assess the relationship between MRI-PDFF and liver histology findings, including steatosis grade, NAFLD activity score (NAS), and fibrosis stage.
Methods
Design
This study was a Health Insurance Portability and Accountability Act–compliant, retrospective analysis of baseline (pretreatment) data acquired prospectively during the course of 3 independent NAFLD/NASH clinical trials and was approved by our institutional review board. The clinical trials contributing data to this study (termed the “parent trials”) were approved by the local institutional review boards at each participating institution. Written informed consent was obtained from all subjects before enrollment and data collection for the parent trials. The sponsors of the parent trials gave permission to the authors to analyze the baseline data and prepare the manuscript. The authors who are not employees of the company sponsors had full control over the data analysis and manuscript preparation.
Study Population
The current study population was composed of subjects with clinical suspicion for NAFLD or NASH who were screened for inclusion in 1 of 3 different phase IIa clinical trials of NAFLD or NASH pharmacotherapies, designated trials 1, 2, and 3. All 3 parent trials were registered with ClinicalTrials.gov. The 3 parent trials shared some similarities in inclusion and exclusion criteria. For inclusion criteria, subjects were required to be at least 18 years old, able to provide written informed consent for the study, and not be pregnant. Patients with cirrhosis were excluded. All 3 parent trials also required pretreatment MRI for PDFF estimation, with the minimum PDFF threshold for trial inclusion/enrollment ranging from 6% to 15% depending on the trial.
Exclusion criteria included a history of any chronic liver disease other than NAFLD/NASH and uncontrolled diabetes with hemoglobin A1c >8.5% to 9.0% (depending on the parent trial). One important difference between parent trials was that trial 1 allowed for the diagnosis of NAFLD using computed tomography, ultrasound, MRI, or biopsy in combination with elevated liver aminotransferases, but did not require histopathological confirmation of NASH for study participants. Trial 2 required histopathological confirmation of NASH. Using the NASH Clinical Research Network’s NAS, a minimum NAS of 4 with at least 1 point in hepatocyte ballooning, as well as fibrosis stage 1 to 3 was needed for enrollment. Trial 3 required histopathological confirmation of NASH, a minimum NAS of 4 with at least 1 point in each category of hepatocyte ballooning, inflammation, and steatosis, as well as fibrosis stage 1 to 3. For the 2 parent trials that required liver biopsy, central pathologists reviewed and assessed biopsy slides, and those central review results were used in the current study. Key inclusion and exclusion criteria for the 3 parent trials are summarized in Supplementary Table 1.
For this retrospective analysis, we included all participants who were screened for enrollment in one of the parent trials and completed the pretreatment MRI for PDFF estimation, even if they were ultimately excluded from the trial. Because our retrospective analysis included all screened subjects from the 3 parent trials who had undergone an MRI, including screening failures, our study included subjects with PDFF values <6% as well as subjects with NAS <4.
Baseline Clinical, Laboratory, and Histopathological Data
Only baseline data from the parent trials were included in the current analysis. Baseline clinical, laboratory, and histopathological data were provided by the parent trial sponsors for all subjects who had successfully undergone an MRI for one of the parent trials, regardless of ultimate enrollment. Data included age, sex, race, ethnicity, body mass index, diagnosis and duration of diagnosis of type 2 diabetes mellitus, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, glycosylated hemoglobin (HbA1c), homeostatic model assessment of insulin resistance value, aspartate aminotransferase to platelet ratio index score, and, for trials 2 and 3, centrally scored histopathological features according to the Brunt classification.15 Because some subjects did not ultimately enroll in one of the parent trials, not all assessments were available for all subjects. The choice of laboratory parameters included in the analysis was supported by a recent consensus statement on essential parameters for NASH clinical trials.16
Baseline MRI Data
Each liver MRI examination was performed at 1 of 44 clinical trial sites located in the United States or Australia. MRI examinations were performed on 1.5T or 3T imaging systems from various manufacturers, including Siemens Healthineers (Erlangen, Germany), GE Healthcare (Waukesha, WI), Philips Healthcare (Best, Netherlands), and Hitachi Medical Corporation (Tokyo, Japan). MRI manuals and imaging protocols were developed by 1 of 2 academic MRI core labs located at Duke University and the University of California-San Diego, respectively. The MRI manuals were distributed to each site, and each site was required to submit a qualification scan that passed a quality assurance process before being certified to perform study MRI examinations for the respective parent trial.
A 6-echo chemical-shift-encoded gradient-echo sequence was used at most sites, when this was available. For sites that were not able to obtain a 6-echo sequence, a dual-dual echo technique was used, which has been shown to provide nearly identical results to the 6-echo acquisition.17 Image acquisitions used low flip angles and relatively long relaxation times to avoid T1 bias as well as corrections for T2*-related signal decay and the spectral complexity of fat.18,19
PDFF calculations were performed centrally at 1 of the 2 MRI core laboratories, depending on the parent trial. Deidentified magnitude images in DICOM format were transmitted to the core laboratories. The core laboratory at Duke University used a PDFF reconstruction algorithm implemented within an image viewer (OsiriX version 7.5.1; Pixmeo Sarl, Geneva, Switzerland), whereas the core laboratory at the University of California-San Diego used a calculation algorithm implemented in MATLAB (The Mathworks, Natick, MA). Readers at each core laboratory placed at least 3 regions of interest within the liver with a total cross-sectional area of at least 6 cm2 and reported the average PDFF values from these regions of interest. PDFF estimations were performed using 1 of 2 techniques that have been previously described and validated against MR spectroscopy, and have been found to be similarly accurate and reproducible.10,20–22
Statistical Analysis
Patient demographics, laboratory values, and PDFF values were summarized using median and interquartile range for continuous variables and counts and proportions for categorical variables. We compared values among the 3 parent trials using the Kruskal-Wallis test for continuous and ordinal variables and the χ2 test for proportions. Next, we assessed for correlations between PDFF values and all other variables using Spearman’s correlation or, in the case of rate of diabetes diagnosis, logistic regression. We performed a receiver operating characteristic analysis to evaluate the diagnostic performance of PDFF for detecting liver injury via the NAS and fibrosis. Specifically, we assessed the performance of PDFF for predicting NAS ≥4 and fibrosis stage ≥3, as these criteria may be used to define patients with more clinically severe disease. Optimal thresholds for predicting NAS ≥4 and fibrosis grade ≥3 were determined based on the Youden index (the single point on the receiver operating characteristic curve with highest overall performance), and performance statistics were subsequently calculated.
Finally, because these data originated from NAFLD/NASH treatment trials incorporating variable PDFF thresholds for inclusion, we investigated the effects of PDFF thresholds on trial populations. First, we calculated potential trial exclusion rates based on a variety of potential PDFF thresholds. Then, we assessed for changes in population characteristics induced by applying several commonly used PDFF thresholds, using the Mann-Whitney U or χ2 test.
In all analyses, a 2-tailed P ≤ .05 was considered statistically significant. Statistical analyses were conducted using R (r-project.org).
Results
Baseline Characteristics
A total of 370 subjects were included in our retrospective analyses. Baseline characteristics of the overall study population and differences among the 3 parent trials are summarized in Table 1. Sex, ethnicity, and baseline PDFF values were similar across the 3 trials. Small but statistically significant differences were observed among the 3 trials for age, body mass index, race, and a variety of laboratory and histological parameters (Table 1).
Table 1.
Baseline Features of the Study Population and Comparison Among the 3 Parent Trials
| Total (n = 370) | Trial 1 (n = 165) | Trial 2 (n = 74) | Trial 3 (n = 131) | P | |
|---|---|---|---|---|---|
| Liver PDFF, % | 16.0 (10.3–23.48) | 15.9 (10.9–24.4) | 16.1 (9.7–22.6) | 15.9 (10.4–23.8) | .80 |
| Demographic data (n = 370) | |||||
| Age,* y | 51 (21–74) | 47 (39–56) | 51 (42–59) | 55 (48–62) | <.001 |
| Sex, % female | 60.1 | 56.4 | 66.2 | 61.8 | .32 |
| BMI, kg/m2 | 33.3 (23.4–67.9) | 32.3 (24.1–44.4) | 34.6 (23.5–52.7) | 34.3 (23.4–67.9) | <.005 |
| Race, % white | 88.4 | 90.9 | 85.1 | 87.8 | <.05 |
| Ethnicity, % Hispanic | 29.4 | 30.3 | 31.8 | 34.4 | .18 |
| Liver function tests (n = 370) | |||||
| Total bilirubin, mg/dL | 0.50 (0.37–0.67) | 0.55 (0.44–0.69) | 0.53 (0.42–0.74) | 0.40 (0.25–0.54) | <.001 |
| ALT, IU/dL | 52.15 (35.2–79.9) | 42.0 (30.0–70.0) | 77.0 (57.0–102.5) | 51.2 (38.3–82.4) | <.001 |
| AST, IU/dL | 36.0 (24.05–53.85) | 28.0 (21.0–41.0) | 50.0 (42.5–71.0) | 39.9 (27.9–60.7) | <.001 |
| Alkaline phosphatase, IU/L | 77.0 (62.25–97.0) | 73.0 (58.0–90.0) | 72.0 (60.5–91.5) | 89.2 (73.0–107.5) | <.001 |
| Histologic features (n = 170) | |||||
| Steatosis grade, points | 2 (2–3) | n/a | 2 (1–3) | 2 (2–3) | .66 |
| Lobular inflammation, points | 2 (1–2) | n/a | 2 (1–2) | 2 (1–2) | <.01 |
| Fibrosis stage, points | 2 (1–3) | n/a | 2 (1–3) | 2 (1–2) | .17 |
| Hepatocyte ballooning, points | 1 (1–2) | n/a | 2 (1–2) | 1 (1–2) | <.001 |
| NAS, points | 5.0 (4–6) | n/a | 5 (5–6) | 5 (4–6) | <.001 |
| Metabolic features | |||||
| Diabetes diagnosis, % | 27.8 | 7.3 | 44.6 | 44.3 | <.001 |
| Duration of diabetes diagnosis, y | 4 (2–8) | 2 (1–3.5) | 6 (3–7) | 4 (2–7.5) | <.05 |
| Glycosylated hemoglobin (HbA1c), % | 5.8 (5.5–6.6) | 5.6 (5.3–5.9) | 6.3 (5.7–7) | 6.1 (5.7–6.9) | <.001 |
| Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) | 6.0 (3.4–9.8) | n/a | 8.1 (4.8–13.7) | 5.0 (3–8.2) | <.001 |
| AST to Platelet Ratio Index (APRI) Score, U/L | 0.36 (0.24–0.54) | 0.30 (0.2–0.47) |
NOTE. Continuous variables are summarized as median (interquartile range). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index.
Relationship Between PDFF and Select Histologic Features
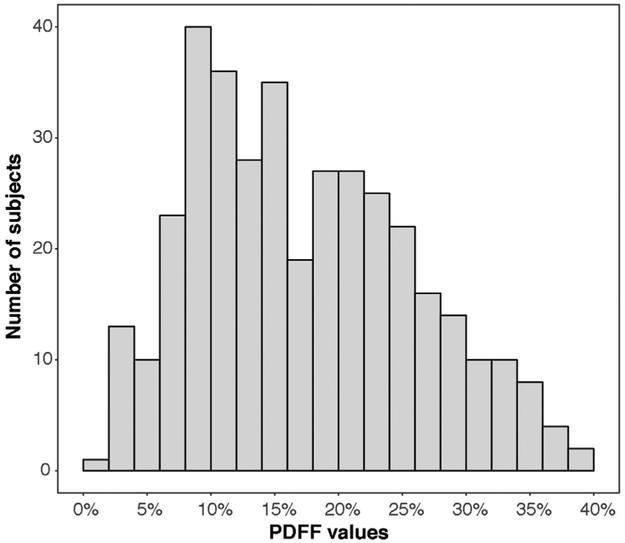
For the combined study population, median baseline PDFF value was 16.0% with an interquartile range of 10.3% to 23.48%. Figure 1 shows the distribution of baseline PDFF values for all subjects. Histological data were available for 45.9% of subjects (170/370). Baseline PDFF values were significantly correlated with several histological values, including positive correlations with steatosis grade (r = 78, P < .001) and NAS (r = 0.54, P < .001) and a negative correlation with fibrosis stage (r = −0.59, P < .001). Baseline PDFF was not significantly correlated with ballooning or lobular inflammation. Additional correlations are summarized in Table 2.
Figure 1.
Histogram of baseline PDFF values for all included subjects.
Table 2.
Correlations Between Baseline PDFF and Other Variables
| Correlation coefficient (r) |
P | |
|---|---|---|
| Liver function tests | ||
| Total bilirubin | 0.32 | <.05 |
| ALT | 0.40 | <.002 |
| AST | 0.22 | .30 |
| Alkaline phosphatase | 0.17 | .60 |
| Histopathologic features | ||
| Steatosis grade | 0.78 | <.001 |
| Lobular inflammation | 0.22 | .49 |
| Fibrosis stage | −0.58 | <.001 |
| Hepatocyte ballooning | 0.37 | .08 |
| NAS | 0.54 | <.001 |
| Metabolic features | ||
| Diabetes diagnosis | n/a | .87 |
| Duration of diabetes diagnosis | 0.39 | .15 |
| Glycosylated hemoglobin (HbA1c) | 0.24 | .26 |
| Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) | 0.32 | .17 |
| AST to Platelet Ratio Index (APRI) Score | 0.14 | .72 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
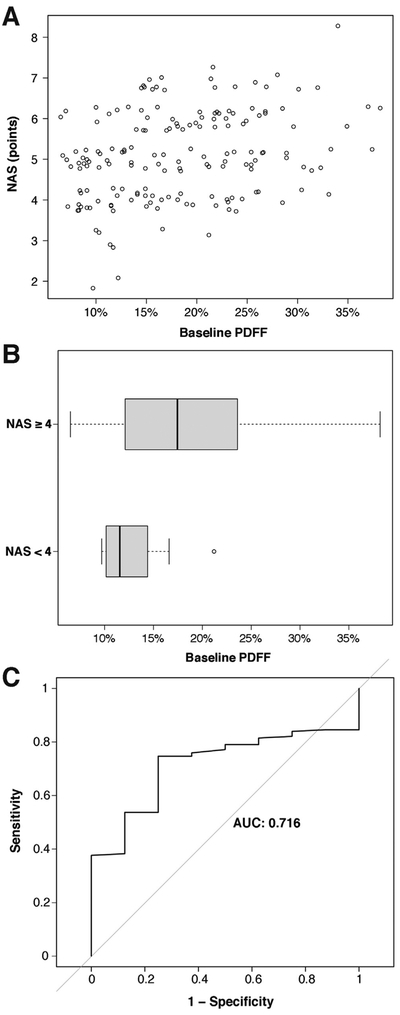
Although significantly correlated, there was substantial overlap between PDFF values for low and high NAS (Figure 2A and B). The AUROC for predicting an NAS ≥4 using PDFF was 0.72 (95% confidence interval [CI] 0.59–0.84) (Figure 2C). The optimal PDFF threshold (Youden index) for predicting an NAS ≥4 was 12.4%, which resulted in a sensitivity of 0.75 (95% CI 0.35–0.97), specificity of 0.75 (95% CI 0.67–0.81), positive predictive value of 0.13 (95% CI 0.05–0.26), and negative predictive value of 0.98 (95% CI 0.94–1).
Figure 2.
(A) Scatterplot of NAS vs baseline liver PDFF value for subjects with histopathological data. (B) Distribution of PDFF values for subjects with baseline NAS ≥4 vs NAS <4. (C) Receiver operating characteristic curve for the prediction of NAS ≥4 using PDFF value as the predictor.
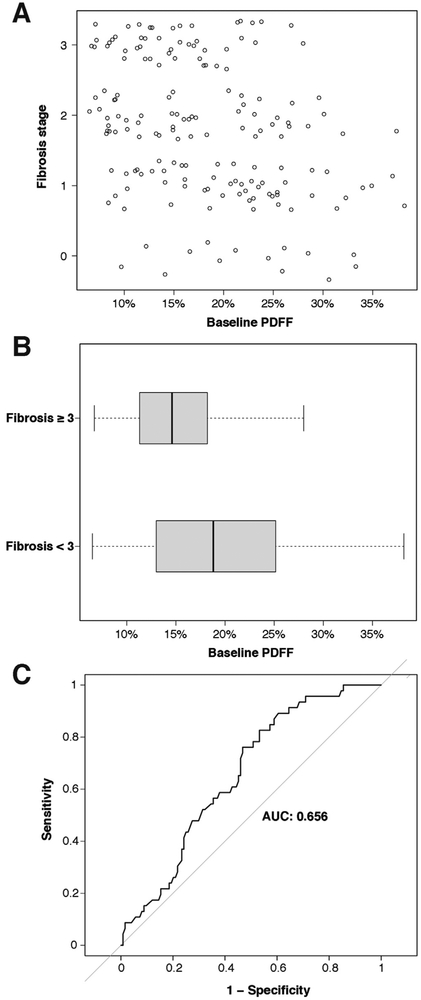
As with NAS, although PDFF and fibrosis stage were significantly (negatively) correlated, there was substantial overlap between PDFF values for early and advanced fibrosis stages (Figure 3A and B). The AUROC for predicting fibrosis stage ≥3 using PDFF was 0.66 (95% CI 0.57–0.74) (Figure 3C). The optimal PDFF threshold for predicting fibrosis stage ≥3 was 20.4%, which resulted in a sensitivity of 0.53 (95% CI 0.44–0.62), specificity of 0.17 (95% CI 0.08–0.31), positive predictive value of 0.63 (95% CI 0.53–0.73), and negative predictive value of 0.14 (95% CI 0.06–0.22).
Figure 3.
(A) Scatterplot of fibrosis stage vs baseline liver PDFF value for subjects with histopathological data. (B) Distribution of PDFF values for subjects with baseline fibrosis stage ≥3 vs fibrosis stage <3. (C) Receiver operating characteristic curve for the prediction of fibrosis stage ≥3 using PDFF value as the predictor.
PDFF Thresholds and Study Population Characteristics
When different PDFF value thresholds were applied to the study population, progressively larger numbers of subjects were excluded as threshold values increased. Supplementary Figure 1 illustrates the exclusion rates associated with various potential PDFF thresholds.
When comparing groups of subjects who would have been included vs excluded from a trial based on a given PDFF threshold, none of the demographic variables were significantly affected by the tested PDFF thresholds. There were some statistically significant differences in various laboratory and histopathologic variables, although the differences were small. These results are summarized in Supplementary Table 2.
Discussion
In this retrospective analysis of data obtained from 3 Phase IIa NAFLD/NASH pharmacotherapy trials, we assessed the relationship between MRI-PDFF and a variety of clinical, laboratory, and histopathological features. We found that PDFF is significantly correlated with steatosis grade and NAS and inversely correlated with fibrosis stage. There was substantial overlap in PDFF values between patients with NAFLD vs NASH as well as between NAFLD patients with vs without fibrosis. As a result, PDFF does not appear to be a strong predictor of advanced liver fibrosis or NASH for an individual patient, but may be more useful for risk stratification.
In clinical practice, MRI-based measures of hepatic steatosis may be useful for the noninvasive detection of NAFLD due to the strong association between PDFF and histopathological steatosis.9,13,14,23–25 But beyond detection and quantification of fat, PDFF may not be a reliable assessment of a given patient’s liver histology. For example, our results showed a statistically significant and moderately strong negative correlation between PDFF and fibrosis stage that contrasts with some prior reports, but not others.25,26 Moreover, our results showed substantial overlap in PDFF values for patients with vs without fibrosis and for patients with or without NAS ≥4.
Clinically, such overlap can create a conundrum in both diagnosis and management of these patients. Our data suggest that at the time of diagnosis, a patient with high liver fat content may or may not have NASH. These results suggest that for optimal patient management, techniques other than (or combined with) MRI-PDFF, such as ultrasound- or MRI-based elastography or liver biopsy, remain necessary for assessing hepatic parenchymal changes other than steatosis. Consequently, an MRI performed to assess for hepatic steatosis may be helpful if negative, but an examination demonstrating abnormally high fat content does not imply NASH or the presence of liver fibrosis, and further assessment using noninvasive tools or biopsy may be needed.
In the research arena, many early-stage clinical trials accept steatosis improvement (as measured noninvasively by MRI-PDFF) as a surrogate endpoint for NASH improvement. However, our data suggest it remains to be seen whether histological response to drug therapy will be well-represented by PDFF changes. Although some studies have shown correlation between improvements in steatosis and improvements in other histological features of NASH, other results remain mixed.11,13,14
One important drawback of pooling data from multiple clinical trials stems from differences between inclusion and exclusion criteria for these trials, as well as differences in the populations screened for those trials. On detailed assessment of the individual parent trials, we found that the most important potential difference was that liver biopsy was required for only 2 of the trials. However, when we directly compared patient characteristics among the 3 parent trials, there were only minor differences, suggesting that these data can be reasonably pooled and the results generalized. Additionally, patient demographics in our study were similar to those reported in other trials of NAFLD pharmacotherapies.12,27–30 This mechanism of pooling data from different early-stage clinical trials provided a relatively large patient pool of data for analysis. Moreover, pooling data from 3 separate studies enhances the geographic diversity of the combined population, which contributes to generalizability. It also allows for an improved understanding of the characteristics of clinical trial populations and whether they are representative of patients undergoing clinical assessments for NAFLD/NASH.
However, there remain a few potential limitations to this work. First, neither biopsy results nor all laboratory data were available for all subjects. This is due to the stepwise nature of the screening process for clinical trials, wherein subjects who screen fail at any point will not undergo the assessments planned at later stages of the screening process. Patients with cirrhosis were excluded from the parent trials, thus our study findings may not be generalizable to patient populations with cirrhosis. Additionally, although the presence and severity of liver iron would have been of interest in the context of MRI-based PDFF estimation, histopathological assessment of liver iron was available in a very small number of patients, as this did not contribute to their clinical trial eligibility and was not a required histological feature. As a result, we did not systematically evaluate the interaction between liver iron and other factors. Similarly, MR elastography values were available for only a minority of subjects, and these data were insufficient to analyze associations between MR elastography and histopathology. In general, this study carries the limitations of a retrospective analysis in which data collection could not be prospectively designed to address the study questions. However, our study population does appear to be representative of an adult clinical population at risk for NAFLD/NASH (without uncontrolled diabetes).
In conclusion, although MRI-PDFF is a useful tool for the noninvasive detection of NAFLD and quantification of steatosis, we found that PDFF was not accurate enough to distinguish among NAFLD, NASH with early fibrosis, and NASH with advanced fibrosis in individual patients.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Patients with NAFLD/NASH are often evaluated using liver biopsy for histologic assessment. MRI-proton density fat fraction (PDFF) has recently emerged as a reliable, noninvasive measure of liver fat, though the relationship between PDFF and other histologic features is less well established.
NEW FINDINGS
MRI-PDFF correlates with several histopathology features but is nonetheless a suboptimal predictor of high fibrosis stage and elevated NASH activity score (NAS).
LIMITATIONS
These data were acquired retrospectively by pooling data from three clinical trials.
IMPACT
These data add to the growing understanding of how MRI-PDFF values relate to liver histology features but suggest that liver biopsy should remain the gold standard.
Acknowledgments
This study was supported in part by NGM Biopharmaceuticals Inc., TaiwanJ Pharma, and NuSirt Biopharma through the conduct of phase 2 studies in NAFLD/NASH from which baseline data were used for the purpose of this analysis. We also thank the site investigators and study participants for their contributions to these clinical trials; without their support, such studies would not have been possible.
Funding
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper:
- AUROC
area under the receiver operating characteristic
- CI
confidence interval
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- PDFF
proton density fat fraction
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at http://www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2018.07.018.
Conflicts of interest
These authors disclose the following: Michael M. Middleton: Funding was provided by NuSirt Biopharma to Michael Middleton’s institution as a lab services agreement to support core lab activities related to one of the parent clinical trials. Cynthia A. Moylan: Funding was provided by NGM Biopharmaceuticals and TaiwanJ Pharma to Cynthia Moylan’s institution as research grants to support effort related to 2 of the parent clinical trials. Cynthia Moylan also received funding for consulting work from NuSirt Biopharma. Stephen Rossi: Stephen Rossi is an employee of NGM Biopharmaceuticals. Omar Flores: Omar Flores is an employee of NuSirt Biopharma. Zac Anchi Chang: Zac Chang was previously an employee of TaiwanJ Pharmaceuticals. Manal F. Abdelmalek: Funding was provided by NGM Biopharmaceuticals and TaiwanJ Pharma to Manal Abdelmalek’s institution as research grants to support effort related to 2 of the parent clinical trials. Claude B. Sirlin: Funding was provided by NuSirt Biopharma to Claude Sirlin’s institution as a lab services agreement to support core lab activities related to one of the parent clinical trials. Mustafa R. Bashir: Funding was provided by NGM Biopharmaceuticals and TaiwanJ Pharma to Mustafa Bashir’s institution as research grants to support core lab activities related to 2 of the parent clinical trials. Benjamin Wildman-Tobriner discloses no conflicts.
References
- 1.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9:524–530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682–689. [DOI] [PubMed] [Google Scholar]
- 6.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500. [DOI] [PubMed] [Google Scholar]
- 7.Hernando D, Sharma SD, Aliyari Ghasabeh M, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med 2017;77:1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 2010;18:337–357, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoo T, Serai SD, Pirasteh A, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology 2018;286:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong X, Nickel MD, Kannengiesser SA, et al. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 2014;72:1353–1365. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton MS, Heba ER, Hooker CA, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017;153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel YA, Imperial JC, Muir AJ, et al. Baseline parameters in clinical trials for nonalcoholic steatohepatitis: recommendations from the Liver Forum. Gastroenterology 2017;153:621–625.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton MS KM, Flores O, Zemel M, Sirlin CB. Agreement of six- and composite three-echo magnitude PDFF-estimation MRI sequences in a multi-center clinical trial. Paper presented at: Radiological Society of North America (RSNA); December 2, 2016; Chicago, IL. [Google Scholar]
- 18.Bashir MR, Zhong X, Nickel MD, et al. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol 2015;204:297–306. [DOI] [PubMed] [Google Scholar]
- 19.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 2011;258:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011;258:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofue K, Mileto A, Dale BM, et al. Interexamination repeatability and spatial heterogeneity of liver iron and fat quantification using MRI-based multistep adaptive fitting algorithm. J Magn Reson Imaging 2015;42:1281–1290. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi A, Yeganeh O, Levin Y, et al. Intra- and inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging 2015;40:3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton MS, Heba ER, Hooker CA, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017;153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwimmer JB, Lavine JE, Wilson LA, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology 2016;151:1141–1154.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147–1159.e5. [DOI] [PubMed] [Google Scholar]
- 29.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial [published online ahead of print September 11, 2017]. Hepatology 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.